Abstract
Background:
In general, organic solvents are inhibiting many physiological enzymes and alter the behavioural functions, but the available scientific knowledge on laboratory solvent induced organ specific toxins are very limited. Hence, the present study was planned to determine the sub-chronic toxic effects of petroleum ether (boiling point 40–60°C), a laboratory solvent in Sprague-Dawley (SD) rats.
Materials and Methods:
The SD rats were divided into three different groups viz., control, low exposure petroleum ether (250 mg/kg; i.p.) and high exposure petroleum ether (500 mg/kg; i.p.) administered group. The animals were exposed with petroleum ether once daily for 2 weeks. Prior to the experiment and end of the experiment animals behaviour, locomotor and memory levels were monitored. Before initiating the study animals were trained for 2 weeks for its learning process and its memory levels were evaluated. Body weight (BW) analysis, locomotor activity, anxiogenic effect (elevated plus maze) and learning and memory (Morris water navigation task) were monitored at regular intervals. On 14th day of the experiment, few ml of blood sample was collected from all the experimental animals for estimation of biochemical parameters. At the end of the experiment, all the animals were sacrificed, and brain, liver, heart, and kidney were collected for biochemical and histopathological analysis.
Results:
In rats, petroleum ether significantly altered the behavioural functions; reduced the locomotor activity, grip strength, learning and memory process; inhibited the regular body weight growth and caused anxiogenic effects. Dose-dependent organ specific toxicity with petroleum ether treated group was observed in brain, heart, lung, liver, and kidney. Extrapyramidal effects that include piloerection and cannibalism were also observed with petroleum ether administered group. These results suggested that the petroleum ether showed a significant decrease in central nervous system (CNS) activity, and it has dose-dependent toxicity on all vital organs.
Conclusion:
The dose-dependent CNS and organ specific toxicity was observed with sub-chronic administration of petroleum ether in SD rats.
Keywords: Cannibalism, petroleum ether, piloerection
Introduction
Polycyclic aromatic hydrocarbons are universal environmental contaminants and get exposed from chemical laboratories and petrochemical industries. Inhalation of aromatic hydrocarbons or hydrocarbons may cause hemorrhaging, central nervous system (CNS) toxicities, craniofacial malformation and cardiac defects, which includes elongated heart. In laboratories, methanol, petroleum ether, diethyl ether and acetonitrile are most commonly used for extraction of plant materials/biological and chromatographic separations.[1] The toxicity of methanol and diethyl ether is well-established and both the solvent produce toxicity in digestive and nervous systems. Moreover, methanol boiling point (BP) is around 65°C ± 1°C, and it is not volatile in ambient temperature. The low BP solvents such as diethyl ether, petroleum ether, etc. has high risk to produce inhalation toxicity. Inhalation of diethyl ether produce hepatotoxicity and this solvent was not recommended for clinical and preclinical experiments.[2] Petroleum ether is one of the most commonly used solvent in chemical and pharmaceutical industries/laboratories and it is volatile in nature. In a pharmacogency laboratory, petroleum ether is used for extraction of plant materials and chromatographic separations. Sometime petroleum ether traces present in herbal extract was not completely removable and which may affect the pharmacological activity of the herb. In pharmacogenetics study, petroleum ether is used as a solvent for extraction of plant, but the toxic effect of petroleum ether is unclear and available toxicity information for petroleum ether is very limited. In some laboratory experiments, petroleum ether herbal extract showed piloerection and cannibalism during repeated dose administration and this was not observed in other solvents[3].
The reported LD50 value of petroleum ether is 3400 mg/l (oral) in 4 h and the major toxic effect of petroleum ether observed in central nervous and dermatological systems. Since petroleum ether is volatile in normal ambient temperature, and inhalation of petroleum ether vapor may cause central nervous, respiratory and digestive systems toxicities. Moreover, petroleum ether is reported to have CNS toxicity and no data available on its effect on learning and memory, neuronal and metabolic functions. Hence, the present study is planned to study the sub-acute effect of petroleum ether exposure on rodent behaviour, locomotor activity, learning and memory process and organ specific changes.
Materials and Methods
Animals
Healthy, adult, either gender of Sprague-Dawley (SD) rats, weighing 140–160 g were obtained from Central Animal house, AIMST University, Malaysia. The animals were housed in large, spacious poly acrylic cages at an ambient room temperature with 12-h-light/12-h-dark cycle. The animals were fed with water, and normal rats pellet fed ad libitum. The study was approved by AIMST University Human and Animal Ethics Committee (AUHAEC10/FOP/2013) and the study was conducted according to Animal Research Review Panel guidelines.
Chemicals
An alkaline phosphatase (ALP), glucose serum glutamic-oxalocetic transaminase (SGOT), serum glutamic-pyruvic transaminase (SGPT), total protein, urea and creatinine analytical strips (Reflotron, USA) were purchased from Chempex Sdn. Bhd., Malaysia. Analytical grade of petroleum ether (BP 40–60°C) was purchased from Bendosen laboratory chemicals, Malaysia. Analytical grade of disodium ethylenediaminetetraacetic acid (EDTA), diethyl ether, and formaldehyde were obtained from RM chemicals/HmbG chemicals, Malaysia and used for the experiment.
