Abstract
The polycomb group protein, enhancer of zeste homolog 2 (EZH2), is a transcriptional repressor involved in cell cycle regulation and has been linked to aggressive breast cancer. We examined the clinical and biological significance of EZH2 expression in triple-negative breast cancers. Tissue microarrays were constructed with invasive breast cancer cases and stained with EZH2, cytokeratin 5/6, epidermal growth factor receptor 1(EGFR) and p53. The expression of these markers was correlated with clinicopathologic variables and patients’ outcome. Furthermore, in vivo EZH2 gene silencing was achieved using siRNA incorporated into chitosan nanoparticles. Out of 261 cases of invasive breast cancer, high expression of EZH2 was detected in 87 (33%) cases, and it was strongly associated with a triple-negative breast cancer phenotype (P<.001) compared to all other non-triple negative breast cancers. Furthermore, high EZH2 was significantly associated with high histologic grade (P=.01), estrogen receptor negativity (P<.001), progesterone receptor negativity (P<.001), EGFR positivity (P=.04), and high p53 expression (P<.001). Survival analysis demonstrated that patients with high EZH2 had a poorer overall survival, compared to those with low EZH2 (P=.03), and it retained its significance as an independent prognostic factor (P=.02). In addition, EZH2 gene silencing resulted in significant reduction in tumor growth (P<.01) in the orthotopic MB-231 mouse model of breast carcinoma. Our results show that high EZH2 expression is significantly associated with triple-negative breast cancer and decreased survival. EZH2 may represent a potential therapeutic target for this aggressive disease, which warrants further investigation.
INTRODUCTION
The enhancer of zeste homolog 2 (EZH2) is a member of the polycomb group of genes (PcG), which are important for transcriptional regulation through nucleosome modification, chromatin remodeling, and interaction with other transcription factors. EZH2 serves as a histone methyl transferase, and disruption of EZH2 expression may lead to dysregulation of genes critical for the G2-M transition. EZH2 was shown to be overexpressed in many malignancies including breast, prostate, and endometrial cancers, and was suggested as a candidate for targeted treatment. In breast cancer, Kleer et al showed that EZH2 expression was increased in malignant tumors and was further associated with larger tumor size, negative estrogen receptor (ER), negative progesterone receptor (PR), advanced stage of disease, and reduced survival.
Triple-negative breast cancers (TNBCs), i.e. ER negative, PR negative, and human epidermal growth factor 2 (HER2) negative, comprise approximately 15% of all breast cancers and have an aggressive clinical course with high rates of local and systemic relapse. The clinical course appears to reflect the intrinsic biology of this group of tumors as well as the absence of specific hormonal or targeted treatments to supplement conventional cytotoxic chemotherapy. In addition, these cancers may have different sensitivities to common chemotherapeutic agents. Identification of new biological key pathways driving TNBCs might aid in finding new targets of potential interest for therapeutic blockade. Given the paucity of data regarding EZH2 in TNBC, we examined the clinical and biological role of EZH2 in this disease.
