Abstract
The biosynthesis of melanin has been linked with virulence in diverse pathogenic fungi. Penicillium marneffei, a dimorphic fungus, is capable of melanization in both mycelial and yeast phases, and the pigment may be produced during infection to protect the fungus from the host immune system. To investigate the impact of yeast morphological transformation on antifungal susceptibility, P. marneffei was cultured on various media including minimal medium, 1% tryptone, brain heart infusion broth, and malt extract broth by using the standardized susceptibility protocol (the M27-A protocol, RPMI medium) for yeasts. We also investigated whether P. marneffei melanization affected its susceptibility to antifungal drugs by adding L-DOPA into culture broths. There were no differences in the minimum inhibitory concentrations (MICs) of P. marneffei yeast cells previously grown in various culture broths with or without L-DOPA using the M27A protocol (into which no melanin substrate can be added due to a rapid color change of the RPMI medium to black) for testing amphotericin B, clotrimazole, fluconazole, itraconazole and ketoconazole. However, both melanized and non-melanized P. marneffei displayed increased resistance to antifungal drugs when L-DOPA was added into a selected assay medium, 0.17% yeast nitrogen base, 2% glucose, and 1.5% agar. Hence, active melanin formation appears to protect P. marneffei by enhancing its resistance to antifungal drugs.
1. Introduction
Penicilliosis marneffei, caused by the dimorphic fungus Penicillium marneffei, recently changed to Talaromyces marneffei (Samson et al. 2011), is a systemic human mycosis geographically restricted to Southeast Asia and southern China (Supparatpinyo et al. 1994). It is believed that the infectious process involves the inhalation of small conidia produced by the environmental mold form of the fungus that subsequently undergo morphogenic transformation into yeast within the lungs. At present, P. marneffei affects mainly patients with advanced HIV infection living within the endemic area, particularly in northern Thailand (Supparatpinyo et al. 1992). In AIDS patients, infection with P. marneffei typically presents as a disseminated illness characterized by fever, cough, weight loss, skin lesions and pancytopenia (Tsui et al. 1992; Sirisanthana and Sirisanthana 1995), and it is fatal without antifungal treatment.
P. marneffei is unique in its genus in being dimorphic, and the capacity of P. marneffei to grow at 37°C facilitates its infectivity (Hamilton 2003). When grown at 37°C, the yeast-like form of P. marneffei is properly described as existing as fission arthroconidia. It is notable that the morphology of yeast cells grown in vitro differs from that found in vivo (Cánovas and Andrianopoulos 2007). The morphology of the yeast cells is significantly impacted by the nutritional conditions of the growth environment (Tongchusak et al. 2004). We investigated the effect of different morphological yeast cells on antifungal susceptibility.
Melanins are of considerable interest as putative virulence factors in many fungal pathogens (Hamilton and Gómez 2002). We have previously shown that P. marneffei can produce melanin or melanin-like compounds in vitro and during infection (Youngchim et al. 2005; Liu et al. 2014). In this respect, melanin presumably contributes to P. marneffei virulence by promoting survival within host tissue by protecting the fungus from oxidative damage, microbicidal peptides, etc as well as providing structural support against osmotic and other stresses in the cell wall (Nosanchuk and Casadevall 2003, 2006). Given the potential role of melanin in virulence of P. marneffei, the impact of melanin on the susceptibility of the fungus to antifungals was studied.
2. Material and Methods
2.1 Chemicals
Glycine, NaCl, RPMI 1640 medium (with L-glutamine and without sodium bicarbonate), vitamin B1, amphotericin B (AMB), morpholinepropanesulfonic acid (MOPS), and L-3,4-dihydroxyphenylalanine (L-DOPA) were purchased from Sigma Chemical Co. (Cleveland, Ohio). Four azole derivatives were used in this study; ketoconazole (KTC, Janssen, Beerse, Belgium), itraconazole (ITC, Janssen), clotrimazole (CLT, Sigma) and fluconazole (FLC, Sigma).
2.2 Production of P. marneffei conidia
P. marneffei ATCC 200051 was isolated from a bone marrow sample of an HIV infected patient at Maharaj Nakorn Chiang Mai University, Chiang Mai, Thailand (Hamilton et al. 1999). The P. marneffei isolate was maintained by monthly subculture onto Malt Extract Agar (MEA; Oxoid). For conidial isolation, P. marneffei was grown on MEA for 7–10 days at 25°C, and conidia were isolated by the addition 5 ml of sterile PBS onto the surface followed by gentle scraping of the mycelia growth with a cotton swab. The conidia were collected by filtration through sterile glass wool, centrifuged at 5000 g for 15 min, and then washed three times with sterile PBS.
