Abstract
AIM: To investigate the feasibility and efficacy of the combination of S-1 with gemcitabine followed by oral S-1 with concurrent radiotherapy (intensity modulated radiotherapy, IMRT) and maintenance therapy with S-1 for locally advanced pancreatic cancer.
METHODS: Subjects selected in the study were patients who had unresectable and locally advanced pancreatic cancer without distant metastases, adequate organ and marrow functions, an Eastern Cooperative Oncology Group performance status of 0-1 and no prior anticancer therapy. Initially the subjects received two cycles of chemotherapy, oral administration of S-1 40 mg/m2 twice daily from day 1 to day 14 of a 21-d cycle, with 30-min intravenous infusions of gemcitabine 1000 mg/m2 on day 1 and day 8. Two weeks after the completion of chemotherapy, S-1 was administered orally with concurrent IMRT. Oral S-1 was administered at a dose of 80 mg/m2 per day twice daily from day 1 to day 14 and from day 22 to day 35. Radiation was concurrently delivered at a dose of 50.4 Gy (1.8 Gy/d, 5 times per week, 28 fractions). One month after the completion of chemotherapy and radiotherapy, S-1 was administered orally at a dose of 80 mg/m2 per day twice daily for 14 d, followed by a 14-d rest period. This cycle was repeated as maintenance therapy, until unacceptable toxicity occurred or the disease worsened. Thirty-two patients were involved in this study. The median follow-up was 15.6 mo (range: 8.6-32.3 mo).
RESULTS: Thirty-two patients completed the scheduled course of chemotherapy, while 30 patients (93.8%) received chemoradiotherapy with two patients ceasing to continue with radiotherapy. The major toxic effects were nausea and leukopenia. There was no grade 4 toxicity or treatment-related death. According to the Response Evaluation Criteria in Solid Tumors criteria, the objective tumor response was partial response in 17 (53.1%) patients, stable disease in 9 (28.1%), and progressive disease in 6 (18.8%). The median overall survival and median progression-free survival were 15.2 mo and 9.3 mo, respectively. The survival rates at 1 year and 2 years were 75% and 34.4%, respectively.
CONCLUSION: The combination of S-1 with gemcitabine followed by oral S-1 with IMRT and maintenance therapy with S-1 alone in patients with locally advanced pancreatic cancer may be considered a well-tolerated, promising treatment regimen.
Keywords: Chemoradiotherapy, Radiosensitizer, S-1, Pancreatic cancer, CA19-9
Core tip: The article describes a study of the combination of S-1 with gemcitabine S-1 followed by oral S-1 with concurrent radiotherapy and maintenance therapy with S-1 for locally advanced pancreatic cancer. It is considered a well-tolerated, promising and effective treatment for unresectable advanced pancreatic cancer.
INTRODUCTION
The prognosis of pancreatic cancer (PC) patients remains very poor, with a 5-year survival rate less than 5% after diagnosis[1-4]. PC is one of the leading causes of death worldwide. Most historical studies show that the median survival ranges from about 8 to 12 mo[5-8] for patients with locally advanced PC.
Importance should be attached to the order in which radiation and chemotherapy are given to patients with locally advanced PC. 20% of patients who received initial chemoradiation experienced immediate metastases after therapy[9,10], or even experienced higher toxicity levels than patients who only received chemotherapy. Because of such levels of toxicity, subsequent chemotherapy that is needed to delay and/or prevent metastatic disease may be involved. Therefore, this research was intended to present an alternative approach to cope with this issue. The approach involves several cycles of induction chemotherapy (2 to 4 cycles) before revaluing the patients. If the patients do not suffer from distant metastases on restaging while maintaining a good performance status, their conditions may be consolidated by chemoradiation. The alternative approach may benefit those patients who will receive the chemoradiation, because it will help to delay or prevent locoregional progression which could lead to duodenal obstruction or result in pain (of the celiac plexus, specifically). Some studies have also shown that when compared with chemotherapy alone, chemoradiation-based control of local progression can improve survival and prevent the development of metastatic disease[5,11].
