Abstract
AIM: To evaluate the efficacy, safety and feasibility of endoscopic full-thickness resection (EFR) for the treatment of gastric submucosal tumors (SMTs) arising from the muscularis propria.
METHODS: A total of 35 gastric SMTs arising from the muscularis propria layer were resected by EFR between January 2010 and September 2013. EFR consists of five major steps: injecting normal saline into the submucosa; pre-cutting the mucosal and submucosal layers around the lesion; making a circumferential incision as deep as the muscularis propria around the lesion using endoscopic submucosal dissection and an incision into the serosal layer around the lesion with a Hook knife; a full-thickness resection of the tumor, including the serosal layer with a Hook or IT knife; and closing the gastric wall with metallic clips.
RESULTS: Of the 35 gastric SMTs, 14 were located at the fundus, and 21 at the corpus. EFR removed all of the SMTs successfully, and the complete resection rate was 100%. The mean operation time was 90 min (60-155 min), the mean hospitalization time was 6.0 d (4-10 d), and the mean tumor size was 2.8 cm (2.0-4.5 cm). Pathological examination confirmed the presence of gastric stromal tumors in 25 patients, leiomyomas in 7 and gastric autonomous nerve tumors in 2. No gastric bleeding, peritonitis or abdominal abscess occurred after EFR. Postoperative contrast roentgenography on the third day detected no contrast extravasation into the abdominal cavity. The mean follow-up period was 6 mo, with no lesion residue or recurrence noted.
CONCLUSION: EFR is efficacious, safe and minimally invasive for patients with gastric SMTs arising from the muscularis propria layer. This technique is able to resect deep gastric lesions while providing precise pathological information about the lesion. With the development of EFR, the indications of endoscopic resection might be extended.
Keywords: Submucosal tumor, Stomach, Endoscopic full-thickness resection, Muscularis propria layer, Excision
Core tip: We used endoscopic full-thickness resection (EFR) to remove gastric submucosal tumors (SMTs) arising from muscularis propria layer. EFR removed all the 35 SMTs successfully. The mean operation time was 90 min (60-155 min). The mean hospitalization time was 6.0 d (4-10 d). The mean tumor size was 2.8 cm (2.0-4.5 cm). Pathological examination confirmed gastric stromal tumors in 25 cases, leiomyoma in 7 and Schwannomas in 2. No gastric bleeding, peritonitis or abdominal abscess occurred. EFR is efficacious, safe and minimally invasive for patients with gastric SMTs arising from the muscularis propria layer, is able to resect deep gastric lesion and provide precise pathological information about the lesion.
INTRODUCTION
Perforation is the main complication of endoscopic resections, and concerns about perforation have largely limited endoscopic resection treatments to the mucosal and submucosal layers. In an attempt to extend the applicability of endoscopic resections, the technique of endoscopic submucosal dissection (ESD) was recently developed for use on large, flat lesions at the mucosal layer[1-3]. With the development of and accumulated experience with ESD, gastric submucosal tumors (SMTs) can be excavated by a method called endoscopic submucosal excavation (ESE)[4-6]. In recent years, based on the practices of ESD and ESE, we have achieved good efficiency using endoscopic full-thickness resection (EFR) to treat gastric SMTs arising from the muscularis propria layer.
MATERIALS AND METHODS
Clinical data
We retrospectively analyzed 35 patients with gastric SMTs arising from the muscularis propria layer. All of the patients were diagnosed by endoscopic ultrasound and contrast-enhanced CT at the Yantai Yu Huang Ding Hospital Affiliated to the Medical College of Qingdao University, China, between January 2010 and September 2013. The 35 patients consisted of 24 males and 11 females, ranged in age between 24 and 67 years, and had a mean age of 41.3 years. Twenty-one tumors were present in the corpus, and 14 in the fundus. All patients had a single tumor, and none had any metastasis on computed tomography (CT) examination. Before EFR, all of the patients underwent routine blood tests for coagulation enzymes and liver and kidney function, as well as electrocardiography, abdominal CT scan, and other tests. All patients and their families were informed of the benefits and risks of EFR and provided written informed consent.