2.3 Preparation of melanized yeast cells in P. marneffei ATCC 200051
In contrast to the conidia, melanization of P. marneffei yeast cells requires the addition of a phenolic substrate; hence, L-DOPA was utilized for melanin induction in yeast cells. P. marneffei conidia, at a concentration 106 cells/ml, were inoculated in a defined liquid minimal medium (MM; 15.0 mM glucose, 10.0 mM MgSO4, 29.4 mM KH2PO4, 13.0 mM glycine, 3.0 μM vitamin B1 [pH 5.5]) with or without 1.0 mM L-DOPA (Sigma) for 7–15 days at 37°C in a rotary shaker at 150 rpm. All cultures were performed in the dark to prevent photopolymerization. To compare the effect of culture broth, P. marneffei conidia were also similarly cultivated in different liquid growth mediums−1% tryptone (Difco), Brain heart infusion (BHI) broth (Difco), and Malt Extract (ME) broth. The yeast cells were harvested by centrifugation at 5000 g for 15 min at 4°C and washed three times with sterile PBS.
2.4 Immunofluorescence analysis of melanin expression in P. marneffei.
To confirm melanization of P. marneffei, slide cultures of this dimorphic fungus were prepared as described (Youngchim et al. 2005). To detect melanin in mycelial phase of P. marneffei, the fungus was cultured on MEA and incubated at 25°C for 7 days at which time conidiogenesis was complete. In addition, yeast cells of P. marneffei were cultured in MM with or without 1.0 mM L-DOPA at 37°C for 7–10 days. The yeast cells were harvested by centrifugation at 5000 g for 15 min. The suspensions of yeast cells from P. marneffei were air-dried on poly-L-lysine slides. The conidia and yeast slides were blocked with Superblock Blocking Buffer in PBS (Pierce) for 2 h at 37°C or overnight at 4°C to block non-specific binding, washed three times with PBS, and then incubated for 1.5 h at 37°C with 10 mg/ml anti-melanin monoclonal antibody (MAb) 8D6, an IgM melanin-binding MAb previously generated against A. fumigatus conidial melanin (Youngchim et al. 2004). The slides were washed in PBS and incubated in a 1: 1000 dilution of FITC-conjugated goat anti-mouse IgM Alexa Fluor 488 conjugate (Invitrogen) for 2 h at 37°C. The slides were washed three times with PBS to eliminate unbound antibody and then examined under a Nikon Eclipse 50i fluorescence microscope (Nikon, Tokyo, Japan). For a negative control, conjugated goat-anti mouse IgM alone without primary MAb was included in the experiments. All images were captured and analyzed with a digital camera (Nikon DS-Fi 1) and imaging software (NIS-D, Nikon).
2.5 MIC determination
The minimal inhibitory concentrations (MICs) of P. marneffei for AMB, ITC, KTC, CLT and FLC were determined using the standardized protocol (the M27-A protocol) for yeasts developed by the National Committee for Clinical Laboratory Standards (NCCLS, 1997) microdilution method (M27-A). Briefly, melanized or nonmelanized yeast cells of P. marneffei were suspended in sterile normal saline and diluted to a concentration of 1 × 106 to 5 × 106 cells/ml. Cell counts were determined with a hemocytometer. Viability was confirmed as >95% by Trypan blue staining. Yeast cell suspensions were made by a 1:100 dilution followed by a 1:20 dilution in RPMI 1640 broth medium, which results in 5.0 × 102 to 2.5 × 103 cells/ml. Polystyrene plates (Corning) containing 0.1 ml aliquots of an antifungal at 2 times the final drug concentrations were inoculated with 0.1 ml of the diluted suspensions. Each micro dilution well containing 100 μl of the diluted drug concentrations was inoculated with 100 μl of the diluted yeast inoculum suspensions (final volume in each well was 200 μl). Final drug concentrations ranged from 0.0313 to 16 μg/ml for AMB, ITC, KTC, and CLT whereas the range was 0.125 to 16 μg/ml for FLC. The MICs were recorded after incubation of the plates at 35°C for 48 hours. The MIC for AMB was defined as the lowest concentration with no visible growth. The MICs for azoles (ITC, KTC, CLT and FLC) were read as the lowest concentration with 80% growth reduction compared with control (drug-free) well. Tests were performed in duplicate and all experiments were repeated three times. Candida albicans ATCC 900028, reference strain, was included to confirm the reproducibility of the results.
2.6 Effects of melanin synthesis on antifungals
The assay media, 0.17 % yeast nitrogen base (YNB; Difco), 2% glucose, and 1.5% agar, with and without 1.0 mM L-DOPA were added with various concentrations of antifungal agents (AMB, CLT, KTC, ITC and FLC). Ten fold dilutions of P. marneffei yeast cells (melanized or non-melanized cells) at a concentration 5 × 106 cells/ml were prepared and then 10 μl of each dilution were inoculated onto assay media with various concentrations of antifungals. The plates were incubated at 28°C for 5 to 7 days. The assay media with or without L-DOPA in the absence of antifungals was used as control.
3. Results
3.1 Melanization of P. marneffei in both mycelial and yeast forms in vitro.
Both P. marneffei mycelial grown on MEA and yeast cells cultivated with L-DOPA were reactive with anti-melanin MAb 8D6 (Fig. 1). In mold phase, conidia, phialides, and hyphae were all labeled by the anti-melanin MAb 8D6 (Fig. 1 A, B). In addition, yeast cells of P. marneffei cultured in a defined liquid MM with L-DOPA were bound by the anti-melanin MAb 8D6 (Fig. 1C, D). There was no antibody binding with P. marneffei yeast cells cultured in a defined liquid MM without L-DOPA. No reactivity with pigmented cells was observed when FITC-labeled goat anti-mouse IgM was used alone.