Some current single phase II studies have shown obvious increases in median survival by using targeted agents, radiosensitizers, and/or combination chemotherapy, in conjunction with radiation therapy in the induction phase of treatment[12-15], which can produce the equivalent effect of S-1, a new oral fluoropyrimidine formulation that combines tegafur, 5-chloro-2,4-dihydroxypyridine (CDHP), and potassium oxonate at a molar ratio of 1:0.4:1.
Sudo et al[9] reported promising outcomes: 70.6% investigated reached a 1-year survival with a median OS of 16.8 mo and acceptable toxicity rates (grade 3-4), after S-1 was given concurrently with radiation (total dose 50.4 Gy) followed by oral S-1 maintenance in patients with locally advanced PC.
Many modern studies have reported the increase in survival rate and the relief of toxicity[16-18] by using aggressive induction therapy and/or dose escalated radiation therapy including stereotactic body radiation therapy (SBRT), 3D conformal radiotherapy and intensity modulated radiotherapy (IMRT).
MATERIALS AND METHODS
Patient eligibility
Entry patients were those who had been confirmed cytologically or histologically as sufferers of locally unresectable advanced pancreatic cancer. The patients whose estimated life expectancy was 12 wk after the study begins were aged between 46 and 69 years. The entry patients also need to meet the following requirements: written informed consent, no evidence of distant metastasis, Karnofsky performance status of 70-100 points, no earlier treatment for pancreatic cancer, adequate oral intake, adequate hepatic and renal function, and adequate haematological function.
Exclusion criteria were active gastroduodenal ulcers, pleural effusion or ascites, watery diarrhea, active infection, complications such as active concomitant malignancy, history of drug hypersensitivity, mental disorders, heart disease or renal disease, females of childbearing age unless using effective contraception, and pregnant and lactating females.
For pretreatment staging, chest and abdomen computed tomography was needed to exclude the presence of distant metastasis and to assess the local extension of the tumor. Tumor unresectability criteria of computed tomography included tumor encasement of the bilateral portal vein, superior mesenteric artery, common hepatic artery or the celiac trunk. Before treatment, all patients with obstructive jaundice underwent an endoscopic retrograde biliary drainage or percutaneous transhepatic.
Characteristics of patients
Thirty-two patients were involved in the study from March 2010 to December 2012 at the Oncology Hospital of Jingzhou, China. The patients’ characteristics are listed in Table 1. Karnofsky performance status was 80 in 3 (9.4%), 90 in 9 (28.1%), and 100 in 20 patients (62.5%). The median age was 55 years (range: 50-69). The median planning target volume was 255 cm3 (range: 149-398) and the median maximum tumor size was 36 mm (range: 24-57). There were 11 patients suffering from the invasion of the superior mesenteric artery, 16 patients suffering from the invasion of the celiac trunk, and 5 patients suffering from invasion of both regions.
Table 1.
Characteristics of the patients n (%)
| Characteristic | Number of patients |
| Age (yr) | |
| Range | 50-69 |
| Median | 55 |
| Gender | |
| Female | 17 (53.12) |
| Male | 15 (46.88) |
| Karnofsky performance status | |
| 100 | 20 (62.5) |
Treatment schedule
Thirty-two patients received two cycles of induction chemotherapy; S-1 was administered at 40 mg/m2 twice daily from day 1 to day 14, and gemcitabine was administered by 30-min intravenous infusions of 1000 mg/m2 on day 1 and day 8 of a 21-d cycle.
Two weeks after the completion of the induction chemotherapy, the patients were treated with S-1 and concurrent radiation. S-1 was given at 80 mg/m2 from day 1 to day 14, from day 22 to day 35 and orally twice daily on the day of irradiation during radiotherapy. One month after chemoradiotherapy was completed, S-1 was administered at 80 mg/m2 per day twice daily for 14 d, followed by a 14-d rest period. The cycle was repeated as maintenance therapy until the toxicity was unacceptable or the disease deteriorated.
