Abstract
Australia has had a government subsidized universal system of pharmaceutical provision for 50 years. The Pharmaceutical Benefits Scheme (PBS) consumes around 14 percent of total government health care expenditures and has grown substantially in both range of drugs covered, and expenditure since it was first introduced in 1950. It incorporates patient copayments (with differentials for the general population compared with concessional beneficiaries). Prior to listing a drug on the PBS it is subject to a rigorous cost-effectiveness analysis.
Introduction
Australia is a federation of six States and two territories with a national (Commonwealth) government In 2003, the population was just over 20 million, mostly concentrated on the eastern side of the continent.
Australia has a mixed government and private health care system. In 2000-2001, they spent approximately 9 percent of its gross domestic product on health services—70 percent by the government and 7 percent mediated by private health insurance organizations and the remainder by other arrangements, including private out-of-pocket costs. There is a universal health insurance arrangement that provides government rebates for the costs of private medical practice and for stays in public hospitals. Approximately 45 percent of the population has private health insurance covering the costs of stays in private hospitals. Persons taking out private health insurance are eligible for a government rebate of 30 percent of the cost of that insurance. The third element of government protection against health costs is in the form of the PBS that predated the major expansion of government support for hospital and medical care. Expenditure on pharmaceuticals is 12.4 percent of total health expenditure and 14.1 percent of government health expenditure. In 2000, Australia spent approximately $292 per person, per year on pharmaceutical services (adjusted to U.S. dollars using purchasing parities), compared with U.S. expenditure of $541 per capita (Organisation for Economic Co-operation and Development, 2003).
Taking a prescription or non-prescription medication is the most common health care activity among Australians. Australian Health Survey data show that almost 70 percent of the population used some form of medication 2 weeks prior to the interview (Australian Bureau of Statistics, 1997). The most common forms of medication usage was vitamins or minerals (258 persons per 1,000).
The second most common form was in pain relievers (236.2 persons per 1,000) followed by heart problems or blood pressure medications (105.8 persons per 1,000). Use of these medications is not necessarily preceded by any form of medical or other health professional advice, and many of these medications (e.g., vitamins and herbal medications) do not have any form of government rebate.
Over two-thirds of doctor visits involve recommendations about medication, most of which result in a prescription drug (Britt et al., 2001), 38.7 percent involved one, 13.6 percent involved two, 7.5 percent more than two, and 40.2 percent involved no prescription.
Australia has adopted a National Medicines Policy to guide policy development of pharmaceuticals (Harvey and Murray, 1995). Key elements are:
Timely access to the medicines that Australians need, at a cost to individuals and the community can afford.
Medicines meeting appropriate standards of quality, safety, and efficacy.
Quality use of medicines.
Maintaining a responsible and viable medicine industry. (Commonwealth Department of Health and Aged Care, 1999).
The principal mechanism for ensuring access to medicines is the PBS. Quality, safety, and efficacy of medicines are regulated through the Therapeutic Goods Administration, with policies and processes similar to the United States' Food and Drug Administration. Quality use of medicines involves a range of policies in terms of educational programs, consumer information, etc. The responsible and viable medicines industry component is achieved through the pharmaceutical industry support program. This article reviews and outlines Australia's policies for ensuring access and promoting quality use of medicines.
Ensuring Access
The PBS was introduced on July 1, 1948, but relatively few prescriptions were covered under it because of opposition from the medical profession. The new (conservative) government elected in 1949 revised the PBS on September 4, 1950, introducing a list of 139 “life saving and disease preventive drugs” that were provided free of charge to the entire community (Sloan, 1995). Since then, the range of drugs covered by the PBS has increased dramatically and by August 2003, it covered 601 generic products, available in 1,469 forms or strengths, and marketed as 2,602 different brands. Some of these items are restricted, requiring some form of preauthorization to prescribe them (over and above medical registration). Obtaining this preauthorization requires contact with the administering agency of the PBS, the Health Insurance Commission and may, for example, require the medical practitioner to certify that specific indications for prescribing the medication are present. Obtaining authorization is not well received by doctors and is seen as bureaucratic and not evidence based (Liaw et al., 2003).
The major types of drugs prescribed under the PBS are shown in Table 1. Drugs are grouped using the Anatomical Therapeutics Chemical Code (ATC). There are five levels to the ATC: anatomical main group, therapeutic main group, therapeutic subgroup, chemical/therapeutic subgroup, and generic drug name.
Table 1. Number, Total Cost, Average Price, and Percent Change of Prescriptions Issued under Australia's Pharmaceutical Benefits Scheme, by Anatomical Therapeutics Chemical Code: July 1, 2000, to June 30, 2002.