Instruments
The instruments used included an Olympus GIF-Q260J gastroscope (Olympus, Japan), a D-201-11304 transparent cap (Olympus, Japan), a KD-1L-1 needle knife (Olympus, Japan), a KD-611L IT knife (Olympus, Japan), a KD-620LR hook knife (Olympus, Japan), an NM-200L-0525 injection needle (Olympus, Japan), AS-1-S and ASJ-1-S snares (Cook Company, United States), an FD-410LR hot biopsy forceps (Olympus), an HX-610-90 (Olympus, Japan), an HX-600-135 (Olympus, Japan), a Boston Resolution hemostat (Boston Company, United States), an ERBE VIO 200S high-frequency electrosurgical unit and ERBE APC2 argon plasma coagulator (Erbe Company, Germany), an Olympus OEV191H laparoscope (Olympus, Japan), and an SBQ 80 HY linear stapler (Johnson and Johnson, United States).
EFR method
Before EFR, a transparent cap was mounted on the end of the gastroscope. Following intravenous anesthesia with propofol, argon plasma coagulation was used to mark the edge of the stromal tumor. The marked submucosal positions were each injected with 2-3 mL of a solution containing 2-3 mL indigo carmine, 1 mL epinephrine, and 100 mL saline. A hook knife was used to precut the surrounding mucosa and submucosa along the marked points and thus expose the stromal tumor. A hook knife or IT knife was used to isolate the tumor body along the capsule from the muscularis propria down to the serosal layer. The serosa was cut along the edge of the tumor; in most cases, the serosa was tightly adherent to the tumor body, making it impossible to remove the tumor directly using an IT knife. Therefore, a needle knife or hook knife was used to penetrate the serosa, resulting in an “artificial perforation”. The liquid in the gastric cavity was fully absorbed, and an IT or hook knife was used to cut the serosa along the edge of the tumor, removing the tumor completely. When a perforation was encountered, the operator had to use an IT knife carefully to avoid injuries to the surrounding organs, such as the transverse colon and pancreas. Under endoscopic guidance, the incisions on the gastric body from the two ends to the middle were fully closed with titanium clips, and the gastric wound was sealed. For wounds that were too large to seal directly, negative pressure was applied to suck the omentum into the gastric cavity, and titanium clips were used to seal the wound by clipping the omentum to the gastric mucosa (Figure 1).
Figure 1.
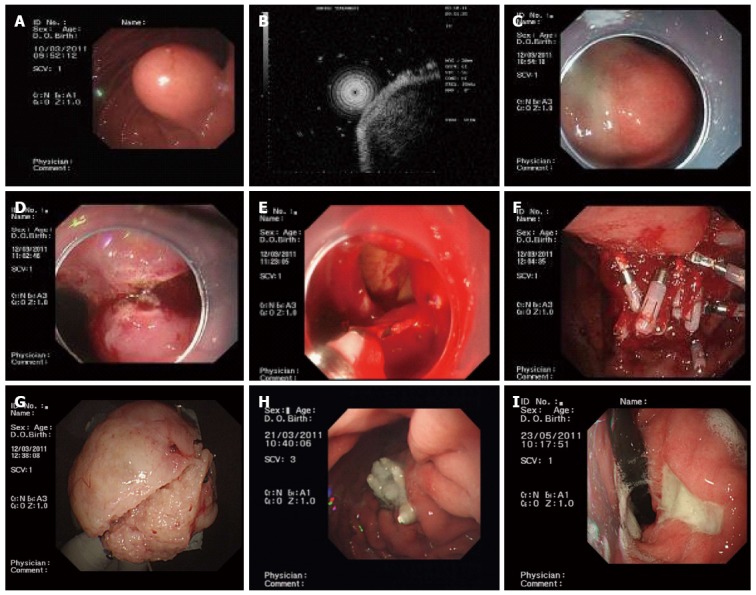
Endoscopic full-thickness resection of gastric stromal tumors arising from the muscularis propria. A: A protruding submucosal lesion in the gastric corpus; B: Endoscopic ultrasound showing that the lesion arose from the muscularis propria; C: Submucosal injection of saline containing adrenaline and indigo carmine; D: Application of the IT knife to isolate the stromal tumor along its periphery; E: An “artificial perforation” was observed after stromal tumor resection and was sealed using titanium clips; F: Sealing of a perforation with multiple titanium clips; G: Resected tumor after the mucosa was removed (4.5 cm in diameter); H: View 9 d after operation, showing that the perforation healed well; I: View 72 d after the operation, showing that the perforation healed well, with only ulcer residues remaining.
Sample processing
Resected samples were fixed with neutral formalin and sent for pathological examination; the samples were immunohistochemically stained for CD34, CD117, Dog-1, S-100, and SMA[7-10].