MATERIAL AND METHODS
Patients and clinicopathologic characteristics
We identified 523 consecutive cases of invasive breast cancer in the database of the department of pathology at Wayne State University diagnosed between 2004 and 2006 for which paraffin blocks and follow up data were available. After obtaining approval from the Institutional Review Board, a retrospective chart review of the patients’ demographic, clinical, and pathological data was performed. Patients who received preoperative treatment were excluded from this study. Tumor grade, tumor histology, lymph node status, ER, PR, and HER2 status were determined from the original pathology reports. The diagnosis was made by experienced pathologists using standard criteria for histology and modified Scarff-Bloom-Richardson criteria for grade. Based on the histologic subtype, tumors were assigned to one of the following groups: 1- invasive ductal carcinoma not otherwise specified, or any other special type of invasive ductal carcinoma, 2-Invasive lobular carcinoma, 3- Mixed ductal and lobular carcinoma, 4- Adenocarcinoma with spindle cell metaplasia and metaplastic carcinoma. Tumors were considered to be positive for ER and PR when nuclear reactivity was observed in more that 1% of neoplastic cells with an intensity of 3+. The expression of Her2 was classified according to the Hercept Test® assay’s scoring system, which includes four categories, namely; 0, 1+, 2+, and 3+, based on the intensity and extent of membrane staining in tumor cells. Positivity was defined as a Her2 score of 3+ for immunostaining (more than 30% of the tumor cells show circumferential intense and uniform staining) or a ≥2.2-fold increase in Her2 gene amplification, as determined by fluorescence in situ hybridization (FISH) using the Vysis PATHVYSION Her2 DNA probe kit (Abbott Molecular Inc). ER, PR, and Her2 tests were done at the time of initial diagnosis on needle core biopsies or excision/mastectomy specimens. The methodology and cut-offs for ER, PR, and Her2 were the same for all the cases included in this study. The stage of the tumor at diagnosis was assigned according to the American Joint Committee on Cancer (AJCC) staging criteria. Follow up information for patients were obtained from our Computer Information System (CIS) records, and the Surveillance Epidemiology and End Results (SEER) database. The overall survival was calculated as the time, in months, from the date of the primary surgery to the time of breast cancer-related death.
Tissue microarray
The hematoxylin and eosin slides of all the cases were reviewed and appropriate areas from the tumors were selected for tissue micro-array (TMA) construction. TMAs were prepared in the department of pathology at Wayne State University using selected paraffin embedded blocks of tumor from each case. Two 1 mm cores were obtained from each block. Each TMA consists of 62 cores containing 2 cores from each patient, and normal kidney and lymph node tissue as controls. This procedure has been validated in previous breast cancer studies.
Immunohistochemistry
TMA sections were stained immunohistochemically by antibodies to EZH2 (Zymed; Carlsbad, CA, USA), cytokeratin 5/6 (Cell Marque; Rocklin, CA, USA), epidermal growth factor receptor 1 (Ventana; Oro Valley, AZ, USA), and p53 (Ventana; Oro Valley, AZ, USA). All the antibodies were monoclonal and prediluted. Standard laboratory protocols according to the laboratory manual were established using the avidin-biotin complex staining procedure. Initial trials used the manufacturer’s suggested specimen preparation and staining conditions. Each protocol was optimized for antigen retrieval, antibody dilution, and incubation conditions. A known positive tissue for the antigen of interest was used to titer the antibody and, subsequently, was stained with each investigative case for the current study. Immunohistochemical staining was performed as follows: tissue sections were deparaffinized, hydrated to phosphate-buffered saline buffer (pH 7.4) and pretreated with hydrogen peroxide (3%) for 10 minutes to remove endogenous peroxidase. This was followed by antigen retrieval through steam bath for 20 minutes with citrate buffer. The slides were then incubated with the primary antibody at ambient temperature and washed with phosphate-buffered saline followed by incubation with biotin-labeled secondary antibody for 30 minutes at room temperature. Finally, slides were developed with 0.05% 3′,3-diaminobenzidine tetrahydrochloride and then counterstained with Mayer hematoxylin, dehydrated, and mounted.
Evaluation of staining results
EZH2 nuclear staining was scored based on intensity (0–3+) and it was categorized into low and high expression. Scores of 0 and 1 were considered low, and scores of 2 and 3 were considered high. The expression of cytokeratin 5/6 (CK5/6), and epidermal growth factor 1 (EGFR) were designated as positive if any cytoplasmic and/or membranous staining was observed. The cutoff point between low and high p53 expression was 50%. Negative controls were performed using phosphate buffered saline in place of the primary antibodies. A positive control with a tissue sample known to express the antigen of interest was included with each stain. For EZH2 stain, tonsil tissue was used a positive control.