| Code | Year Ended June 30, 2002 | Percent Change 2000 to 2002 | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Number of Prescriptions | Total Cost (In Millions) |
Average Price | Number of Prescriptions | Total Cost | Average Price | |
| Total | 154,970,262 | 5003.3 | 32.29 | 4.7 | 9.6 | 4.7 |
| Alimentary Tract and Metabolism | 19,082,701 | 692.4 | 36.28 | 6.4 | 7.4 | 0.9 |
| Blood and Blood Forming Organs | 4,023,864 | 111.9 | 27.8 | 12.3 | 38.5 | 23.3 |
| Cardiovascular System | 46,587,011 | 1556.9 | 33.42 | 5.3 | 8.7 | 3.2 |
| Dermatologicals | 2,934,367 | 81.2 | 27.68 | -2.7 | 1.1 | 3.9 |
| Genito Urinary System and Sex Hormones | 6,323,714 | 159.8 | 25.27 | 2.7 | 20.4 | 17.3 |
| Systemic Hormonal Preparations, Excluding Sex Hormones | 2,304,729 | 30.2 | 13.09 | 3.9 | 8.1 | 4.0 |
| General Anti-Infectives for Systemic Use | 12,550,089 | 273.1 | 21.76 | -1.1 | 2.9 | 4.0 |
| Antineoplastic and Immunomodulating Agents | 961,818 | 317.7 | 330.35 | 8.0 | 17.2 | 8.5 |
| Musculo-Skeletal System | 10,709,738 | 340.1 | 31.75 | 27.2 | 14.4 | 10.0 |
| Nervous System | 30,564,051 | 906.5 | 29.66 | 3.4 | 8.6 | 5.1 |
| Antiparasitic Products, Insecticides and Repellants | 910,580 | 8.9 | 9.81 | 0.4 | 2.4 | 2.0 |
| Respiratory System | 10,341,286 | 374.0 | 36.16 | -6.5 | 7.3 | 14.8 |
| Sensory Organs | 6,779,904 | 104.0 | 15.33 | 4.4 | 8.1 | 3.6 |
| Various | 602,127 | 43.6 | 72.41 | 5.7 | 5.4 | -0.3 |
| Not Otherwise Classified | 294,283 | 3.1 | 10.58 | -6.5 | 0.0 | 6.9 |
SOURCE: Duckett, S.J.: estimates derived from http://www.hic.gov.au/statistics/dyn_pbs/forms/pbsgtab1.shtml (Accessed 2004.)
The most frequently prescribed group of drugs are those for the cardiovascular system, accounting for just over 30 percent of all prescriptions and costs. Twenty percent of both prescriptions and costs are for the nervous system. Antineoplastic and immunomodulating agents, although accounting for less than 1 percent of prescriptions, account for 6 percent of costs. These cost, on average, ten times as much as the average drug dispensed under the PBS.
The PBS initially required no patient copayment, then on March 1, 1960, a 50-cent copayment was introduced for general beneficiaries under the PBS. A copayment for pensioners of A$2.501 per prescription was introduced on November 1, 1990. The copayment amounts are indexed for inflation and, by 2003, the copayment for pensioners had increased to A$3.70 per prescription and for general beneficiaries to A$23.10. The PBS provides some protection from the cumulative impact of these copayments through a safety net threshold that is set for pensioners at 52 times the copayment. If pensioners require more than 52 prescriptions in any one CY, they can obtain a safety net card that entitles them to further prescriptions without any copayment. The safety net threshold for general beneficiaries applies after they (or their immediate family) purchase PBS items that total a copayment of at least A$708.40 in any CY, after that, prescriptions are supplied for the concessional copayment.
Where a pharmaceutical is listed on the PBS under more than one brand name, pharmacists may dispense generic forms of the drug unless specifically directed not to do so by the prescribing medical practitioner on the prescription form. If generic equivalents are available, the PBS will only pay for the least-costly product and the consumer pays any additional costs for a specific brand-name alternative in addition to the copayments previously discussed. This brand name equal is not counted as part of the safety net arrangements. An additional alternative is also payable if other pharmaceuticals in the same therapeutic class are deemed to be equivalent, and an exemption on clinical grounds has not been granted for that patient. This policy (known as Therapeutic Group Premiums) applies only to items in three therapeutic groups: H2-receptor antagonists; calcium channel blockers; and ACE inhibitors.
The generic substitution policy is facilitated by a government requirement that, where computer software used by medical practitioners to generate prescriptions for the PBS has a default preferred drug, it defaults automatically to the generic form of a drug rather than a proprietary form of the drug. As of May 2002, 293 products had a brand premium, with the premium ranging from 1 cent to A$79.48. Over 30 million prescriptions had been dispensed with a brand-premium, being about 50 percent of all prescriptions covered by the brand-name premium policy (Lofgren, 2002).
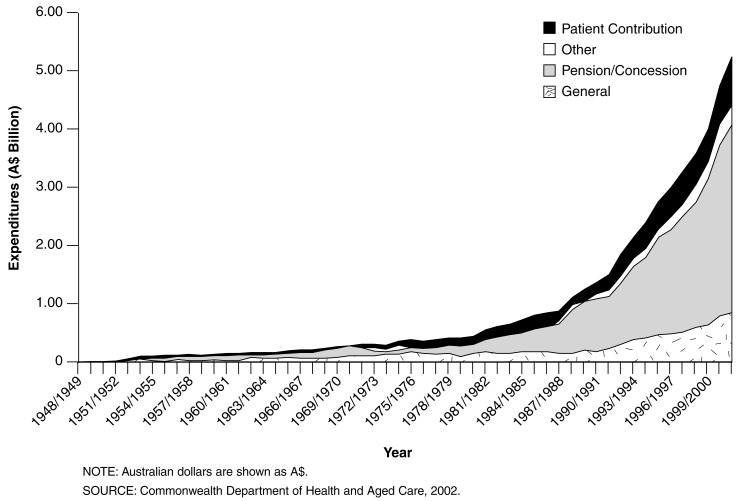
Figure 1 shows the growth in pharmaceutical benefits expenditure since the inception of PBS. Expenditure on pharmaceutical benefits has increased exponentially since the beginning of the program, with particularly rapid growth in expenditure on drugs used by pensioners and concessional cardholders. More importantly, 73 percent of government pharmaceutical benefits prescription expenditure is for pensioners, severely limiting the ability of government to curtail expenditure using the strategy of shifting costs to consumers. People age 65 or over have a 50-percent higher prescription rate than those under age 45. This high percentage of use for pensioners should not be surprising because the elderly generally have poorer health status than younger persons and have higher hospital utilization rates.