Fig. 1.
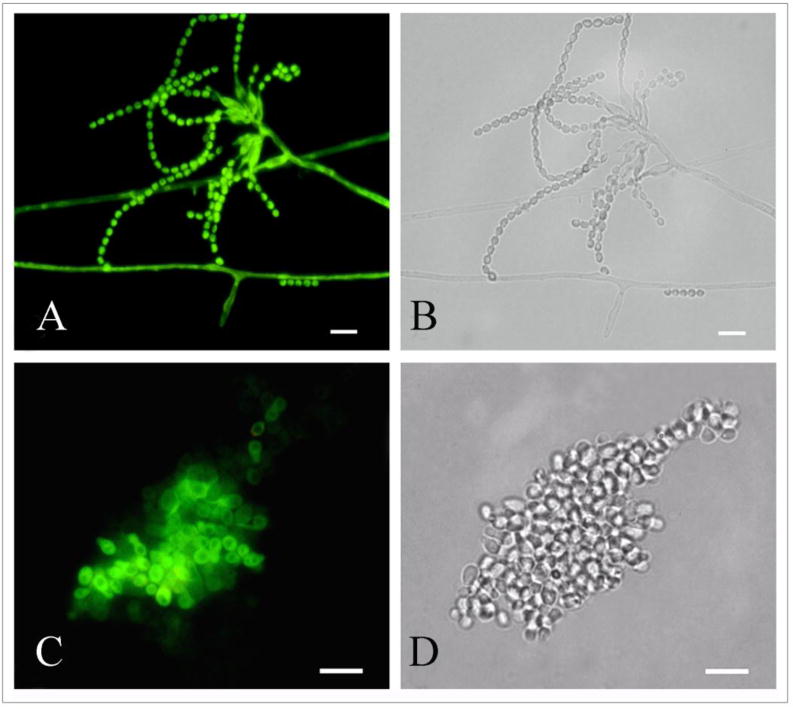
Corresponding immunofluorescence (A,C) and bright field (B,D) microscopy images demonstrating the labeling of mycelial phase (A,B) and yeast cells of P. marneffei by the melanin-binding MAb 8D6. No reactivity was observed when FITC labeled goat-anti mouse IgM alone was used (not shown). Bar, 5 μm.
3.2 P. marneffei yeast cells in different culture broth
The morphology of P. marneffei yeast cells cultured in MM, 1% tryptone, BHI or ME broth at 37°C was examined (Fig. 2). First, we assessed morphology in the absence of L-DOPA. P. marneffei conidia inoculated in MM and 1 % tryptone broth produced yeast cells with internal septa, fission yeast similar to yeast cells found in clinical specimens (Fig. 2A, B). P. marneffei conidia cultured in BHI and ME broth transformed into rectangular or rounded cells resembling arthroconidia (Fig. 2C, D). The majority of cells in BHI were in the large rectangular form whereas most of the cells were rounded in ME broth. Interestingly, the addition of L-DOPA in culture media had no gross effects on the morphology of P. marneffei as cells grown in each culture media with or without L-DOPA were morphologically similar (data not shown).
Fig. 2.
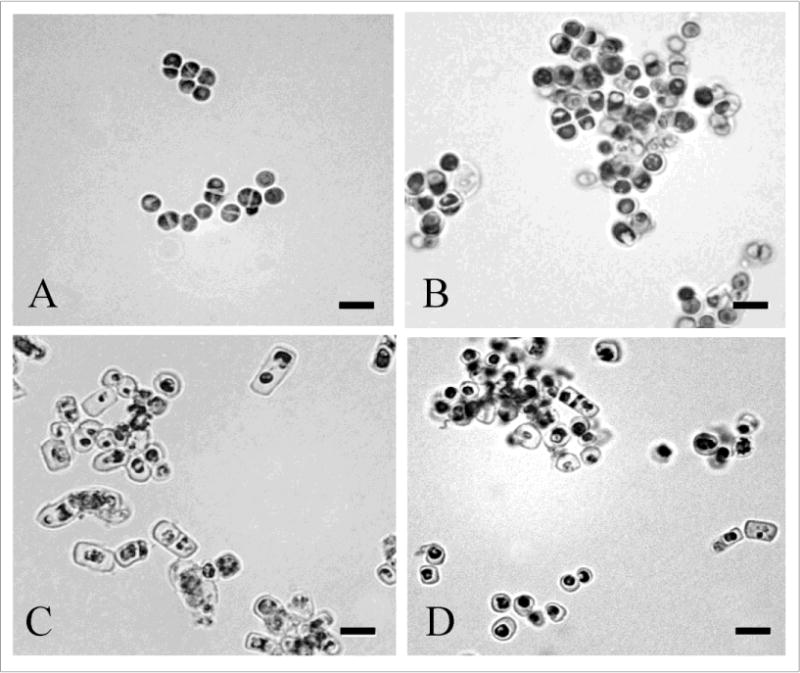
Morphology of P. marneffei yeast cells grown in various culture media: A, minimal medium; B, 1% tryptone; C, BHI broth; D, ME broth. Bar, 5 μm.