Acute toxicity testing
The fixed dose method (FDM) was used for acute toxicity testing. The acute toxicity testing of petroleum ether was carried out at the dose levels of 5, 50, 500 and 2000 mg/kg body weight (BW), according to the Organization for Economic Cooperation Development (OECD) guidelines (new OECD FDM test guideline 420). Three female rats were sequentially dosed (single intraperitoneal injection) at intervals of 24 h and observed for toxicity signs (behavioural functions: alertness, restlessness, irritability, and fearfulness; neurological activity: spontaneous activity, reactivity, touch response, pain response, and gait; and autonomic profiles: defecation and urination) for at least once daily for 14 days.[4]
Sub-chronic toxicity testing
Repeat-dose oral toxicity study of petroleum ether was carried out at 250 and 500 mg/kg BW, according to OECD guideline 407. The rats of SD strain were divided into three groups of 8 animals each (4 males and 4 females). Group 1 received 1 ml/kg BW, of normal saline and served as control. Groups 2 and 3 received petroleum ether doses of 250 and 500 mg/kg BW, respectively. Petroleum ether and normal saline were administered intraperitoneally, once daily for 14 days at morning times. The animals were observed at least twice for toxicity signs and mortality, cannibalism and other behavioural abnormalities. The BW variations of all the experimental animals were monitored at regular intervals. During the study, regular food and water intake were also monitored. The locomotor action, water navigation and grip strength, were monitored on prestudy day, 7th and 14th day of the experiment. On 14th day of the experiment, few ml of blood sample was collected from all the experimental animals for estimation of biochemical parameters. At the end of the study, all the animals were sacrificed, gross pathology was observed and brain, liver, kidney, lung, and heart were isolated and organ weights were measured. Part of the brain tissue was preserved in − 80°C for estimation of glutamate and glutathione (GSH). Liver, lungs, heart, kidney and part of the brain samples were preserved in 10% formalin for histopathological analysis.[4,5,6]
Body weight analysis
The BW of each rat in each group was recorded initially and at weekly intervals. The percentage change in BW was calculated and recorded.
Locomotor activity
The activity of the rats were recorded in a rodent activity cage (actophotometer) provided with an acrylic cage and 16 beams of infrared light along both the x- and y-axis. The activity of each rat was monitored at room temperature over 10 min.
Anxiogenic effect of petroleum ether (elevated plus maze)
The rodent elevated plus maze consists of two open and enclosed arms with the dimension of 50 × 10 (L × W) and 40 cm height of enclosed arms. Two open and enclosed arms are opposite to each other and maze is elevated to a height of 60 cm. The rats were handled by two of the investigator to reduce animal handling induced stress. Prior to the experiment, at 7th and 14th day of the experiment, the rat was placed in the center of the maze, facing one of the enclosed arms and monitored for 10 min. During observation, number of the entries to each arms and time spent in each arms were recorded. The experiment was conducted at sound free environment.[7]
Effect of petroleum ether on learning and memory (Morris water navigation task)
Water navigation test is employed as a method to test spatial learning and memory parameters to evaluate the spatial learning and memory functions.[8] The water maze consists of circular take with 90 cm diameter and wall 20 cm above the water level with 25°C water. A square platform (10 × 5 cm) is hidden 2 cm below the water level. Training was taken place for 3 consecutive days, with a 4 consecutive trials/day for each experimental rat at the inter-trial interval of 30 min. The tank was divided into 4 equal quadrants and trail was started from one of four assigned polar positions with different sequence each day. The latency was measured by finding the time reach to the platform. If any animal fails to reach the platform in any trial within 3 min was excluded from the study.[9]
Effect of petroleum ether on wire grip strength
The string is made up of a plastic or metallic material with 1 cm2 grids, which is suitable for the rats to grip (approximately 0.3–0.5 mm).[10] Training was taken place for 3 consecutive days. Controlled rats as well as petroleum ether induced rats were timed to observe the duration taken by rats to support their weight holding onto a metal rail suspended in midair about 30 cm height from ground. The string was tied into a support by the investigator using a Burette stand. The investigator held the rats by the tail and allowed the rats to grasp the metal rail. Each rat was subjected to five trials with at least 10 min rest period in between tests.[11]
Effect of petroleum ether on biochemical parameter
A blood sample (1 ml) was collected on prestudy day and end of the experiment in disodium EDTA tubes.[12] The plasma and blood matrix were separated by centrifugation at 2500 RPM for 10 min at 4°C, and stored at − 80°C until further biochemical and genotyping analysis (data not presented). The plasma sample was used for estimation of ALP, glucose, SGOT, SGPT, total protein, bilirubin, creatinine and urea levels using a biochemical analyzer (Reflotron Plus System, Hoffmann-La Roche, USA). The activity of brain glutathione peroxidase (GPx) and reduced GSH was estimated by the method described elsewhere.[13,14,15,16,17]
Estimation of glutathione peroxidase
Glutathione peroxidase activity was measured by method described elsewhere and modified by Pasupathi et al. in 2009.[14] GSH reductase activity was expressed as μmol of NADPH oxidized/hour/mL. The animal brain tissue was homogenized using tris buffer. In 0.5 ml of tissue homogenate, 0.2 ml of EDTA, 0.01 ml of sodium azide, 0.2 ml of tris buffer were added. To the mixture 0.2 ml of GSH followed by 0.1 ml of H2O2 was added, mixed and incubated at 37°C for 10 min. After 10 min, the reaction was arrested by adding 0.5 mL of 10% TCA, and the content was centrifuged at 2000 RPM for 20 min. The blank sample was prepared without adding the animal brain tissue homogenate. The remaining GSH activity in the supernatant was measured calorimetrically at 340 nm. The enzyme activity was calculated as nmol NADPH oxidized/minutes/mg protein, using a molar extinction co-efficient of 6.22 × 103/M/cm.
