Summary
The relative importance of Lewy- and Alzheimer-type pathologies to dementia in Parkinson’s disease remains unclear. We have examined the combined associations of α-synuclein, tau and amyloid-β accumulation in 56 pathologically confirmed Parkinson’s disease cases, 29 of whom had developed dementia. Cortical and subcortical amyloid-β scores were obtained, while tau and α-synuclein pathologies were rated according to the respective Braak stages. Additionally, cortical Lewy body and Lewy neurite scores were determined and Lewy body densities were generated using morphometry. Non-parametric statistics, together with regression models, receiver-operating characteristic curves and survival analyses were applied. Cortical and striatal amyloid-β scores, Braak tau stages, cortical Lewy body, Lewy neurite scores and Lewy body densities, but not Braak α-synuclein stages, were all significantly greater in the Parkinson’s disease-dementia group (P < 0.05), with all the pathologies showing a significant positive correlation to each other (P < 0.05). A combination of pathologies [area under the receiver-operating characteristic curve = 0.95 (0.88–1.00); P < 0.0001] was a better predictor of dementia than the severity of any single pathology. Additionally, cortical amyloid-β scores (r = −0.62; P = 0.043) and Braak tau stages (r = −0.52; P = 0.028), but not Lewy body scores (r = −0.25; P = 0.41) or Braak α-synuclein stages (r = −0.44; P = 0.13), significantly correlated with mini-mental state examination scores in the subset of cases with this information available within the last year of life (n = 15). High cortical amyloid-β score (P = 0.017) along with an older age at onset (P = 0.001) were associated with a shorter time-to-dementia period. A combination of Lewy- and Alzheimer-type pathologies is a robust pathological correlate of dementia in Parkinson’s disease, with quantitative and semi-quantitative assessment of Lewy pathology being more informative than Braak α-synuclein stages. Cortical amyloid-β and age at disease onset seem to determine the rate to dementia.
Keywords: lewy bodies, amyloid-β; tau, Parkinson’s disease, dementia
Introduction
Dementia is frequent in the terminal phases of Parkinson’s disease (Aarsland et al., 2003), but its anatomical and pathological substrate remains unclear. Limbic and neocortical Lewy body pathology has been claimed to be the main determinant of the development of cognitive impairment in a number of studies (Hurtig et al., 2000; Mattila et al., 2000; Harding and Halliday, 2001; Kövari et al., 2003; Aarsland et al., 2005). A synergistic interaction between the Alzheimer’s disease-related protein aggregates (amyloid-β and tau) and α-synuclein-containing inclusions (perikaryal Lewy bodies and Lewy neurites) has also been proposed as a critical determinant (Masliah et al., 2001; Apaydin et al., 2002; Giasson et al., 2003; Pletnikova et al., 2005; Lashley et al., 2008, Clinton et al., 2010), and a link between concomitant Alzheimer’s disease pathology and faster progression to dementia has been suggested (Ballard et al., 2006; Halliday et al., 2008; Sabbagh et al., 2009). Subcortical amyloid-β pathology has also been reported in patients with Parkinson’s disease and cognitive impairment (Kalaitzakis et al., 2008, 2009), but it remains uncertain whether the striatal amyloid-β deposition alone could drive the progression of dementia in Parkinson’s disease independently of cortical amyloid-β lesions and cortical Lewy body burden (Lashley et al., 2008; Dickson et al., 2009).
Variants of the APOE and the MAPT gene have been respectively associated with the presence of amyloid-β aggregates (Polvikoski et al., 1995) and with neuronal degeneration related to increased tau protein expression (Laws et al., 2007), and both genes have been linked with an increased risk of dementia in Parkinson’s disease (Goris et al., 2007; Williams-Gray et al., 2009); but a connection between these genetic variants and the burden of Lewy body- and Alzheimer’s disease-type pathologies in Parkinson’s disease has not been studied in detail.
Given that cognitive impairment usually occurs in the later phase of the disease and that advanced age increases the risk of multiple pathologies (Kovacs et al., 2008; Matthews et al., 2009), brains of patients with Parkinson’s disease with dementia (PDD) are likely to show various degrees of amyloid-β and tau pathologies, in addition to α-synuclein pathology leading to difficulties in determining their relative contribution to the clinical picture.
In this study, we investigated the combined effect of Lewy body- and Alzheimer’s disease-type (amyloid-β and tau) pathologies on the presence of and progression to dementia in Parkinson’s disease.
Materials and methods
Case selection
Two hundred and three patients with an initial clinical diagnosis of parkinsonism and a pathologically proven diagnosis of Parkinson’s disease, according to widely accepted neuropathological criteria (Ince et al., 2008), were identified from the records of donors to the Queen Square Brain Bank for Neurological Disorders, UCL Institute of Neurology (QSBB) from the period 2000 to 2008. Cases were collected using a protocol approved by a London Multicentre Research Ethics Committee and are stored under a licence issued by the Human Tissue Authority. The main selection criterion was the availability of reliable clinical information on the presence or the absence of cognitive impairment and features associated with dementia in Parkinson’s disease, such as visual hallucinations and fluctuating confusion. Cases were excluded if the clinical records were incomplete as to whether the cognitive changes were related to alternative causes (i.e. drug-related) rather than to disease progression. Subsequently, the cases were retrospectively classified as Parkinson’s disease without dementia (PDND) or PDD by consensus between three neurologists with expertise in movement disorders (Y.C., S.O.S., A.J.L.); who independently applied the functional rule of the presence of substantial and apparently permanent impairment of ability to perform tasks of daily living because of cognitive disability (Diagnostic and Statistical Manual of Mental Disorders, DSM-IV, 1995), and the recently proposed Movement Disorder Society clinical criteria for patients with PDD, which partly rely on the afore-mentioned dementia-associated features (Emre et al., 2007).
Demographic and clinical data included gender, age at onset, age at death and disease duration, along with time to dementia for patients with PDD cases. The score on mini-mental state examination (MMSE) within the last year of life was also included, where available (Folstein et al., 1975).
Neuropathological assessment
After fixation in 10% buffered formalin, the brains were examined by a neuropathologist and sampled in accordance with the standardized protocols of the Queen Square Brain Bank. In compliance with established criteria for the neuropathological diagnosis of Parkinson’s disease (Ince et al., 2008) and Alzheimer’s disease (Mirra et al., 1991; Ball et al., 1997; Braak et al., 2006), brain samples from selected regions were embedded in paraffin. Tissue sections (8 μm thick) were cut, deparaffinized and rehydrated, followed by pretreatment with formic acid and/or pressure-cooking in citrate buffer at pH 6.0, as appropriate. Following epitope unmasking, monoclonal antibodies to amyloid-β, recognizing residues 17–26, (clone 4G8, dilution 1:3000; Cell Sciences Inc), phospho-tau recognizing phosphorylated serine 202/threonin 205 (clone AT8, dilution 1:600; Autogen Bioclear) and α-synuclein (clone KM51, dilution 1:1000; Novocastra) were applied and incubated at room temperature for 1 h or at 4°C overnight for α-synuclein. Subsequently, the secondary antibody (Dako, 1:200) was applied, followed by incubation with avidin-biotin peroxidase complex (ABC) kit (Elite PK-6100 Standard Vectastain® ABC kit, Vector Laboratories). Colour was developed with diaminobenzidine/H2O2 (DAB, Sigma) and with Romulin AEC chromogen (Biocare Medical) for α-synuclein.