Breast cancer model
Female athymic nude mice (NCr-nu) were purchased from the NCI-Frederick Cancer Research and Development Center (Frederick, MD) and maintained as previously described. All mouse studies were approved by the Institutional Animal Care and Use Committee. Mice were cared for in accordance with guidelines set forth by the American Association for Accreditation of Laboratory Animal Care and the US Public Health Service Policy on Human Care and Use of Laboratory Animals. For therapy experiments, each siRNA was given twice weekly at a dose of 150 mg/kg body weight. At the time of sacrifice, mouse and tumor weight, number, and distribution of tumors were recorded. Individuals who performed the necropsies were blinded to the treatment group assignments. MB231 triple-negative breast cancer cells (5 × 106) were injected into the mammary fat pad of mice as described previously. For systemic delivery of siRNA, we utilized chitosan nanoparticles (CH) as described previously. We used siRNA sequences targeted to the human (Hs) or mouse (Mm) EZH2. Ten days after injection of tumor cells, 10 mice were randomized to each of the following groups according to the specific treatment: 1) control siRNA/CH 150 μg/kg, 2) EZH2 Hs siRNA/CH (human sequence) 150 μg/kg, 3) EZH2 Mm siRNA/CH (mouse sequence) 150 μg/kg, 4) EZH2 Hs siRNA/CH + EZH2 Mm siRNA/CH. The siRNA treatments were given intravenously. Following 4 weeks of therapy, a necropsy was performed to assess the aggregate tumor weight.
Statistical methods
Statistical analysis was performed using SPSS version 17.0 (Chicago, IL, USA). Chi-square and Fisher’s exact tests were used to study the statistical association between clinicopathologic and immunohistochemical variables. The unpaired t-test was used for the analysis of continuous variables. Overall survival times were estimated in months from the date of diagnosis to the date of death or last follow-up. Survival curves were plotted using the Kaplan– Meier method, and comparisons for the various prognostic variables were made with the log-rank test. Eight variables were analyzed including age, race, grade, stage, nodal status, triple-negative breast cancer (TNBC vs non-TNBC), EZH2, EGFR, HER2 and p53 expression. Statistical significance was defined as a P-value less than .05.
RESULTS
EZH2 expression and its correlation with clinicopathologic characteristics
EZH2 immunohistochemical stain was available for 261 cases. The interpretable cases were representative of the total cases in terms of various clinicopathologic variables including age, tumor grade, stage, and hormonal status; however, Her2 overexpression was seen less frequently among the interpretable cases (14/261; 5.4%) compared to the original cohort (60/523; 11.5%).
High EZH2 expression was seen in 87 out of 261 (33%) cases. In cases where EZH2 expression was high, the staining was noted to be diffuse, involving the vast majority of tumor cells (more than 75%). Positive staining of EZH2 is illustrated in figure 1. The detailed clinical and pathological characteristics of the patients and their correlation with EZH2 expression are detailed in table 1. Breast cancer patients who had high EZH2 expression were significantly younger than those with low EZH2 expression (mean age: 54.9 versus 59.4 years; P=.01). In addition high EZH2 expression was significantly more frequent in invasive ductal carcinoma and mixed ductal and lobular carcinoma compared to invasive lobular carcinoma (P<.001). There was a trend toward an association between high EZH2 and tumors larger than 2 cm in size but this association did not reach statistical significance (P=.06). High EZH2 expression was significantly associated with markers of poor prognosis such as high histologic grade (P=.01), ER negativity (P<.001), PR negativity (P<.001), EGFR positivity (P=.04), and high p53 expression (P<.001). There was no statistically significant association between increased EZH2 expression and any of the following: race, lymph node status, pathologic stage, Her2 status, and CK5/6. Among the 261 cases, 57 (21%) cases were categorized as TNBC and 204 (79%) as non-TNBC. High EZH2 expression was strongly associated with a triple negative phenotype (P<.001) compared to all other non-triple negative tumors. High EZH2 expression was noted in 41 out of 57 (72%) of TNBC versus 47 out of 204 (23%) of non-TNBC.
Figure 1.

EZH2 nuclear immunohistochemical staining in a high grade invasive ductal carcinoma, not otherwise specified, that is triple-negative. X200
Table 1.