Figure 1. Trends in Expenditures for Australia's Pharmaceutical Benefits Scheme: 1948-1949 to 2001-2002.
Expenditure on pharmaceuticals has been growing faster than the economy as a whole in recent years. Since the late 1970s, pharmaceutical expenditure has grown from 0.6 percent of gross domestic product to 1.1 percent in 2000-2001. For most of the last decade patient contributions, which account for 15 percent of the PBS expenditure, have been increasing at 5 to 8 percent per year in real terms. Government expenditure has been increasing faster than this at 7 to 14 percent per year. This growth in expenditure is partly driven by increased utilization and by increased prices for dispensed medications.
Expenditure on the PBS is the fastest growing component of health expenditure, growing at 15- to 20-percent per year. If the current rates of growth continue, expenditure on the PBS will exceed that on public hospitals by 2007-2008 and on all hospitals by 2010-2011 (Walker, Percival, and Harding, 1998).
Growth in expenditure is distributed unevenly across the PBS with use of newer drugs increasing faster than older drugs. The average price of a new drug listed on the PBS is about two times that of all drugs on the PBS (Sweeny, 2002).
For most of the last decade, the growth in PBS expenditure has primarily been driven by increases in the number of prescriptions that had been listed on the schedule for at least 12 months rather than by the cost of new drug listings (Sweeny, 2002). For the last 5 years, price effects of drugs that have been listed for more than 12 years have acted to moderate growth in expenditure rather than contribute to it.
The decision to list an item on the PBS can lead to commitment of significant government expenditure, and since 1993 has involved a decision not only about whether the drug is an effective complement to existing items on the PBS, but also an assessment of whether the drug is cost effective. The legislation to require cost-effectiveness analysis was passed in 1987; draft guidelines on how listing submissions were to incorporate cost-effectiveness analysis were published in 1990, with definitive guidelines in 1992. (These guidelines are updated regularly on http://www.health.gov.au/pbs/general/pubs/guidelines [accessed 2004]).
The guidelines provide that a drug will be listed on the PBS if it is:
Needed for the prevention or treatment of significant medical conditions not already covered, or inadequately covered, by drugs in the existing list and is of acceptable cost effectiveness.
More effective, less toxic (or both) than a drug already listed for the same reasons and is of acceptable cost effectiveness.
At least as effective and safe as a drug already listed for the same reasons and is similar or shows more cost effectiveness.
Under the cost-effectiveness arrangements, the pharmaceutical manufacturer needs to present cost-effectiveness data, usually based on randomized controlled trial evidence against a designated comparator, to the Pharmaceutical Benefits Advisory Committee (PBAC) (Henry, 1992; Harris, 1994; Hailey, 1997; Hill et al., 1997a; Salkeld, Mitchell, and Hill, 1999; Mitchell, 1996, Hill, Mitchell, and Henry, 2000). A retrospective study of the decisionmaking process showed that new pharmaceuticals costing more than A$68, 913 for each life year saved are generally not listed on the PBS, while those costing less than A$36, 450 for each life year saved are listed. No apparent decision rules have been found to apply to the intermediate zone (George, Harris, and Mitchell, 1998).
The operation of PBAC and the economic evaluation policies of the PBS have not been entirely without problems or controversy. Almost two-thirds of submissions to PBAC have contained significant problems from the point of view of the PBAC evaluators, most of which were seen as avoidable (Hill, Mitchell, and Henry, 2000).
In a controversial move early in 2001, the government restructured the membership of PBAC to include a person with strong industry links. This initiative was believed to be in response to industry pressure to water down the strong emphasis on economic evaluation followed by PBAC and was seen in the public debate as weakening PBAC (Goddard, Henry, and Birkett, 2001). These fears do not appear to have translated into reality and economic evaluation still appears to be a central component of the listing decisions (Aroni, deBoer, and Harvey, 2003).
As part of the listing decision, the government establishes a price for the drug after advice from the Pharmaceutical Benefits Pricing Authority (PBPA). In determining the price, the PBPA takes into account:
The PBAC comments on clinical and cost effectiveness.
Prices of alternative brands of a drug.
Comparative prices of drugs in the same therapeutic group.
Cost information, whether provided by the supplier or estimated by the PBPA.
Prescription volumes, economies of scale, and other factors such as date expiration, storage requirements, product stability, and special manufacturing requirements.
Prices of the drug in relevant overseas countries.