Using tau immunohistochemistry, neurofibrillary tangle and neuropil thread pathology was staged (Braak stages I–VI) by Y.C., J.L.H. and T.R. in sections from the medial temporal lobe comprising the hippocampal formation with the entorhinal and transentorhinal cortices and fusiform gyrus, first and second temporal gyri and occipital cortex (including striate, peri- and para-striate cortices), according to recommended criteria (Braak et al., 2006).
Semi-quantitative assessment of α-synuclein-immunoreactive Lewy body-type pathology was carried out in five cortical regions (frontal, temporal, parietal, entorhinal and cingulate cortices) based on the recommendation by the consensus diagnostic criteria for dementia with Lewy bodies (McKeith et al., 2005) and rated by L.P. and C.C., as follows: 1 = mild (sparse Lewy bodies at ×100 magnification); 2 = moderate (1-3 Lewy bodies at ×100 magnification); 3 = severe (≥4 Lewy bodies at ×200 magnification); 4 = very severe (numerous Lewy bodies and neurites at ×200 magnification). The α-synuclein-immunoreactive Lewy neurites were rated semi-quantitatively as: 0 = absent, 1 = sparse; 2 = moderate; and 3 = frequent. Each subject was given a cortical Lewy body and Lewy neurite score, calculated as the sum of all semi-quantitative regional scores in the above mentioned cortical areas (McKeith et al., 2005). In addition, all Lewy bodies were systematically counted within the same five cortical areas of interest and these counts were adjusted to the surface area (Lewy body/mm2) using Image-Pro Plus software package (Media Cybernetics). Finally, each case was also classified according to Braak Parkinson’s disease stage (ranging from 0 to 6) depending on the topographic distribution of α-synuclein-immunoreactive inclusions (Braak et al., 2003).
Analysis of cortical amyloid-β immunoreactive plaques was performed by Y.C., J.L.H. and T.R. using a previously described protocol, based on the principles of ‘The Consortium to Establish a Registry for Alzheimer’s disease’ (CERAD) (Mirra et al., 1991; Lashley et al., 2008). Both amyloid-β immunoreactive diffuse and mature plaques were scored semi-quantitatively as: 0 = absent, 1 = sparse; 2 = moderate; and 3 = frequent in the six selected cortical regions (frontal, temporal, parietal, occipital and cingulate cortices, and entorhinal gyrus). The corresponding subtotal score, ranging from 0 to 18, for both diffuse and mature plaques was calculated as the sum of the semi-quantitative scores of the six areas. We subsequently calculated the total (diffuse + mature) cortical amyloid-β score (from 0 to 36). In addition, the diffuse amyloid-β immunoreactive plaques were assessed in the striatum (with caudate and putamen being rated separately and together) and the claustrum by L.P. using a semi-quantitative scale ranging from 0 to 3 (absent to frequent).
The extent and severity of amyloid-β-cerebral amyloid angiopathy was examined as described elsewhere (Lashley et al., 2008) by Y.C., J.L.H. and T.R. In brief, a semi-quantitative approach was used in the same six cortical areas used to determine the severity of amyloid-β plaque load as follows: 0 = none; 1 = mild (non-circumferential involvement of the vessel wall); 2 = moderate (involving the whole vessel wall circumference); 3 = severe (degenerative changes as vessel wall disruption or blood leakage out of the vessel). Leptomeningeal and parenchymal cerebral amyloid angiopathy were scored separately in each cortical area and, as with cortical plaques, the subtotal and total scores were subsequently calculated.
Genetic studies
For the genetic analysis of the tau (MAPT) and APOE genes, DNA was available for 46 cases. The MAPT H1 and H2 haplotypes were determined by genotyping the 238 bp insertion-deletion in intron 9 as previously described (Baker et al., 1999). APOE was genotyped for the ε2, ε3 and ε4 variants following a published protocol using polymerase chain reaction amplification and HhaI restriction digestion and fragment analysis on a 6% Tris-borate-EDTA-polyacrylamide gel (Hixson and Vernier, 1990).
Statistical analysis
Statistical analysis was performed using Statistical Package for the Social Sciences 15.0 software (SPSS Inc). Intra- and inter-rater reliability was examined for all neuropathological procedures with Cohen’s κ coefficient and intra-class correlation. To assess univariate associations, chi-square test was used for comparisons of proportions, and Student’s t-test or Mann–Whitney U test for comparison of frequencies depending on the applicability conditions. Bivariate correlations between the pathological variables were assessed by means of Spearman’s coefficient. To investigate how well the three pathologies could classify cases as demented or not, the area under the receiver operating characteristic was used. Firstly, a binary regression model was used to determine the estimated probability for each case to belong to the dementia group, according to the burden of the three studied types of pathologies, both separately and altogether. Then, the estimated probabilities were used to obtain the area under the receiver operating characteristic curves (the closer to 1, the stronger the ability to classify cases) and its 95% confidence interval (CI) for cortical Lewy body scores, Braak tau stages, and cortical amyloid-β plaque scores, both separately and in combination.
Two types of analysis were undertaken to assess the possible differential relationship of the Alzheimer’s disease and Lewy body pathologies with clinical variables related to dementia. Firstly, we performed correlation analyses between the severity of each pathology type with MMSE scores where available (Spearman’s test). Secondly, Kaplan–Meier survival curves and Cox regression models (which provide estimates of the risk for the outcome with the corresponding 95% CI), were used to assess the effect of the cortical pathologies on time to dementia.
The statistical significance level was established at P ≤ 0.05 (two-tailed analyses). All results were uncorrected for multiple comparisons, as the study was driven by a priori biologically and clinically plausible hypotheses (Perneger, 1998).
Results
Demographic, clinical and genetic information
Of the 203 potentially eligible cases, 147 were excluded due to incomplete medical records or inadequate brain sampling not meeting the criteria of this study. Of the 56 studied cases, 23 had been partially assessed in a previous study on the correlation between cortical α-synuclein and amyloid-β burden in Parkinson’s disease (Lashley et al., 2008). The included and excluded cases did not significantly differ in either gender distribution, age at onset, age at death or disease duration (Supplementary Table 1). Of the 56 included patients with Parkinson’s disease, 29 (52%) were classified as demented (PDD) and 27 as non-demented (PDND), with no significant demographical or clinical differences between these two groups (Table 1). The H1/H2 haplotypes and APOE status were determined in 46 cases. The proportion of these genotypes in PDND and PDD groups are shown in Table 1 as descriptive information only, as the sample size was too small to perform any comparative statistical analysis of the genetic data.
Table 1.
Basic demographic and clinical data of patients with Parkinson’s disease (n = 56) classified according to lack or presence of dementia status upon their death
| PDND (n = 27) | PDD (n = 29) | P-value | |
|---|---|---|---|
| Gender (%) | 12 (44) female | 8 (38) female | 0.19 |
| Age at onset (years) | 59.41 ± 13.66 | 60.06 ± 11.37 | 0.98 |
| Age at death (years) | 73.22 ± 9.85 | 75.75 ± 8.23 | 0.25 |
| Disease duration (years) | 13.78 ± 6.85 | 15.69 ± 7.50 | 0.25 |
| Age at dementia (years) | - | 72.92 ± 8.43 | NA |
| Time to dementia (years) | - | 12.99 ± 7.57 (2–27.4) | NA |
| H1H1 carriers (%) | 13/21(62) | 12/24 (50) | NA |
| APOE4 allele carriers (%) | 6/22 (27) | 10/24 (42) | NA |
Data expressed as mean ± SD, except for gender as n (percentage) females. Mann–Whitney test (PDND versus PDD) was applied, except for gender (chi-square test). NA = not applicable.