Correlation of EZH2 expression with clinicopathologic characteristics.
| Variable | Patients 261 | Low EZH-2 (n=173), n (%) | High EZH-2 (n=88), n (%) | P value |
|---|---|---|---|---|
| Mean Age (mean ± SD) | 57.85±13.5 | 59.4 ±13.5 | 54.9 ± 13.5 | .01 |
| Race* | ||||
| AA | 147 (56.8%) | 93 (63.3%) | 54 (36.7%) | .30 |
| White | 110 (42.5%) | 77 (70%) | 33 (30%) | |
| others | 2 (0.8%) | 2 (100%) | 0 (0%) | |
| Histologic grade * | ||||
| 1 | 21 (9.2%) | 17 (81%) | 4 (19%) | .01 |
| 2 | 70 (30.6%) | 53 (75.7%) | 17 (24.3%) | |
| 3 | 138 (60.2%) | 79 (57.2%) | 59 (42.8%) | |
| Histologic subtype | ||||
| IDC | 223 (85.4%) | 146 (65.5%) | 77 (34.5%) | <.001 |
| ILC | 21 (8%) | 19 (90.5%) | 2 (9.5%) | |
| Mixed | 10 (3.8%) | 8 (80%) | 2 (20%) | |
| Spindle cell & metaplastic carcinoma | 7 (2.7%) | 0 (0%) | 7 (100%) | |
| Tumor size* | ||||
| ≤2 cm | 84 (48.3%) | 62 (73.8%) | 22 (26.2%) | .06 |
| > 2 cm | 90 (51.7%) | 54 (60%) | 36 (40%) | |
| Lymph node* | ||||
| Negative | 106 (56.7%) | 68 (64.2%) | 38 (35.8 %) | .50 |
| Positive | 81 (43.3%) | 56 (69.1%) | 25 (30.9%) | |
| AJCC stage* | ||||
| 1 | 77 (35.3%) | 50 (64.9%) | 27 (35.1%) | .2 |
| 2 | 96 (44%) | 62 (64.6%) | 34 (35.4%) | |
| 3 | 32 (14.7%) | 20 (62.5%) | 12 (37.5%) | |
| 4 | 13 (6%) | 12 (92.3%) | 1 (7.7%) | |
| ER | ||||
| Negative | 63 (24.1%) | 19 (30.2%) | 44 (69.8%) | <.001 |
| Positive | 198 (75.9%) | 154 (77.8%) | 44 (22.2%) | |
| PR | ||||
| Negative | 135 (51.7%) | 70 (51.9%) | 65 (48.1%) | <.001 |
| Positive | 126 (48.3%) | 103 (81.7%) | 23 (18.3%) | |
| Her2 | ||||
| Negative | 247 (94.6%) | 163 (66%) | 84(34%) | .77 |
| Positive *** | 14 (5.4%) | 10 (71.4%) | 4 (28.6%) | |
| CK5/6 ** | ||||
| Negative | 157 (67.1%) | 108 (68.8%) | 49 (31.2%) | .11 |
| Positive | 77 (32.9%) | 46 (59.7%) | 31 (40.3%) | |
| EGFR ** | ||||
| Negative | 170 (73.6%) | 118 (69.4%) | 52 (30.6%) | .04 |
| Positive | 61 (26.4%) | 34 (55.7%) | 27 (44.3%) | |
| p53 ** | ||||
| Low (≤50%) | 169 (75.8%) | 118 (69.8%) | 51 (30.2%) | .01 |
| High (>50%) | 54 (24.2% | 26 (48.1%) | 28 (51.9%) | |
| Triple-negative status | ||||
| Triple-negative | 57 (21.8%) | 16 (28.1%) | 41 (71.9%) | <.001 |
| Non Triple-negative | 204 (78.2%) | 157 (77%) | 47 (23%) |
Data are lacking for some patients
some tissue cores are missing due to processing
Among the 14 Her2 overexpressing cases, 3 cases (21.4%) were positive by fluorescent in situ hybridization.
AA, African American; CK5/6, cytokeratin5/6; EGFR, epidermal growth factor receptor; ER, estrogen receptor; EZH2, enhancer of zeste homolog 2; Her2, human epidermal growth factor-2; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; PR, progesterone receptor.