The Australian Orphan Drug Program provides support for registration and listing of new therapeutic agents for rare diseases through waived application fees. The purchasing arrangements for pharmaceuticals covered under the PBS involve a government agreed-upon price (90 percent of which is for the supplier, and 10 percent for the wholesaler). The government also undertakes postmarketing surveillance to ensure that the volumes of listed drugs are close to those predicted in the cost-effectiveness analyses and other submissions on which the pricing negotiations were based.
Historically, the government has been able to use its monopsonistic purchasing strength to achieve lower prices relative to those paid in international markets. The ability to do this appears to be weakening as other countries establish schemes similar to the PBS and monitor international pricing negotiations (Löfgren, 1998). However, Bessell, Hiller, and Sansom (1999) have documented an example where the market price rose when the product was deleted from the PBS.
The pharmaceutical industry is regularly ranked as the most profitable of all industries and pricing decisions of the PBS have significant implications for profit that pharmaceutical manufacturers obtain from selling their products in Australia. One of the major costs of bringing a drug on the market are in the research and development that can occur over a long period of time. Of course, research and development costs associated with failed research or research that does not lead to a drug that comes on to the market needs to be spread over the costs of pharmaceuticals which do come on to the market. In many cases the drug research is subsidized by national research organizations, such as the National Health and Medical Research Council.
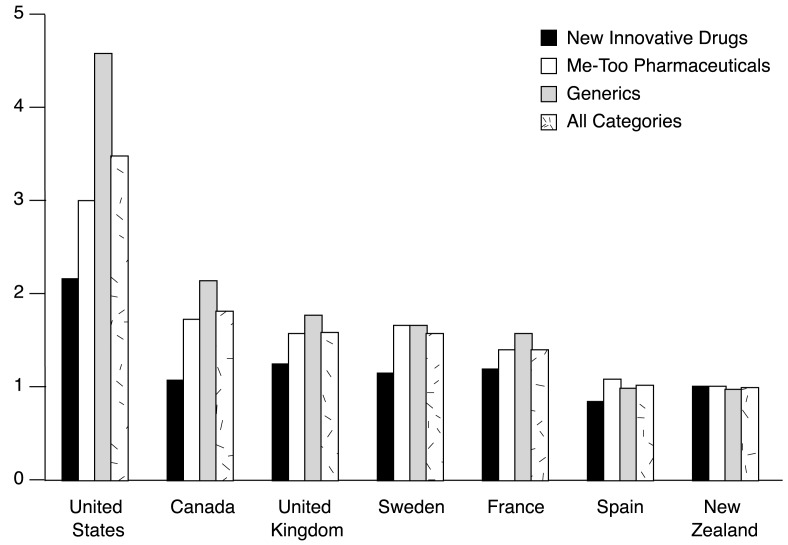
International comparison of pricing is difficult, in part because of the existence of discounting arrangements in several countries: list prices may not reflect prices actually paid in the market place (Productivity Commission, 2001). Accordingly, in its major study of comparative prices of drugs, the Productivity Commission reported high and low estimates of the difference between costs in other countries and costs in Australia (Figure 2). The high estimate is the greatest difference likely to be found.
Figure 2. Ratio of Prevailing Prices for Pharmaceuticals in Selected Countries in Comparison to Australia's1 Pharmaceutical Benefits Scheme: 2000.
1 PBS equals 1.0. NOTE: Me-too pharmaceuticals are pharmaceuticals for which an alternative is already available.
SOURCE: (Productivity Commission, 2001.)
There are a number of broad, general conclusions that can be drawn from Figure 2. Prices in Australia are certainly substantially less than prices in the U.S. On the other hand, prices in Australia are similar to prices in other countries. The success of the PBS in restraining prices has attracted the attention of the U.S. pharmaceutical manufacturing industry (Lokuge and Denniss, 2003). However, listing on the PBS reduces the effective price faced by consumers, and hence economic theory would predict that it is likely to lead to increased demand and sales against a situation of unsubsidized prices. This in turn suggests that even with the relatively lower prices paid by the PBS, pharmaceutical manufacturers may be better off because of the increased sales than they would be in the absence of the PBS (Wright, 2003, In press).
There are significant differences in price relativities for different types of drugs. The differences between new and innovative pharmaceuticals are much lower than for all pharmaceuticals suggesting that the Australian purchasers (particularly those involved in setting prices under the PBS) are not able to extract the same price discounts for newer drugs relative to older drugs.
Because listing under the PBS provides a significant marketing boost (by reducing the effective price faced by consumers to the copayment), the government is in a strong position to negotiate over price. However, where a pharmaceutical company has a new medication significantly superior to others, or which is unique, it can threaten to rely on consumers paying the full price for the drug rather than accept a lower price from the government. In these circumstances, consumers are likely to place significant pressure on the government to list the product.
Quality Use of Medicines
The fourth arm of National Medicines Policy is to promote the quality use of medicines. Although such a policy could involve a mix of strategies, the emphasis in Australia has been on sporadic educational programs rather than systematic use of incentives on manufacturers or medical practitioners. Such campaigns encourage consumers to be more judicious in use of medication and/or attempt to educate medical practitioners to reduce their prescribing. The high rate of growth in the cost of pharmaceuticals has led the government in Australia (and around the world) to develop a number of strategies to limit this expenditure (Saltman and Figueras, 1997; Maynard and Bloor, 2003). Table 2 shows some of the strategies that have been utilized in Australia.
