Abstract
Comparing data from both the 1999 MCBS and drug utilization data supplied by the survey respondents' pharmacies, the author details the methods used to determine the level of misreporting of drug expenditures in the MCBS. Findings suggest that prescription drug expenditures are underreported by 17 percent and the number of prescriptions used is underreported by 17.7 percent. The data also identify demographic factors that predict a beneficiary's likelihood to either overreport or underreport his or her medications, as well as the extent to which beneficiaries misreport their drug use and spending.
Background
Interest in prescription drug expenditures, as they relate to high out-of-pocket costs and possible drug coverage expansion, remains high. Prescription drug spending rose almost 16.0 percent in 2001 and is projected to rise an average of 11.1 percent per year between 2002 and 2012 (Heffler et al., 2003). Senior citizens are particularly vulnerable to these rising costs, partially due to higher incidence of chronic disease, many of which can be effectively treated with prescription medication.
Adding a prescription drug benefit to Medicare has been the focus of debate on Capitol Hill for several years and remains a foremost policy issue. After controlling for factors like age, supplementary insurance status, and income, recent findings suggest that Medicare beneficiaries without drug coverage fill fewer prescriptions than their covered counterparts (Poisal and Murray, 2001). Moreover, many beneficiaries skip dosages or avoid filling prescriptions entirely due to prohibitively high drug costs (Steinman, Sands, and Covinsky, 2001). These findings emphasize the importance of prescription drug coverage for the Medicare population.
In response to legislative proposals to add a drug benefit to Medicare, CMS's Office of the Actuary and the U.S. Congressional Budget Office are regularly asked to make cost projections, many of which rely on survey prescription drug costs and utilization data. Surveys, however are subject to various kinds of error, including the tendency of respondents to misreport their usage of medical services (Groves, 1989). When using survey data for estimating costs, several assumptions must be made to accurately project these expenditures including adjustments for survey misreporting, institutional drug usage, and the degree to which demand would increase with the passage of a new benefit or alteration of an existing one.
Different agencies have arrived at different results from the same survey, specifically because of differing assumptions about the accuracy of reporting. This article reports primarily on the methods, but also on the results, of an attempt to quantify the extent to which prescription drug expenditures are misreported in one such survey—the MCBS.1 The MCBS is an ongoing household panel survey of about 13,000 Medicare beneficiaries, conducted by CMS (Adler, 1994). Annually, CMS produces two files: the Access to Care File and the Cost and Use File. The Cost and Use File contains data on health care utilization and expenditures for beneficiaries “ever enrolled” in Medicare, including persons who enrolled in the program or died during the year. This file also includes data on Medicare covered services, as well as those not covered by Medicare, such as prescription drugs.
Household surveys of health and health expenditures, such as the MCBS, are subject to non-response and misreporting of medical events (Eppig and Chulis, 1997). As a general rule, health events that are farther removed in time and those that are less prominent are less likely to be recalled at the time of interview (Cohen and Burt, 1985). Prescription drug purchases are no exception. During each interview, respondents are asked about all of their medication use since their last interview. The MCBS takes steps to minimize recall error by beneficiaries. For example, respondents are asked to retain and bring to their interview any prescription bottles, packages, or receipts associated with their medication use. They are also encouraged to make notes on calendars, provided by the survey, to record all of their health care events. Finally, utilizing computer-assisted personal interviews, MCBS interviewers are furnished with a list of all prescription drugs reported in previous interviews so they can ask whether the respondent has taken any of those drugs during the most recent reporting period. However, to date, there have been no efforts to assess whether or how much misreporting occurs.
This article provides an answer to that question via a multi-step process. First, the author collected and compared data from a survey of MCBS beneficiaries and the pharmacies they used. Then the author determined the differences in reporting rates for MCBS respondents for whom there was complete survey and pharmacy data. Finally, the author generalized the results to the entire non-institutionalized MCBS population, based on a series of micro-simulation models. This effort culminated in an estimate of the direction and magnitude of reporting errors, as well as the identification of the social, economic, and demographic correlates of those errors.
Data
Collecting Pharmacy Data
To test the extent of misreported prescription drug use and spending in the MCBS, a pharmacy follow-back study was designed and conducted in the first 4 months of 2000.
Four types of MCBS respondents were omitted from the study. They included the following:
Respondents who were institutionalized for all of CY 1999 were not asked to participate.
Persons who lived in the community during 1999, but were institutionalized at the time of their spring interview were excluded because facility interviews are conducted with a representative of the institution, not with the beneficiary.2
Respondents for whom a proxy answered on their behalf.3
Beneficiaries who were not enrolled in Medicare for all 12 months of 1999 (including deaths).
The remaining survey participants (n=9,384) were asked to request patient profiles from all the pharmacies where they obtained their drugs in 1999.4 Sample persons who had not reported any medication use in 1999 were also asked to participate in the study; beneficiaries were asked to identify the pharmacies that they normally used to fill a prescription.