Correlation of EZH2 expression with patient survival
Follow up data was available for 251 patients. The median follow-up time was 42.3 months (range, 0–217 months). The overall survival rate was 76%. In univariate analysis, high EZH2 expression showed a significant negative impact on patient overall survival (P=.03) (Figure 2). At 5 years, 79% of patients with low EZH2 tumors were alive, compared to 73% of patients with High EZH2 tumors. In the multivariate Cox-regression analysis; after controlling for the other variables; EZH2 (hazard ratio, 2.8; 95 % CI, 1.2–6.6; P=.02), grade (hazard ratio, 3.1; 95 % CI, 1.1–9; P=.04), stage (P=.001), and triple-negative status (hazard ratio, 2.8; 95 % CI, 1.2–6.2; P=.01) retained their significance as independent poor prognostic factors.
Figure 2.

Kaplan Meier graph showing the correlation between EZH2 expression and overall survival.
Effects of EZH2 gene silencing on tumor growth
Next, we examined the biological effects of EZH2 gene silencing using siRNA incorporated into chitosan nanoparticles. Greater than 80% EZH2 gene silencing was achieved as assessed by qRT-PCR (data not shown). Ten days following tumor cell injection, the siRNA treatments were initiated according to the following groups: 1) control siRNA/CH, 2) EZH2 Hs siRNA/CH, 3) EZH2 Mm siRNA/CH, 4) EZH2 Hs siRNA/CH + EZH2 Mm siRNA/CH. There was a 38% reduction with EZH2 Hs siRNA/CH and 51% reduction with EZH2 Mm siRNA/CH (P=<05; Figure 3). Moreover, there was a 73% reduction in tumor growth with the combined EZH2 silencing in tumor and endothelial cells (P<.01). There were no significant differences in body weight between the treatment groups, suggesting that there were no obvious toxicities.
Figure 3.
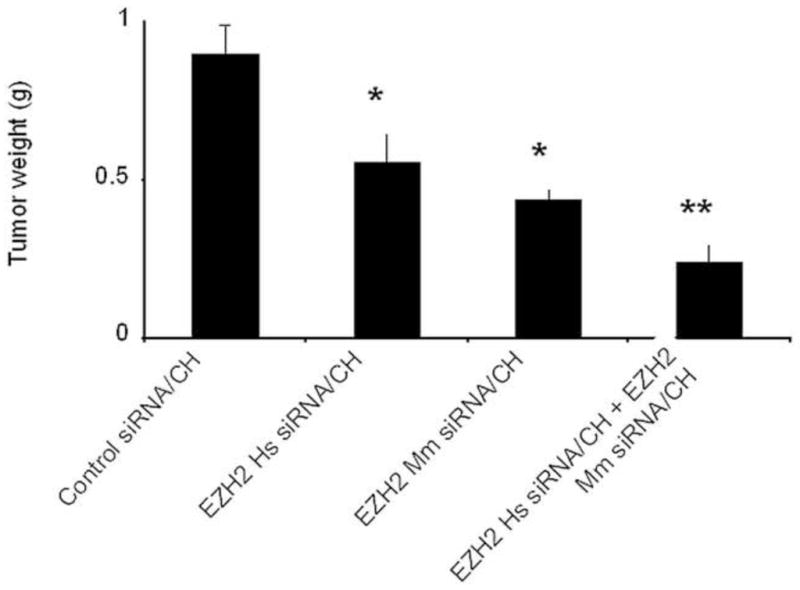
EZH2 down-regulation inhibits breast tumor growth in vivo. Ten days following tumor cell injection, the siRNA treatments were started according to the following groups: 1) control siRNA/CH, 2) EZH2 Hs siRNA/CH, 3) EZH2 Mm siRNA/CH, 4) EZH2 Hs siRNA/CH + EZH2 Mm siRNA/CH. There was a 38% reduction with EZH2 Hs siRNA/CH and 51% reduction with EZH2 Mm siRNA/CH (p<.05). Moreover, there was 73% reduction in tumor growth with the combined EZH2 silencing in tumor and endothelial cells (p<.01).
