Abstract
Intramedullary spinal cord metastasis (ISCM) is very rare and its optimal treatment remains controversial. Pancreatic neuroendocrine tumor (pNET) is a rare tumor that usually presents with hepatic metastasis. Hepatic failure due to tumor progression is the major cause of death in cases of pNET. To date, no report has described a case of ISCM from pNET. Although spinal cord metastasis of a solid tumor is uncommon, it is a critical condition that can cause a potentially irreversible loss of neurologic function. Here, we report the case of a 45-year-old man who presented with leg weakness and voiding difficulty, and was found to have ISCM from pNET. Surgical treatment prevented further neurological deterioration. This is the first case report of ISCM from pNET.
Keywords: Intramedullary, Spinal cord neoplasm, Metastasis, Neuroendocrine tumor, Pancreas
Core tip: We report the first case of intramedullary spinal cord metastasis (ISCM) from a pancreatic neuroendocrine tumor. Despite its rarity, ISCM is a significant clinical condition that can cause critical neurologic issues. We suggest that taking an immediate surgical approach can increase the chances of restoring neurologic deficits and improving quality of life in cases of pancreatic neuroendocrine tumor with ISCM. Our report includes a review of previous studies of surgical and non-surgical approaches to ISCM.
INTRODUCTION
When advanced cancers show metastasis to the spine, the major sites of metastasis are the vertebral body and the extradural space of the spinal cord. However, metastasis to the spinal cord parenchyma is rare, accounting for only 4.2%-8.5% of all central nervous system (CNS) metastasis[1].
Intramedullary spinal cord metastasis (ISCM) accounts for less than 5% of all spinal metastasis[1]. There have only been a small number of case series and retrospective reports concerning ISCM[1]. Despite the rarity of ISCM as a complication of malignant tumors, it should be handled as a critical condition that can potentially cause an irreversible loss of neurologic function. ISCM most commonly originates from lung cancer. However, breast cancer, renal cell carcinoma, malignant lymphoma, and melanoma have also been reported to be major causes of ISCM.
Neuroendocrine tumors (NET) comprise a heterogeneous group of tumors that originate from various organs. Pancreatic neuroendocrine tumor (pNET) is a rare cancer, accounting for only 1%-2% of all cancers of the pancreas[2]. The most common metastatic site of pNET is the liver[3]. In cases of pNET, the major cause of death is hepatic failure due to the progression of cancer with hepatic metastasis[4]. To the best of our knowledge, no reports have discussed cases of ISCM from pNET. Here, we report the first case of ISCM from pNET.
CASE REPORT
A 45-year-old man was transferred to our center. At the hospital that he had initially visited, the patient underwent liver biopsy due to multiple metastastic masses of the pancreas tail, gallbladder, liver, and bone. Based on the results of the liver biopsy, he was diagnosed with pNET. Immunohistochemical staining showed that the tissue was positive for synaptophysin, chromogranin, and CD45, as well as being negative for mucicarmin. The Ki67 labeling index was 50%. Because of his poor performance status, chemotherapy was attempted at the initial hospital. After the patient was transferred to our center for supportive care, his performance status fortunately improved, and it was determined that he could begin palliative chemotherapy. Palliative chemotherapy was initiated 15 d after the pNET had been confirmed based on biopsy samples. The patient received the following regimen, which was repeated every 3 wk: 100 mg/m2 of etoposide on days 1-3 and 70 mg/m2 of cisplatin on day 1. Long-acting octreotide was also administered monthly. After 3 cycles of chemotherapy, response was evaluated as stable disease.
However, on the fifth day of the fourth cycle of chemotherapy, the patient visited the emergency room (ER), presenting with voiding difficulty and right leg weakness. His anal sphincter tone was becoming weak and he complained of encopresis. The weakness of his right leg had begun 5 h before visiting the ER and he could not ambulate well.
His vital signs were normal, including a blood pressure of 100/70 mmHg, a pulse rate of 105 per minute, a respiratory rate of 18/min, and a body temperature of 36.4 °C. Blood tests showed a leukocyte count of 6800/μL, an absolute neutrophil count of 6430/μL (segmented neutrophil, 95.3%), 9.7 g/dL hemoglobin, a platelet count of 131000/μL, 4.7 mg/dL total bilirubin, 250 IU/L aspartate aminotransferase, 304 IU/L alanine aminotransferase, 1855 IU/L alkaline phosphatase, 35.0 mg/dL blood urea nitrogen, and 1.5 mg/dL creatinine. Motor power was normal for both upper extremities. However, the motor power of the right leg was grade III and the motor power of the left leg was grade V, as assessed following the British Medical Council grading system.