Estimation of glutathione reductase
A GSH reductase level in the brain was determined by method described by Ellman in 1959, and modified by Hissin and Hilf in 1973. Known weight of brain sample was homogenized in PBS. In 1 ml brain homogenate 0.5 ml of 5% TCA was added to the centrifuge at 3000 RPM for 20 min to remove the protein. In 1 ml of supernatant, 0.5 ml of 5% of Ellmans reagent (19.8 mg of 5,5’-dithiobisnitro benzoic acid in 100 ml of 0.1% sodium nitrate) and 3.0 ml of phosphate buffer (0.2 M, ph 8.0) were added, and absorbance was measured at 412 nm on a spectrophotometer. Standards (different concentration of the GSH solution) were also prepared as described above (without tissue homogenate) and absorbance was measured at 412 nm. A GSH reductase levels in brain tissue homogenate was calculated by interpolation method and expressed as μmol/mg protein.[15,16,17]
Histopathological analysis
Part of the brain, heart, liver and lungs samples were preserved in 10% neutral formalin for histopathological analysis. The brain, heart, liver, and lungs sample were embedded in paraffin after being dehydrated in alcohol and subsequently cleared with xylene. Five micrometer thickness of liver section were prepared from paraffin blocks and stained with hematoxylin and eosin and mounted in neutral DPX medium, and the sections were examined under light microscope.[18]
Statistical analysis
The mean ± standard error of the mean values was calculated for each group. Statistical differences among the groups were determined using One-way ANOVA followed by Bonferroni post-hoc test. P < 0.05 was considered to be significant.
Results
Effect of petroleum ether on acute toxicity testing
Acute toxicity testing of single intraperitoneal injection of petroleum ether in rats did not show any mortality, behavioural changes upto 2000 mg/kg. Hence further sub-chronic toxicity studies were carried out at the dose levels of 250 and 500 mg/kg dose levels.
Effect of petroleum ether on sub-chronic toxicity testing
Effect of petroleum ether on body weight
Petroleum ether significantly (P < 0.01) inhibited the regular BW growth at the dose level of 500 mg/kg. In 250 mg/kg administered group reduction in regular BW growth observed, but it was not significant [Table 1]. When compared with control, female rats treated with petroleum ether (500 mg/kg) showed a significant reduction (P < 0.05) in BW at end of the study. The effect of petroleum ether on BW was summarized in Figure 1.
Table 1.
Effect of petroleum ether on food and water intake of SD rats
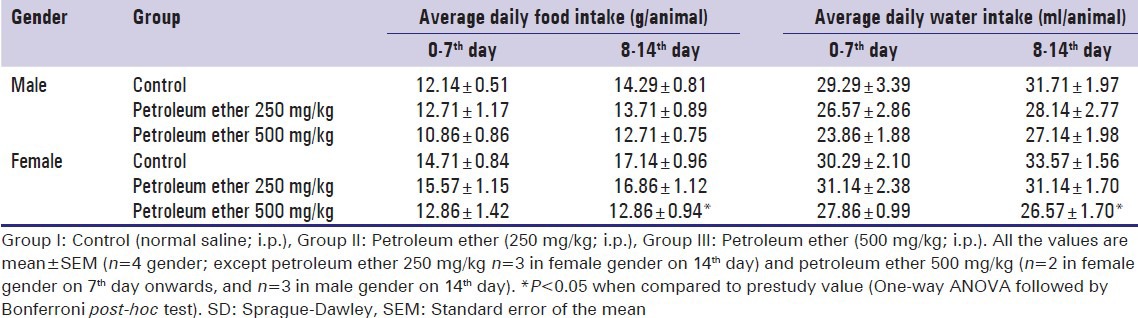
Figure 1.
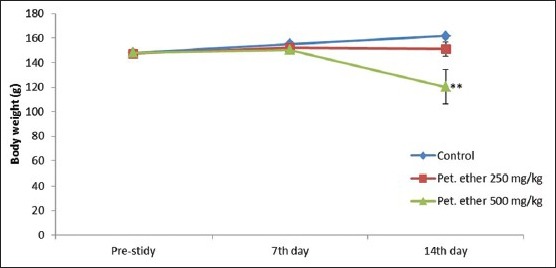
Effect of petroleum ether on weekly body weight of Sprague-Dawley rats. All the values are mean ± standard error of the mean (n = 8; except petroleum ether 250 mg/kg [n = 7 on 14th day] and petroleum ether 500 mg/kg [n = 6 on 7th day onwards and n = 5 on 14th day]). **P < 0.01 when compared to prestudy value (One-way ANOVA followed by Bonferroni post-hoc test)
Effect of petroleum ether on water and food intake
In the control group, the females animals showed an increase in food and water intake than male rats. Regular, age-depended on increase in food intake was observed in the control group, whereas petroleum ether administered group showed a significant decrease in food intake at the end of the study [Table 1]. The decrease in food intake may affect regular metabolic function and BW growth.
Effect of petroleum ether on behaviour of Sprague-Dawley rats
The animals were observed for any behaviour changes from the beginning of the study onwards. The animals were observed for motor activity, grooming, touch response, pain response, tremors, convulsion, righting reflux, writing, urination, salivation and skin color. The control group was found to have no marked change on the cholinergic and adrenergic system throughout the study. On 7th day of the experiment, petroleum ether (250 and 500 mg/kg) administered group, salivation, diarrhea and cannibalism were observed.