Univariate analysis of α-synuclein, tau and amyloid-β pathologies
Intra- and inter-rater Cohen’s κ and intra-class correlation coefficients were >0.75 for all the ratings performed, thus reflecting high reliability.
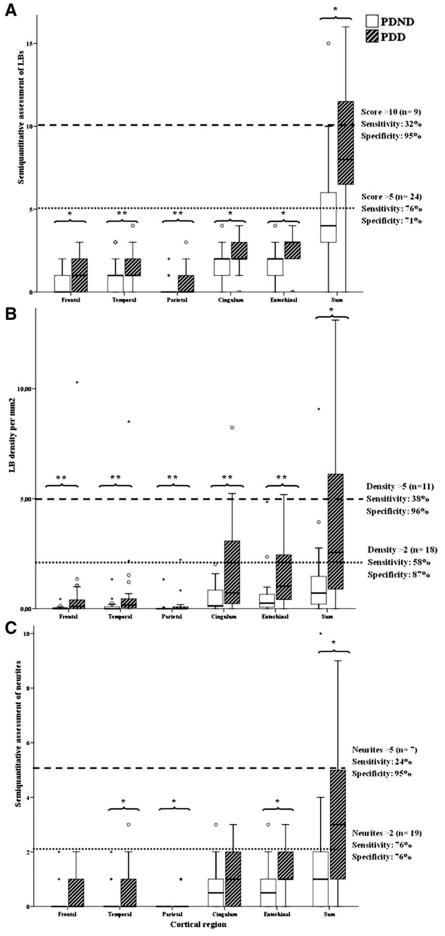
The proportion of subjects with Braak Alzheimer’s disease Stage >II (median value) was greater in the PDD group (Table 2; Fig. 1A). In most of the cases (90%) with moderate to severe Alzheimer’s disease tau pathology and widespread cortical Lewy pathology the clinical information indicated cognitive decline. This contrasted with the group with neocortical Lewy pathology and mild or absent Alzheimer’s disease tau pathology, in which a statistically smaller proportion of cases (<30%) (P = 0.0002) had dementia (Table 3). No distinction could be made between the PDD and the PDND groups on the basis of the Braak Parkinson’s disease stages as the median value calculated in the entire cohort was 6 and the inter-quartile range was 3–6 for both groups (Table 2), with Stage 5 being more frequent in PDND and Stage 6 in PDD cases (P = 0.001) (Fig. 1B). However, the semi-quantitative cortical Lewy body and Lewy neurite scores, together with the Lewy body densities, were more sensitive measures of cognitive decline in life as these were significantly higher in the PDD than in the PDND group (Table 2; a detailed regional assessment is shown for cortical Lewy body and Lewy neurite scores and Lewy body densities in Fig. 2 and Supplementary Table 2). Similarly, both cortical and striatal amyloid-β scores, together with total cerebral amyloid angiopathy scores were significantly higher in the PDD than in the PDND cases (Table 2; a detailed regional assessment is shown for cortical and subcortical amyloid-β plaque scores in Fig. 3 and Supplementary Tables 3 and 4; and for leptomeningeal, parenchymal and combined cerebral amyloid angiopathy scores in Supplementary Fig. 1 and Supplementary Table 5). Overall, diffuse plaques were more common than mature plaques in the entire cohort (the mean of the sum of diffuse plaque scores: 9.05 ± 7.02, the mean of the sum of mature plaque scores 4.39 ± 4.40; P = 0.0035), whereas there was no significant difference between the means of cerebral amyloid angiopathy leptomeningeal and parenchymal scores (3.05 ± 4.67 versus 1.49 ± 3.00; P = 0.16).
Table 2.
Summary of the comparisons between PDND and PDD cases of the severity of the three pathologies: total cortical and striatal plaque scores along with total cerebral amyloid angiopathy score for amyloid-β; Braak tau stage; and semi-quantitative scoring of the Lewy body, Lewy neurite assessment and Lewy body densities for a-synuclein
| Total cortical amyloid-β plaque score | Total striatal amyloid-β plaque score | Total cerebral amyloid angiopathy score | Braak Alzheimer’s disease stage | Braak Parkinson’s disease stage | Total cortical Lewy body score | Total cortical Lewy neurite score | Total cortical Lewy body densities | |
|---|---|---|---|---|---|---|---|---|
| n | 17 | 24 | 18 | 23 | 23 | 21 | 21 | 23 |
| PDND | 6.65 ± 7.66 | 1.21 ± 1.86 | 2.00 ± 5.15 | 0–III (3) | 3–6 (NA) | 4.71 ± 2.43 | 1.67 ± 2.29 | 1.29 ± 1.95 |
| n | 21 | 23 | 23 | 28 | 26 | 25 | 25 | 26 |
| PDD | 18.95 ± 10.41 | 2.74 ± 2.47 | 6.52 ± 8.5 | I–V (20) | 3–6 (NA) | 9.17 ± 3.81 | 3.64 ± 3.21 | 4.86 ± 6.19 |
| P-value | 0.0005* | 0.018* | 0.009* | 0.0001* | NA | 0.001* | 0.015* | 0.002* |
Data expressed as mean ± SD except for Braak Alzheimer’s disease and Parkinson’s disease stages, as inter-quartile range with the number of cases above the median value (Stages II and 6, respectively), within parentheses. Mann–Whitney test (PDND versus PDD) was applied, except for chi-square test for high versus low Braak Alzheimer’s disease and Parkinson’s disease stages.
Statistically significant values.
Figure 1.
Distribution of cases with each of the Braak Alzheimer’s disease (AD) tau stages (A) and Braak Parkinson’s disease (PD) stages (B) as represented by empty (PDND) and striped bars (PDD). Asterisk denotes significant differences. αS = α-synuclein.
Table 3.
Likelihood that pathological findings of dementia are associated with a Lewy body pathology and not Alzheimer’s disease (according to McKeith etal., 2005)
| McKeith stage | Braak Alzheimer’s disease stage | ||
|---|---|---|---|
| Braak stage O–II | Braak stage III–IV | Braak stage V–VI | |
| n = 24 | n = 17 | n = 3 | |
| Brainstem predominant | Low | Low | Low |
| n = 5 | n = 4 | n = 1 | n = 0 |
| (4 PDND + 1 PDD) | (3 PDND + 1 PDD) | (1 PDND) | |
| Limbic transitional | High | Intermediate | Low |
| n = 22 | n = 11 | n = 6 | n = 0 |
| (14 PDND + 8 PDD) | (10 PDND + 1 PDD) | (all 6 =DD) | |
| Diffuse neocortical | High | High | Intermediate |
| n = 22 | n = 9 | n = 10 | n = 3 |
| (5 PDND + 17 PDD) | (4 PDND + 5 PDD) | (1 PDND + 9 PDD) | (all 3 PDD) |
| Proportion of demented cases* (%) | 7 out of 24 (29) | 18 out of 20 (90) | |
chi-square test; P = 0.0002.
Figure 2.
(A) Bar diagrams of regional and total semi-quantitative Lewy body (LB) scores with dashed and dotted lines, respectively, delineating cut-off values of 10 and 5 (score >10: 88% PDD; score >5: 79% PDD). (B) Bar diagrams of regional and total Lewy body (LB) densities with dashed and dotted lines, respectively, delineating cut-off values of 5 and 2 (scores >5: 91% PDD; scores >2: 83% PDD). (C) Bar diagrams of the regional and total semi-quantitative Lewy neurite scores with dashed and dotted lines, respectively, delineating cut-off values of 5 and 2 (scores >5: 86% PDD; scores >2: 74% PDD). *P < 0.005; **P < 0.05.