Respondents who agreed to participate were demographically similar to those who declined participation with respect to age, sex, race, and metropolitan/non-metropolitan status. They were asked to supply the names and addresses of every pharmacy they used during 1999. To help beneficiaries recall their pharmacies, interviewers suggested referring to medicine labels, receipts, telephone books, and pharmacy directories. Each respondent was asked to sign a pre-printed letter, requesting a profile of his or her 1999 drug utilization from each pharmacy on the list.
Of those asked to participate in the pharmacy follow-back study (Table 1), more than one-half were complete responses (e.g., not only did they participate, but all of their reported pharmacies submitted prescription profiles on their behalf). Only respondents for whom all pharmacies returned usable profiles were examined in this analysis. Thus, the effective response rate was 57 percent (Table 1).
Table 1. Pharmacy Follow-Back Study Status of Sample Persons in the Medicare Cost and Use File: CY 1999.
| Follow-Back Category | Sample Persons | MCBS Respondents | Study Participants or Non-Participants |
|---|---|---|---|
|
| |||
| Percent | |||
| Total Sample | 13,106 | 100 | NA |
| Excluded from the Follow-Back Study | 3,722 | 28 | 100 |
| No 1999 Event Level Drug Data Collected in Facility for All of 1999 | 946 | NA | 25 |
| New Enrollee in 1998 or 1999 | 638 | NA | 17 |
| Didn't Receive Round 26 Interview | |||
| Proxy Interviews | 1,162 | NA | 31 |
| Spring Interview Was Facility Interview | 227 | NA | 6 |
| Deaths and Refusals | 749 | NA | 20 |
| Asked to Participate in Follow-Back Study | 9,384 | 72 | 100 |
| Refused | 570 | NA | 6 |
| No Pharmacies Reported by Beneficiary | 408 | NA | 4 |
| Reported 1 or More Pharmacies | |||
| Partial Complete1 | 813 | NA | 9 |
| Pharmacy Non-Response or Unusable Data2 | 2,291 | NA | 24 |
| All Pharmacies Reported Usable Data | 5,302 | NA | 57 |
At least 1, but not all pharmacies responded.
For example, missing or invalid dates.
NOTES: Numbers may not add to totals due to rounding. NA is not applicable.
SOURCE: Centers for Medicare & Medicaid Services: Office of Research, Demonstrations and Information: Data from the Medicare Current Beneficiary Survey, Pharmacy Follow-Back Study, 1999.
Including the Proper Medications for Analysis
A number of edits were necessary prior to analysis of the data. From the MCBS, all beneficiary-reported drug names were standardized; misspelled words were corrected and drug names were reformatted. Over-the-counter medications reported by the respondent were dropped.6
In preparing the pharmacy profile data, beneficiaries were dropped from the analysis if any of their profiles contained prescription drug events with a day or month missing. As with the beneficiary-reported data, all drug names were standardized and any over-the-counter medications were deleted.
The next step was to ensure that beneficiary-reported drugs and pharmacy-reported drugs were from exactly the same time periods. Unlike the pharmacy profile data, beneficiary-reported data do not record dates of purchase. Nevertheless, the survey establishes a recall reference period with beginning and ending dates. Drug purchases for CY 1999 were recorded in 4 rounds of interviews, numbered 23, 24, 25, and 26. Because the reference period for any interview is the previous 4 months, drug purchases recalled in rounds 23 and 26 could have occurred during the end of 1998 or the beginning of 2000. All reported drugs for rounds 24 and 25 (June-December) were purchased in 1999; therefore, the survey data analyzed were limited to those rounds.
Including the proper drugs from the pharmacy reports involved a simple process of date comparisons. For each person, all beneficiary-reported drugs collected in rounds 24 and 25 were included; all pharmacy-reported drugs that fell between the beginning date of the round 24 reference period and the ending date of the round 25 interview were included. The results were a total of 101,144 pharmacy-reported drug events and 96,878 survey-reported drug events.
Matching Medications from Beneficiary and Pharmacy
An initial attempt was made to match beneficiary-reported drugs to pharmacy-reported drugs electronically. For each event in the survey-reported file, a variable, MATCH_KEY, was created that contained the sample person's personal identification code, the drug name, and a sequence number; there was one record per beneficiary, per drug, per purchase (including refills).
The same process was carried out on the pharmacy-report file; the records from the two files were matched on the variable MATCH_KEY. The automated merge produced 64,273 matches; 36,871 events appeared only in the pharmacy file and 32,605 events appeared only in the survey file.
Examination of the pharmacy-only and survey-only records revealed many missed matches. There were many events in which a generic name was reported by one party while the brand name was reported by the other. There were also events in which the drug name was converted to different standardized names. For instance, if a beneficiary reported the name, “Cardizem®,” and the pharmacy reported the drug, “Cardizem® SR,” then the prescriptions failed to match during the electronic merge.