The IMRT was administered by three-dimensional treatment planning using 10 or 15 MV photons. The total dose was 50.4 Gy delivered in 28 fractions in about 5.5 wk. The area of solid macroscopic tumors that was contrast enhanced on MR and CT-imaging and/or PET positive was defined as the gross tumor volume (GTV). The GTV plus a margin of at least 5 mm, including any areas of microscopic spread and the regional lymph nodes, was defined as the clinical target volume (CTV). The CTV plus a 10 mm margin in the craniocaudal direction and a 5 mm margin in the lateral direction to account for daily set-up error and respiratory organ motion was defined as the planning target volume (PTV). The dose received by ≥ 50% of the liver was limited to ≤ 30Gy, and that received by ≥ 50% of both kidneys was limited to ≤ 20Gy. The spinal cord dose was maintained below 45Gy.
Evaluation
All the eligible patients must be included in the toxicity and response evaluations. During chemotherapy, biochemistry tests, along with complete blood cell counts and physical examination were assessed on day 1 and day 8 in each cycle. According to the Response Evaluation Criteria in Solid Tumors version 1.0, objective tumor response was evaluated every 4-6 wk by magnetic resonance imaging or computed tomography. CA 19-9 (tumor marker carbohydrate antigen) was measured every 4-6 wk. In this study, the CR (the complete response), PR (partial response) and SD (stable disease) were assessed at an interval of at least 4 wk to confirm the objective response. The interval from the first documentation of response (CR or PR) to the first documentation of tumor progression was defined as the response duration. According to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0, adverse events were evaluated. Objective responses and adverse events were confirmed by an external review committee. The date of treatment initiation to the censored date of follow-up or death was calculated as overall survival (OS). The date of the initiation of treatment until documented disease progression or death due to any cause was calculated as progression-free survival (PFS).
RESULTS
Efficacy
All of the patients were included in the response evaluation. Based on the Response Evaluation Criteria in Solid Tumors version 1.0), 17 (53.1%) patients were categorized as partial response, 9 (28.1%) stable disease and 4 (12.5%) progressive disease; while tumor response in only two (6.3%) patients could not be evaluated due to the fact that they had ceased treatment. After the completion of the treatment, none of the patients’ conditions improved to the extent that they can be operated on or the tumors resected. The serum CEA level was reduced by more than 50%, relative to the pretreatment level in three of four (75%) patients who had a pretreatment level of 10 ng/mL or greater; the serum CA19-9 level was reduced by more than 50%, relative to the pretreatment level in 24 of 28 (85.7%) patients who had shown a pretreatment level of 100 U/mL or greater. At the time of analysis, 29 of the 32 patients had disease progression. The disease progression recurred locoregionally in three patients (9.38%), general condition deteriorated in four (12.5%) and distant metastases occurred in 10 (31.3%). Median overall survival and median progression-free survival were 15.2 mo and 9.3 mo, respectively. The survival rates at 1 year and 2 years were 75% and 34.4%, respectively (Figure 1).
Figure 1.
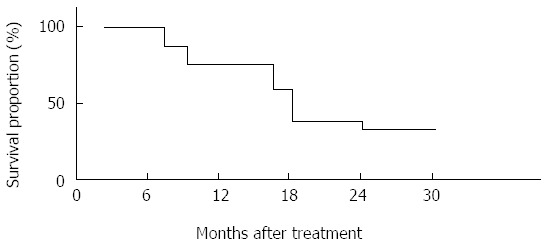
Progression-free survival and overall survival curves of the 32 patients.