Figure 3.
(A) Bar diagrams of regional and total amyloid-β diffuse plaque scores with dashed and dotted lines, respectively, delineating 10 and 5 cut-offs (score >10: 79% PDD; score >5: 71% PDD). (B) Bar diagrams of regional and total amyloid-β mature plaque scores with dashed and dotted lines, respectively, delineating 10 and 5 cut-offs (score >10: 100% PDD; score >5: 93% PDD). (C) Regional and total amyloid-β diffuse + mature plaque scores with dashed and dotted lines, respectively, delineating 18 and 12 (score >18: 100% PDD; score >12: 76% PDD). (D) amyloid-β diffuse plaque scores in the caudate and the putamen separately and together (striatum) with a dashed line delineating cut-off value of 3 for the total striatal score (73% PDD), as well as in the claustrum, with a dotted line delineating cut-off value of 2 (90% PDD). *P < 0.005; **P < 0.05.
The sensitivity and specificity calculated for each pathological variable using a series of cut-off points of varying stringency are illustrated in Figs 1-3. These cut-offs were chosen on the basis of their degree of overlap or lack of overlap between PDND and PDD when analysing the raw data, as described elsewhere (Lashley et al., 2008).
Correlations and combined analysis of α-synuclein, tau and amyloid-β pathologies
The severity of the studied pathologies (Lewy body and Lewy neurite densities, amyloid-β plaque and cerebral amyloid angiopathy scores, and tau stages) showed a significant correlation with each other, except total cortical Lewy body densities with cerebral amyloid angiopathy and cortical Lewy neurite scores with both cortical and striatal amyloid-β scores and cerebral amyloid angiopathy (Table 4). Confirming similar previous observations (Lashley et al., 2008; Dickson et al., 2009), when cases were separated into two groups according the severity of the cortical amyloid-β score (cut-off value of total cortical amyloid-β score = 18) (Fig. 3C), there was a significant correlation between cortical amyloid-β score and cortical α-synuclein load (Lewy body densities) in the group with high total cortical amyloid-β scores in contrast to the group with low cortical amyloid-β scores (Supplementary Fig. 2).
Table 4.
Correlations between the different pathologies across the entire Parkinson’s disease cohort
| Total cortical amyloid-β score | Total striatal amyloid-β score | Total cerebral amyloid angiopathy score | Braak Alzheimer’s disease stage | Braak Parkinson’s disease stage | Lewy body Score | Lewy neurite score | Lewy body densities | ||
|---|---|---|---|---|---|---|---|---|---|
| Total cortical Aβ | r | 0.88 | 0.40 | 0.56 | 0.43 | 0.51 | 0.19 | 0.42 | |
| score | P-value | <0.001* | 0.01* | <0.001* | 0.011* | 0.002* | 0.24 | 0.008* | |
| Total striatal Aβ | r | 0.36 | 0.47 | 0.43 | 0.46 | 0.17 | 0.35 | ||
| score | P-value | 0.032* | 0.001* | 0.004* | 0.003* | 0.25 | 0.015* | ||
| Total cerebral | r | 0.57 | 0.27 | 0.47 | 0.19 | 0.23 | |||
| amyloid angiopathy score | P-value | <0.001* | 0.12 | 0.005* | 0.22 | 0.15 | |||
| Braak | r | 0.35 | 0.54 | 0.27 | 0.39 | ||||
| Alzheimer’s disease stage | P-value | 0.02* | <0.001* | 0.06 | 0.004* | ||||
| Braak | r | 0.8 | 0.52 | 0.66 | |||||
| Parkinson’s disease stage | P-value | <0.001* | <0.001* | <0.001* | |||||
| Lewy body score | r | 0.69 | 0.90 | ||||||
| P-value | <0.001* | <0.001* | |||||||
| Lewy neurite | r | 0.75 | |||||||
| score | P-value | <0.001* | |||||||
| Lewy body | r | ||||||||
| densities | P-value |
Bivariate, pair-wise parametric (Spearman) correlation analysis between the three types of pathologies across the whole Parkinson’s disease sample (PDND + PDD). r = correlation coefficient;
significant correlations.
The binary regression model with dementia as an outcome and Braak Alzheimer’s disease tau stages and total cortical amyloid-β scores as covariates showed that the severity of both tau [odds ratio (OR) = 3.92; immunoreactive = 292%; 95% CI = 1.29–11.91; P = 0.016] and amyloid-β plaque (OR = 1.12; increased risk (IR) = 12%; 95% CI = 1.01–1.24; P = 0.037) pathologies significantly increased the risk of belonging to the PDD group, with age at onset having no significant influence (OR = 0.92; 95% CI = 0.85–1.00; P = 0.061). This effect was lost when cortical Lewy body scores were added as a further covariate, due to the observed correlation between all these variables, with such colinearity precluding further multivariate analyses (Harrell, 2001).
Using the estimated probabilities for each case to belong to the dementia group as calculated with the binary regression model, we created the receiver operating characteristic curves for the currently accepted and most widely used staging or scoring systems for each pathology type: cortical Lewy body scores alone [area under the curve with 95% CI: 0.83 (0.70–0.97), P = 0.001]; Braak Alzheimer’s disease tau stages alone [0.82 (0.70–0.93); P = 0.0001]; cortical total amyloid-β scores alone [0.83 (0.69–0.97), P = 0.001]; all the three pathologies in combination [0.95 (0.88–1.00); P < 0.0001]. In short, the ability of any of these pathologies alone to classify a given case as PDD was improved when all three pathologies were taken into consideration (Fig. 4A-D). The receiver operating characteristic curves for the other pathological variables related to Lewy-type and amyloid-β pathologies did not add any further information as a means of classifying cases as demented or not [total Lewy body densities: 0.76 (0.63–0.89), P = 0.002; Lewy neurite scores: 0.71 (0.56–0.860, P = 0.016; striatal amyloid-β scores: 0.69 (0.53–0.84), P = 0.028; total cerebral amyloid angiopathy scores: 0.72 (0.56–0.88), P = 0.017] (Supplementary Fig. 3).
Figure 4.
Receiver operating characteristic (ROC) curves for ability of pathology to classify cases as demented or non-demented created using the probabilities obtained in the binary regression models. (A) Cortical Lewy body (LB) scores alone (area under the curve = 0.83, 95% CI = 0.70–0.97, P = 0.001); (B) tau stages alone (area under the curve = 0.82, 95% CI = 0.70–0.93, P = 0.0001); (C) cortical amyloid-β (A-β) scores alone (area under the curve = 0.83, 95% CI = 0.69–0.97, P = 0.001); and (D) all three pathologies in combination (area under the curve = 0.95, 95% CI = 0.88–1.00, P = 0.000003). AD = Alzheimer’s disease.
Association of α-synuclein, tau and amyloid-β pathologies with the severity of cognitive impairment and progression to dementia
The subset of cases with MMSE performed within the year before death (n = 15; seven PDND and eight PDD) did not significantly differ in any demographic or clinical features from the other 41 cases (Supplementary Table 6) and showed pathological findings in line with those from the entire cohort (Supplementary Table 7). The analysis of this subset revealed that the Braak tau stages along with the parietal, cingulate, entorhinal and total cortical amyloid-β scores negatively correlated with MMSE score ante-mortem (that is, the higher the pathological stage or burden, the lower the MMSE score), whereas such correlation was not found for the Lewy body score or Braak Parkinson’s disease stage (Table 5).
Table 5.