This manual review of the electronic match improved the agreement between pharmacy- and survey-reported events in the aggregate. The matched figure increased by 9,246 to 73,519. The pharmacy-only figure fell to 27,625 and the survey-only figure dropped to 23,359.
Unmatched survey-reported drugs were further classified into one of two categories: survey overreports or omitted-pharmacy underreports. A prescription was assigned survey overreport status if there was any mention of that drug in the pharmacy file. For instance, if a beneficiary reported four prescriptions of verapamil and his or her pharmacy reported at least one verapamil script, then the unmatched survey reported drugs were classified as survey overreports. The author assumed that survey overreports were possible if a beneficiary “telescoped” a refill; that is, the beneficiary reported a refill which occurred in an earlier round or which never occurred at all. Of the 23,359 survey-only drugs, 12,779 were assigned survey over-report status.
A drug was assigned omitted-pharmacy underreport status if that drug name did not appear in the pharmacy data. Borrowing information from the previously mentioned example, if a beneficiary reported four verapamil purchases, but the pharmacy did not report any verapamil refills, those survey-reported medications were classified as omitted-pharmacy under reports. The author assumed that this situation was possible if a beneficiary failed to report all of his or her pharmacies and the beneficiary's unmatched prescriptions were filled in one of these “omitted” pharmacies. In total, 10,580 survey-only drugs were assigned omitted-pharmacy underreport status.
Methods
Model: Misreporting Prescription Drug Utilization
The author explored several misreporting models, which varied in their assumptions regarding the source and nature of survey-only events. One model (not shown) assumed all drugs and all pharmacies were perfectly reported, resulting in a net-adjusted under-reporting rate of 4.2 percent (1-(96,878/101,144)) among follow-back participants. Provided that surveys are subject to non-response and misreporting of events, these two assumptions did not appear to be reasonable. Another model (not shown) assumed all overreported survey drugs were filled in omitted pharmacies (e.g., those pharmacies whose names were not given to MCBS interviewers), but were perfectly reported, resulting in a net-adjusted underreporting rate of 22.2 percent (1-(96,878/(101,144 + 23,359)). This model did not allow for the possibility that some beneficiaries may report more prescriptions than they actually filled from the pharmacies they identified. Once more, the assumption of perfectly-reported survey drugs was questionable.
The misreporting model shown here was deemed most appropriate for this estimate. It allowed for the possibility that some beneficiaries may have misreported their drug experience and also misreported the number of pharmacies they used in 1999. The model assumed the same rate of over- or underreporting of drug use in omitted pharmacies as in reported pharmacies.
The formula resulted in a net-adjusted underreporting rate of 14.7 percent among follow-back participants and may be written as follows:
Misreporting Model
With the numbers:
Where:
P=Sum of all prescriptions reported by beneficiary's pharmacies7
M=Number of matched prescriptions
O1=Number of non-matched survey-only prescriptions that were deemed a result of survey overreports
O2=Number of non-matched survey-only prescriptions that were deemed a result of omitted-pharmacy underreports
S=All survey-reported prescriptions or M+O1+O2, and
R=Net-adjusted underreporting rate.
Working backwards through the equation, the assumptions contained in the model may be explained in the following three steps:
P/(M+O1): Estimates the reporting rate for the beneficiary for all of the pharmacies that the beneficiary reported in the follow-back study (recall that O1 represents unmatched survey-reported refills of a drug located in the beneficiary's pharmacy-reported data).
(O2 * P/(M+O1)): Multiplying the reporting rate calculated in step 1 by O2 (or those unmatched survey-reported medications that were not encountered in any of the pharmacy-supplied drugs) results in the number of drugs that would have been reported by unidentified pharmacies, had they been queried. For beneficiaries without O2-classified drugs, this section of the equation was set to 0.
1-(S/(P+(O2 * P/(M+O1)))): Estimates the total reporting rate by dividing all of the beneficiary-reported drugs by the estimated number of pharmacy-reported drugs. Subtracting that total from 1 calculates the net-adjusted underreporting rate.
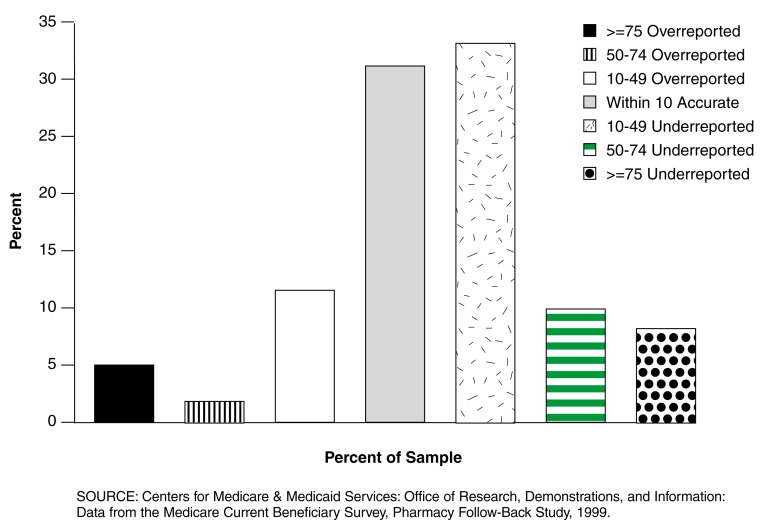
The distribution of net-adjusted utilization underreporting rates, using the misreporting model, appears in Figure 1.