At the end of the experiment, petroleum ether (250 and 500 mg/kg) exposed animals were observed with decrease BW, decrease food intake, salivation, cannibalism, hair fall, reduced muscular activity, straub tail phenomenon and piloerection. More number of cannibalism were observed with petroleum ether 500 mg/kg administered group [Table 2].
Table 2.
Effect of petroleum ether on cannibalism of SD rats
Effect of petroleum ether on locomotor activity
The male and female rats in the control group do not have any significant change in locomotor activity at the course of the experiment. The rats treated with 250 mg/kg, and 500 mg/kg of petroleum ether showed a significant decrease in locomotor activity at end of the study when compared with the control group (P < 0.05) [Figure 2].
Figure 2.
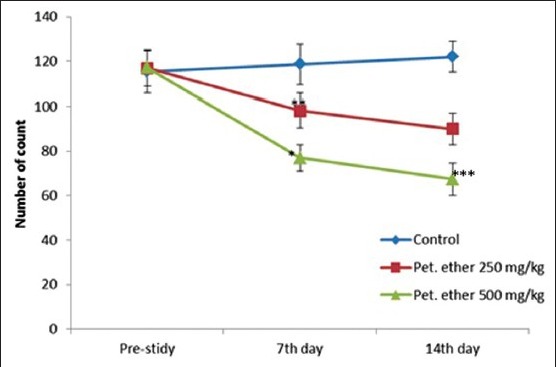
Effect of petroleum ether on locomotor activity of Sprague-Dawley rats. All the values are mean ± standard error of the mean (n = 8; except petroleum ether 250 mg/kg [n = 7 on 14th day] and petroleum ether 500 mg/kg [n = 6 on 7th day onwards and n = 5 on 14th day]). *P < 0.05; **P < 0.01 and ***P < 0.001 when compared to prestudy value (One-way ANOVA followed by Bonferroni post-hoc test)
Anxiogenic effects of petroleum ether
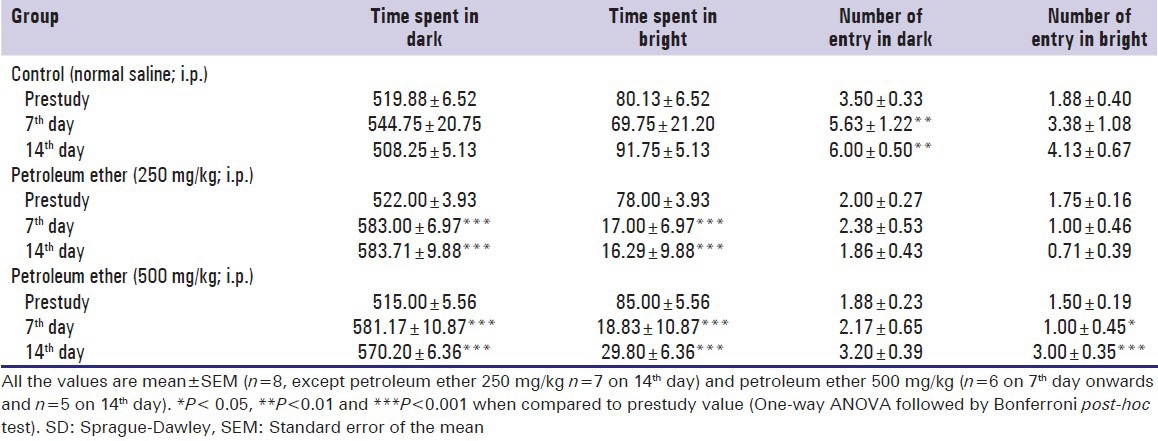
The normal control animals did not show any alterations in behaviour whereas petroleum ether administered group showed significant alterations in anxiogenic behaviour [Table 3]. The rats administer with 250 and 500 mg/kg petroleum ether spend more time in closed space to ratio of time spend in open space, when compare to the control group.
Table 3.
Effect of petroleum ether on anxiety (elevated plus maze) of SD rats
Effect of petroleum ether on water navigation of Sprague-Dawley rats
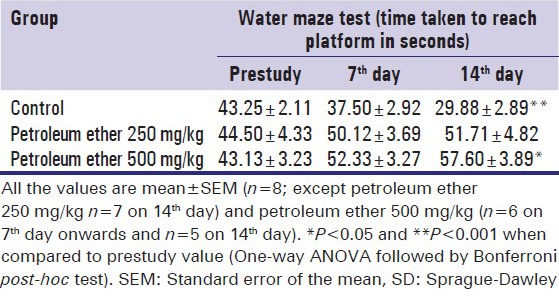
In post training administration of normal saline into the Control group, the animals showed reduced immobilization time, increased swimming speed to reach the platform and reduced traveled distance. Whereas petroleum ether administered group showed increased immobilization time decreased swimming speed to reach the platform and increased traveled distance [Table 4]. Petroleum ether 500 mg/kg administered animals showed a significant reduction in spatial learning and memory at the end of the study.
Table 4.
Effect of petroleum ether on water navigation of SD rats
Effect of petroleum ether on grip strength of Sprague-Dawley rats
Petroleum ether at the doses of 250 and 500 mg/kg showed a significant reduction in catching reflex at the end of the study when compared to the control [Figure 3]. Compare to male animal female animals which are received petroleum ether showed a significant reduction in catching reflex from 7th days onwards (P < 0.01).
Figure 3.