Postoperative treatment
Patients with an artificial perforation and significant pneumoperitoneum were submitted to a puncture of the right upper quadrant of the abdomen with an abdominal puncture needle to reduce bloating during or after surgery. After surgery, these patients were placed in a semi-supine position. Drinking and eating were forbidden, and gastrointestinal decompression was applied. The patients were closely monitored to determine whether any abdominal pain, bloating, or peritoneal irritation was present. Proton pump inhibitors and antibiotics were administered postoperatively to prevent abdominal infection. Three days after the surgery, oral diatrizoate was administered to determine whether any contrast agent extravasation or gastric motility was present. In addition, an ultrasound examination was performed to determine whether effusions could be found in the abdominal or pelvic cavity. One month after the surgery, an endoscopic examination was performed to observe wound healing and to determine whether any residual or recurrent tumors were present. All of the patients were followed for 6 mo after EFR.
Statistical analysis
Data are expressed as mean ± SD. The SPSS for Windows Version 17.0 software was used for all statistical analyses.
RESULTS
Complete resection rate and operative time
All 35 gastric SMTs arising from the muscularis propria layer were removed successfully by EFR in a single operation, and “artificial gastric perforation” was performed. The complete resection rate was 100%. All perforation wounds were sealed with titanium clips. The operative time ranged from 60 to 155 min, with a mean of 90 ± 17 min.
Hospital stay
The duration of hospitalization ranged from 4 to 10 d, with a mean of 6.0 ± 1.8 d.
Number of titanium clips used
To completely close the perforation, between 6 and 25 titanium clips, with a mean of 12 clips, were used. In 8 cases, some of the normal mucosa at the periphery was also sutured to reduce the wound size so that the remaining perforation could be closed with clips.
Number of patients requiring puncturing to resolve pneumoperitoneum
In total, 5 patients had serious pneumoperitoneum that required puncturing for its resolution.
Maximum diameter of the resected tumors
The maximum diameter of the resected tumors ranged from 2.0-4.5 cm, with a mean of 2.8 cm. The maximum size of the resected specimens was 4.5 cm. The operation demanded that the specimen be cut into several pieces in the stomach so that it could be pulled out through the esophagus.
Postoperative pathology
The pathological examination confirmed gastric stromal tumors in 25 cases, leiomyomas in 7 and gastric autonomous nerve tumors in 2.
Complications
Upper gastroenterography with meglumine diatrizoate 3 d after the operation showed no leakage of the contrast agent in any patient. Postoperative reexamination showed good wound healing. There were no complications, such as bleeding, signs of peritonitis, and/or abdominal abscesses, in any patient.
Post-procedure morbidity
All patients recovered well, and the morbidity was zero.
Recurrence or residual rate
During the 6-mo follow-up after the EFR, no residual or recurrent tumors were observed.
DISCUSSION
Most gastric SMTs grow expansively in the gastric wall. Because lymph node metastasis occurred rarely, resection with a laparoscope was often used[11-13]. Although resection with a laparoscope is simple, without the assistance of a gastroscope, the resected range cannot easily be determined, resulting in frequent resection of normal gastric tissue. For lesions at the esophagus-stomach conjunction or pyloric ring, operating the laparoscope proved difficult. Large gastric SMTs growing in the gastric cavity could not be observed from the serosa[14,15].
Although endoscopic snares and ligation are widely used in the clinic, the snare can be easily detached, making it difficult to resect the lesion completely. Meanwhile, the rate of complications due to residual tumors, bleeding and perforation was high. The rate of complications due to perforation and bleeding was high using the ligation method. At the same time, certain lesions were difficult to ligate, and the results of the pathologic diagnosis could not be obtained with the ligation method[16,17].
Gastric stromal tumors arising from muscularis propria layer are the most common type of gastric SMTs. Because of their potential malignant tendencies, the resection of gastric stromal tumors is recommended[18]. Because gastric stromal tumors arising from the muscularis mucosa are located superficially, their endoscopic resection or ligation is not difficult; these procedures are often performed in clinical practice. However, stromal tumors arising from the muscularis propria are located within deeper layers, especially those tumors that do not grow within cavities. Endoscopic resection can easily lead to perforation, and the tumor excision is often not complete. Hence, stromal tumors arising from the muscularis propria are often considered a contraindication for endoscopic resection and are therefore removed surgically or laparoscopically, especially when they are larger than 2 cm in diameter[19,20]. In recent years, based on endoscopic submucosal dissections and endoscopic submucosal excavations, and on improvements in the application of titanium clips during endoscopy and nylon purse-string suture techniques, the EFR treatment of gastrointestinal tumors arising from the muscularis propria has become possible.