3.3. MICs of P. marneffei with melanized and non-melanized yeast cells
Table 1 shows the susceptibilities of melanized and non-melanized cells of P. marneffei for AMB and the azoles. MICs were within the range of values previously reported for this fungus (McGinnis et al. 2000; Sar et al. 2006). No significant differences of melanized and non-melanized cells in the different culture broths were observed between MICs of antifungal drugs.
Table 1.
MICs for P. marneffei with melanized and nonmelanized yeast cells in different culture broth.
| P. marneffei in | MIC (μg/ml) | ||||
|---|---|---|---|---|---|
| Culture broth | AMB | ITC | CTM | FCN | KTC |
| Minimal medium with L-DOPA without L-DOPA |
1.0 1.0 |
0.25 0.25 |
0.125 0.250 |
4.0 4.0 |
0.125 0.125 |
| 1% Tryptone broth with L-DOPA without L-DOPA |
0.5 0.5 |
<0.0313 <0.0313 |
0.0625 0.0625 |
8.0 8.0 |
0.25 0.25 |
| Brain Heart infusion with L-DOPA without L-DOPA |
0.5 0.5 |
<0.0313 <0.0313 |
0.0625 0.0625 |
8.0 8.0 |
0.125 0.125 |
| Malt Extract broth with L-DOPA without L-DOPA |
0.5 0.5 |
<0.0313 <0.0313 |
0.0625 0.0625 |
4.0 4.0 |
0.125 0.125 |
3.4. Effects of melanin synthesis on antifungals
To further study the effect of melanization on susceptibility to antifungals, L-DOPA was added into the antifungal assay medium, YNB agar, with various concentrations of antifungals. In the study of AMB susceptibility, melanin influenced susceptibility (Fig. 3). When L-DOPA was added to the medium, initially melanized and non-melanized yeast cells were more resistant compared to these cells grown on medium without L-DOPA. In the presence of L-DOPA, both initially melanized and non-melanized cells displayed similar resistance to AMB. In contrast, the melanized cells were significantly more resistant than the non-melanized cells in the absence of L-DOPA in the medium.
Fig. 3.
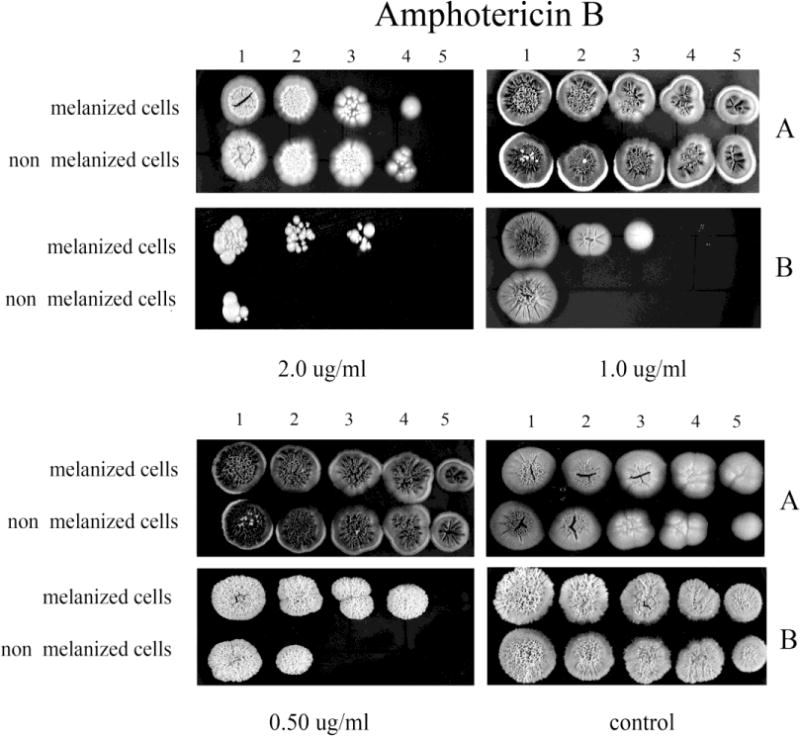
The effect of melanization of P. marneffei yeast cells on susceptibility to AMB. Ten fold dilutions of melanized and non-melanized cells, at a concentration 5 × 106 cells/ml, were prepared and then 10 μl of each dilution were inoculated onto YNB agar containing AMB with or without L-DOPA (A, and B, respectively).