DISCUSSION
The aim of this study, was to investigate the clinical and the biological role of EZH2 in TNBC compared to non-TNBCs, in addition to the association of EZH2 with various clinicopathologic characteristics and overall patient survival. We found that high expression of EZH2 was associated with features of aggressive tumors such as high histologic grade, ER and PR negativity, EGFR positivity, high p53 expression. There was a significant association between high EZH2 expression and TNBC phenotype compared to non-TNBC. Furthermore, increased expression of EZH2 was significantly associated with poor overall survival on both univariate and multivariate analyses. Another significant finding of this study is that EZH2 knockdown in MB-231triple-negative breast cancer cell lines significantly decreased cancer growth and proliferation.
Our findings regarding the association of high EZH2 expression with features of aggressive breast cancers is consistent with previous reports. Kleer et al showed that EZH2 expression was associated with higher histologic grade, increased tumor diameter, negative ER, negative PR, and poor survival [2]. In another study, Raaphorst et al found that EZH2 expression was associated with poorly differentiated breast carcinomas. Furthermore, Collett et al showed a significant association between EZH2 expression and high histologic type, invasive ductal carcinoma histology, ER negativity, PR negativity, and poor survival. However, in contrast to our study and the Kleer et al study, EZH2 expression was significantly associated with Her2 overexpression. The reason for this disparity might be due to the small number of cases overexpressing Her2 in our study.
Whereas previous reports showed an association between high EZH2 expression and individual markers of triple negative breast cancer such as ER negativity and or PR negativity, our study points to the specific association between high EZH2 expression and TNBCs as a separate category in a cohort of breast cancer patients from a single institution. This finding suggests that EZH2 might play a role in the biology of this aggressive disease. In this report, we also found a significant correlation between high EZH2 expression and EGFR reactivity (a marker commonly expressed in basal-like breast cancer); however, there was no association with CK5/6 (another basal cell marker).
We also found a significant correlation between high EZH2 expression and the overexpression of p53 that might reflect p53 mutation. This is consistent with previous experimental findings that suggest a role of p53 in regulating EZH2 via its promoter. Furthermore, EZH2 expression is also known to be under the transcriptional control of E2F, which are transcription factors located downstream of the retinoblastoma protein (Rb) known to be involved in p53- regulated cell cycle control.
The association of high EZH2 expression with poor clinicopathologic risk factors in our study translated into a poorer overall survival for patients with tumors over expressing EZH2. At 5 years, 79% of patients with low EZH2 tumors were alive, compared to 73% of patients with High EZH2 tumors. Even though the difference in survival was statistically significant, it might not have a clinical implication since it is small and could potentially be explained by differences in the response to different treatment regimens.
Another finding of this study is the ability of EZH2 to modulate breast cancer growth in vivo. EZH2 gene silencing resulted in significant reduction in tumor growth in the orthotopic MB-231 mouse model of triple-negative breast carcinoma. Bracken et al demonstrated that ectopic overexpression of EZH2 promotes cell proliferation in human diploid fibroblasts, that EZH2 siRNA inhibits BrdU incorporation suggesting a role for EZH2 for progression through the cell cycle. Varambally et al reported that EZH2 siRNA induces a G2/M arrest in prostate cell lines. Similar to our finding, Gonzalez et al showed that EZH2 knockdown significantly decreased the rate of ER negative breast cancer growth, caused a delay in the G2/M transition of the cell cycle and improved survival of mice.
In the absence of therapeutic targets in TNBC, chemotherapy has remained the only therapeutic option available for patients with TNBC. Research during recent years has focused on increasing knowledge about key pathways in TNBC aimed at discovery of new treatment options. Novel targeted therapies (e.g., EGFR, c-kit, and VEGF inhibitors alone or in combination with chemotherapy) have been investigated with mixed results. The association of high EZH2 expression with TNBC has practical applications. In addition to its prognostic value, EZH2 was suggested in previous studies as a candidate for targeted therapy. Gonzalez et al recently showed that down-regulation of EZH2 decreased the growth of ER negative invasive breast carcinoma and required BRCA1. BRCA1 mutations and protein deficiency are frequent in TNBC. Down-regulation of EZH2 in TNBC cell lines was also shown to be sufficient to restore BRCA1 protein levels in vivo and in vitro, suggesting that therapies targeting EZH2 protein may restore BRCA1 expression and function in triple-negative breast cancers and may decrease tumor progression.