Table 2. Targets for Control of Government Pharmaceutical Expenditure Used in Australia.
| Demand | Supply | |
|---|---|---|
| Price | Patient co-payments. Encourage prescription of “generic” (non-brand name) drugs. Encourage prescription of alternative medication in same therapeutic class. |
Negotiate prices based on lowest in other countries. |
| Volume | Patient education programs. | Incentives on medical profession as a whole to limit prescribing. Practice guidelines. Limit inclusion on the approval list through use of cost effectiveness analysis. Provide education program to pharmacists, doctors. |
SOURCE: Stephen J. Duckett, M.H.A., Ph.D., La Trobe University, Victoria, Australia, 2004.
In broad general terms, the government can shape pharmaceutical policy by intervening at any point of the drug distribution chain with initiatives targeted at manufacturers, medical practitioners who prescribe drugs, pharmacists who dispense drugs, and consumers.
Different policy initiatives will have different equity effects and will have different impacts. Policies to reduce consumption of pharmaceuticals could involve a mix of price strategies on the demand side, increasing consumer copayments or, alternatively, acting on supply by attempting to change the behavior of manufacturers by reducing the incentives to manufacturers to promote increased use of pharmaceuticals. One of the most powerful supply-side strategies is using price volume contracts to reduce the price paid under the PBS as volume increases. The two different strategies have different equity impacts and different effectiveness. Organizational and systemwide change by changing incentives on the manufacturers will have a far more pervasive effect than educational strategies on medical practitioners.
Despite the regulation of claims made by pharmaceutical manufacturers in their advertising campaign to medical practitioners, manufacturers advertising to medical practitioners do not promote the scientific basis of their products (Loke et al., 2002). Because there are few price volume agreements in place under the PBS, manufacturers have a very strong incentive to increase sales when the marginal cost production of the additional drugs is well below the revenue from the PBS. These incentives on manufacturing companies overwhelm the educational campaigns on medical practitioners. Pharmaceutical marketing budgets are large and this results in entanglement between doctors and the manufacturing companies as the manufacturers encourage medical practitioners to prescribe their products through enticements (Moynihan, 2003). Manufacturing companies also support consumer organizations to lobby for inclusion of relevant drugs on the PBS and use the consumer organizations to advocate use of the manufacturer's product (Herxheimer, 2003).
The primary interest of the pharmaceutical industry is in maximizing sales and profits, and in Australia this usually translates into maximizing government expenditure. Australia uses a range of strategies to rein in pharmaceutical benefits expenditure, for example, providing feedback to general practitioners on costs (Beilby and Silagy, 1997; Hill, Henry, and Smith, 1997b; Harvey and Murray, 1995; National Health Strategy, 1992). Despite government programs to promote evidence-based prescribing and improve consumer information (Shenfield and Tasker, 1997), the pharmaceutical industry has been remarkably successful in creating needs (Moynihan, Heath, and Henry, 2002), persuading medical practitioners to prescribe the latest drug, even if benefits to the consumer are marginal and practitioners do not profit directly from increased sales to their patients (Moulds, 1992; Day, 1998; Roughead et al., 1998, 1999).
An important contributor to Australia's quality use of medicines strategy is the government-funded National Prescriber Service (NPS) (http://www.nps.org.au) which provides evidence-based information to health providers and consumers about medicines, ostensibly independently of industry and government. But even this independent service can be subject to government and/or industry conniving, with government recently asking the NPS not to educate doctors about two newly listed diabetes medications (Marino, 2003).
Supply Arrangements
Payments to pharmacists for dispensing prescriptions under the PBS are set following negotiations between the Commonwealth government and the Pharmacy Guild of Australia. The contemporary arrangements are incorporated in the Third Community Pharmacy Agreement for the 5-year period from July 1, 2000, to June 30, 2005 (http://www.health.gov.au/pbs/healthpro/pharmacy/pharmacyagree.pdf. The agreement provides for differential base dispensing fees for dispensing ready prepared items (A$4.40 fee in 2000-2001) and extemporaneously prepared items (A$6.28). In either case the pharmacist also receives a markup of 10 percent on the agreed-upon price if that price is less than A$180, A$18 if the price is between A$180 and A$450, and 4 percent if the price is more than A$450. The base fees are indexed annually. The agreement also provides for upward or downward adjustment of payments if the average markup increases by more than 4 percent over that projected in the agreement of if prescriptions increase faster or slower than projected. The operation of these claw-back and supplementation arrangements both protect the government from additional expenditure on pharmacists simply because of increases in average prescription prices and protects pharmacists from the impact on total remuneration if prescription volume declines. The agreement also provides that pharmacists can receive a range of other miscellaneous fees and allowances.
Evaluation of The PBS
Australia's arrangements for the supply of pharmaceuticals are the envy of many other developed countries. The PBS performs well in terms of criteria of equity, efficiency, quality, and acceptability. In terms of equity, the structure of the PBS minimizes the financial barriers to access to pharmaceuticals. The low copayment for concession cardholders ensure that they have access to the medication prescribed for them: only 2 percent of people age 65 or over have reported that they did not fill a prescription because of cost (Schoen, 2003). However, copayments for the general population are much higher than for concession cardholders, and may create a barrier, especially for people with low incomes. Blendon et al. (2002) has reported that 23 percent of Australians did not fill a prescription because of cost.
