Abstract
Context:
Cross infection remains one of the major challenges in the dental profession, especially in field settings. Transmission of hepatitis B, hepatitis C, and human immunodeficiency virus have raised a major concern for patients and dental staff. These risks can be eliminated by effective sterilization and disinfection techniques.
Aim:
The aim was to compare the disinfecting efficacy of three chemical disinfectants on contaminated diagnostic instruments.
Settings and Design:
This was a randomized, cross over trial conducted among three participants selected from a research laboratory, Bhopal, Madhya Pradesh, India.
Materials and Methods:
The study participants were examined 4 times on different days. Each time, the coded mouth mirrors of different make were used, and the disinfection was accomplished using coded disinfectants. The reduction in total viable count was compared between the three groups (2% glutaraldehyde, 6% hydrogen peroxide (H2O2) and 99.9% ethyl alcohol) with distilled water as negative control and autoclaving as a positive control. Furthermore, the predisinfection count was compared between the instruments of different make.
Statistical Analysis Used:
Statistical analysis was performed using paired t-test and One-way ANOVA. The statistical significance was fixed at 0.05.
Results:
Autoclaved instruments resulted in complete elimination of viable micro-organisms. Maximum reduction in microbial load was observed after disinfection with H2O2 followed by glutaraldehyde, ethyl alcohol and distilled water in descending order. Furthermore, maximum microbial contamination was recorded on locally manufactured mirrors, while standard plain mirrors showed least contamination.
Conclusions:
Although, a significant reduction in total viable count was observed with all the disinfectants evaluated in the present study, none of the disinfectants was successful in completely eliminating the viable micro-organisms.
Keywords: Cross infection, disinfectants, disinfection, ethanol, glutaraldehyde, hydrogen peroxide
Introduction
The epidemiology of a disease or condition includes the prevalence and the characteristics of people at risk for the disease.[1] The science of epidemiology has enhanced our knowledge and improved the standard of public health. These epidemiological principles and methods have been vital in the study of oral conditions. Current data available for oral conditions relied on descriptive surveys intended to measure disease experience through clinical examinations in field.[2]
A major issue in these settings is infection control. This is an area where blood or saliva contamination can easily occur. As there is use of small, sharp instruments contaminated with blood or other fluids, there is ample opportunity for transmission of hepatitis B, hepatitis C and human immunodeficiency virus (HIV). Dentistry potentially exposes much of the population to blood contact with infected patients. Thus, a wide range of dental equipment may pose an unacceptable risk of cross infection.[3]
In dentistry, re-sterilization of used instruments for reuse on another patient has been a common practice. Although, single-use devices have been promoted as a strategy to prevent cross-infection among patients, re-sterilization of previously used instruments still continues to be a common practice as cost is a significant factor.[4]
The term “disinfection” is defined as a process that kills only vegetative organisms, whereas, sterilization kills spores as well.[5] Sterilizing instruments is a time-consuming process that requires careful attention. If the protocol is not followed strictly, contamination of instruments may result. Interruption of the cycle results in inadequately sterilized instruments that cannot be considered safe.[6] After the sterilization cycle, the sterilizer must depressurize, and the packs remain in the sterilizer for drying. The drying phase may take an additional 20-45 min. The unit must only be opened after completion of the drying cycle making it more time consuming in field settings.[6] Improperly sterilized instruments utilized in patient care can result into surgical site infection and pose a serious threat to the patient's safety leading to life-threatening infection or even death.[7]
In general, heat sterilization method is preferred to chemical disinfection. However, with certain instruments that are repeatedly used, frequent chemical disinfection may be necessary since heat sterilization can lead to corrosion.[8] Further, it is not possible to carry out heat sterilization in field surveys due to time constraints.