The inclusion of L-DOPA in medium also significantly enhanced the resistance of both initially melanized and nonmelanized cells against the azole drugs tested compared to cells tested in the absence of L-DOPA (Figs. 4–7). However, the benefit of melanization prior to drug screening varied. For CTL, melanized cells were more resistant by one dilution compared to initially non-melanized cells at 0.0313 and 0.125 μg/ml in the presence of L-DOPA in the YNB agar and at 0.0313 and 0.0625 μg/ml in the absence of L-DOPA (Fig. 4). For FLC, colony formation was impaired in the absence of L-DOPA at all concentrations tested; however, initially melanized cells were ~1 dilution more resistant relative to those not previously melanized (Fig. 5). The presence of L-DOPA appeared to benefit previously melanized cells more than those not initially melanized, albeit to a small extent. ITC was less effective in the presence of L-DOPA and initially melanized cells were more resistant in this condition by 1 or 2 dilutions (Fig. 6). Colony formation was more robust on medium with L-DOPA in the presence of KTC (Fig. 7). Notably, previously melanized cells were more resistant by 1 dilution at 1.0 μg/ml and by 2 dilutions at 2.0 μg/ml in the mediums with or without L-DOPA.
Fig. 4.
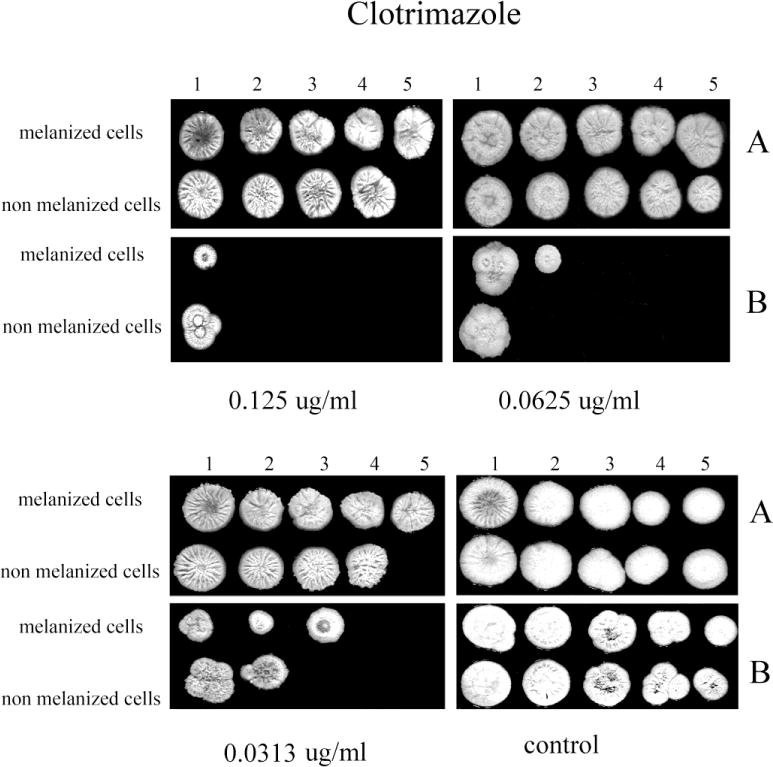
The effect of melanization of P. marneffei yeast cells on susceptibility to CLT. Ten fold dilutions of melanized and non-melanized cells, at a concentration 5 × 106 cells/ml, were prepared and then 10 μl of each dilution were inoculated onto YNB agar containing CLT with and without L-DOPA (A, and B, respectively).
Fig. 7.
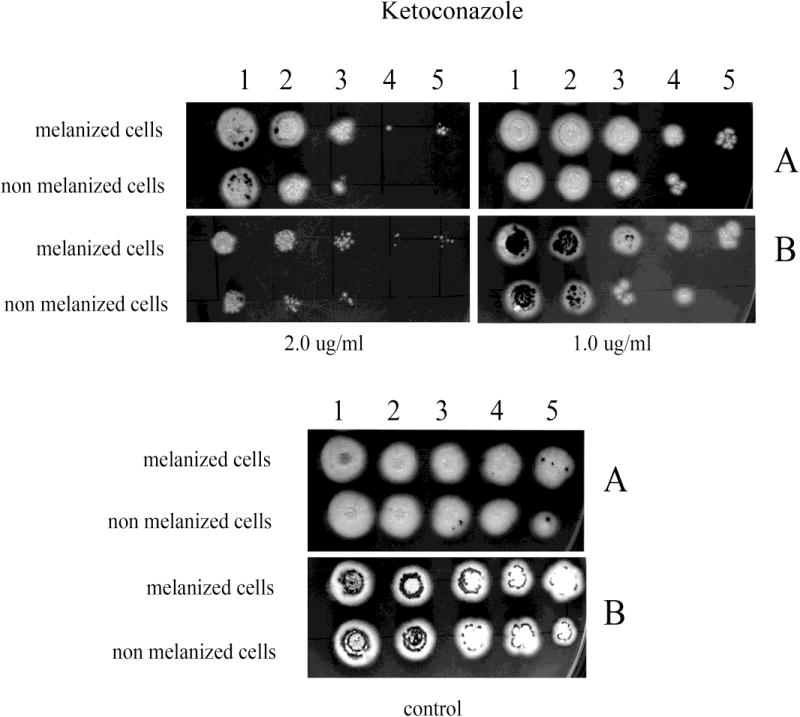
The effect of melanization of P. marneffei yeast cells on susceptibility to KTC. Ten fold dilutions of melanized and non-melanized cells, at a concentration 5 × 106 cells/ml, were prepared and then 10 μl of each dilution were inoculated onto YNB agar containing KTC with and without L-DOPA (A and B, respectively).