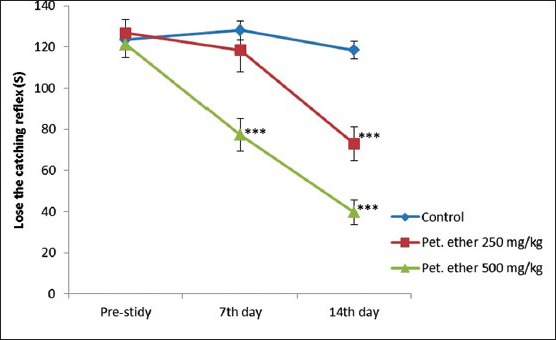
Effect of petroleum ether on grip strength of Sprague-Dawley rats. All the values are mean ± standard error of the mean (n = 8; except petroleum ether 250 mg/kg [n = 7 on 14th day] and petroleum ether 500 mg/kg [n = 6 on 7th day onwards and n = 5 on 14th day]). *P < 0.05 and **P < 0.001 when compared to prestudy value (One-way ANOVA followed by Bonferroni post-hoc test)
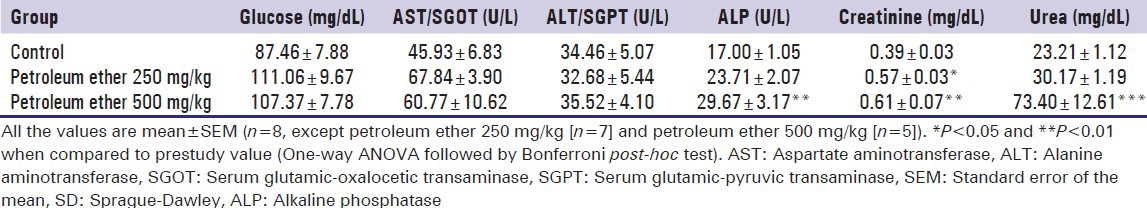
Effect of petroleum ether on biochemical parameters
The effect of petroleum ether on biochemical parameters (glucose, creatinine, urea, aspartate aminotransferase [AST], alanine aminotransferase and ALP) were summarized in Table 5. At the end of the study, petroleum ether at the dose of 250 mg/kg significantly increased creatinine level when compared with control group. Whereas petroleum ether at the dose of 500 mg/kg significantly increased the levels of ALP, creatinine and urea levels. On 14th day male rats showed significant increased level of urea and ALP, whereas female rats showed significant increased levels of AST (P < 0.05) when compared to the control group.
Table 5.
Effect of petroleum ether on plasma biochemical parameters of SD rats
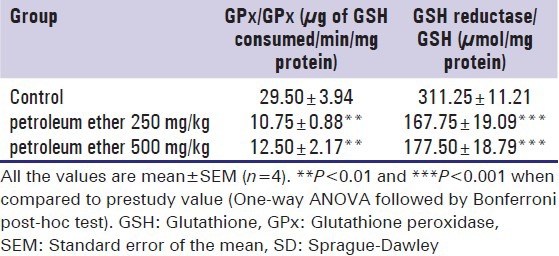
Petroleum ether at the dose levels of 250 and 500 mg/kg showed significantly increased levels of GSH and GPx in brain tissue homogenate at the end of the study [Table 6].
Table 6.
Effect of petroleum ether on GSH and GPx in brain tissue homogenate of SD rats
Effect of petroleum ether on absolute and relative organ weight
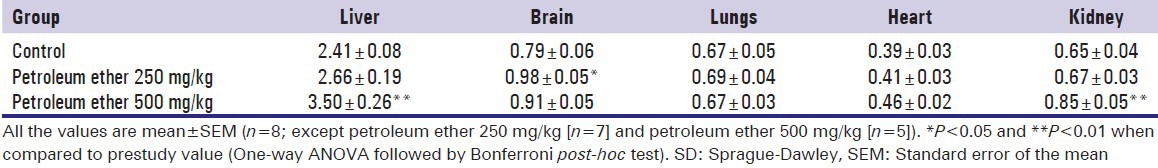
Petroleum ether at 500 mg/kg showed significant increase of absolute organ weight of liver and relative organ weight of liver and kidney when compared to the control animals, whereas petroleum ether at 250 mg/kg administered group showed a significant increase of relative organ weight of the brain when compare to the control animals [Tables 7 and 8].
Table 7.
Effect of petroleum ether on absolute organ weight of SD rats
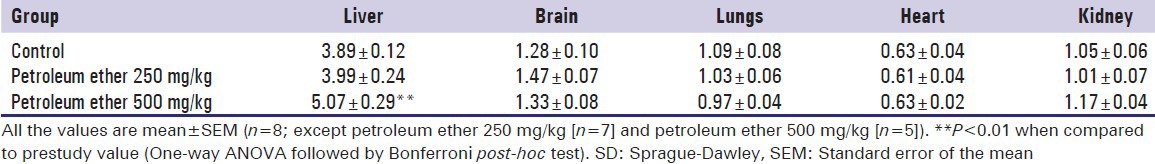
Table 8.
Effect of petroleum ether on relative organ weight of SD rats
Histopathological analysis
The histopathological analysis showed dose-dependent morphological changes in the brain, lungs, liver, kidney and heart when compared to control group. Effect of petroleum ether at 250 and 500 mg/kg on histopathology of brain, lungs, liver, kidney, and heart were summarized in Table 9, Figures 4 and 5.
Table 9.