Toxicities
The toxicities observed in the 32 enrolled patients are listed in Table 2. Acute non-haematological toxicities including grade 3 AST elevation (two patients), grade 3 vomiting (four patients), grade 3 anorexia and nausea (eight patients) and grade 3 haemorrhagic gastritis (one patient) were observed. With regard to acute haematological toxicities, grades 4 toxicities were not observed and grade 3 neutropenia was observed in only two patients. We observed late toxicity duodenal ulcer 7 mo after treatment in one patient, but no other late toxicities occurred. No treatment-related deaths or other grades 3-4 non-haematological toxicities occurred in the study. Because of grade 3 anorexia, the treatment was stopped in two patients. During the therapy they did not receive S-1 on day 3 and day 12. The compliance rate of the patients taking S-1 reached as high as 93.8%. Among the 32 patients engaged in this study, two had to abandon this treatment due to their progressive diseases.
Table 2.
Toxicities
|
Number of patients |
||||
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Leucocytes | 10 | 6 | 2 | 0 |
| Neutrophils | 5 | 3 | 0 | 0 |
| Haemoglobin | 3 | 2 | 0 | 0 |
| Platelets | 2 | 1 | 0 | 0 |
| Anorexia | 9 | 7 | 5 | 0 |
| Nausea | 4 | 7 | 3 | 0 |
| Vomiting | 8 | 6 | 4 | 0 |
DISCUSSION
The prognosis of patients with locally advanced pancreatic adenocarcinoma is extremely poor. Because many patients with locally advanced PC are not curable through multidisciplinary treatment, it is necessary to optimize patient selection that can balance toxicity, quality of life and disease control.
Many previous random trials showed that concurrent radiotherapy with 5-FU has become a frequently employed treatment for locally advanced PC[19]. Despite the fact that no marked improvement of survival has been observed, many investigators are still pursuing phase I and II clinic trials of radiotherapy with new chemotherapeutic agents, such as bevacizumab, erlotinib, gefitinib, gemcitabine, capecitabine, oxaliplatin and paclitaxel, because 5-FU-based chemoradiotherapy has a modest survival benefit[20]. The agent S-1 is an oral fluoropyrimidine derivative, which has demonstrated mild toxicity and excellent efficacy in patients with metastatic and locally advanced PC[21-24]. Therefore, it can be recommended as an effective treatment for locally advanced PC since many clinical trials of concurrent chemoradiotherapy with S-1 for locally advanced PC consider it a well-tolerated, promising regimen[25-29].
However, many patients with locally advanced PC who received upfront chemoradioation experienced metastases soon after they completed therapy. On the contrary, in some clinical trials, including this trial, induction chemotherapy such as gemcitabine and S-1 followed by chemoradiotherapy demonstrated promising activity in treatment of locally advanced PC. Therefore, further consideration of radiation schedule and duration of induction chemotherapy is required to enhance the efficacy of this treatment strategy[30-34].
In this study, the combination of S-1 with gemcitabine followed by oral S-1 with concurrent radiotherapy and maintenance with S-1 in the patients who suffered from locally advanced pancreatic cancer was tested. It was easy to administer and keep toxicities at a relatively low level when the standard-dose IMRT (50.4 Gy/28 fractions) and full-dose S-1 (80 mg/m2) were combined. Moreover, since this regimen can improve local control and prevent tumors from spreading, it may benefit patients with locally advanced pancreatic cancer. The study demonstrated that there were 17 patients (53.1%) with partial response, 9 (28.1%) with stable disease and 4 (12.5%) with progressive disease. Median overall survival and median progression-free survival were 15.2 mo and 9.3 mo, respectively. The survival rates at 1 year and 2 years were 75% and 34.4%, respectively. As for acute haematological toxicity, grade 4 toxicities were not observed and grade 3 neutropenia was observed in only two patients. For acute nonhaematological toxicity, grade 3 AST elevation (two patients), grade 3 vomiting (four patients), grade 3 anorexia and nausea (eight patients) and grade 3 haemorrhagic gastritis (one patient) were observed. Duodenal ulcer as a late toxicity was observed 7 mo after treatment in one patient, but no other late toxicities occurred.
Thus, with regard to the antitumor activity of this treatment, S-1 at a daily dose of 80 mg/m2 was considered well tolerated and this dose was deemed recommendable.