Figure 1. Distribution of Net-Adjusted Underreporting Rates for Follow-Back Participants: CY 1999.
AWP Expenditures
In order to estimate expenditure misreporting, all survey- and pharmacy-reported events were electronically passed through a published industry source First DataBank's Bluebook (www.FirstDataBank.com) that assigns a unit average wholesale price (AWP) to each prescription. This step was required for two important reasons: (1) the expenditure data collected in the survey, as well as data collected in the pharmacy follow-back study, were often missing and (2) a total drug price reported by a beneficiary may exclude certain price adjustments reflected in the pharmacy-reported data. For these reasons, expenditures were standardized to AWP.
Depending on the completeness of the drug data record, the author used a variety of techniques to impute an event price or total AWP for that prescription.8 The average event price for all survey-reported drugs was $50.31; the average for all pharmacy-reported drugs was $49.62.
In order to pass the results of the imputation through the misreporting model, all survey- and pharmacy-reported drugs were electronically organized into the same categories, as described in the misreporting model (P, M, O1, O2). Their averages appear in Table 2.
Table 2. Pharmacy Follow-Back Study Unweighted and Weighted Results of AWP Imputation, by Category: CY 1999.
| Survey Reported | Number of Prescriptions | AWP | |
|---|---|---|---|
|
| |||
| Unweighted Average | Weighted Average | ||
| All Matched (M) | 73,519 | $49.30 | $49.47 |
| Unmatched | 23,359 | 53.45 | 53.62 |
| Survey Overreports (O1) | 12,779 | 50.94 | 51.53 |
| Pharmacy Underreports (O2) | 10,580 | 56.48 | 56.08 |
| Pharmacy Reported | |||
| All (P) | 101,144 | 49.62 | 45.85 |
NOTES: AWP is average wholesale price. MCBS weights reflect the probabilities of selection for the MCBS, adjusted for under coverage and non-response. They have been post-stratified for age, sex, region, metropolitan residence, and year of entry into the sample. (M) is the number of matched prescriptions. (P) is the sum of all prescriptions reported by the beneficiary's pharmacies.
SOURCE: Centers for Medicare & Medicaid Services: Office of Research, Demonstrations and Information: Data from the Medicare Current Beneficiary Survey, Pharmacy Follow-Back Study, 1999.
After classifying and pricing each drug and in preparation for the micro-simulation phase of the estimate, the total survey-reported AWP expenditures and the total adjusted pharmacy-reported AWP expenditures were summed to the person level. Total survey-reported AWP expenditures were also estimated for all of the non-follow-back participants. For this cohort, event prices were derived by passing their reported prescriptions through First DataBank's Bluebook, using the identical event price algorithm that was used to estimate total AWP levels for the drugs collected in the follow-back study.
Determining Factors Predictive of Expenditure Misreporting
A multi-step process was used to determine the overall net-adjusted underreporting of prescription drug expenditures in the MCBS to include both follow-back and non-follow-back participants. The author began by analyzing the demographic data of the follow-back participants to determine those factors that were predictive of a person's AWP expenditure-reporting status. Next, the author determined those factors that were predictive of the degree to which a person under- or overreports his or her prescription expenditures. After these models were developed, they were applied to beneficiaries not in the follow-back study so that an aggregate estimate of expenditure misreporting could be made.
Multiple demographic and socioeconomic variables were examined, using multinomial logistic regression to test their predictive power of a person's expenditure-reporting status. The original model tested the following variables: number of beneficiary-reported prescriptions, total AWP, age category, ethnicity, health status, number of chronic conditions, income, number of inpatient hospitalizations, number of doctor visits, number of home health visits, number of outpatient procedures, sex, prescription drug coverage status, and supplementary health insurance status.
All but two variables were collected in the survey; the exceptions were AWP and drug coverage. Drug coverage, a derived field, was based on responses to several drug coverage-related questions in the survey. For the purposes of this analysis, drug coverage was assigned if a beneficiary had at least 1 month of drug coverage in 1999. Beneficiaries were categorized as covered if one or more of the following occurred:
M+C Beneficiaries—They belonged to a plan that offered prescription drug coverage as part of its basic benefit package or they purchased such coverage via an added premium.
Medicaid Beneficiaries—They were fully entitled, as determined by CMS administrative data, or they self-identified Medicaid drug coverage.
Privately-Insured Beneficiaries—They reported a private plan (employer sponsored or individually purchased) that covered their prescription drugs.