In these situations, the disinfection using chemical disinfectants may be considered an alternate for heat sterilization to reduce the risk of cross contaminations. Glutaraldehyde is a dialdehyde that displays potent bactericidal, fungicidal, mycobactericidal, sporicidal and virucidal activities. Mechanism of its action is based on its interaction with amino groups in proteins and enzymes.[9] Glutaraldehyde is normally used as a 2% solution, which is sufficient to achieve a sporicidal effect.[10] It is used as an as immersion solution for metallic instruments, face masks, heat sensitive plastic rubbers, and fiber optics.[11]
Hydrogen peroxide (H2O2) is commonly employed for disinfection, sterilization, and antisepsis and is effective against bacteria, viruses, yeast and spores. It is commercially available in concentrations ranging from 3% to 90%. H2O2 is environmental friendly, because it can rapidly degrade into harmless products that is, water and oxygen. H2O2 acts as an oxidant by producing hydroxyl free radicals (•OH), which attack cell components, including lipids, proteins, and DNA. Proposed mechanism of action is based on its ability to target exposed sulfhydryl groups and double bonds.[10]
Alcohol is an effective skin antiseptic and disinfectant for medical instruments. A number of alcohols have shown effective antimicrobial activity but, ethyl alcohol, isopropyl alcohol and n-propanol are the most widely used. Alcohols exhibit rapid broad-spectrum antimicrobial activity against vegetative bacteria (including mycobacteria), fungi, and viruses but they lack sporicidal activity hence are not recommended for sterilization. In general, the antimicrobial activity of alcohols is optimum in the range of 60-90%, but it becomes significantly lower at concentrations below 50%. The exact mode of action of alcohols is unclear, but it is generally believed that they cause membrane damage leading to cell lysis and result into a rapid denaturation of proteins.[10]
The lack of published literature comparing the effectiveness of various chemical disinfectants has prompted us to undertake this study to evaluate the disinfecting efficacy of three chemical solutions in reducing the contamination of diagnostic instruments that are routinely used in dental screening camps.
Materials and Methods
Selection of the study participants
This randomized cross over type of trial was conducted among three participants selected from a research laboratory, Bhopal. The permission to conduct the study was obtained from the administrative authorities of the concerned research laboratory and the ethical clearance from the institutional ethics committee. A total of 30 participants was interviewed and screened in an attempt to identify the eligible participants for the study. A face-to-face interview was conducted by one investigator to collect the desired information using a checklist. The selection of participants into the study was based on the following inclusion and exclusion criteria.
Inclusion criteria
The participants:
Willing to offer voluntary informed consent to participate in the study
Aged 25-30 years
Available in the laboratory for the entire study duration.
Exclusion criteria
Presence of advanced periodontal disease (Periodontal pockets, mobility, gingival recession, furcation involvement, etc)
Presence of any grossly decayed teeth
Absence of first or second molar teeth in both the arches
Presence of dental appliances (removable or fixed)
Individual using mouth rinses containing chemical agents or interdental cleaning aids on a regular basis
Presence of any systemic diseases
Presence of malocclusion traits
Deleterious habits like smoking or use of other tobacco related habits
Individuals who are not willing to offer written informed consent.
At the time of interviewing, the informed consent was obtained from each eligible participant after explaining the entire research protocol. Three participants who fulfilled the inclusion and exclusion criteria were selected for the study.
Procedure for clinical examination
An intra-oral examination was conducted among the selected study participants by a single investigator. The clinical examination was carried out in the Research Laboratory on a plastic chair under natural daylight using a mouth mirror and explorer. All the teeth in the following four segments were examined by retracting the cheek, lip and the tongue using the mouth mirror and explorer.
Segment I: Upper right posterior segment – comprising of upper right first pre molar (14) to the upper right second molar (17)
Segment II: Upper left posterior segment – comprising of upper left first pre molar (24) to the upper left second molar (27)
Segment III: Lower left posterior segment – comprising of lower left first pre molar (34) to the lower left second molar (37)
Segment IV: Lower right posterior segment – comprising of lower right first pre molar (44) to the lower right second molar (47).
First, the maxillary teeth were examined starting from the maxillary right second molar (17) to maxillary right first pre molar (14) followed by examination of the maxillary left posterior segment starting from maxillary left second molar (27) to maxillary left second pre molar (24). After the completion of the maxillary arch, the mandibular arch was examined starting with the left posterior segment, followed by the right posterior segment. In each segment, the explorer tip was first passed on the occlusal surfaces, followed by buccal and lingual surfaces. It took approximately 5 min for the investigator to complete the examination of one individual. The time duration was kept constant for the subsequent examinations to ensure uniformity in the sample collection.
Group allocation
The three eligible study participants were assigned a unique subject I.D which was maintained throughout the study. These participants were examined 4 times on different days. Each time two sets of coded mouth mirrors of different make were used.
First examination: Locally manufactured mouth mirror
Second examination: Standard plain mirror
Third examination: Rhodium coated mirror
Fourth examination: Disposable mouth mirror.
The clinical oral examination of the study participants was done randomly without any prior information to the participant regarding the day of examination. This was done to reduce the Hawthorne effect. The prior information on the examination date could have made these participants put in extra efforts in oral hygiene practices. This in turn could have reduced the plaque on the teeth surfaces.
Each time, the disinfection of one set of instruments was accomplished using coded disinfectants and another set was subjected to autoclaving.
Group 1: Distilled water
Group 2: 2% glutaraldehyde solution
Group 3: 6% H2O2 solution
Group 4: 99.9% ethyl alcohol
Group 5: Autoclaving.