Histopathological summary report for evaluation organ specific toxicity of petroleum ether
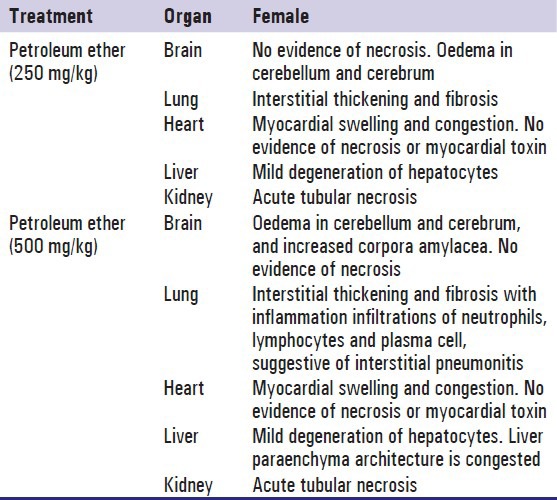
Figure 4.
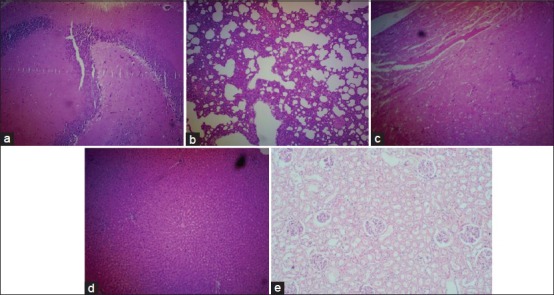
Histological features of the various organs of rat treated with petroleum ether 250 mg/kg. (a) section from brain shows edema in cerebellum and cerebrum, H and E, ×100, (b) section from lung shows interstitial thickening and fibrosis, H and E, ×100, (c) section from heart shows myocardial swelling and congestion, H and E, ×100, (d) section from liver shows mild degeneration of hepatocytes, H and E, ×100, (e) section from kidney shows acute tubular necrosis, H and E, ×100
Figure 5.
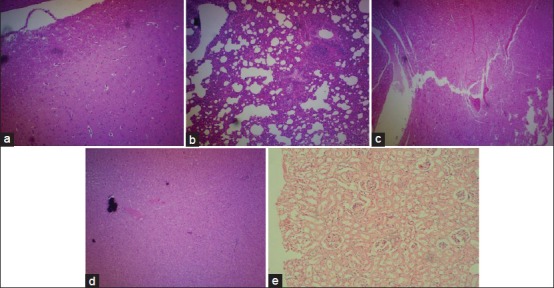
Histological features of the various organs of rat treated with petroleum ether 500 mg/kg. (a) Section from brain shows edema in cerebellum and cerebrum, and increased corpora amylacea, H and E, ×100, (b) section from lung shows interstitial thickening and fibrosis with inflammation infiltrations of neutrophils, lymphocytes and plasma cell, H and E, ×100, (c) section from heart shows myocardial swelling and congestion, H and E, ×100, (d) section from liver shows mild degeneration of hepatocytes and congested liver paraenchyma architecture, H and E, ×100, (e) section from kidney shows acute tubular necrosis, H and E, ×100
Discussion
Petroleum ether administered group showed a significant reduction in regular food intake and this may be because of petroleum ether induced loss of appetite. The animals were given normal rats pellet fed ad libitum. Each animal has free access to 20 g of food and could regulate their intake according to their biological needs. In both preclinical and clinical studies, loss of appetite and anorexia wereobserved with petroleum ether.[19,20] The loss of appetite is may be because of neurotoxic effect of petroleum ether and this may lead to development of anorexia and others such as malaise, muscle pain and headache.[20,21] The neurotoxins also affects the most of the sympathetic neurons, which innervate internal organs regulating their function for instance gut motility, salivation, piloerection, pupil diameter and vasoconstriction.[21]
Diarrhea and increased salivary secretion were observed in petroleum ether administered group and this may be due to an alteration in the cholinergic system. The cholinergic stimulation may cause the above-specified effect, and its confirmed that petroleum ether stimulates the cholinergic innervation there by causing diarrhea and increased salivary secretion. Piloerection is also observed in petroleum ether administered animals, and this may be due to sympathetic stimulation or by dopamine-induced localized cutaneous vasoconstriction.[22,23,24]
Cannibalism was observed in petroleum ether 250 and 500 mg/kg administered group and this may be due to the psychostimulant properties of petroleum ether. Common adverse effects of psychostimulants include appetite suppression, weight loss, edginess and stereotyped movements.[25] Cannibalism is a complex behaviour pattern by urgent reactions such as fear, the need to escape and influenced by many environmental factors such as disturbance by noise (high frequency) or rough handling, light intensity, housing system/density, group size, shortage of water or food and relative excess of Vitamin B1.[26,27] However in this study, cannibalism occurred under sufficient food and water supply as well as sufficient spacing. Thus these factors can be omitted. It may be due to the psychoactive neurochemical component related to cannibalistic behaviour in rodents, which present as a constituent in the petroleum ether extract.[28] Rats fed on this extract may exhibit schizophrenic properties, including thought disturbance, delusions, hallucinations and loss of reality, leading to killing and cannibalistic action among the animals.