We found that artificial perforation was necessary in all 35 patients with stromal tumors who were treated by EFR. Titanium clips were used to seal the wound, abdominal puncturing was applied to relieve intra-abdominal pressure, and conservative methods were followed for postoperative care. The key to preventing complications was the proper use of titanium clips to seal the wounds under endoscopy. Based on our experience, wounds from large perforations should be sutured from both ends toward the middle; in some cases, normal mucosa at the periphery may require suturing to reduce the wound size. Successful treatment using EFR required a successful repair of the perforation that could avoid the need for additional surgical repair and postoperative peritonitis[21,22]. The most common method used to repair perforations required titanium clips[23,24]. For small perforations, one or a few titanium clips would be sufficient. For larger perforations, the limited spans of the titanium clips would require the air in the gastric lumen to be sucked out to narrow the perforation as much as possible before the application of multiple titanium clips. If the perforation is too large for the direct application of titanium clips, an omental patch technique is used[25]. In this method, negative pressure is continuously applied to suck the air from the gastric lumen until the adipose tissue outside the gastric wall covers the perforation, followed by the application of the titanium clips. In addition, a nylon string-purse suture technique can be used to suture overly large perforations. During EFR, perforations will cause pneumoperitoneum, which blocks the operative field in the stomach, making endoscopic operations difficult. Thus, during EFR, abdominal palpation should be performed repeatedly. If the abdominal pressure increases, timely exhaustion should be applied. The puncture site should be located in the right lower quadrant of the abdomen, and a 20 mL injection needle may be used as the puncture needle. After puncturing, the abdomen should be manipulated to exhaust the air, and the puncture needle should be positioned at the puncture site until the wounds are completely sealed and the pneumoperitoneum has significantly improved. The puncture needle should be extracted after confirming that no air continues to flow through it. Other key elements for successful EFR treatment include avoiding the introduction of excessive gastric acid into the abdominal cavity, thereby preventing postoperative infection; hemostasis to avoid repeated rinsing during the excision[26]; the complete removal of gas and fluid from the gastric lumen prior to incision of the serosa; continuous effective gastrointestinal decompression postoperatively; and the administration of proton pump inhibitors and antibiotics postoperatively to prevent abdominal infection. None of the patients in our EFR group experienced peritonitis or intra-abdominal abscesses.
We found that the complete resection rate in our EFR group was 100%, with no recurrent or residual tumors. One advantage of EFR is its accurate localization of the tumor. Without the assistance of the gastroscope, it can be difficult during laparoscopic surgery to determine the extent of the excision; thus, excess normal gastric tissue is removed. In patients with giant stromal tumors protruding into the gastric lumen, it is difficult for the laparoscope to distinguish the tumor from the serosal layer or to pull and remove the tumor body. Therefore, gastroscope-assisted laparoscopic EFR may be favorable for treating patients with gastric stromal tumors arising from the muscularis propria.
EFR was performed under general anesthesia. The total cost of this procedure was less than that of LECS (laparoscopy and endoscopy cooperative surgery).
In conclusion, EFR can completely remove gastric SMTs arising from the muscularis propria. This technique can replace certain surgical and laparoscopic procedures, and its application should be recommended.
Because the present follow-up period is relatively short (6 mo), we will follow the patients over the next 5 years to observe any iatrogenic peritoneal seeding after EFR.
COMMENTS
Background
Gastric stromal tumors arising from the muscularis propria layer are the most common type of gastric submucosal tumors (SMTs). The purpose of this study was to evaluate the efficacy, safety and feasibility of endoscopic full-thickness resection (EFR) for the treatment of gastric SMTs arising from the muscularis propria.
Research frontiers
With the development of and accumulated experience with ESD, gastric SMTs can be excavated using the endoscopic submucosal excavation (ESE) technique. In recent years, based on ESD and ESE, EFR has been used to treat gastric SMTs arising from the muscularis propria layer.
Innovations and breakthroughs
EFR can completely remove gastric SMTs arising from the muscularis propria; therefore, this technique should be considered to replace certain surgical and laparoscopic procedures.