In conclusion, a regimen of combination of S-1 with gemcitabine followed by oral S-1 with IMRT and maintenance therapy with S-1 in patients having locally advanced pancreatic cancer is considered a well-tolerated, promising regimen, which can be recommended as an effective treatment for locally advanced PC.
COMMENTS
Background
Currently, unresectable advanced pancreatic cancer is one of the leading causes of death among the cancer patients. Many patients who received upfront chemoradiation have been found to have immediate metastases after therapy. Furthermore, the patients who received initial chemoradiation may experience much higher levels of toxicity than the patients who only received chemotherapy. As a result of such toxicity, the subsequent chemotherapy may be involved, and thus it is important to take into account the order in which radiation and chemotherapy are given to the patients with locally advanced pancreatic cancer (PC). In addition to this, several cycles of induction chemotherapy at the initial phase of treatment are needed before the patients are restaged. The conditions of the patients without distant metastases on restaging but with a good performance status, may be consolidated by chemoradiation.
Research frontiers
This article could contribute to the progress of the treatment for patients with locally advanced disease.
Innovations and breakthroughs
S-1, which has been proved to possess excellent efficacy with mild toxicity, is a type of oral fluoropyrimidine derivative which could be used for patients with metastatic and locally advanced PC. Therefore, the concurrent radiotherapy with S-1 therapy could be considered a promising and well-tolerated regimen for locally advanced PC. Although it has been found that many patients will suffer from metastases soon after their initial chemoradiation completes, the induction chemotherapy proposed in the study, the adoption of S-1 and gemcitabine followed by chemoradiotherapy, proves to be effective in treating locally advanced pancreatic cancer. However, further consideration of duration of induction chemotherapy and the radiation schedule is required to enhance the efficacy of this treatment strategy. Overall, the induction chemotherapy with S-1 and gemcitabine followed by concurrent IMRT with S-1 and maintenance therapy with S-1 could be considered a well-tolerated and promising regimen for patients with locally advanced PC.
Applications
This procedure has proved to be feasible. A larger sample of patients is needed to confirm the metabolic advantages of the regimen.
Terminology
Stereotactic body radiation therapy (SBRT) or intensity modulated radiation therapy (IMRT) are the latest technologies. They can deliver higher doses of radiation to the tumor bed than traditional methods and limit the dose to normal structures (the liver, kidneys, and bowel), since they employ modulated, multiple beams of radiation that are likely to be more effective and safer than the traditional radiation techniques. These technologies have been proved to be effective in patients with locally advanced cancers.
Peer review
Series of contemporary studies focusing on using aggressive induction chemotherapy with S-1 and gemcitabine, then combining therapy with S-1 and IMRT and maintenance S-1 in patients with locally advanced pancreatic cancer.
Footnotes
P- Reviewer: Tamori A S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Liu XM
References
- 1.Colucci G, Labianca R, Di Costanzo F, Gebbia V, Cartenì G, Massidda B, Dapretto E, Manzione L, Piazza E, Sannicolò M, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer: the GIP-1 study. J Clin Oncol. 2010;28:1645–1651. doi: 10.1200/JCO.2009.25.4433. [DOI] [PubMed] [Google Scholar]