Petroleum ether showed a significant reduction in locomotor activity from 1st week of the study onwards. This may be because of increasing the activity of gamma-aminobutyric acid and thus depressing the CNS or increasing the concentration of cathecholamines.[29,30] Das et al. studied the pharmacological properties of petroleum ether extracts of Amorphophallus paeoniifolius in rodents, and they observed decrease in locomotor activity with petroleum ether extracts of A. paeoniifolius administered group.[31]
The elevated plus maze result showed that after being exposed to petroleum ether, time spent in the open space and the number of entries into the open arm decreased in the petroleum ether administered group. The anxiogenic effect of petroleum ether is probably due to the elevation in 5-HT.[32] Ai et al., studied the effect of petroleum ether extract from Maca (Lepidium meyenii) in mice that are exposed to chronic unpredictable mild stress. The mice treated with Maca (Lepidium meyenii) showed increase in noradrenaline and dopamine level in the mouse brain tissue and this may be because of either disease model or solvent which used for extraction of Maca or direct of phytoconstituents of Maca.[33]
Petroleum ether also affected the spatial learning and memory. In water navigation test, control animals showed a significant increase in memory function, whereas petroleum ether administered animals showed a significant increase in “time taken to reach platform” compare to prestudy values. This may be due petroleum ether induced neurotoxicity. Neurotoxicity of laboratory solvents such as petroleum ether, benzene and n-hexane is well-known.[34]
The administration of petroleum ether results in loss of rigidity and decreased grip strength. This may be due to nondepolarizing neuromuscular blocking effect of petroleum ether. In many studies, petroleum ether extract of plants showed loss of grip strength and this may be due to ether solvent or plant extract effect.[35]
In some animal petroleum ether showed significant respiratory, cardiovascular and CNS toxicities. In petroleum ether 250 mg/kg administered group lung damage is more prominent in male and severe necrosis and accumulation of peritoneal fluid were observed. Severe polyneuropathy (with motor paralysis) and loss of motor coordination was seen immediately after 48 of petroleum ether 500 mg/kg administered group. Some of the female animals exhibited signs of severe tachycardia after 1 week of exposure. This was supported by organ weight changes, histopathology of the organs and alterations in biochemical parameters.
Conclusion
Petroleum ether showed a significant CNS depressant action and dose-dependent organ specific toxic effects in SD rats at the dose levels of 250 and 500 mg/kg. Marked alterations in the behaviour of the rats, locomotor activity, BW, grip strength, learning and memory process, and anxiogenic behaviour were observed with the induction of study dose levels of petroleum ether.
Acknowledgment
The authors are grateful to AIMST University for financial support (Project Code: AURGC/11/FOP/2013) to carry out toxicological study.
Footnotes
Source of Support: AIMST University, Malaysia
Conflict of Interest: No.
References
- 1.Tiem V, Anne L. Molecular mechanisms of polycyclic aromatic dydrocarbon-induced teratogenesis in Zebrafish (Danio rerio) [Last accessed on 2013 Jun 05]. Available from: http://www.dukespace.lib.duke.edu/dspace/handle/10161/5705; http://www.hdl.handle.net/10161/5705 .
- 2.Stevens WC, Eger EI, 2nd, White A, Halsey MJ, Munger W, Gibbons RD, et al. Comparative toxicities of halothane, isoflurane, and diethyl ether at subanesthetic concentrations in laboratory animals. Anesthesiology. 1975;42:408–19. doi: 10.1097/00000542-197504000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Parasuraman S, Thing GS, Christapher PV, Dhanaraj SA. Petroleum ether, a laboratory solvent induced cannibalism, piloerection and straub tail reaction in rodents. Res Rev: J Pharmacogn. 2014;1:6–10. [Google Scholar]
- 4.Parasuraman S. Toxicological screening. J Pharmacol Pharmacother. 2011;2:74–9. doi: 10.4103/0976-500X.81895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park JH, Choi KH, Kwak HS. Single- and 14-day repeat-dose toxicity of cross-linked ß-cyclodextrin in rats. Int J Toxicol. 2011;30:700–6. doi: 10.1177/1091581811419678. [DOI] [PubMed] [Google Scholar]
- 6.Liu T, Li L, Teng X, Huang X, Liu H, Chen D, et al. Single and repeated dose toxicity of mesoporous hollow silica nanoparticles in intravenously exposed mice. Biomaterials. 2011;32:1657–68. doi: 10.1016/j.biomaterials.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 7.Zarrindast MR, Khalifeh S, Rezayof A, Rostami P, Aghamohammadi Sereshki A, Zahmatkesh M. Involvement of rat dopaminergic system of nucleus accumbens in nicotine-induced anxiogenic-like behaviors. Brain Res. 2012;1460:25–32. doi: 10.1016/j.brainres.2012.04.036. [DOI] [PubMed] [Google Scholar]
- 8.Terry AV., Jr . Spatial navigation (Water Maze) tasks. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2nd ed. Ch 13. Boca Raton (FL): CRC Press; 2009. [Last accessed on 2013 Jun 05]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK5217/ [PubMed] [Google Scholar]