Fig. 5.
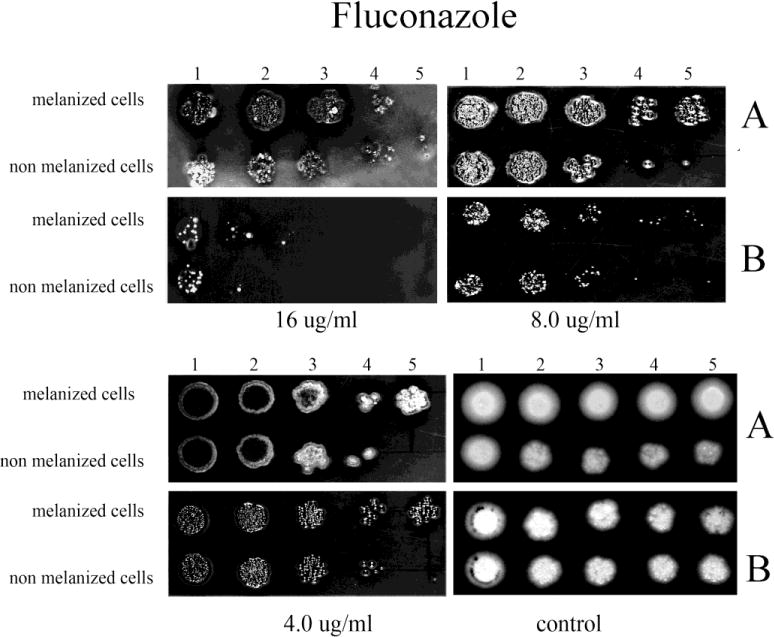
The effect of melanization of P. marneffei yeast cells on susceptibility to FLC. Ten fold dilutions of melanized and non-melanized cells, at a concentration 5 × 106 cells/ml, were prepared and then 10 μl of each dilution were inoculated onto YNB agar containing FLC with and without L-DOPA (A, and B, respectively).
Fig. 6.
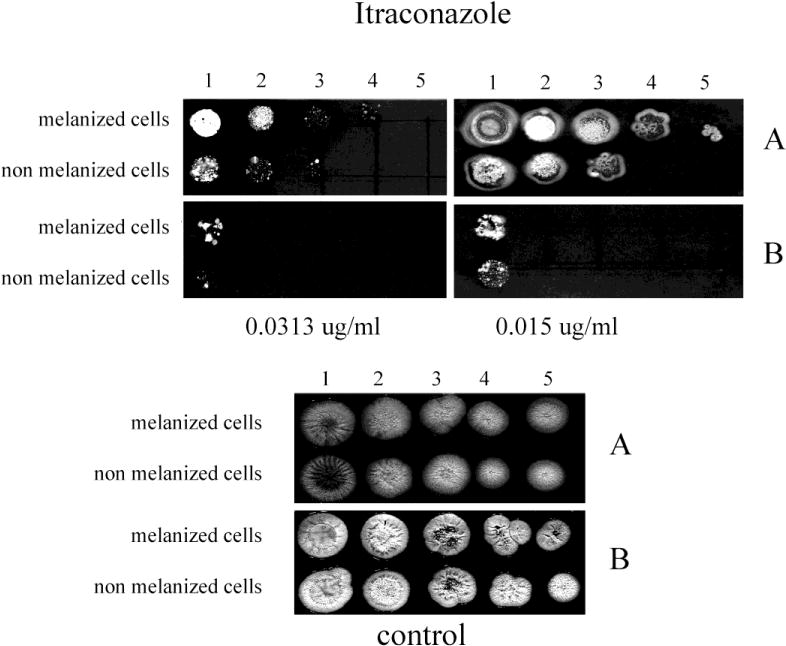
The effect of melanization of P. marneffei yeast cells on susceptibility to ITC. Ten fold dilutions of melanized and non-melanized cells, at a concentration 5 × 106 cells/ml, were prepared and then 10 μl of each dilution were inoculated onto YNB agar containing ITC with and without L-DOPA (A, and B, respectively).