Other Public Insured Beneficiaries—They reported drug coverage from State-based pharmaceutical assistance programs, the Department of Veterans Affairs, the Department of Defense, or any other public source.
All Beneficiaries—They reported any third-party reimbursements for a prescription drug.9
Expenditure-reporting status for follow-back participants was one of three types:
Underreporters were defined as those beneficiaries whose survey-reported total AWP expenditures were less than the estimated total AWP expenditures from pharmacies.
Overreporters were those enrollees who reported total AWP expenditures greater than pharmacy estimates.
Persons were labeled perfect reporters if their survey-reported AWP drug expenditures matched the total estimated AWP expenses from pharmacies. The unweighted frequencies of each category among the pharmacy follow-back participants were 3,221 (60.8 percent), 1,564 (29.5 percent), and 511 (9.7 percent), respectively.
Study members averaged 365 days in the community during 1999 while non-members spent 332 days in the community.10 In order to adjust for this experience, for modeling development purposes, total AWP expenditures per beneficiary were standardized to annual figures by dividing 365 days by each beneficiary's community days.
As determined by multi-nomial logistic regression applied to pharmacy follow-back participants relative to perfect reporters, there were 10 factors that were statistically significant and predictive of reporting status. Not all variables were significant for both under- and overreporters. Those variables included:
Annualized total prescriptions.
Annualized total AWP.
Level of self-reported health status.
Number of chronic conditions.
Number of doctor visits.
Other race/ethnicity (1=yes, 0=no).11
Medicare risk plan (1=yes, 0=no).
Medicaid (1=yes, 0=no).
Employer sponsored (1=yes, 0=no).
Female (1=yes, 0=no).
Generalizing Results to the Full Population
To apply these results to the entire population, the results from the multi-nomial regression model, as applied to the follow-back members, were used to impute reporting status for non-follow-back beneficiaries.12 Following the assignment of reporting status, the author estimated the degree to which respondents either underor overreported their drug expenses for the entire year, using separate models for each category. Using only follow-back participants, an inflation factor was computed at the person level to annualize the level of AWP expenditures that had been identified as either under- or overreported. The factor was computed by dividing the sum of AWP expenses estimated for all of the beneficiary's CY 1999 purchases by the sum of his or her AWP expenditures from the period covered by the follow-back study. The mechanics of this operation, as applied only to follow-back participants, appear in Table 3.
Table 3. Pharmacy Follow-Back Study Examples of Calculations to Estimate Annualized Under- and Overreported AWP Expenditures: CY 1999.
| Person's Identification | Person's Identification Number1 | ||
|---|---|---|---|
|
| |||
| 00000100 | 00000200 | 00000300 | |
|
| |||
| Status | Overreporting | Underreporting | Perfect |
| Total AWP | $1,000 | $1,200 | $400 |
| Total AWP from Follow-Back Period | 800 | 800 | 240 |
| Underreported AWP from Follow-Back Period | NA | $200 | NA |
| Overreported AWP from Follow-Back Period | 100 | NA | NA |
| Annualized Underreported AWP | NA | 300 | NA |
| Annualized Overreported AWP | 125 | NA | NA |
| Factor | 1.25 | 1.50 | 1.67 |
Beneficiaries are typically identified with an alpha-numeric field unique to them.
NOTES: AWP is average wholesale price. NA is not applicable.
SOURCE: Centers for Medicare & Medicaid Services: Office of Research, Demonstrations, and Information: Data from the Medicare Current Beneficiary Survey, Pharmacy Follow-Back Study, 1999.
Restricted to underreporting follow-back participants, a multi-linear regression model was developed to determine which factors were predictive of the level of annualized underreported AWP. The model produced an R2 of 13.8 percent and showed that the following variables significantly increased the level of underreporting:
Annualized AWP.
Number of chronic conditions.
Number of doctor visits.
Drug coverage (1=yes, 0=no).
African American (1=yes, 0=no).
Other public coverage (1=yes, 0=no).
Being an aged beneficiary (age 65 and over) significantly decreased the level of expenditure underreporting.
The model was then used to assign an annualized amount of underreported dollars to all non-follow-back members designated to be underreporters.
Identical processes were adopted for predicting the annualized level of overreported AWP expenses among overreporters in the follow-back study, as well as assigning annualized overreported dollars to non-follow-back respondents. That model produced an R2 of 12.9 percent. Annualized AWP and chronic conditions were shown to significantly increase the level of expenditure overreporting. Being an aged beneficiary as well as an increasing number of home health visits had the opposite effect.
Calculating Final Expenditure Misreporting Rate
The next step was to convert the annualized level of over- or underreported expenditures to reveal the actual experience of each beneficiary. Reduction ratios were developed by dividing the respondent's total CY 1999 AWP expenditures by his or her annualized AWP expenditures. Table 4 illustrates how the annualized levels of under- and overreported AWP for all beneficiaries (follow-back and non-follow-back participants included) were then multiplied by these ratios, resulting in their corresponding adjusted levels. Recall that follow-back participants had 365 days of community exposure, meaning their reduction ratios were equal to 1.