- 9.Bromley-Brits K, Deng Y, Song W. Morris water maze test for learning and memory deficits in Alzheimer's disease model mice. Vis Exp. 2011:2920. doi: 10.3791/2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butchbach ME, Edwards JD, Burghes AH. Abnormal motor phenotype in the SMNDelta7 mouse model of spinal muscular atrophy. Neurobiol Dis. 2007;27:207–19. doi: 10.1016/j.nbd.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vogel HG, editor. 2nd ed. Berlin: Springer; 2002. Drug Discovery and Evaluation: Pharmacological Assays. [Google Scholar]
- 12.Parasuraman S, Raveendran R, Kesavan R. Blood sample collection in small laboratory animals. J Pharmacol Pharmacother. 2010;1:87–93. doi: 10.4103/0976-500X.72350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar EP, Rajan VR, Kumar AD, Parasuraman S, Emerson SF. Hepatoprotective activity of Clearliv a polyherbal formulation in Wistar rats. Arch Med Health Sci. 2013;1:120–5. [Google Scholar]
- 14.Pasupathi P, Deepa M, Rani P, Sankar RR. Circulating lipid peroxidation, plasma and erythrocyte antioxidant status in patients with rheumatoid arthritis. Bangladesh Med Res Counc Bull. 2009;35:57–62. doi: 10.3329/bmrcb.v35i2.2798. [DOI] [PubMed] [Google Scholar]
- 15.Peshenko IV, Shichi H. Oxidation of active center cysteine of bovine 1-Cys peroxiredoxin to the cysteine sulfenic acid form by peroxide and peroxynitrite. Free Radic Biol Med. 2001;31:292–303. doi: 10.1016/s0891-5849(01)00579-2. [DOI] [PubMed] [Google Scholar]
- 16.Pari L, Latha M. Protective role of Scoparia dulcis plant extract on brain antioxidant status and lipidperoxidation in STZ diabetic male Wistar rats. BMC Complement Altern Med. 2004;4:16. doi: 10.1186/1472-6882-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adewole SO, Salako AA, Doherty OW, Naicker T. Effect of melatonin on carbon tetrachloride induced kidney injury in Wistar rats. Afr J Biomed Res. 2009;10:153–64. [Google Scholar]
- 18.Parasuraman S, Raveendran R, Rajesh NG, Nandhakumar S. Sub-chronic toxicological evaluation of cleistanthin A and cleistanthin B from the leaves of Cleistanthus collinus (Roxb.) Toxicol Rep. 2014 doi: 10.1016/j.toxrep.2014.08.006. In press. DOI: 10.1016/j.toxrep.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arlien-Soborg P. Boca Raton, USA: CRC Press; 1992. Solvent Neurotoxicity; p. 159. [Google Scholar]
- 20.Chang LW, Slikker W. California, USA: Academic Press; 1995. Neurotxicology: Approaches and Methods; p. 633. [Google Scholar]
- 21.Furlan A, Lübke M, Adameyko I, Lallemend F, Ernfors P. The transcription factor Hm ×1 and growth factor receptor activities control sympathetic neurons diversification. EMBO J. 2013;32:1613–25. doi: 10.1038/emboj.2013.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer E, Heller B. Pharmacology of the mechanism of certain effects of reserpine in the rat. Nature. 1967;216:1221–2. doi: 10.1038/2161221a0. [DOI] [PubMed] [Google Scholar]
- 23.Pramanik D. 2nd ed. Kolkatha, India: BK Dhur of Academic Publisher; 2007. Principles of Physiology; p. 292. [Google Scholar]
- 24.Khanorkar SV. 1st ed. New Delhi, India: Jaypee Brothers Medical Publishers (P) Ltd; 2012. Insights in Physiology; p. 748. [Google Scholar]
- 25.Tseng AL, Foisy MM. Significant interactions with new antiretrovirals and psychotropic drugs. Ann Pharmacother. 1999;33:461–73. doi: 10.1345/aph.18240. [DOI] [PubMed] [Google Scholar]
- 26.Lane-Petter W. Cannibalism in rats and mice. Proc R Soc Med. 1968;61:1295–6. doi: 10.1177/003591576806101228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parasuraman S, Babuji SS, Thing GS, Kumari KS, Yoganishalini A, Lian CW, et al. Antihyperlipidemic effect of Angiosifa, a polyherbal formulation, in Sprague Dawley rats. Pharmacogn J. 2013;5:221–7. [Google Scholar]
- 28.Worthington MK. Stocksfield: Oriel Press Ltd; 1977. Behavioural Problems of Farm Animals: Discussion and Conclusions. [Google Scholar]
- 29.Kolawole OT, Makinde JM, Olajide OA. Central nervous system depressant activity of Russelia equisetiformis. Niger J Physiol Sci. 2007;22:59–63. [PubMed] [Google Scholar]
- 30.White HS. Remington: The Science and Practice of Pharmacy. In: Gennaro AR, editor. II. Easton, Pennsylvania: Mack Publishing Co; 1995. p. 1233. [Google Scholar]
- 31.Das SS, Sen M, Dey YN, De S, Ghosh AK. Effects of petroleum ether extract of Amorphophallus paeoniifolius tuber on central nervous system in mice. Indian J Pharm Sci. 2009;71:651–5. doi: 10.4103/0250-474X.59547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasture VS, Deshmukh VK, Chopde CT. Anticonvulsant and behavioral actions of triterpene isolated from Rubia cordifolia Linn. Indian J Exp Biol. 2000;38:675–80. [PubMed] [Google Scholar]
- 33.Ai Z, Cheng AF, Yu YT, Yu LJ, Jin W. Antidepressant-like behavioral, anatomical, and biochemical effects of petroleum ether extract from Maca (Lepidium meyenii) in mice exposed to chronic unpredictable mild stress. J Med Food. 2014;17:535–42. doi: 10.1089/jmf.2013.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ono Y, Takeuchi Y, Hisanaga N, Iwata M, Kitoh J, Sugiura Y. Neurotoxicity of petroleum benzine compared with n-hexane. Int Arch Occup Environ Health. 1982;50:219–29. doi: 10.1007/BF00378084. [DOI] [PubMed] [Google Scholar]
- 35.Sravani KM, Kuma CA, Lakshmi MS, Rani GS. Psychopharmacological profiles of Pergularia daemia (Forsk.) Chiov. Asian J Pharm Clin Res. 2012;5:112–4. [Google Scholar]