4. Discussion
The production of melanin in fungal pathogens has been associated with their capacity to survive in difficult environments and combat host effector responses (Nosanchuk and Casadevall 2003). For adaptation to harsh environments, melanin can protect fungi against ultraviolet (UV) light, extreme temperatures, heavy metals, lysing enzymes and radiation (Jacobson 2000; Dadachova et al. 2008). Melanization has even more pleotrophic protective effects for fungal pathogens during mammalian infection (Nosanchuk and Casadevall 2003). Melanin pigments can protect pathogenic fungi from the host immune responses by scavenging reactive oxygen species from leukocytes as well as by binding microbicidal peptides. Melanin has been called “an antifungal resistance factor”, given its ability to reduce the susceptibilities of melanized cells to antifungal drugs (Nosanchuk and Casadevall 2006; Ikeda et al. 2003). Here, we investigated the role of melanin of P. marneffei in resistance to antifungal agents. Our results demonstrated no differences in susceptibility between melanized and nonmelanized P. marneffei yeast cells by MIC assays of the M27A protocol for yeasts. These results were similar to those reported for other pathogenic fungi, C. neoformans and H. capsulatum (van Dulin et al. 2002), Paracoccidioides brasiliensis (da Silva et al. 2006), and Blastomyces dermatitidis (Nosanchuk et al. 2004). Similarly, no difference in the susceptibilities of melanized and nonmelanized P. brasiliensis to antifungal drugs was observed using the minimum inhibitory concentration (MIC) method. This may have been affected by the lack of substrates for melanization in the medium used in the M27A protocol. With this protocol, the addition of L-DOPA to the RPMI medium results in L-DOPA auto-polymerization, which makes it unusable for MIC testing. Hence, we utilized our dilution assay on YNB agar with or without L-DOPA to evaluate whether or not melanin had a protective role in the presence of the antifungal drugs studied. The presence of L-DOPA in the medium enhanced the resistance of both initially melanized and non-melanized P. marneffei yeast cells to each of the antifungal agents tested. The degree to which prior melanization further protected the yeast cells varied according to the antifungal examined, but, overall, prior melanization enhanced fungal resistance compared to initially non-melanized cells by at least 1 dilution at most drug concentrations tested. To our knowledge, this is the first study demonstrating that melanin production of P. marneffei has an impact on enhancing the resistance of fungal cells to antifungal agents.
The production of P. marneffei as uniform fission yeast was achieved in MM with 1% tryptone. Similar results have previously been observed in 1% peptone, although the growth rate was significantly slower than other laboratory media tested (Tongchusak et al. 2004, 2006). To study the effect of melanization on drug susceptibility, we selected MM with 1% tryptone for culturing fission yeasts of P. marneffei as this most closely resembles this fungus’ form in vivo. Furthermore, the addition of the melanin substrate L-DOPA into this culture media has no effect on the morphology of P. marneffei yeast cells.
To summarize, P. marneffei melanization provides protection against antifungal drugs used clinically to treat this fungus. It is noteworthy that prior melanization of P. marneffei yeast cells is protective against antifungals, but, significantly, access to a phenolic substrate, such as L-DOPA, during exposure to antifungals promotes the resistance of both previously melanized and non-melanized yeast cells. Due to the protective role against antifungal drugs in P. marneffei, melanin is therefore an appropriate target for therapeutic intervention.
Acknowledgments
We would like to thank the National Research University Project under Thailand’s Office of the Higher Education Commission for financial support. JDN is supported in part by NIH AI52733.
Footnotes
Conflicts of interest
The authors have declared that no competing interests exist.
References
- Cánovas D, Andrianopoulos A. The Biology of the Thermally Dimorphic Fungal Pathogen Penicillium marneffei. In: Kavanagh K, editor. New Insights in Medical Mycology. Springer; Netherlands: 2007. pp. 213–226. [Google Scholar]
- da Silva MB, Marques AF, Nosanchuk JD, Casadevall A, Travassos LR, Taborda CP. Melanin in the dimorphic fungal pathogen Paracoccidioides brasiliensis: effects on phagocytosis, intracellular resistance and drug susceptibility. Microbes Infect. 2006;8:197–205. doi: 10.1016/j.micinf.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Dadachova E, Bryan RA, Howell RC, Schweitzer AD, Aisen P, Nosanchuck JD, Casadevall A. The radioprotective properties of fungal melanin are a function of its chemical composition, stable radical presence and spatial arrangement. Pigment Cell Melanoma Res. 2008;21:192–199. doi: 10.1111/j.1755-148X.2007.00430.x. [DOI] [PubMed] [Google Scholar]