Table 4. Pharmacy Follow-Back Study Examples of Transforming Annualized AWP Estimates into Actual AWP Estimates: CY 1999.
| Person's Identification | Person's Identification Number1 | ||
|---|---|---|---|
|
| |||
| 00000400 | 00000500 | 00000600 | |
|
| |||
| Status | Overreporting | Underreporting | Perfect |
| Total AWP | $1,500.00 | $2,000.00 | $400.00 |
| Annualized AWP | 1,800.00 | 2,500.00 | 600.00 |
| Annualized Undereported | NA | $800.00 | NA |
| Annualized Overreported | 400.00 | NA | NA |
| Adjusted Underreported | NA | 640.00 | NA |
| Adjusted Overreported | 333.33 | NA | NA |
| Ratio | 0.83 | 0.80 | 0.67 |
Beneficiaries are typically identified with an alpha-numeric field unique to them.
NOTES: AWP is average wholesale price. NA is not applicable.
SOURCE: Centers for Medicare & Medicaid Services: Office of Research, Demonstrations, and Information: Data from the Medicare Current Beneficiary Survey, Pharmacy Follow-Back Study, 1999.
Next, the author calculated the level of AWP expenditures believed to have been purchased by the beneficiary (Table 5). A comparison between the total AWP collected in the MCBS and the estimated level of AWP expenses showed a net-adjusted expenditure underreporting rate of 17 percent.
Table 5. Pharmacy Follow-Back Study Example Calculations of Estimated Purchases: CY 1999.
| Person's Identification | Person's Identification Number1 | ||
|---|---|---|---|
|
| |||
| 00000400 | 00000500 | 00000600 | |
|
| |||
| Status | Overreporting | Underreporting | Perfect |
| Total AWP | $1,500.00 | $2,000.00 | $400.00 |
| Annualized AWP | 1,800.00 | 2,000.00 | 600.00 |
| Annualized Underreported | NA | $800.00 | NA |
| Annualized Overreported | 400.00 | NA | NA |
| Adjusted Underreported | NA | 640.00 | NA |
| Adjusted Overreported | 333.33 | NA | NA |
| Estimated AWP Purchases | 1,667.67 | 2,640.00 | 400.00 |
| Ratio | 0.83 | 0.80 | 0.67 |
Beneficiaries are typically identified with an alpha-numeric field unique to them.
NOTES: AWP is annual wholesale price. NA is not applicable.
SOURCE: Centers for Medicare & Medicaid Services: Office of Research, Demonstrations, and Information: Data from the Medicare Current Beneficiary Survey, Pharmacy Follow-Back Study, 1999.
Discussion
This analysis addresses the important issue of adjusting survey-reported drug use and expenditure data to account for survey underreporting. It has demonstrated several critical findings regarding prescription drug data collection among the Medicare elderly and disabled populations.
Utilization
Medicare beneficiaries, on average, underreport 17.7 percent of their drug utilization, as measured in the number of prescriptions filled or refilled (average survey reported = 23.3, average estimated = 28.3).
Nearly one-quarter (23 percent) of beneficiaries actually overreport their drug utilization in surveys.
Adjusted for underreporting (Table 6), the data show that approximately 25 percent of Medicare beneficiaries filled more than 40 prescriptions in CY 1999.
The probability of misreporting drug use increases with utilization and the number of chronic conditions.
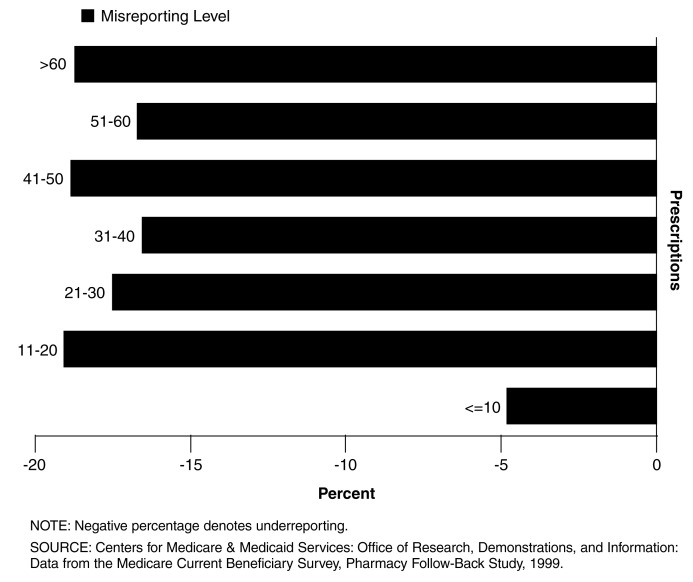
The most accurate utilization reporters (Figure 2) filled between 1 and 10 prescriptions in CY 1999.