Correlation between amyloid-β, tau and a-synuclein pathology with MMSE scores
| r | P | |
|---|---|---|
| Braak Alzheimer’s disease (tau) stage | −0.565 | 0.028* |
| Cortical amyloid-β score | ||
| Mature parietal | −0.575 | 0.031* |
| Subtotal parietal | −0.558 | 0.035* |
| Diffuse cingulate | −0.595 | 0.032* |
| Mature cingulate | −0.579 | 0.038* |
| Subtotal cingulate | −0.61 | 0.027* |
| Mature entorhinal | −0.56 | 0.030* |
| Total cortical amyloid-β load | −0.62 | 0.043* |
| Total cortical Lewy body (a-synuclein) score | −0.25 | 0.41 |
| Braak Parkinson’s disease (a-synuclein) stage | −0.44 | 0.13 |
Spearman’s non-parametric correlation analysis between each pathological variable and the MMSE scores within the last year of life (n = 15).
Significant correlations.
On survival analysis with time to dementia as time variable and dementia as outcome, high cortical amyloid-β scores implied shorter time to dementia than low scores (P = 0.017). In contrast, cases with Braak Alzheimer’s disease tau stages >II, and those with high cortical Lewy body scores were not associated with significantly faster progression to dementia (P = 0.83 and 0.055, respectively) (Table 6, Fig. 5). Of the demographic data, only older age at onset of Parkinson’s disease had a significant effect (estimated relative hazard = 1.09; increased risk = 9%; 95% CI = 1.04–1.14; P = 0.001). When both amyloid-β and age at onset were included in the analysis as covariates, such significant effect was lost due to the fact that they were related to each other, as further confirmed by means of a post hoc Mann–Whitney comparison, which revealed that cases with greater cortical amyloid-β burden were those with higher age at onset (P = 0.048).
Table 6.
Cox regression analyses for the effect of cortical amyloid-β scores, Braak Alzheimer’s disease and Parkinson’s stages and cortical Lewy body scores on time to dementia
| n (high versus with low burden or stage) | Estimated relative hazard | 95% CI (lower–upper) | P-value | |
|---|---|---|---|---|
| Total cortical amyloid-β score >18 | 13 versus 6 | 6.43 | 1.33–111.67 | 0.017* |
| Braak Alzheimer’s disease stage >11 | 19 versus 7 | 1.10 | 0.46–2.66 | 0.83 |
| Brook Porkinson’s disease stage >5 | 10 versus 5 | 2.15 | 0.76–6.07 | 0.15 |
| Total cortical Lewy Body score >10 | 10 versus 13 | 2.53 | 0.98–6.52 | 0.055 |
Significant findings.
Figure 5.
Survival analysis of time to dementia by means of Kaplan–Meier curves. (A) Effect on time to dementia of cortical amyloid-β (A-β) score; (B) effect on time to dementia of Braak Alzheimer’s disease (AD) tau stage; and (C) effect on time to dementia of cortical Lewy body (LB) load.
Genetic pathology association studies
Tau gene (MAPT) H1 homozygotes and APOE ε4 carriers did not show higher Braak Alzheimer’s disease tau stages or cortical Lewy body scores. In contrast, APOE ε4 carriers featured higher total cortical amyloid-β plaque scores in entorhinal, temporal and parietal cortices, with no differences in striatal amyloid-β plaque scores, and higher entorhinal, occipital and total cerebral amyloid angiopathy scores (Supplementary Fig. 3, Supplementary Tables 8 and 9).
Discussion
We have explored the individual and combined roles of Lewy body- and Alzheimer’s disease-type pathologies in Parkinson’s disease-related dementia in a single large cohort. Our study indicates that a combined high burden of all three (Lewy body, amyloid-β and tau) pathologies is the best neuropathological correlate of patients with PDD, although marked severity of any one of these three cardinal pathological features in isolation also showed a good statistical association. Our study has also confirmed that quantitative and semi-quantitative measures of α-synuclein pathology had a better discriminatory ability than currently used staging criteria. Additionally, we were able to show that higher cortical amyloid-β scores were associated with a faster progression to dementia.
In our study, we have found that both Lewy body- (α-synuclein) and Alzheimer’s disease- (amyloid-β and tau) type pathologies are increased in cortical areas of patients with PDD compared to patients with PDND. A number of studies have proposed that limbic and cortical Lewy body pathology is the main and specific pathological correlate of dementia in Parkinson’s disease (Hurtig et al., 2000; Mattila et al., 2000; Harding and Halliday, 2001; Kövari et al., 2003; Aarsland et al., 2005), but other surveys have challenged this view (Apaydin et al., 2002; Colosimo et al., 2003; Pletnikova et al., 2005; Ballard et al., 2006; Kalaitzakis et al., 2008, 2009; Lashley et al., 2008). Furthermore, some clinicopathological studies favour a role for Alzheimer’s disease-type pathological changes driving clinical disease progression to cognitive impairment in Parkinson’s disease (Apaydin et al., 2002; Jellinger and Attems, 2008). However, the topographical distribution of α-synuclein- and tau-immunoreactive lesions behaved differently in our cohort. Tau pathology was most prominent in the entorhinal gyrus in PDND and with increasing Braak Alzheimer’s disease tau stages, it spread out to the rest of the hippocampal formation, fusiform gyrus and the lateral temporal neocortex in patients with PDD. It is of note that with advancing Braak Alzheimer’s disease tau stages there is both topographical progression and an increase in density of tau-positive lesions in affected regions (Braak et al., 2006). Our finding that the higher the Braak Alzheimer’s disease tau stages, the worse the cognitive performance was on the MMSE test, is in keeping with previous studies in Alzheimer’s disease (Giannakopoulos et al., 2003) and is in compliance with findings indicating that the severity of neurofibrillary tangle pathology is a good pathological indicator of dementia in the elderly (Matthews et al., 2009).
In contrast, it was not possible to separate the non-demented patients with Parkinson’s disease from the patients with PDD cases with the help of Braak Parkinson’s disease stages, as most of our Parkinson’s disease cases (94%) had reached the highest cortical Braak Parkinson’s disease stages (Stages 5 and 6). This also explains why we were unable to find a significant correlation between Braak Parkinson’s disease stages and MMSE scores, in contrast to a previous clinicopathological study (Braak et al., 2005), where higher Braak Parkinson’s disease stages were associated with lower MMSE scores. This is in line with studies suggesting an inconstant association between cortical Lewy body pathology and dementia (Colosimo et al., 2003; Parkkinen et al., 2005). In particular, and in contrast to our group, Braak’s study had as many as 50% of cases in Stages 3 and 4, some of whom, nevertheless, displayed very low MMSE scores (Braak et al., 2005). It is of note that one of the only two cases of the cohort with Braak Parkinson’s disease Stage 3 was demented despite having Braak Alzheimer’s disease Stage II, total cortical amyloid-β score as low as 13, and striatal amyloid-β score of 0, confirming that, although significant cortical Lewy pathology with conjoint Alzheimer-type pathology is the characteristic feature of the majority of patients with PDD cases, it is not an obligate post-mortem finding in all such cases. Other contributors including vascular damage (Jellinger and Attems, 2008; Matthews et al., 2009), neuronal loss (Harding et al., 2002), or specific neurotransmitter deficiencies (Cummings, 1988), might play a role in such instances, but their assessment was beyond the scope of our study.