- Hamilton AJ, Jeavons L, Youngchim S, Vanittanakom N. Recognition of fibronectin by Penicillium marneffei conidia via a sialic acid-dependent process and its relationship to the interaction between conidia and laminin. Infect Immun. 1999;67:5200–5205. doi: 10.1128/iai.67.10.5200-5205.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AJ, Gómez BL. Melanins in fungal pathogens. J Med Microbiol. 2002;51:189–191. doi: 10.1099/0022-1317-51-3-189. [DOI] [PubMed] [Google Scholar]
- Hamilton AJ. Penicillium marneffei: Penicilliosis and the read peril in the east. Mycologist. 2003;17:84–85. doi: 10.1017/S0269-915X(03)00218-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda R, Sugita T, Jacobson ES, Shinoda T. Effects of melanin upon susceptibility of Cryptococcus to antifungals. Microbiol Immunol. 2003;47:271–277. doi: 10.1111/j.1348-0421.2003.tb03395.x. [DOI] [PubMed] [Google Scholar]
- Jacobson ES. Pathogenic roles for fungal melanins. Clin Microbiol Rev. 2000;13:708–717. doi: 10.1128/cmr.13.4.708-717.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Wei L, Guo T, Tan W. Detection of DOPA-melanin in the dimorphic fungal pathogen Penicillium marneffei and its effect on macrophage phagocytosis in vitro. PLoS One. 2014;9(3):e92610. doi: 10.1371/journal.pone.0092610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis MR, Nordoff NG, Ryder NS, Nunn GB. In vitro comparison of terbinafine and itraconazole against Penicillium marneffei. Antimicrob Agents Chemother. 2000;44:1407–1408. doi: 10.1128/aac.44.5.1407-1408.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCCLS. Approved standard M27-A. National Committee for Clinical Laboratory Standards; Villanova, Pa: 1997. Reference method for broth dilution antifungal susceptibility testing for yeasts. [Google Scholar]
- Nosanchuk JD, Casadevall A. The contribution of melanin to microbial pathogenesis. Cell Microbiol. 2003;5:203–223. doi: 10.1046/j.1462-5814.2003.00268.x. [DOI] [PubMed] [Google Scholar]
- Nosanchuk JD, van Duin D, Mandal P, Aisen P, Legendre AM, Casadevall A. Blastomyces dermatitidis produces melanin in vitro and during infection. FEMS Microbiol Lett. 2004;239:187–193. doi: 10.1016/j.femsle.2004.08.040. [DOI] [PubMed] [Google Scholar]
- Nosanchuk JD, Casadevall A. Impact of melanin on microbial virulence and clinical resistance to antimicrobial compounds. Antimicrob Agents Chemother. 2006;50:3519–3528. doi: 10.1128/AAC.00545-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson RA, Yilmaz N, Houbraken J, Spierenburg H, Seifert KA, Peterson SW, Varga J, Frisvad JC. Phylogeny and nomenclature of the genus Talaromyces and taxa accommodated in Penicillium subgenus Biverticillium. Stud Mycol. 2011;70:159–183. doi: 10.3114/sim.2011.70.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sar B, Boy S, Keo C, Ngeth CC, Prak N, Vann M, Monchy D, Sarthou JL. In vitro antifungal-drug susceptibilities of mycelial and yeast forms of Penicillium marneffei isolates in Cambodia. J Clin Microbiol. 2006;44:4208–4210. doi: 10.1128/JCM.00902-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirisanthana V, Sirisanthana T. Disseminated Penicillium marneffei infection in human immunodeficiency virus-infected children. Pedriatr Infect Dis. 1995;14:935–940. doi: 10.1097/00006454-199511000-00003. [DOI] [PubMed] [Google Scholar]
- Supparatpinyo K, Chiewchanvit S, Hirunsri P, Baosoung V, Uthammachai C, Nelson KE, Sirisanthana T. Penicillium marneffei infection in patients infected with human immunodeficiency virus. Clin Infect Dis. 1992;14:871–874. doi: 10.1093/clinids/14.4.871. [DOI] [PubMed] [Google Scholar]
- Supparatpinyo K, Khamwan C, Baosoung V, Nelson KE, Sirisanthan T. Disseminated Penicillium marneffei infection in Southeast Asia. Lancet. 1994;344:110–113. doi: 10.1016/s0140-6736(94)91287-4. [DOI] [PubMed] [Google Scholar]
- Tongchusak S, Pongtanalert P, Pongsunk S, Chawengkirttikul R, Chaiyaroj SC. Distinct immunologic properties of Penicillium marneffei yeasts obtained from different in vitro growth conditions. Asian Pac J Allergy Immunol. 2004;22:229–235. [PubMed] [Google Scholar]
- Tongchusak S, Pongsunk S, Watkins P, Chaiyaroj SC. In vitro growth characterization of Penicillium marneffei morphotypic conversion. Science Asia. 2006;32:385–393. [Google Scholar]
- Tsui WMS, Ma KF, Tsang DNC. Disseminated Penicillium marneffei infection in HIV-infected subject. Histopathology. 1992;20:287–293. doi: 10.1111/j.1365-2559.1992.tb00985.x. [DOI] [PubMed] [Google Scholar]
- van Duin D, Casadevall A, Nosanchuk JD. Melanization of Cryptococcus neoformans and Histoplasma capsulatum reduces their susceptibilities to amphotericin B and caspofungin. Antimicrob Agents Chemother. 2002;46:3394–3400. doi: 10.1128/AAC.46.11.3394-3400.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngchim S, Morris-Jones R, Hay RJ, Hamilton AJ. Production of melanin by Aspergillus fumigatus. J Med Microbiol. 2004;53:175–181. doi: 10.1099/jmm.0.05421-0. [DOI] [PubMed] [Google Scholar]
- Youngchim S, Hay RJ, Hamilton AJ. Melanization of Penicillium marneffei in vitro and in vivo. Microbiol. 2005;151:291–299. doi: 10.1099/mic.0.27433-0. [DOI] [PubMed] [Google Scholar]