Applications
EFR can be used for the treatment of gastric SMTs arising from the muscularis propria.
Terminology
EFR is a method for removing a whole gastric submucosal tumor, which creates an artificial perforation that is later closed with clips.
Peer review
This paper is about the efficacy, safety and feasibility of endoscopic full-thickness resection for the treatment of gastric submucosal tumors arising from the muscularis propria. The authors have a great deal of experience, and the paper is nicely composed.
Footnotes
Supported by Natural Science Foundation of Shandong Province, No. ZR2013HM004
P- Reviewer: Endo I, Ramia JM S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Ma S
References
- 1.Gotoda T, Ho KY, Soetikno R, Kaltenbach T, Draganov P. Gastric ESD: current status and future directions of devices and training. Gastrointest Endosc Clin N Am. 2014;24:213–233. doi: 10.1016/j.giec.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Boda T, Ito M, Oka S, Kitamura Y, Numata N, Sanomura Y, Matsuo T, Tanaka S, Yoshihara M, Arihiro K, et al. Characteristics of metachronous gastric tumors after endoscopic submucosal dissection for gastric intraepithelial neoplasms. Gastroenterol Res Pract. 2014;2014:863595. doi: 10.1155/2014/863595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimamura Y, Ishii N, Nakano K, Ikeya T, Nakamura K, Takagi K, Fukuda K, Suzuki K, Fujita Y. Repeat endoscopic submucosal dissection for recurrent gastric cancers after endoscopic submucosal dissection. World J Gastrointest Endosc. 2013;5:600–604. doi: 10.4253/wjge.v5.i12.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi WB, Wang ZH, Qu CY, Zhang Y, Jiang H, Zhou M, Chen Y, Xu LM. Comparison between air and carbon dioxide insufflation in the endoscopic submucosal excavation of gastrointestinal stromal tumors. World J Gastroenterol. 2012;18:7296–7301. doi: 10.3748/wjg.v18.i48.7296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Ye LP, Zhu LH, Zhou XB, Mao XL, Ding JX. Endoscopic muscularis excavation for subepithelial tumors of the esophagogastric junction originating from the muscularis propria layer. Dig Dis Sci. 2013;58:1335–1340. doi: 10.1007/s10620-012-2487-7. [DOI] [PubMed] [Google Scholar]
- 6.Fukami N. ESD around the world: United States. Gastrointest Endosc Clin N Am. 2014;24:313–320. doi: 10.1016/j.giec.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Kang YN, Jung HR, Hwang I. Clinicopathological and immunohistochemical features of gastointestinal stromal tumors. Cancer Res Treat. 2010;42:135–143. doi: 10.4143/crt.2010.42.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kara T, Serinsoz E, Arpaci RB, Gubur O, Orekici G, Ata A, Colak T, Arican A. Contribution of DOG1 expression to the diagnosis of gastrointestinal stromal tumors. Pathol Res Pract. 2013;209:413–417. doi: 10.1016/j.prp.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer. 2011;11:865–878. doi: 10.1038/nrc3143. [DOI] [PubMed] [Google Scholar]
- 10.Pelz AF, Agaimy A, Daniels M, Evert M, Schulz HU, Lüders P, Müller G, Lasota J, Röpke A, Wieacker P, et al. Gastrointestinal stromal tumor presenting as a rectovaginal mass. Clinicopathologic and molecular-genetic characterization of a rare tumor with a literature review. Hum Pathol. 2011;42:586–593. doi: 10.1016/j.humpath.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Kasetsermwiriya W, Nagai E, Nakata K, Nagayoshi Y, Shimizu S, Tanaka M. Laparoscopic surgery for gastric gastrointestinal stromal tumor is feasible irrespective of tumor size. J Laparoendosc Adv Surg Tech A. 2014;24:123–129. doi: 10.1089/lap.2013.0433. [DOI] [PubMed] [Google Scholar]
- 12.Feng Y, Yu L, Yang S, Li X, Ding J, Chen L, Xu Y, Shi R. Endolumenal endoscopic full-thickness resection of muscularis propria-originating gastric submucosal tumors. J Laparoendosc Adv Surg Tech A. 2014;24:171–176. doi: 10.1089/lap.2013.0370. [DOI] [PubMed] [Google Scholar]