Conversely, using semi-quantitative (Lewy bodies and Lewy neurites) and quantitative measures (Lewy body densities per mm2) of cortical α-synuclein lesions, we observed a higher burden of α-synuclein pathologies in patients with PDD than in non-demented patients with Parkinson’s disease (PDND). This suggests that quantitative or semi-quantitative measures of α-synuclein-immunoreactive inclusions are more informative than the topographical assessment of Lewy bodies and Lewy neurites, perhaps because such quantitative approaches can overcome the ceiling effect of the Braak Parkinson’s disease staging system, in which a single Lewy body in first order sensory association areas of the neocortex or premotor areas is enough to upgrade a given case from Stage 5 to 6 (Braak et al., 2003). In our cohort, there was no correlation between the cortical Lewy body scores and the MMSE scores either, which is in contrast to a recent survey where quantitative assessment and an alternative staging system for α-synuclein were used, showing a significant but mild correlation between α-synuclein lesion densities and MMSE scores in Parkinson’s disease, with no mention of the association with concurrent Alzheimer’s disease pathology, present in up to 62% of the studied patients with PDD (Beach et al., 2009).
We found higher amyloid-β scores in cortical and subcortical structures in the PDD group compared to PDND cases, along with a significant correlation between the MMSE scores and the amyloid-β scores in several different cortical areas as well as the total cortical amyloid-β score. In Alzheimer’s disease, some studies have suggested that insoluble amyloid-β load, inferred by plaque density at post-mortem, is a poor correlate of the degree of cognitive dysfunction (Giannakopoulos et al., 2003; Guillozet et al., 2003), while the cerebral levels of soluble amyloid-β (McLean et al., 1999) as well as the topographical progression and the severity of tau pathology are better correlates of dementia (Yamaguchi et al., 2001). However, there is also evidence from recent studies using positron emission tomography probes for amyloid-β parenchymal amyloid deposits that the degree of uptake of the tracer 11C-Pittsburgh Compound-B in several cortical areas and the putamen is closely associated with the cognitive performance in Alzheimer’s disease (Grimmer et al., 2009). In Parkinson’s disease, the association between the severity of amyloid-β pathology and cognitive performance might be further explained by an apparent interaction between amyloid-β and α-synuclein (Masliah et al., 2001; Pletnikova et al., 2005; Lashley et al., 2008; Dickson et al., 2009), but such an interaction could not be tested further by statistical means in our sample due to the colinearity between these variables (Harrell, 2001).
We also found a strong correlation between cortical and striatal amyloid-β burden suggesting that these are closely related pathological processes, which contrasts with the conclusions of a previous study (Kailatzakis et al., 2008). Interestingly, the burden of diffuse plaques was higher than that of mature plaques, but showed more overlap between PDND and PDD. It has been suggested that diffuse plaques represent an earlier event in amyloid-β aggregation preceding mature plaque formation (Yamaguchi et al., 2001), so that diffuse plaques could already be seen in Parkinson’s disease in the absence of dementia, whereas mature ones would be mostly observed in the setting of Parkinson’s disease-dementia.
All the cortical pathologies were significantly correlated to each other in the bivariate analysis. We also replicated our earlier finding of significant correlation between cortical Lewy body densities and total cortical amyloid-β scores in the subset of Parkinson’s disease patients with high cortical amyloid-β burden (Lashley et al., 2008). This raises the possibility that there may be two pathological subsets of Parkinson’s disease cases—one with severe cortical Lewy body in close correlation with the cortical amount of amyloid-β pathology, and another where both Lewy body and amyloid-β pathologies are relatively mild and unrelated to each other (Lashley et al., 2008).
The receiver operating characteristic curves and the descriptive cut-offs for each pathological variable reflect the fact that a single pathology is not as reliable as a combination of all three in classifying Parkinson’s disease cases as demented or not. This finding underlines the heterogeneous pathological substrate of Parkinson’s disease-related dementia and the importance of the detailed assessment of all three pathologies. Large epidemiological-pathological surveys have also emphasized the importance of multiple pathologies in dementia (Kovacs et al., 2008; Matthews et al., 2009).
Genetic association studies have shown that common variation in the α-synuclein gene (SNCA) and the MAPT H1 haplotype contribute to sporadic Parkinson’s disease risk. This has been confirmed in recent genome-wide association studies, which have confirmed SNCA gene polymorphisms and the MAPT H1 haplotype as the strongest genetic risk factors for Parkinson’s disease (Pankratz et al., 2009; Simon-Sanchez et al., 2009). We did not find any association between the MAPT H1/H2 haplotype status and presence of dementia or the degree of tau or α-synuclein pathology probably due to underpowered analysis in the setting of numbers too small for a genetic association study. However, the MAPT H1 haplotype could be implicated in Parkinson’s disease and related dementia through mechanisms influencing neuronal vulnerability without the necessary formation of tau fibrillar aggregates or independent of α-synuclein aggregation. In contrast, and not surprisingly, we have replicated the association between the APOE ε4 allele and the severity of amyloid-β pathology (Lashley et al., 2008). The fact that this was limited to the entorhinal, temporal and parietal cortices and did not occur in the striatum might suggest that factors other than APOE ε4 may also play a role in the increased cortical and striatal amyloid-β pathology in Parkinson’s disease. Perhaps α-synuclein could be one such factor, in light of the suggested synergism between the aggregation of these proteins (Masliah et al., 2001; Gallardo et al., 2008; Pletnikova et al., 2008; Clinton et al., 2010).
The wide range of time to dementia in our PDD cases reflected the presence of different rates of progression to dementia and allowed us to perform a survival analysis between the three variables of pathology (tau, α-synuclein and amyloid-β) and time to dementia. Besides a borderline significant association with cortical Lewy body burden, the main finding was that of greater cortical amyloid-β and older age at disease onset significantly accelerating the rate of dementia in Parkinson’s disease. This result reinforces the notion that there are two subsets of patients with PDD, as suggested by previous clinicopathological studies (Ballard et al., 2006; Halliday et al., 2008; Kempster et al., 2010): those who are older at disease onset, develop dementia earlier in the disease course and have more mixed Alzheimer’s disease-Lewy body pathology, as opposed to those who are younger at disease onset, present with cognitive decline after a much longer duration of disease and have a more pure Lewy body phenotype. The fact that amyloid-β and age at onset separately influenced the rate to dementia, but their significant effect was lost when assessed in combination suggests that amyloid-β deposition and ageing are closely related and together influence the time course to dementia. In contrast, age at onset did not imply higher risk of dementia in the binary regression model, in agreement with previous studies, where it was the general effect of age that was associated with dementia in Parkinson’s disease (Aarsland et al., 2007), whereas age at onset rather influenced the time interval between disease onset and recording of milestones as dementia (Kempster et al., 2010).
The strict inclusion of a large number of cases for which detailed clinical information was available, the independent evaluation of clinical notes by neurologists experienced in Parkinson’s disease by applying an accepted definition of Parkinson’s disease-related dementia (Emre et al., 2007), and the comprehensive and combined assessment of different pathologies potentially relevant to dementia in Parkinson’s disease in a single group of patients represent strengths of the present study. Although findings from community- and hospital-based studies demonstrating the presence of these pathologies in cognitively intact elderly individuals (Esiri et al., 2001; Parkkinen et al., 2005) cast some doubt on their primary pathogenic role, there is growing evidence that amyloid-β, tau and α-synuclein act synergistically to enhance the aggregation of each other and to promote cognitive decline (Masliah et al., 2001; Gallardo et al., 2008; Pletnikova et al., 2008; Clinton et al., 2010). The retrospective nature of the study and the lack of assessment of other potentially relevant contributors, as discussed above, are shortcomings.