In terms of efficiency, the PBS again performs well in the sense that all new drugs are subject to economic evaluation prior to listing. Because new drugs listed on the PBS have been evaluated for their cost effectiveness, it could be argued that any increased expenditure on the PBS is worthwhile in terms of savings in other sectors of the health care industry (for example, hospitals), in increased productivity (reduced time off work), or in terms of improved quality of life generally. Thus, even where there is increased expenditure on these drugs, this may be efficient and cost effective when viewed in a wider economic context.
The success of the cost-effectiveness strategy relies on rigorous standardized cost-effectiveness analyses undertaken in accordance with published guidelines. It also relies on ensuring that pre-listing estimates of demand (based on the indications tested in the cost-effectiveness analyses) hold true once the drug is marketed. However, pharmaceutical companies are normally the sponsors of cost-effectiveness analyses submitted for review. Friedberg et al. (1999) and Neumann et al. (2000) have shown that drug company funded cost-effectiveness analyses are more likely than studies funded from other sources to report favorable cost-effectiveness results. There is also a tendency for drug company sponsored studies to overstate qualitative conclusions (that is, a favorable qualitative conclusion in the face of neutral or negative quantitative results). Studies sponsored by pharmaceutical manufacturers are also more likely to report outcomes favoring the sponsor than research funded from other sources (Lexchin et al., 2003).
The Commonwealth government's Department of Health and Ageing undertakes post-marketing surveillance of new PBS listings, but renegotiation of prices in the face of unexpected increased sales volumes is relatively unusual. A weakness of the PBS in terms of efficiency lies in this area of post-market surveillance: the economic evaluation is based on a particular range of conditions and evidence from particular research studies, and that prescription use of the drug in practice, and its promotion by the manufacturers may not be in accordance with the research and the basis of the listing decision. Efficiency of the scheme would be promoted if there were stronger incentives on manufacturers to restrain use in line with the research evidence, possibly by expanded use of pricing arrangements.
Although the PBS incorporates incentives for the quality use of medicines, the incentives are primarily directed at the weakest points of the supply chain in terms of the effectiveness of policy instruments. Educational strategies aimed at consumers and medical practitioners should be supplemented by stronger incentives on manufacturers to encourage quality use of medicines. Evidence of over-prescription can be seen in the rate of admissions to hospitals for drug-related adverse events.
Finally, the PBS appears to be popular and so would rate highly in terms of the criterion of acceptability. As almost 80 percent of pharmaceutical benefits expenditure is for pensioners and concessional beneficiaries, the program is already highly targeted. As a corollary, in 1989-1990, 26 percent of persons in the lowest income decile benefited from the PBS subsidy for pharmaceuticals compared with 5 percent of persons in the highest income decile (Schofield, 1998). Further use of demand side strategies, such as patient copayments, would thus have an adverse impact on equity, but this effect has not precluded advocacy of this policy option, especially by pharmaceutical manufacturers (Löfgren, 1998). It should therefore be anticipated that expenditure on the PBS will continue to rise as new therapeutic agents are developed and replace older, less expensive products.
Footnotes
Stephen J. Duckett is with La Trobe University, Victoria, Australia. The views expressed in this article are those of the author and do not necessarily reflect the views of La Trobe University or the Centers for Medicare & Medicaid Services (CMS).
Australian dollars are shown as A$.
Reprint Requests: Stephen J. Duckett, M.H.A, Ph.D., La Trobe University, Victoria, Australia 3086. E-mail: sduckett@latrobe.edu.au
References
- Aroni R, de Boer R, Harvey K. The Viagra Affair: Evidence as the Terrain for Competing Partners. In: Lin V, Gibson B, editors. Evidence-Based Health Policy. Oxford University Press; South Melbourne, Victoria, Australia: 2003. [Google Scholar]
- Australian Bureau of Statistics. 1995 National Health Survey: Summary of Results, Australia. Australian Bureau of Statistics; Canberra, Australia: 1997. Number 4364.0. [Google Scholar]
- Beilby JJ, Silagy CA. Trials of Providing Costing Information to General Practitioners: A Systematic Review. Medical Journal of Australia. 1997 Jul 21;167(2):89–92. doi: 10.5694/j.1326-5377.1997.tb138787.x. [DOI] [PubMed] [Google Scholar]
- Bessell TL, Hiller JE, Sansom LN. Pharmacist Only Medicines. Australian and New Zealand Journal of Public Health. 1999;23(6):661–662. doi: 10.1111/j.1467-842x.1999.tb01557.x. [DOI] [PubMed] [Google Scholar]
- Blendon RJ, Schoen C, DesRoches CM, et al. Inequities in Health Care: A Five-Country Survey. Health Affairs. 2002 May-Jun;21(3):182–191. doi: 10.1377/hlthaff.21.3.182. [DOI] [PubMed] [Google Scholar]
- Britt H, Miller GC, Knox S, et al. General Practice Activity in Australia 2000-01. Internet address: http://www.aihw.gov.au/publications/index.cfm?type=detail&id=7280 (Accessed 2004.)