- 13.Mori H, Kobara H, Kobayashi M, Muramatsu A, Nomura T, Hagiike M, Izuishi K, Suzuki Y, Masaki T. Establishment of pure NOTES procedure using a conventional flexible endoscope: review of six cases of gastric gastrointestinal stromal tumors. Endoscopy. 2011;43:631–634. doi: 10.1055/s-0030-1256227. [DOI] [PubMed] [Google Scholar]
- 14.Toyota K, Sugawara Y, Hatano Y. Recurrent upside-down stomach after endoscopic repositioning and gastropexy treated by laparoscopic surgery. Case Rep Gastroenterol. 2014;8:32–38. doi: 10.1159/000358553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koh CE, Martin DJ, Cavallucci DJ, Becerril-Martinez G, Taylor CJ. On the road to single-site laparoscopic adjustable gastric banding: lessons learned from 60 cases. Surg Endosc. 2011;25:947–953. doi: 10.1007/s00464-010-1259-8. [DOI] [PubMed] [Google Scholar]
- 16.Schlag C, Wilhelm D, von Delius S, Feussner H, Meining A. EndoResect study: endoscopic full-thickness resection of gastric subepithelial tumors. Endoscopy. 2013;45:4–11. doi: 10.1055/s-0032-1325760. [DOI] [PubMed] [Google Scholar]
- 17.Chugh R. Current directions in systemic therapy for gastrointestinal stromal tumors. Curr Probl Cancer. 2011;35:255–270. doi: 10.1016/j.currproblcancer.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Tabrizian P, Sweeney RE, Uhr JH, Nguyen SQ, Divino CM. Laparoscopic resection of gastric and small bowel gastrointestinal stromal tumors: 10-year experience at a single center. J Am Coll Surg. 2014;218:367–373. doi: 10.1016/j.jamcollsurg.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 19.Sorour MA, Kassem MI, Ghazal Ael-H, El-Riwini MT, Abu Nasr A. Gastrointestinal stromal tumors (GIST) related emergencies. Int J Surg. 2014;12:269–280. doi: 10.1016/j.ijsu.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Talathi NP, Telavane PP, Adhikari DR, Singh R, Joshi RM. Gastrointestinal stromal tumours: a series of 12 cases. Indian J Surg. 2013;75:134–140. doi: 10.1007/s12262-012-0530-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park JY, Eom BW, Yoon H, Ryu KW, Kim YW, Lee JH. Transumbilical single-incision laparoscopic wedge resection for gastric submucosal tumors: technical challenges encountered in initial experience. J Gastric Cancer. 2012;12:173–178. doi: 10.5230/jgc.2012.12.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou PH, Yao LQ, Qin XY, Cai MY, Xu MD, Zhong YS, Chen WF, Zhang YQ, Qin WZ, Hu JW, et al. Endoscopic full-thickness resection without laparoscopic assistance for gastric submucosal tumors originated from the muscularis propria. Surg Endosc. 2011;25:2926–2931. doi: 10.1007/s00464-011-1644-y. [DOI] [PubMed] [Google Scholar]
- 23.Mori H, Rafiq K, Kobara H, Tsushimi T, Fujihara S, Nishiyama N, Matsunaga T, Ayaki M, Yachida T, Tani J, et al. Development of pure endoscopic full-thickness resection with mechanical countertraction and double-armed bar suturing systems. Gastrointest Endosc. 2014;79:24–25. doi: 10.1016/j.gie.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 24.Joensuu H, DeMatteo RP. The management of gastrointestinal stromal tumors: a model for targeted and multidisciplinary therapy of malignancy. Annu Rev Med. 2012;63:247–258. doi: 10.1146/annurev-med-043010-091813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu BR, Song JT, Kong LJ, Pei FH, Wang XH, Du YJ. Tunneling endoscopic muscularis dissection for subepithelial tumors originating from the muscularis propria of the esophagus and gastric cardia. Surg Endosc. 2013;27:4354–4359. doi: 10.1007/s00464-013-3023-3. [DOI] [PubMed] [Google Scholar]
- 26.Fritscher-Ravens A, Cuming T, Jacobsen B, Seehusen F, Ghanbari A, Kahle E, von Herbay A, Koehler P, Milla P. Feasibility and safety of endoscopic full-thickness esophageal wall resection and defect closure: a prospective long-term survival animal study. Gastrointest Endosc. 2009;69:1314–1320. doi: 10.1016/j.gie.2008.09.031. [DOI] [PubMed] [Google Scholar]