The predisinfection count was compared between the instruments of different make. The mean reduction in the microbial count following disinfection was compared between different disinfectant groups.
Procedure for laboratory investigation
The diagnostic instruments following the completion of clinical examination were submitted immediately to the laboratory assistant to prepare the predisinfection stock solution. The diagnostic instruments were gently stirred for 5 min in normal saline in an attempt to obtain the sample of plaque micro-organisms. Later, the instruments were taken back and disinfected using a designated disinfectant for 30 min. The postdisinfection stock solution was prepared using the methodology described earlier. The stock solution was diluted further to obtain 10− 2 and 10− 3 serial dilutions. Nutrient agar was used as a media to elicit the growth of micro-organisms. Pour plate technique was employed to uniformly dispense the diluted samples on the Petri plates (Autoclavable petri plates, HiMedia Laboratories, Mumbai, India) containing the nutrient agar. These Petri plates were then inoculated and incubated at 37°C for 24 h. The Petri plates were examined for recording the total viable count following incubation. The number of colony forming units (CFU's) of the viable micro-organisms on the Petri plates was counted using a digital colony counter. This laboratory procedure was employed on each of the four occasions where instruments of different make were used for clinical oral examination and different disinfectants were used for disinfection purpose.
Blinding
The study participants and laboratory assistant who carried out the microbiological assay were not having any information on the type of instruments used and the disinfectant employed. The laboratory assistant entered the code written on the stock solution on the data collection sheet following the completion of microbial count on each occasion.
Statistical analysis
The data were analyzed using SPSS version 20 (IBM. Chicago, USA). The total viable count (number of CFU) was presented as mean and standard deviation (SD). Paired t-test was used to compare the mean number of CFUs between pre and post disinfection within each disinfectant group. The pre disinfection mean CFU between the instruments of different make as well as the mean CFU following disinfection between different disinfectant groups was compared using One-way ANOVA and Tukey's post-hoc test. The statistical significance was fixed at 0.05.
Results
Three participants who consented and fulfilled the inclusion and exclusion criteria were considered for clinical oral examination. The participants were examined to simulate patient examination in a field setting. Later, the same three participants were re-examined on 3 different days for comparing the level of contamination between instruments of different make as well as effectiveness of three disinfecting solutions using distilled water as a negative control [Table 1]. Autoclaving eliminated the viable micro-organisms completely and considered the gold standard procedure to be followed whenever feasible. Autoclaving offered significantly superior benefits compared with all other methods and hence, the comparison was made between other disinfectant groups.
Table 1.
Details of instruments and disinfectants used in four clinical oral examinations
Comparison of the pre disinfection microbial contamination between instruments (mouth mirrors) of different make
The total viable count was significantly high [P: 0.006, Table 2] following use of locally manufactured mirrors (365 CFU/ml ± 48.42) (mean ± SD) followed by Rhodium coated mirrors (258.33 CFU/ml ± 52.44) and disposable mirrors (224.33 CFU/ml ± 34.82) in the descending order. Least contamination was observed on Standard Plain mirrors (Mouth Mirror Top Plane- MT001, Number 5, GDC Marketing, Punjab, India) (208.00 CFU/ml ± 23.30) at 10− 2 serial dilution. Post-hoc comparison revealed statistically significant difference between locally manufactured mouth mirror and Standard plain mirror (P: 0.007), Rhodium coated mirror (P: 0.052) and Disposable mouth mirror (P: 0.013). No significant differences were observed between Standard plain mirror and Rhodium coated mirror (P: 0.485), Standard Plain mirror and Disposable mirror (P: 0.961), as well as between Rhodium coated mirror and Disposable mirror [P: 0.751, Table 2].
Table 2.
Comparison of the pre-disinfection microbial contamination between the diagnostic instruments (mouth mirrors) of different make
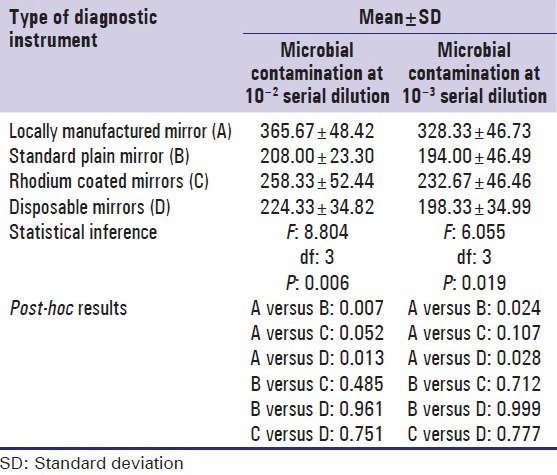
The locally manufactured mirrors showed maximum microbial contamination (328.33 CFU/ml ± 46.73) even at 10-3 serial dilution. This was followed by Rhodium coated mirrors (232.67 CFU/m l ± 46.46), disposable mirrors (198.33 CFU/ml ± 34.99) and Standard Plain mirrors (194.00 CFU/ml ± 46.49) in the descending order. The difference was statistically significant [P: 0.019, Table 2]. Post-hoc comparison revealed statistically significant difference between locally manufactured mirror and Standard Plain mirrors (P: 0.024), locally manufactured mirror and disposable mirror (P: 0.028). However, no significant difference was observed when locally manufactured mirror was compared with Rhodium coated mirror (P: 0.107), Standard Plain mirror and Rhodium coated mirror (P: 0.712), Standard Plain mirror and disposable mirror (P: 0.999) as well as between Rhodium coated mirror and disposable mirror [P: 0.777, Table 2].