- 2.Crane CH, Varadhachary GR, Yordy JS, Staerkel GA, Javle MM, Safran H, Haque W, Hobbs BD, Krishnan S, Fleming JB, et al. Phase II trial of cetuximab, gemcitabine, and oxaliplatin followed by chemoradiation with cetuximab for locally advanced (T4) pancreatic adenocarcinoma: correlation of Smad4(Dpc4) immunostaining with pattern of disease progression. J Clin Oncol. 2011;29:3037–3043. doi: 10.1200/JCO.2010.33.8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crane CH, Winter K, Regine WF, Safran H, Rich TA, Curran W, Wolff RA, Willett CG. Phase II study of bevacizumab with concurrent capecitabine and radiation followed by maintenance gemcitabine and bevacizumab for locally advanced pancreatic cancer: Radiation Therapy Oncology Group RTOG 0411. J Clin Oncol. 2009;27:4096–4102. doi: 10.1200/JCO.2009.21.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duffy A, Kortmansky J, Schwartz GK, Capanu M, Puleio S, Minsky B, Saltz L, Kelsen DP, O’Reilly EM. A phase I study of erlotinib in combination with gemcitabine and radiation in locally advanced, non-operable pancreatic adenocarcinoma. Ann Oncol. 2008;19:86–91. doi: 10.1093/annonc/mdm441. [DOI] [PubMed] [Google Scholar]
- 5.Huguet F, André T, Hammel P, Artru P, Balosso J, Selle F, Deniaud-Alexandre E, Ruszniewski P, Touboul E, Labianca R, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007;25:326–331. doi: 10.1200/JCO.2006.07.5663. [DOI] [PubMed] [Google Scholar]
- 6.Loehrer PJ, Feng Y, Cardenes H, Wagner L, Brell JM, Cella D, Flynn P, Ramanathan RK, Crane CH, Alberts SR, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29:4105–4112. doi: 10.1200/JCO.2011.34.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Philip PA, Benedetti J, Corless CL, Wong R, O’Reilly EM, Flynn PJ, Rowland KM, Atkins JN, Mirtsching BC, Rivkin SE, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol. 2010;28:3605–3610. doi: 10.1200/JCO.2009.25.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sawaki A, Hoki N, Ito S, Matsumoto K, Mizuno N, Hara K, Takagi T, Kobayashi Y, Sawai Y, Kawai H, et al. Clinical impact of radiotherapy for locally advanced pancreatic cancer. J Gastroenterol. 2009;44:1209–1214. doi: 10.1007/s00535-009-0116-9. [DOI] [PubMed] [Google Scholar]
- 9.Sudo K, Yamaguchi T, Ishihara T, Nakamura K, Hara T, Denda T, Tawada K, Imagumbai T, Araki H, Sakai M, et al. Phase II study of oral S-1 and concurrent radiotherapy in patients with unresectable locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2011;80:119–125. doi: 10.1016/j.ijrobp.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 10.Tempero MA, Arnoletti JP, Behrman SW, Ben-Josef E, Benson AB, Casper ES, Cohen SJ, Czito B, Ellenhorn JD, Hawkins WG, et al. Pancreatic Adenocarcinoma, version 2.2012: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2012;10:703–713. doi: 10.6004/jnccn.2012.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krishnan S, Rana V, Janjan NA, Varadhachary GR, Abbruzzese JL, Das P, Delclos ME, Gould MS, Evans DB, Wolff RA, et al. Induction chemotherapy selects patients with locally advanced, unresectable pancreatic cancer for optimal benefit from consolidative chemoradiation therapy. Cancer. 2007;110:47–55. doi: 10.1002/cncr.22735. [DOI] [PubMed] [Google Scholar]