The current study has limitations that include its retrospective design, which is prone to selection bias. The small amount of tissue analyzed using a panel of immunohistochemical stains on TMA, and the loss of some cases either due to loss of tissue cores during processing, or due to lack of tumor cells, might constitute another limitation, although this procedure has been validated in previous breast cancer studies. Another shortcoming of this study is the relatively short follow-up period, which made it difficult to study the impact of EZH2 expression on recurrence and resistance to treatment. Nonetheless, the strength of our study lies in the analysis of a series of breast cancer patients from a single institution, evaluating the association with EZH2 expression by immunohistochemistry and showing the effect of EZH2 gene silencing on TNBC cell line.
In conclusion, our findings point to a strong correlation between high EZH2 expression and TNBC phenotype, asserting further its biological role in this aggressive disease. It also validates the previous findings about its association with other features of aggressive breast cancer such as high grade, high p53 expression, and poor survival. In addition to offering us a better understanding of the molecular mechanisms behind carcinogenesis in TNBC, this study is an eye opener to the potential role of EZH2 as a therapeutic target in TNBC.
Acknowledgments
Part of this research was supported by grants from the National Institutes of Health (CA128797, RC2GM092599, U54 CA151668), Department of Defense (BC085265), and the Laura and John Arnold Foundation.
Footnotes
This work was presented in part at the 100th annual meeting of the United States and Canadian Academy of Pathology, San Antonio, TX, in March 2011.
Disclosure/Conflict of interest
The authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Simon JA, Tamkun JW. Programming off and on states in chromatin: mechanisms of Polycomb and trithorax group complexes. Curr Opin Genet Dev. 2002;12(2):210–218. doi: 10.1016/s0959-437x(02)00288-5. [DOI] [PubMed] [Google Scholar]
- 2.Kleer CG, Cao Q, Varambally S, Shen R, Ota I, Tomlins SA, Ghosh D, Sewalt RG, Otte AP, Hayes DF, et al. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci U S A. 2003;100(20):11606–11611. doi: 10.1073/pnas.1933744100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachmann IM, Halvorsen OJ, Collett K, Stefansson IM, Straume O, Haukaas SA, Salvesen HB, Otte AP, Akslen LA. EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast. J Clin Oncol. 2006;24(2):268–273. doi: 10.1200/JCO.2005.01.5180. [DOI] [PubMed] [Google Scholar]
- 4.Cleator S, Heller W, Coombes RC. Triple-negative breast cancer: therapeutic options. Lancet Oncol. 2007;8(3):235–244. doi: 10.1016/S1470-2045(07)70074-8. [DOI] [PubMed] [Google Scholar]
- 5.Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, Hess KR, Stec J, Ayers M, Wagner P, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11(16):5678–5685. doi: 10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- 6.Bloom HJ, Richardson WW. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer. 1957;11(3):359–377. doi: 10.1038/bjc.1957.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spitale A, Mazzola P, Soldini D, Mazzucchelli L, Bordoni A. Breast cancer classification according to immunohistochemical markers. clinicopathologic features and short-term survival analysis in a population-based study from the South of Switzerland. Annals of Oncology. 2009;20:628–635. doi: 10.1093/annonc/mdn675. [DOI] [PubMed] [Google Scholar]
- 8.Fitzgibbons PL, Murphy DA, Hammond ME, Allred DC, Valenstein PN. Recommendations for validating estrogen and progesterone receptor immunohistochemistry assays. Arch Pathol Lab Med. 2010;134(6):930–935. doi: 10.5858/134.6.930. [DOI] [PubMed] [Google Scholar]
- 9.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25(1):118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 10.Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI, Borgen PI, Clark GM, Edge SB, Hayes DF, et al. Staging system for breast cancer: revisions for the 6th edition of the AJCC Cancer Staging Manual. Surg Clin North Am. 2003;83(4):803–819. doi: 10.1016/S0039-6109(03)00034-3. [DOI] [PubMed] [Google Scholar]