Although magnetic resonance imaging (MRI) of the brain showed multiple metastases in the left cerebellum and the left inferior temporal lobe, those lesions were each less than 5 mm in size and their locations were not correlated with any of the neurologic deficits experienced by the patient. An MRI scan of the spine did not reveal any lesion that compressed the spinal cord, although diffuse vertebral bone metastasis was observed. Further, in the T10/11 level, a 5.9 mm × 5.3 mm × 14.8 mm ISCM was found (Figure 1). Dexamethasone treatment was started immediately and urgent radiotherapy was considered as an empirical treatment. However, the neurologic symptoms rapidly worsened and the motor function of the left leg, which was initially grade V, progressed to grade III.
Figure 1.
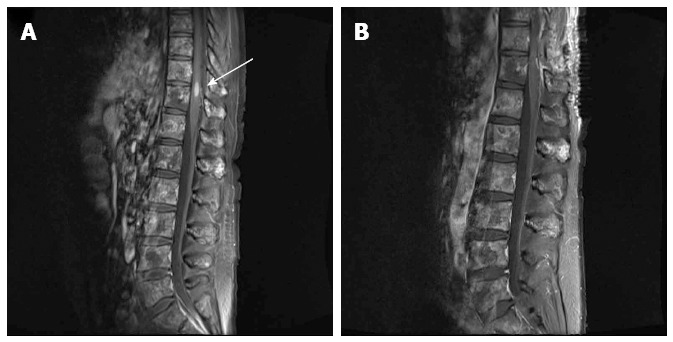
T1-weighted magnetic resonance image of the spine. A: The 5.9 mm × 5.3 mm × 14.8 mm metastatic tumor was observed in the T10/11 level (arrow). Diffuse vertebral metastases were also observed in almost the entire spine; B: The intramedullary tumor was removed by surgical treatment.
We concluded that radiation therapy offered little benefit for ISCM. We chose surgical treatment to address the rapid deterioration of neurologic symptoms. A gross total removal of the metastatic intramedullary tumor at the T10/T11 level was performed. Biopsy tissue from the surgical excision showed positivity for CD56, chromogranin, and synaptophysin (Figure 2), and the Ki67 index was 50%. These findings were consistent with an high grade neuroendocrine tumor (according to World Health Organization classification), indicating that the tumor was a metastasis from pNET[5]. After the operation, motor power was restored to grade IV for both legs. The patient was able to use a walker for assisted ambulation and could control voiding and defecation. After the operation, he received everolimus as palliative chemotherapy and survived 105 additional days before expiring from biliary sepsis with the progression of massive hepatic metastasis.
Figure 2.
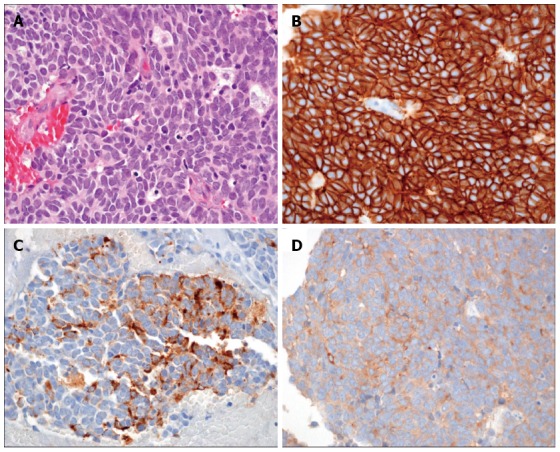
Pathologic findings of surgically removed tissue. A: High-power view of the tumor shows medium to large sized tumor cells with scanty cytoplasm, prominent nucleoli, and coarse “salt and pepper” chromatin. The tumor cells exhibited intense mitosis greater than 10/10 HPF (HE × 400); B-D: Immunohistochemically, tumor cells showed a diffuse and strong membranous positivity for CD56 (B), as well as cytoplasmic positivity for chromogranin (C) and synaptophysin (D) (immunohistochemical staining × 400).