In summary, data from our study suggest that a combination of Lewy body- and Alzheimer’s disease-type pathologies is the most robust pathological correlate of dementia in Parkinson’s disease and that semi-quantitative and quantitative assessment of Lewy body-type pathology are more informative than the currently used topographical staging criteria. All this, along with the relevance of amyloid-β pathology to the rate of dementia in Parkinson’s disease, probably in association with ageing, suggest that both Lewy- and Alzheimer-type pathologies are important in Parkinson’s disease-related dementia.
Supplementary Material
Acknowledgements
The authors would like to thank the patients and their families for their generosity and goodwill, as without their support none of this research would have been possible. This work was undertaken at UCLH/UCL with a proportion of funding from the Department of Health’s NIHR Biomedical Research Centres funding scheme. The Queen Square Brain Bank, UCL Institute of Neurology is supported by the Reta Lila Weston Institute of Neurological Studies and the Progressive Supranuclear Palsy (Europe) Association.
Funding: Y.C. was supported by a grant from the Spanish Neurological Society (Sociedad Española de Neurología; SEN). J.L.H., T.R. and A.J.L. are supported by research grants from the Multiple System Atrophy Trust and the Alzheimer’s Research Trust. T.R. and J.L.H. are recipients of a project grant from Parkinson’s UK. J.L.H. is supported by the Reta Lila Weston Institute for Neurological Studies. R.d.S. is supported by the Medical Research Council UK [G0501560], Cure PSP+ (the Irene & Abe Pollin Fund for CBD Research and the Charles D. Peebler Jr P.S.P. and CBD Genetics Program), the Brain Research Trust, the Alzheimer’s Research Trust and Research into Ageing. This work was supported in part by the Wellcome/MRC Parkinson’s Disease Consortium grant to UCL Institute of Neurology, the University of Sheffield and the MRC Protein Phosphorylation Unit at the University of Dundee.
Abbreviations
- MMSE
mini-mental state examination
- PDD
Parkinson’s disease with dementia
- PDND
Parkinson’s disease without dementia
References
- Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sørensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003;60:387–92. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- Aarsland D, Perry R, Brown A, Larsen JP, Ballard C. Neuropathology of dementia in Parkinson’s disease: a prospective, community-based study. Ann Neurol. 2005;58:773–6. doi: 10.1002/ana.20635. [DOI] [PubMed] [Google Scholar]
- Aarsland D, Kvaløy JT, Andersen K, Larsen JP, Tang MX, Lolk A, et al. The effect of age of onset of PD on risk of dementia. J Neurol. 2007;254:38–45. doi: 10.1007/s00415-006-0234-8. [DOI] [PubMed] [Google Scholar]
- Apaydin H, Ahlskog JE, Parisi JE, Boeve BF, Dickson DW. Parkinson disease neuropathology: later-developing dementia and loss of the levodopa response. Arch Neurol. 2002;59:102–12. doi: 10.1001/archneur.59.1.102. [DOI] [PubMed] [Google Scholar]
- Baker M, Litvan I, Houlden H, Adamson J, Dickson D, Perez-Tur J, et al. Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum Mol Genet. 1999;8:711–5. doi: 10.1093/hmg/8.4.711. [DOI] [PubMed] [Google Scholar]
- Ball M, Braak H, Coleman P, Dickson D, Duyckaerts C, Gambetti P, et al. Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. Neurobiol Aging. 1997;18(4 Suppl):S1–S2. [PubMed] [Google Scholar]
- Ballard C, Ziabreva I, Perry R, Larsen JP, O’Brien J, McKeith I, et al. Differences in neuropathologic characteristics across the Lewy body dementia spectrum. Neurology. 2006;67:1931–4. doi: 10.1212/01.wnl.0000249130.63615.cc. [DOI] [PubMed] [Google Scholar]
- Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, et al. Arizona Parkinson’s Disease Consortium. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009;117:613–34. doi: 10.1007/s00401-009-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Braak H, Rüb U, Jansen Steur ENH, Del Tredici K, de Vos RAI. Cognitive status correlates with neuropathologic stage in Parkinson disease. Neurology. 2005;64:1404–10. doi: 10.1212/01.WNL.0000158422.41380.82. [DOI] [PubMed] [Google Scholar]
- Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del TK. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton LK, Blurton-Jones M, Myczek K, Trojanowski JQ, Lamerla FM. Synergistic Interactions between Abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline. J Neurosci. 2010;30:7281–9. doi: 10.1523/JNEUROSCI.0490-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colosimo C, Hughes AJ, Kilford L, Lees AJ. Lewy body cortical involvement may not always predict dementia in Parkinson’s disease. J Neurol Neurosurg Psychiatr. 2003;74:852–6. doi: 10.1136/jnnp.74.7.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL. Intellectual impairment in Parkinson’s disease: clinical, pathologic, and biochemical correlates. J Geriatr Psychiatry Neurol. 1988;1:24–36. doi: 10.1177/089198878800100106. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Fujishiro H, Orr C, DelleDonne A, Josephs KA, Frigerio R, et al. Neuropathology of non-motor features of Parkinson disease. Parkinsonism Relat Disord. 2009;15(Suppl 3):S1–S5. doi: 10.1016/S1353-8020(09)70769-2. [DOI] [PubMed] [Google Scholar]
- DSM-IV . Diagnostic and statistical manual of mental disorders. American Psychiatric Association; Washington: 1995. [Google Scholar]
- Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2007;22:1689–707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- Esiri M, Matthews F, Brayne C, Ince PG. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Lancet. 2001;357:169–75. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gallardo G, Schlüter OM, Südhof TC. A molecular pathway of neurodegeneration linking alpha-synuclein to ApoE and Abeta peptides. Nat Neurosci. 2008;11:301–8. doi: 10.1038/nn2058. [DOI] [PubMed] [Google Scholar]
- Giannakopoulos P, Herrmann FR, Bussière T, Bouras C, Kövari E, Perl DP, et al. Tangle and neuron numbers, but not amyloid load predict cognitive status in Alzheimer’s disease. Neurology. 2003;60:1495–500. doi: 10.1212/01.wnl.0000063311.58879.01. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Forman MS, Higuchi M, Golbe LI, Graves CL, Kotzbauer PT, et al. Initiation and synergistic fibrillization of tau and alpha-synuclein. Science. 2003;300:636–40. doi: 10.1126/science.1082324. [DOI] [PubMed] [Google Scholar]
- Goris A, Williams-Gray CH, Clark GR, Foltynie T, Lewis SJ, Brown J, et al. Tau and alpha-synuclein in susceptibility to, and dementia in, Parkinson’s disease. Ann Neurol. 2007;62:145–53. doi: 10.1002/ana.21192. [DOI] [PubMed] [Google Scholar]
- Grimmer T, Henriksen G, Wester HJ, Förstl H, Klunk WE, Mathis CA, et al. Clinical severity of Alzheimer’s disease is associated with PIB uptake in PET. Neurobiol Aging. 2009;30:1902–9. doi: 10.1016/j.neurobiolaging.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Guillozet al, Weintraub S, Mash DC, Mesulam MM. Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Arch Neurol. 2003;60:729–36. doi: 10.1001/archneur.60.5.729. [DOI] [PubMed] [Google Scholar]