Although the number of misreported drugs increases with utilization, the rate at which they are misreported (Figure 2) is relatively consistent once the beneficiary exceeds the 10 prescription threshold.
Among utilization overreporters, heavy prescription drug users and racial/ethnic minorities tend to overreport to a greater degree.
Among utilization underreporters, the number of medication purchases that beneficiaries underreport increases as the number of physician visits goes up but decreases for those who are privately insured.
Table 6. Pharmacy Follow-Back Study Distributions of Reported and Estimated Drug Use: CY 1999.
| Percentile | Prescription Drug Use | |
|---|---|---|
|
| ||
| Estimated | Reported | |
| 5 | 0.0 | 0 |
| 10 | 0.0 | 0 |
| 25 | 9.0 | 6 |
| 50 | 21.5 | 17 |
| 75 | 40.3 | 34 |
| 90 | 63.7 | 54 |
| 95 | 80.4 | 70 |
NOTE: Columns are calculated independently.
SOURCE: Centers for Medicare & Medicaid Services: Office of Research, Demonstrations, and Information: Data from the Medicare Current Beneficiary Survey, Pharmacy Follow-Back Study, 1999.
Figure 2. Misreporting Rates, by Estimated Prescriptions Per Beneficiary Category Among Prescription Drug Users: CY 1999.
Expenditures
Medicare beneficiaries, on average, underreport 17 percent of their drug expenses (average survey reported = $1,253.25, average estimated = $1,510.23).
Twenty-eight percent of beneficiaries overreport their prescription drug spending.
As measured by total AWP, the probability of misreporting drug spending increases with the beneficiary's expenditures and his or her enrollment in an M+C plan or Medicaid.
An analysis of the percentile distributions (Table 7) shows that, when adjusted for underreporting, the median spending level exceeded $1,000 in CY 1999, up from an unadjusted figure of $809.
Among prescription drug users, the most accurate expenditure reporters, as shown in Table 8, tend to be those beneficiaries who purchased between $250 and $500 in drugs, as measured by AWP expenses.
Among expenditure overreporters, being an aged beneficiary mitigates the degree of overreporting.
Among expenditure underreporters, the amount of expenses that beneficiaries underreport increases with the number of physician visits.
Beneficiaries frequently report incomplete drug names (eg., Cardizem® instead of Cardizem® CR). As a result, drug cost estimates are below actual total expenditures. This more than offsets the practice of inadvertently reporting more expensive brand-name drugs when, in fact, beneficiaries received less expensive generic drugs.
With respect to average drug prices, Medicare beneficiaries tend to report drug purchases that were, to some extent, higher in cost and not report drug purchases that were somewhat less expensive, marginally offsetting their recall error rate.
Table 7. Pharmacy Follow-Back Study Distributions of Reported and Estimated AWP Expenditures: CY 1999.
| Percentile | Total AWP | |
|---|---|---|
|
| ||
| Estimated | Reported | |
| 5 | $0.00 | $0.00 |
| 10 | 0.00 | 0.00 |
| 25 | 326.85 | 225.03 |
| 50 | 1,028.20 | 809.62 |
| 75 | 2,108.99 | 1,720.67 |
| 90 | 3,468.01 | 2,891.03 |
| 95 | 4,595.98 | 3,936.13 |
NOTES: AWP is average wholesale price. Columns are calculated separately.
SOURCE: Centers for Medicare & Medicaid Services: Office of Research, Demonstrations, and Information: Data from the Medicare Current Beneficiary Survey, Pharmacy Follow-Back Study, 1999.
Table 8. Pharmacy Follow-Back Study Misreporting Rates Among Prescription Drug Users by Estimated AWP Expenditure Category: CY 1999.
| Estimated AWP Range | Average Estimated AWP | Average Reported AWP | Misreporting Level Percent |
|---|---|---|---|
| $1-$250 | $124.60 | $196.46 | 57.7 (+) |
| $251-$500 | 374.93 | 338.91 | 9.6 (-) |
| $501-$750 | 621.85 | 535.95 | 13.8 (-) |
| $751-$1,000 | 873.00 | 715.22 | 18.1 (-) |
| $1,001-$1,250 | 1,122.71 | 930.05 | 17.2 (-) |
| $1,251-$1,500 | 1,369.40 | 1,103.79 | 19.4 (-) |
| $1,501-$1,750 | 1,623.46 | 1,309.45 | 19.3 (-) |
| $1,751-$2,000 | 1,868.18 | 1,530.59 | 18.1 (-) |
| $2,001-$2,250 | 2,125.82 | 1,673.73 | 21.3 (-) |
| $2,251-$2,500 | 2,369.07 | 1,934.03 | 18.4 (-) |
| >$2,501 | 4,258.02 | 3,488.21 | 18.1 (-) |
NOTES: (+) positive percentages denotes overreporting; (-) negative percentages denote underreporting. AWP is average wholesale price.