DISCUSSION
pNET is a rare cancer, having an incidence of approximately 1 per 1000000 individuals per year. In the United States, approximately 1000 patients are newly diagnosed with pNET each year[6]. Many of the patients with malignant pNET are diagnosed with hepatic metastasis[3,7]. In our case, the patient was also diagnosed with pNET and massive hepatic metastasis. For patients who only have limited hepatic metastasis, surgical excision can be considered for both the primary tumor and the liver metastasis. According to recent data, the 10-year overall survival rate reaches 50.4% among patients who undergo curative excision following this method (excision of both lesions)[8]. However, as in our case, patients who have advanced pNET with extensive metastasis are not indicated for curative resection. For these patients, a biological therapy (such as everolimus or sunitinib) or cytotoxic chemotherapy with octreotide treatment would generally be considered[3,9]. ISCM is one of the rarest clinical features of cases of cancer that involve the CNS. Only 8.5% of cases of CNS metastasis show this particular clinical feature and, overall, 0.1%-0.4% of patients with cancer develop ISCM.
Hematogeneous spread via the arterial route is most commonly suggested as a mechanism of ISCM occurrence. The metastases that most commonly accompany ISCM are pulmonary and brain metastases. Particularly, brain metastasis is found in 35% of patients with ISCM. As compared with brain metastasis, ISCM is extremely rare, likely because the cerebral artery that supplies brain is almost a direct extension with high pressure from the aorta. Accordingly, the brain supplied by the cerebral artery is more favor to the embolic seeding of cancer cells[10].
By the time ISCM is identified, the primary cancer has usually reached an advanced state. Patients usually present with rapidly progressing myelopathy due to ISCM[11]. In our case, the primary tumor had already metastasized to multiple sites; a curative treatment approach was not indicated. Further, the progression of myelopathy was so rapid that the patient had to visit emergency room only 5 h after the onset of symptoms.
Cases in which the metastatic tumor involves the spinal cord are quite critical. Usually, primary intramedullary tumor is clinically described as having a slower onset and progression. In contrast, the onset of symptoms is abrupt and progression is very rapid if the intramedullary mass is a metastatic tumor. Therefore, patients experience the rapid progression of neurologic deficits within a short time[12].
Myelopathy due to metastatic spinal cord compression (MSCC) in patients with pNET is also very rare. Although MSCC with pNET is very rare, a case of MSCC due to vertebral body metastasis from pNET has been reported. In that case, metastases were identified in multiple levels of the thoracic vertebral bodies and myelopathy had occurred as a consequence of MSCC[13]. However, excluding our case, there has been no report of ISCM in a patient with pNET.
The optimal treatment of ISCM remains controversial and there is no established consensus. In resemblance to cases of MSCC, an empirical steroid is generally recommended in cases of ISCM, and external beam radiation would also be provided as treatment. In a single-institution study of 12 patients with ISCM, Lee et al[11] reported that 92% of the patients received a combination therapy of radiation and steroids, but no patient underwent surgery. In their study, the median survival time was 3.9 mo, and 17% of the patients showed immediate neurological improvement after the combination radiation and steroid treatment. However, neurological deterioration ultimately progressed in all cases. Despite the rarity of studies concerning ISCM, radiation-based therapy is generally agreed to provide only transient improvements in neurological symptoms[11,14]. Indeed, pNET is known to be a radio-resistant tumor[4] and, accordingly, we do not regard radiation therapy as an optimal treatment for ISCM from pNET.
On the other hand, a few reports have considered the benefits and general role of surgical resection for ISCM[10,15,16]. Gasser et al[15] studied 13 patients with ISCM, each of whom had received surgical treatment. The median survival time was 7.1 mo. They reported that surgical treatment did not restore neurologic deficit. Although 15% of the patients showed ongoing progression of neurologic deficits, 85% of the patients were free from further neurologic deterioration after surgery. Their report provides important evidence that surgical treatment can prevent further neurologic progression due to ISCM. Wilson et al[16] reported the characteristics of 10 ISCM cases from 9 patients, each of whom had received surgical treatment. The median overall duration of post-operative survival was 6.4 mo. The authors reported that further neurologic deterioration was prevented in 8 patients (89%), including 1 patient who was neurologically improved. Only 1 (11%) of the 9 patients showed further neurological deterioration after surgical treatment. The reports of Gasser et al[15] and Wilson et al[16] recommend that clinicians pursue surgical treatment for ISCM, noting that it is a relatively favorable treatment. Nonetheless, their studies are small case series and do not show consistent results. Because ISCM is quite rare, scant data and evidence are available, limiting efforts to establish the optimal treatment for this form of metastasis.