- Halliday G, Hely M, Reid W, Morris J. The progression of pathology in longitudinally followed patients with Parkinson’s disease. Acta Neuropathol. 2008;115:409–15. doi: 10.1007/s00401-008-0344-8. [DOI] [PubMed] [Google Scholar]
- Harding AJ, Halliday GM. Cortical Lewy body pathology in the diagnosis of dementia. Acta Neuropathol. 2001;102:355–63. doi: 10.1007/s004010100390. [DOI] [PubMed] [Google Scholar]
- Harding AJ, Stimson E, Henderson JM, Halliday GM. Clinical correlates of selective pathology in the amygdala of patients with Parkinson’s disease. Brain. 2002;125:2431–45. doi: 10.1093/brain/awf251. [DOI] [PubMed] [Google Scholar]
- Harrell FE. Regression Modelling Strategies. Springer; New York: 2001. pp. 64–65. [Google Scholar]
- Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–8. [PubMed] [Google Scholar]
- Hurtig HI, Trojanowski JQ, Galvin J, Ewbank D, Schmidt ML, Lee VM, et al. Alpha-synuclein cortical Lewy bodies correlate with dementia in Parkinson’s disease. Neurology. 2000;54:1916–21. doi: 10.1212/wnl.54.10.1916. [DOI] [PubMed] [Google Scholar]
- Ince PG, Clark B, Holton JL, Revesz T, Wharton SB. Disorders of movement and system degenerations. In: Love S, Louis DN, Ellison DW, editors. Greenfield’s neuropathology. 8th edn Edward Arnold; London: 2008. pp. 889–1030. [Google Scholar]
- Jellinger KA, Attems J. Prevalence and impact of vascular and Alzheimer pathologies in Lewy body disease. Acta Neuropathol. 2008;115:427–36. doi: 10.1007/s00401-008-0347-5. [DOI] [PubMed] [Google Scholar]
- Kalaitzakis ME, Graeber MB, Gentleman SM, Pearce RKB. Striatal beta-amyloid deposition in Parkinson disease with dementia. J Neuropathol Exp Neurol. 2008;67:155–61. doi: 10.1097/NEN.0b013e31816362aa. [DOI] [PubMed] [Google Scholar]
- Kalaitzakis ME, Pearce RK, Gentleman SM. Clinical correlates of pathology in the claustrum in Parkinson’s disease and dementia with Lewy bodies. Neurosci Lett. 2009;461:12–5. doi: 10.1016/j.neulet.2009.05.083. [DOI] [PubMed] [Google Scholar]
- Kempster PA, O’Sullivan SS, Holton JL, Revesz T, Lees AJ. Relationships between age and late progression of Parkinson’s disease: a clinico-pathological study. Brain. 2010;133:1755–62. doi: 10.1093/brain/awq059. [DOI] [PubMed] [Google Scholar]
- Kovacs GG, Alafuzoff I, Al-Sarraj S, Arzberger T, Bogdanovic N, Capellari S, et al. Mixed brain pathologies in dementia: the BrainNet Europe consortium experience. Dement Geriatr Cogn Disord. 2008;26:343–50. doi: 10.1159/000161560. [DOI] [PubMed] [Google Scholar]
- Kövari E, Gold G, Herrmann FR, Canuto A, Hof PR, Bouras C, et al. Lewy body densities in the entorhinal and anterior cingulate cortex predict cognitive deficits in Parkinson’s disease. Acta Neuropathol. 2003;106:83–8. doi: 10.1007/s00401-003-0705-2. [DOI] [PubMed] [Google Scholar]
- Lashley T, Holton JL, Gray E, Kirkham K, O’Sullivan SS, Hilbig A, et al. Cortical alpha-synuclein load is associated with amyloid-beta plaque burden in a subset of Parkinson’s disease patients. Acta Neuropathol. 2008;115:417–25. doi: 10.1007/s00401-007-0336-0. [DOI] [PubMed] [Google Scholar]
- Laws SM, Friedrich P, Diehl-Schmid J, Müller J, Eisele T, Bäuml J, et al. Fine mapping of the MAPT locus using quantitative trait analysis identifies possible causal variants in Alzheimer’s disease. Mol Psychiatr. 2007;12:510–7. doi: 10.1038/sj.mp.4001935. [DOI] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Veinbergs I, Sagara Y, Mallory M, Hashimoto M, et al. Beta-amyloid peptides enhance alpha-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer’s disease and Parkinson’s disease. Proc Natl Acad Sci USA. 2001;98:12245–50. doi: 10.1073/pnas.211412398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews FE, Brayne C, Lowe J, McKeith I, Wharton SB, Ince P. Epidemiological pathology of dementia: attributable-risks at death in the Medical Research Council Cognitive Function and Ageing Study. PLoS Med. 2009;6:e1000180. doi: 10.1371/journal.pmed.1000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila PM, Rinne JO, Helenius H, Dickson DW, Roytta M. Alpha-synuclein-immunoreactive cortical Lewy bodies are associated with cognitive impairment in Parkinson’s disease. Acta Neuropathol. 2000;100:285–90. doi: 10.1007/s004019900168. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, et al. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol. 1999;46:860–6. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The consortium to establish a registry for Alzheimer’s disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Pankratz N, Wilk JB, Latourelle JC, DeStefano AL, Halter C, Pugh EW, et al. PSG-PROGENI and GenePD Investigators, Coordinators and Molecular Genetic Laboratories. Genome wide association study for susceptibility genes contributing to familial Parkinson disease. Hum Genet. 2009;124:593–605. doi: 10.1007/s00439-008-0582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkkinen L, Kauppinen T, Pirttilä T, Autere JM, Alafuzoff I. Alpha-synuclein pathology does not predict extrapyramidal symptoms or dementia. Ann Neurol. 2005;57:82–91. doi: 10.1002/ana.20321. [DOI] [PubMed] [Google Scholar]
- Perneger TV. What’s wrong with Bonferroni adjustments. BMJ. 1998;316:1236–8. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletnikova O, West N, Lee MK, Rudow GL, Skolasky RL, Dawson TM, et al. Abeta deposition is associated with enhanced cortical alpha-synuclein lesions in Lewy body diseases. Neurobiol Aging. 2005;26:1183–92. doi: 10.1016/j.neurobiolaging.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Polvikoski T, Sulkava R, Haltia M, Kainulainen K, Vuorio A, Verkkoniemi A, et al. Apolipoprotein E, dementia, and cortical deposition of beta-amyloid protein. N Engl J Med. 1995;333:1242–7. doi: 10.1056/NEJM199511093331902. [DOI] [PubMed] [Google Scholar]
- Sabbagh MN, Adler CH, Lahti TJ, Connor DJ, Vedders L, Peterson LK, et al. Parkinson disease with dementia: comparing patients with and without Alzheimer pathology. Alzheimer Dis Assoc Disord. 2009;23:295–7. doi: 10.1097/WAD.0b013e31819c5ef4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simón-Sànchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, et al. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet. 2009;41:1308–12. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi Y, Huchikado H, Dickson DW. Neuropathology of Parkinson’s disease dementia and dementia with Lewy bodies with reference to striatal pathology. Parkinsonism Relat Disord. 2007;13:S221–4. doi: 10.1016/S1353-8020(08)70005-1. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Goris A, Saiki M, Foltynie T, Compston DA, Sawcer SJ, et al. Apolipoprotein E genotype as a risk factor for susceptibility to and dementia in Parkinson’s disease. J Neurol. 2009;256:493–8. doi: 10.1007/s00415-009-0119-8. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Sugihara S, Ogawa A, Oshima N, Ihara Y. Alzheimer beta amyloid deposition enhanced by ApoE epsilon4 gene precedes neurofibrillary pathology in the frontal association cortex of nondemented senior subjects. J Neuropathol Exp Neurol. 2001;60:731–9. doi: 10.1093/jnen/60.7.731. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.