- Commonwealth Department of Health and Aged Care. National Medicines Policy. Internet address: http://www.nmp.health.gov.au/pdf/nmp2000.pdf (Accessed 2004.)
- Day R. Pharmaceutical Company Promotion: Striking a Balance. Australia New Zealand Journal of Medicine. 1998;28:291–93. doi: 10.1111/j.1445-5994.1998.tb01950.x. [DOI] [PubMed] [Google Scholar]
- Friedberg M, Saffran B, Stinson TJ, et al. Evaluation of Conflict of Interest in Economic Analyses of New Drugs Used in Oncology. JAMA. 1999;282(15):1453–1457. doi: 10.1001/jama.282.15.1453. [DOI] [PubMed] [Google Scholar]
- George B, Harris A, Mitchell A. Reimbursement Decisions and the Implied Value of Life: Cost Effectiveness Analysis Decisions to Reimburse Pharmaceuticals in Australia 1993-96. In: Harris A, editor. Economics and Health: 1997 Proceedings of the Nineteenth Australian Conference of Health Economists. School of Health Services Management, University of New South Wales; Sydney, Australia: 1998. [Google Scholar]
- Goddard M, Henry D, Birkett DJ Securing the Future of the Pharmaceutical Benefits Scheme. Daring to Dream. In: The Future of Australian Health Care. Mooney G, Plant A, editors. Black Swan Press; Perth, Australia: 2001. [Google Scholar]
- Hailey D. Australian Economic Evaluation and Government Decisions on Pharmaceuticals, Compared to Assessment of Other Health Technologies. Social Science & Medicine. 1997;45(4):563–581. doi: 10.1016/s0277-9536(96)00397-8. [DOI] [PubMed] [Google Scholar]
- Harris AH. Economic Appraisal in the Regulation of Pharmaceuticals in Australia: Its Rationale and Potential Impact. The Australian Economic Review. 1994:98–105. 2nd Quarter. [Google Scholar]
- Harvey K, Murray M. Medicinal Drug Policy. In: Gardner H, editor. The Politics of Health: The Australian Experience. Churchill Livingstone; Maryborough, Australia: 1995. [Google Scholar]
- Henry D. Economic Analysis As an Aid to Subsidisation Decisions: The Development of Australian Guidelines for Pharmaceuticals. PharmacoEconomics. 1992;1(1):54–67. doi: 10.2165/00019053-199201010-00010. [DOI] [PubMed] [Google Scholar]
- Herxheimer A. Relationships between the Pharmaceutical Industry and Patients' Organisations. BMJ. 2003 May 31;326:1208–1210. doi: 10.1136/bmj.326.7400.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S, Henry D, Pekarsky B, Mitchell A. Economic Evaluation of Pharmaceuticals: What Are Reasonable Standards for Clinical Evidence—The Australian Experience. British Journal of Clinical Pharmacology. 1997a Nov;44(5):421–425. doi: 10.1046/j.1365-2125.1997.t01-1-00597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SR, Henry DD, Smith AJ. Rising Prescription Drug Costs: Whose Responsibility? Medical Journal of Australia. 1997b Jul 7;167(1):6–7. doi: 10.5694/j.1326-5377.1997.tb138752.x. [DOI] [PubMed] [Google Scholar]
- Hill SR, Mitchell AS, Henry DA. Problems with the Interpretation of Pharmacoeconomic Analyses: A Review of Submissions to the Australian Pharmaceutical Benefits Scheme. JAMA. 2000;283(16):2116–2121. doi: 10.1001/jama.283.16.2116. [DOI] [PubMed] [Google Scholar]
- Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical Industry Sponsorship and Research Outcome and Quality: Systematic Review. BMJ. 2003 May 31;326:1167–1177. doi: 10.1136/bmj.326.7400.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw ST, Pearce CM, Chondros P, et al. Doctors' Perceptions and Attitudes to Prescribing within the Authority Prescribing System. Medical Journal of Australia. 2003;178(5):203–206. doi: 10.5694/j.1326-5377.2003.tb05162.x. [DOI] [PubMed] [Google Scholar]
- Löfgren H. The Pharmaceuticals Benefit Scheme and the Shifting Paradigm of Welfare Policy. Australian Health Review. 1998;21(2):111–123. doi: 10.1071/ah980111. [DOI] [PubMed] [Google Scholar]
- Löfgren H. Generic Drugs: International Trends and Policy Developments in Australia. Victoria University of Technology; Melbourne, Australia: 2002. Centre for Strategic Economic Studies Working Paper No. 10. [DOI] [PubMed] [Google Scholar]
- Loke TW, Koh FC, Ward JE. Pharmaceutical Advertisement Claims in Australian Medical Publications. Medical Journal of Australia. 2002;177(6):291–293. doi: 10.5694/j.1326-5377.2002.tb04785.x. [DOI] [PubMed] [Google Scholar]
- Lokuge K, Denniss R. Trading in Our Health System? The Impact of the Australia-US Free Trade Agreement on the Pharmaceutical Benefits Scheme. The Australian Institute; Canberra, Australia: 2003. Australian National University Discussion Paper Number 55. [Google Scholar]
- Marino M. Diabetes Drug Pleas Sparks Row. The Age. 2003. Internet address: http://www.theage.com.au/articles/2003/12/06/1070625578319.html (Accessed 2004.)