Nevertheless, mounting evidence suggests that early surgical treatment (rather than non-surgical treatment) is suitable for preventing neurological deterioration in cases of ISCM. Particularly, there is a growing consensus that surgical treatment can help to preserve ambulatory function in patients who present neurologic symptoms of ISCM and are preoperatively ambulatory[16].
To date, there have been no reports of ISCM from pNET, and no studies have discussed the optimal treatment for such cases. Moreover, NET is known to be radio-resistant[4], so surgical treatment should be regarded as a significant therapeutic option, such as in our case. Although ISCM is a rare complication, it is an important clinical entity that can cause critical neurologic issues. Our experience with this first reported case of ISCM from pNET suggests that surgical management may be favorable. Naturally, further case reports are necessary to grow the evidence base for ISCM from pNET. In the meantime, however, we expect that an immediate surgical approach can increase the chance of restoring neurologic deficits and improve quality of life for patients with ISCM and pNET.
COMMENTS
Case characteristics
A 45-year-old man with pancreatic neuroendocrine tumor (pNET) visited the emergency room, presenting with voiding difficulty and right leg weakness.
Clinical diagnosis
According to the British Medical Council grading system, the motor power of the right leg was grade III and the motor power of the left leg was grade V.
Differential diagnosis
Spinal cord compression of pNET, spine metastasis of malignant tumor.
Laboratory diagnosis
His vital signs were normal, including a blood pressure of 100/70 mmHg, a pulse rate of 105/min, a respiratory rate of 18/min, and a body temperature of 36.4 °C. Blood tests showed a leukocyte count of 6800/μL, an absolute neutrophil count of 6430/μL (segmented neutrophil, 95.3%), 9.7 g/dL hemoglobin, a platelet count of 131000/μL, 4.7 mg/dL total bilirubin, 250 IU/L aspartate aminotransferase, 304 IU/L alanine aminotransferase, 1855 IU/L alkaline phosphatase, 35.0 mg/dL blood urea nitrogen, and 1.5 mg/dL creatinine.
Imaging diagnosis
In a magnetic resonance imaging of the spine, a 5.9 mm × 5.3 mm × 14.8 mm intramedullary spinal cord metastasis (ISCM) was found at the T10/11 level.
Pathological diagnosis
Biopsy tissue from the surgical excision showed positivity for CD56, chromogranin, and synaptophysin, and the Ki67 index was 50%. These findings were consistent with an high grade neuroendocrine tumor, indicating that the tumor was a metastasis from pNET.
Treatment
A gross total removal of the metastatic intramedullary tumor at the T10/T11 level was performed and sequential chemotherapy was continued.
Related reports
To date, there have been no reports of ISCM from NET (including pNET and midgut NET) and no studies have discussed the optimal treatment for such cases.
Term explanation
ISCM is one of the rarest clinical features of cases of cancers that involve the central nervous system.
Experiences and lessons
Although ISCM is a rare complication, it is an important clinical entity that can cause critical neurologic issues. The authors’ experience with this first reported case of ISCM from pNET suggests that surgical management may be favorable.
Peer review
The authors have described the first case of ISCM from a pNET. This is interesting case report describing a new association and suggesting a surgical approach for ISCM.