- 12.Ko AH, Quivey JM, Venook AP, Bergsland EK, Dito E, Schillinger B, Tempero MA. A phase II study of fixed-dose rate gemcitabine plus low-dose cisplatin followed by consolidative chemoradiation for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2007;68:809–816. doi: 10.1016/j.ijrobp.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Moureau-Zabotto L, Phélip JM, Afchain P, Mineur L, André T, Vendrely V, Lledo G, Dupuis O, Huguet F, Touboul E, et al. Concomitant administration of weekly oxaliplatin, fluorouracil continuous infusion, and radiotherapy after 2 months of gemcitabine and oxaliplatin induction in patients with locally advanced pancreatic cancer: a Groupe Coordinateur Multidisciplinaire en Oncologie phase II study. J Clin Oncol. 2008;26:1080–1085. doi: 10.1200/JCO.2007.12.8223. [DOI] [PubMed] [Google Scholar]
- 14.Rich TA, Winter K, Safran H, Hoffman JP, Erickson B, Anne PR, Myerson RJ, Cline-Burkhardt VJ, Perez K, Willett C. Weekly paclitaxel, gemcitabine, and external irradiation followed by randomized farnesyl transferase inhibitor R115777 for locally advanced pancreatic cancer. Onco Targets Ther. 2012;5:161–170. doi: 10.2147/OTT.S33560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spalding AC, Jee KW, Vineberg K, Jablonowski M, Fraass BA, Pan CC, Lawrence TS, Haken RK, Ben-Josef E. Potential for dose-escalation and reduction of risk in pancreatic cancer using IMRT optimization with lexicographic ordering and gEUD-based cost functions. Med Phys. 2007;34:521–529. doi: 10.1118/1.2426403. [DOI] [PubMed] [Google Scholar]
- 16.Wahl RL, Herman JM, Ford E. The promise and pitfalls of positron emission tomography and single-photon emission computed tomography molecular imaging-guided radiation therapy. Semin Radiat Oncol. 2011;21:88–100. doi: 10.1016/j.semradonc.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yovino S, Maidment BW, Herman JM, Pandya N, Goloubeva O, Wolfgang C, Schulick R, Laheru D, Hanna N, Alexander R, et al. Analysis of local control in patients receiving IMRT for resected pancreatic cancers. Int J Radiat Oncol Biol Phys. 2012;83:916–920. doi: 10.1016/j.ijrobp.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katz MH, Wang H, Balachandran A, Bhosale P, Crane CH, Wang X, Pisters PW, Lee JE, Vauthey JN, Abdalla EK, et al. Effect of neoadjuvant chemoradiation and surgical technique on recurrence of localized pancreatic cancer. J Gastrointest Surg. 2012;16:68–78; discussion 78-79. doi: 10.1007/s11605-011-1748-7. [DOI] [PubMed] [Google Scholar]
- 19.Yip D, Karapetis C, Strickland A, Steer CB, Goldstein D. Chemotherapy and radiotherapy for inoperable advanced pancreatic cancer. Cochrane Database Syst Rev. 2006;(3):CD002093. doi: 10.1002/14651858.CD002093.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, Fishman EK, Hruban RH. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013;63:318–348. doi: 10.3322/caac.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikeda M, Okusaka T, Ito Y, Ueno H, Morizane C, Furuse J, Ishii H, Kawashima M, Kagami Y, Ikeda H. A phase I trial of S-1 with concurrent radiotherapy for locally advanced pancreatic cancer. Br J Cancer. 2007;96:1650–1655. doi: 10.1038/sj.bjc.6603788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shinchi H, Maemura K, Mataki Y, Kurahara H, Sakoda M, Ueno S, Hiraki Y, Nakajo M, Natsugoe S, Takao S. A phase II study of oral S-1 with concurrent radiotherapy followed by chemotherapy with S-1 alone for locally advanced pancreatic cancer. J Hepatobiliary Pancreat Sci. 2012;19:152–158. doi: 10.1007/s00534-011-0400-y. [DOI] [PubMed] [Google Scholar]