SOURCE: Centers for Medicare & Medicaid Services: Office of Research, Demonstrations, and Information: Data from the Medicare Current Beneficiary Survey, Pharmacy Follow-Back Study, 1999.
Finally, prior to this analysis, CMS' Information and Methods Group (IMG) recommended using the underreporting estimate for physician visits (30 percent) as a proxy for underreporting prescription drugs.13 Adjusted for a net-expenditure underreporting rate of 17 percent, CY 1999 MCBS data indicate that outpatient prescription drug spending among the non-institutionalized Medicare population totaled approximately $46.7 billion. Given that level of expenditure, precise assumptions about survey misreporting take on added significance; a difference of just 1 percent in the underreporting estimate can change total projected annual outlays by nearly $570 million. The author believes this analysis will help inform policymakers and contribute to more accurate cost estimates in legislative proposals involving prescription drugs.
Acknowledgments
The author wishes to thank Charles Waldron, Dan Waldo, Frank Eppig, Cliff Bailey, and Tom Rice for their input and guidance in the development of this article.
Footnotes
The author is with the Centers for Medicare & Medicaid Services (CMS). The views expressed in this article are those of the author and do not necessarily reflect the views of CMS.
Although this article focuses on the methods used to estimate the misreporting of MCBS's drug expenditures, a similar analysis was conducted on utilization reporting rates (Poisal, 2003).
Spring (round 26) interviews were conducted between January and April 2000.
There are times when a beneficiary is unable to participate in the MCBS interview. Where possible, someone familiar with the beneficiary's health care utilization and expenditures serves as a proxy.
The total number of respondents in the 1999 MCBS Cost and Use File was 13,106; not all were selected to participate in the study.
Demographically, beneficiaries for whom we received complete responses did not significantly differ from the remaining beneficiaries.
MCBS interviewers are instructed not to collect over-the-counter medications.
The author assumed that all pharmacy-reported drugs were accurate.
For the majority of drugs, AWP was imputed based on matched names, forms, and strengths. For others, AWP was imputed based on name only.
There were isolated cases where beneficiaries reported a third-party drug payment made on their behalf, yet they did not identify drug coverage from any private or public insurance.
This variation is explained by inclusion rules: Non-participants included beneficiaries who began receiving Medicare benefits during the year, died during the year, or moved between facilities and the community.
Other categories included Asians, Hispanics, and North American Natives.
None of the beneficiaries in the pharmacy follow-back study had his or her reporting status changed.
IMG is located within the Office of Research, Development, and Information and is responsible for maintaining and analyzing the MCBS.
Reprint Requests: John A. Poisal, M.B.A., Centers for Medicare & Medicaid Services, 7500 Security Boulevard, C3-16-27, Baltimore, MD 21244-1850. E-mail: jpoisal@cms.hhs.gov
References
- Adler G. A Profile of the Medicare Current Beneficiary Survey. Health Care Financing Review. 1994 Summer;15(4):153–163. [PMC free article] [PubMed] [Google Scholar]
- Cohen SB, Burt VL. Data Collection Frequency Effect in the National Medical Care Expenditure Survey. Journal of Economic and Social Measurement. 1985;13:125–151. Internet address: http://www.amstat.org/sections/srms/Proceedings/papers/1985_099.pdf (Accessed 2004) [PubMed] [Google Scholar]
- Eppig FJ, Chulis GS. Matching MCBS and Medicare Data: The Best of Both Worlds. Health Care Financing Review. 1997 Spring;18(3):211–229. [PMC free article] [PubMed] [Google Scholar]
- Groves RM. Survey Errors and Survey Costs. John Wiley and Sons; New York, NY.: 1989. [Google Scholar]
- Heffler S, Smith S, Keehan S, et al. Health Spending Projections for 2002-2012. Health Affairs. 2003 Mar-Apr;22(2):w3-54–w3-65. doi: 10.1377/hlthaff.w3.54. Internet address: http://content.healthaffairs.org/cgi/reprint/hlthaff.w3.54v1.pdf (Accessed 2004) [DOI] [PubMed] [Google Scholar]
- Poisal JA. Misreporting of Drug Expenditures in the Medicare Current Beneficiary Survey. Baltimore, MD.: 2003. Centers for Medicare & Medicaid Services: Unpublished data. [Google Scholar]
- Poisal JA, Murray LA. Growing Differences Between Medicare Beneficiaries With and Without Drug Coverage. Health Affairs. 2001 Mar-Apr;20(2):74–85. doi: 10.1377/hlthaff.20.2.74. [DOI] [PubMed] [Google Scholar]
- Steinman M, Sands L, Covinsky K. Self-Restriction of Medications Due to Cost in Seniors Without Prescription Coverage. Journal of General Internal Medicine. 2001 Dec;16(12):793–799. doi: 10.1111/j.1525-1497.2001.10412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]