Footnotes
Supported by Grant from Gachon University Gil Medical Center, No. 2013-37
P- Reviewer: Rossi RE S- Editor: Gou SX L- Editor: A E- Editor: Ma S
References
- 1.Sung WS, Sung MJ, Chan JH, Manion B, Song J, Dubey A, Erasmus A, Hunn A. Intramedullary spinal cord metastases: a 20-year institutional experience with a comprehensive literature review. World Neurosurg. 2013;79:576–584. doi: 10.1016/j.wneu.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Milan SA, Yeo CJ. Neuroendocrine tumors of the pancreas. Curr Opin Oncol. 2012;24:46–55. doi: 10.1097/CCO.0b013e32834c554d. [DOI] [PubMed] [Google Scholar]
- 3.Cerwenka H. Neuroendocrine liver metastases: contributions of endoscopy and surgery to primary tumor search. World J Gastroenterol. 2012;18:1009–1014. doi: 10.3748/wjg.v18.i10.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eriksson B, Oberg K. Neuroendocrine tumours of the pancreas. Br J Surg. 2000;87:129–131. doi: 10.1046/j.1365-2168.2000.01277.x. [DOI] [PubMed] [Google Scholar]
- 5.Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707–712. doi: 10.1097/MPA.0b013e3181ec124e. [DOI] [PubMed] [Google Scholar]
- 6.Dimou AT, Syrigos KN, Saif MW. Neuroendocrine tumors of the pancreas: what’s new. Highlights from the “2010 ASCO Gastrointestinal Cancers Symposium”. Orlando, FL, USA. January 22-24, 2010. JOP. 2010;11:135–138. [PubMed] [Google Scholar]
- 7.Yao JC, Eisner MP, Leary C, Dagohoy C, Phan A, Rashid A, Hassan M, Evans DB. Population-based study of islet cell carcinoma. Ann Surg Oncol. 2007;14:3492–3500. doi: 10.1245/s10434-007-9566-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glazer ES, Tseng JF, Al-Refaie W, Solorzano CC, Liu P, Willborn KA, Abdalla EK, Vauthey JN, Curley SA. Long-term survival after surgical management of neuroendocrine hepatic metastases. HPB (Oxford) 2010;12:427–433. doi: 10.1111/j.1477-2574.2010.00198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu E, Marincola P, Oberg K. Everolimus in the treatment of patients with advanced pancreatic neuroendocrine tumors: latest findings and interpretations. Therap Adv Gastroenterol. 2013;6:412–419. doi: 10.1177/1756283X13496970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogino M, Ueda R, Nakatsukasa M, Murase I. Successful removal of solitary intramedullary spinal cord metastasis from colon cancer. Clin Neurol Neurosurg. 2002;104:152–156. doi: 10.1016/s0303-8467(02)00004-5. [DOI] [PubMed] [Google Scholar]
- 11.Lee SS, Kim MK, Sym SJ, Kim SW, Kim WK, Kim SB, Ahn JH. Intramedullary spinal cord metastases: a single-institution experience. J Neurooncol. 2007;84:85–89. doi: 10.1007/s11060-007-9345-z. [DOI] [PubMed] [Google Scholar]
- 12.Tan LA, Kasliwal MK, Nag S, O’Toole JE. A rare intramedullary spinal cord metastasis from uterine leiomyosarcoma. J Clin Neurosci. 2013;20:1309–1312. doi: 10.1016/j.jocn.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Eads TA, Hattab EM, Rodgers RB. Metastatic pancreatic endocrine tumor presenting as thoracic spinal cord compression. Spine (Phila Pa 1976) 2010;35:E510–E513. doi: 10.1097/BRS.0b013e3181cb4730. [DOI] [PubMed] [Google Scholar]
- 14.Conill C, Marruecos J, Verger E, Berenguer J, Lomeña F, Domingo-Domènech J, Grau JJ, Casas F. Clinical outcome in patients with intramedullary spinal cord metastases from lung cancer. Clin Transl Oncol. 2007;9:172–176. doi: 10.1007/s12094-007-0031-6. [DOI] [PubMed] [Google Scholar]
- 15.Gasser T, Sandalcioglu IE, El Hamalawi B, van de Nes JA, Stolke D, Wiedemayer H. Surgical treatment of intramedullary spinal cord metastases of systemic cancer: functional outcome and prognosis. J Neurooncol. 2005;73:163–168. doi: 10.1007/s11060-004-4275-5. [DOI] [PubMed] [Google Scholar]
- 16.Wilson DA, Fusco DJ, Uschold TD, Spetzler RF, Chang SW. Survival and functional outcome after surgical resection of intramedullary spinal cord metastases. World Neurosurg. 2012;77:370–374. doi: 10.1016/j.wneu.2011.07.016. [DOI] [PubMed] [Google Scholar]