- 23.Shinchi H, Maemura K, Noma H, Mataki Y, Aikou T, Takao S. Phase-I trial of oral fluoropyrimidine anticancer agent (S-1) with concurrent radiotherapy in patients with unresectable pancreatic cancer. Br J Cancer. 2007;96:1353–1357. doi: 10.1038/sj.bjc.6603735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sudo K, Yamaguchi T, Ishihara T, Nakamura K, Shirai Y, Nakagawa A, Kawakami H, Uno T, Ito H, Saisho H. Phase I study of oral S-1 and concurrent radiotherapy in patients with unresectable locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2007;67:219–224. doi: 10.1016/j.ijrobp.2006.08.046. [DOI] [PubMed] [Google Scholar]
- 25.Eguchi H, Nagano H, Kobayashi S, Kawamoto K, Wada H, Hama N, Tomimaru Y, Akita H, Sakai D, Satoh T, et al. A phase I trial of combination therapy using gemcitabine and S-1 concurrent with full-dose radiation for resectable pancreatic cancer. Cancer Chemother Pharmacol. 2014;73:309–315. doi: 10.1007/s00280-013-2357-9. [DOI] [PubMed] [Google Scholar]
- 26.Ikeda M, Ioka T, Ito Y, Yonemoto N, Nagase M, Yamao K, Miyakawa H, Ishii H, Furuse J, Sato K, et al. A multicenter phase II trial of S-1 with concurrent radiation therapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2013;85:163–169. doi: 10.1016/j.ijrobp.2012.03.059. [DOI] [PubMed] [Google Scholar]
- 27.Kim HM, Bang S, Park JY, Seong J, Song SY, Chung JB, Park SW. Phase II trial of S-1 and concurrent radiotherapy in patients with locally advanced pancreatic cancer. Cancer Chemother Pharmacol. 2009;63:535–541. doi: 10.1007/s00280-008-0836-1. [DOI] [PubMed] [Google Scholar]
- 28.Morizane C, Okusaka T, Mizusawa J, Takashima A, Ueno M, Ikeda M, Hamamoto Y, Ishii H, Boku N, Furuse J. Randomized phase II study of gemcitabine plus S-1 versus S-1 in advanced biliary tract cancer: a Japan Clinical Oncology Group trial (JCOG 0805) Cancer Sci. 2013;104:1211–1216. doi: 10.1111/cas.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ueno H, Ioka T, Ikeda M, Ohkawa S, Yanagimoto H, Boku N, Fukutomi A, Sugimori K, Baba H, Yamao K, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol. 2013;31:1640–1648. doi: 10.1200/JCO.2012.43.3680. [DOI] [PubMed] [Google Scholar]
- 30.Lee GW, Kim HJ, Ju JH, Kim SH, Kim HG, Kim TH, Kim HJ, Jeong CY, Kang JH. Phase II trial of S-1 in combination with gemcitabine for chemo-naïve patients with locally advanced or metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2009;64:707–713. doi: 10.1007/s00280-008-0918-0. [DOI] [PubMed] [Google Scholar]
- 31.Nakachi K, Furuse J, Kinoshita T, Kawashima M, Ishii H, Ikeda M, Mitsunaga S, Shimizu S. A phase II study of induction chemotherapy with gemcitabine plus S-1 followed by chemoradiotherapy for locally advanced pancreatic cancer. Cancer Chemother Pharmacol. 2010;66:527–534. doi: 10.1007/s00280-009-1193-4. [DOI] [PubMed] [Google Scholar]
- 32.Nakai Y, Isayama H, Sasaki T, Sasahira N, Tsujino T, Toda N, Kogure H, Matsubara S, Ito Y, Togawa O, et al. A multicentre randomised phase II trial of gemcitabine alone vs gemcitabine and S-1 combination therapy in advanced pancreatic cancer: GEMSAP study. Br J Cancer. 2012;106:1934–1939. doi: 10.1038/bjc.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozaka M, Matsumura Y, Ishii H, Omuro Y, Itoi T, Mouri H, Hanada K, Kimura Y, Maetani I, Okabe Y, et al. Randomized phase II study of gemcitabine and S-1 combination versus gemcitabine alone in the treatment of unresectable advanced pancreatic cancer (Japan Clinical Cancer Research Organization PC-01 study) Cancer Chemother Pharmacol. 2012;69:1197–1204. doi: 10.1007/s00280-012-1822-1. [DOI] [PubMed] [Google Scholar]
- 34.Satoi S, Toyokawa H, Yanagimoto H, Yamamoto T, Kamata M, Ohe C, Sakaida N, Uemura Y, Kitade H, Tanigawa N, et al. Neo-adjuvant chemoradiation therapy using S-1 followed by surgical resection in patients with pancreatic cancer. J Gastrointest Surg. 2012;16:784–792. doi: 10.1007/s11605-011-1795-0. [DOI] [PubMed] [Google Scholar]