- 11.Camp RL, Charette LA, Rimm DL. Validation of tissue microarray technology in breast carcinoma. Lab Invest. 2000;80(12):1943–1949. doi: 10.1038/labinvest.3780204. [DOI] [PubMed] [Google Scholar]
- 12.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98(19):10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alkushi A, Lim P, Coldman A, Huntsman D, Miller D, Gilks CB. Interpretation of p53 immunoreactivity in endometrial carcinoma: establishing a clinically relevant cut-off level. Int J Gynecol Pathol. 2004;23(2):129–137. doi: 10.1097/00004347-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Landen CN, Jr, Chavez-Reyes A, Bucana C, Schmandt R, Deavers MT, Lopez-Berestein G, Sood AK. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 2005;65(15):6910–6918. doi: 10.1158/0008-5472.CAN-05-0530. [DOI] [PubMed] [Google Scholar]
- 15.Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori M, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12(8):939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 16.Lu C, Han HD, Mangala LS, Ali-Fehmi R, Newton CS, Ozbun L, Armaiz-Pena GN, Hu W, Stone RL, Munkarah A, et al. Regulation of tumor angiogenesis by EZH2. Cancer Cell. 2010;18(2):185–197. doi: 10.1016/j.ccr.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raaphorst FM, Meijer CJ, Fieret E, Blokzijl T, Mommers E, Buerger H, Packeisen J, Sewalt RA, Otte AP, van Diest PJ. Poorly differentiated breast carcinoma is associated with increased expression of the human polycomb group EZH2 gene. Neoplasia. 2003;5(6):481–488. doi: 10.1016/s1476-5586(03)80032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collett K, Eide GE, Arnes J, Stefansson IM, Eide J, Braaten A, Aas T, Otte AP, Akslen LA. Expression of enhancer of zeste homologue 2 is significantly associated with increased tumor cell proliferation and is a marker of aggressive breast cancer. Clin Cancer Res. 2006;12(4):1168–1174. doi: 10.1158/1078-0432.CCR-05-1533. [DOI] [PubMed] [Google Scholar]
- 19.Kleer CG. Carcinoma of the breast with medullary-like features: diagnostic challenges and relationship with BRCA1 and EZH2 functions. Arch Pathol Lab Med. 2009;133(11):1822–1825. doi: 10.1043/1543-2165-133.11.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang X, Milyavsky M, Shats I, Erez N, Goldfinger N, Rotter V. Activated p53 suppresses the histone methyltransferase EZH2 gene. Oncogene. 2004;23(34):5759–5769. doi: 10.1038/sj.onc.1207706. [DOI] [PubMed] [Google Scholar]
- 21.Bracken AP, Pasini D, Capra M, Prosperini E, Colli E, Helin K. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J. 2003;22(20):5323–5335. doi: 10.1093/emboj/cdg542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt RG, Otte AP, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419(6907):624–629. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez ME, Li X, Toy K, DuPrie M, Ventura AC, Banerjee M, Ljungman M, Merajver SD, Kleer CG. Downregulation of EZH2 decreases growth of estrogen receptor-negative invasive breast carcinoma and requires BRCA1. Oncogene. 2009;28(6):843–853. doi: 10.1038/onc.2008.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corkery B, Crown J, Clynes M, O’Donovan N. Epidermal growth factor receptor as a potential therapeutic target in triple-negative breast cancer. Ann Oncol. 2009;20(5):862–867. doi: 10.1093/annonc/mdn710. [DOI] [PubMed] [Google Scholar]
- 25.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357(26):2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 26.Bengala C. Recent advances in managing triple-negative breast cancers. F1000 Med Rep. 2009:1. doi: 10.3410/M1-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirmizis A, Bartley SM, Farnham PJ. Identification of the polycomb group protein SU(Z)12 as a potential molecular target for human cancer therapy. Mol Cancer Ther. 2003;2(1):113–121. [PubMed] [Google Scholar]