- Maynard A, Bloor K. Dilemmas in Regulation of the Market for Pharmaceuticals. Health Affairs. 2003 May-Jun;22(3):31–41. doi: 10.1377/hlthaff.22.3.31. [DOI] [PubMed] [Google Scholar]
- Mitchell A. Update and Evaluation of Australian Guidelines: Government Perspective. Medical Care. 1996;34(12):DS216–DS25. [PubMed] [Google Scholar]
- Moulds RFW. Promoting and Advertising Therapeutic Goods: Trade Practices Commission Report on Self-Regulation. Medical Journal of Australia. 1992 Oct;157(8):513–514. [PubMed] [Google Scholar]
- Moynihan R. Who pays for the Pizza? Redefining the Relationships Between Doctors and Drug Companies 1: Entanglement. BMJ. 2003 May 31;326:1189–1192. doi: 10.1136/bmj.326.7400.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynihan R, Heath I, Henry D. Selling Sickness: The Pharmaceutical Industry and Disease Mongering. BMJ. 2002 Apr 13;324:886–891. doi: 10.1136/bmj.324.7342.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Health Strategy. Issues in Pharmaceutical Drug Use in Australia. Canberra, Australia: 1992. National Health Strategy Issues Paper Number 4. [Google Scholar]
- Neumann PJ, Sandberg EA, Bell CM, et al. Are Pharmaceuticals Cost-Effective? A Review of the Evidence. Health Affairs. 2000 Mar-Apr;19(2):92–109. doi: 10.1377/hlthaff.19.2.92. [DOI] [PubMed] [Google Scholar]
- Organisation for Economic Co-operation and Development. OECD Health Data 2003(Computer Package) OECD; Paris, France: 2003. [Google Scholar]
- Productivity Commission. Research Report. Ausinfo; Canberra, Australia: 2001. International Pharmaceutical Price Differences. [Google Scholar]
- Roughead EE, Gilbert AL, Primrose JG, et al. Report of the National Indicators: Evaluating the Quality Use of Medicines Component of Australia's National Medicines. Publication Production Unit, Commonwealth Department of Aged Care; Canberra, Australia: 1999. [Google Scholar]
- Roughead EE, Gilbert AL, Primrose JG, Sanson LN. Drug-Related Hospital Admissions: A Review of Australian Studies Published 1988-1996. Medical Journal of Australia. 1998 Apr 20;168(8):405–408. doi: 10.5694/j.1326-5377.1998.tb138996.x. [DOI] [PubMed] [Google Scholar]
- Salkeld G, Mitchell A, Hill S. Pharmaceuticals. In: Mooney R, Scotton R, editors. Economics and Australian Health Policy. Allan and Unwin; St Leonards, Australia: 1999. [Google Scholar]
- Saltman RB, Figueras J. European Health Care Reform: Analysis of Current Strategies. WHO Regional Publications; Copenhagen, Denmark: 1997. (European Series Number 72). [Google Scholar]
- Schoen C. A Disease-Based Comparison of Health Systems. What Is Best and at What Cost? Paris, France: OECD; 2003. Ageing and Health Policy: The Value of International Comparisons and the Potential of Surveys to Add a Missing Perspective; pp. 339–350. Internet address: http://www.oecdwash.org/PUBS/BOOKS/RP033/rp033hlth.htm (Accessed 2004.) [Google Scholar]
- Schofield D. Re-Examining the Distribution of Health Benefits in Australia: Who Benefits from the Pharmaceutical Benefits Scheme. National Centre for Social and Economic Modelling, University of Canberra; Canberra, Australia: 1998. Discussion Paper Number 36. [Google Scholar]
- Shenfield GM, Tasker JL. History in the Making: The Evolution of Consumer Product Information (CPI) Medical Journal of Australia. 1997 Apr 21;166(8):425–428. doi: 10.5694/j.1326-5377.1997.tb123195.x. [DOI] [PubMed] [Google Scholar]
- Sloan C. A History of the Pharmaceutical Benefits Scheme 1947-1992. Commonweath Department of Human Services and Health; Canberra, Australia: 1995. [Google Scholar]
- Sweeny K. Trends in the Use and Cost of Pharmaceuticals under the Pharmaceutical Benefits Scheme. Victoria University of Technology; Melbourne, Australia: 2002. Centre for Strategic Economic Studies Working Paper Number 5. [Google Scholar]
- Walker A, Percival R, Harding A. The Impact of Demographic and Other Changes on Expenditure on Pharmaceutical Benefits in 2020 in Australia. National Centre for Social and Economic Modelling, Faculty of Management. University of Canberra; Canberra, Australia: 1998. Discussion Paper Number 31. [Google Scholar]
- Wright DJ. Profitability and Leakage under the PBS. The Drawing Board Digest. 2003 Dec; Internet address: http://www.econ.usyd.edu.au/drawingboard (Accessed 2004.)
- Wright DJ. The Drug Bargaining Game: Pharmaceutical Regulation in Australia. Journal of Health Economics. doi: 10.1016/j.jhealeco.2003.11.003. in press. [DOI] [PubMed] [Google Scholar]