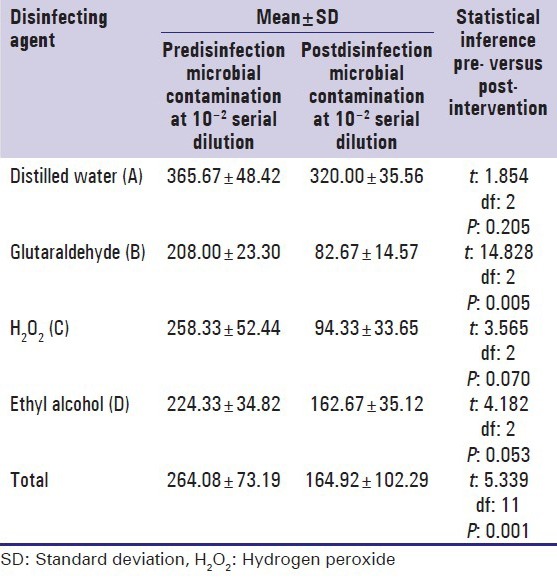
Comparison of the pre and post disinfection microbial contamination using different disinfecting solutions at 10−2 and 10−3 serial dilutions
A reduction in the total viable count following disinfection compared to baseline levels was noted with all the three disinfectants as well as with distilled water at 10−2 serial dilution. However, a statistically significant reduction in the total viable count was observed following disinfection with Glutaraldehyde (P: 0.005) and Ethyl Alcohol (P: 0.053). The reduction following disinfection with H2O2 (P: 0.070) and distilled water (P: 0.205) was not statistically significant [Table 3].
Table 3.
Comparison of the pre- and post-disinfection microbial contamination using different disinfecting solutions at 10−2 serial dilutions
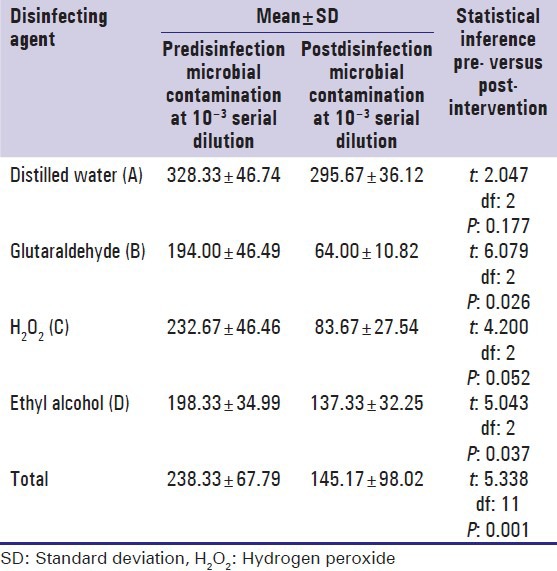
The reduction in the total viable count following disinfection compared to baseline levels was noted with all the three disinfectants as well as with distilled water even at 10−3 serial dilution. However, a statistically significant reduction in the total viable count was observed with Glutaraldehyde (P: 0.026), H2O2 (P: 0.052) and Ethyl Alcohol (P: 0.037), but not with Distilled Water (P: 0.177) [Table 4].
Table 4.
Comparison of the pre- and post-disinfection microbial contamination between different disinfecting solutions at 10−3 serial dilutions
Comparison of the mean reduction in microbial contamination following disinfection between different disinfecting solutions
The mean reduction in the total viable count following disinfection was significantly higher in the groups involving the use of chemical disinfectants compared with one achieved with distilled water [P: 0.050, Table 5]. However, the post-hoc comparison revealed no statistically significant difference between the various disinfectant groups at 10−2 serial dilution.
Table 5.
Comparison of mean reduction in microbial contamination following disinfection between different disinfecting solutions
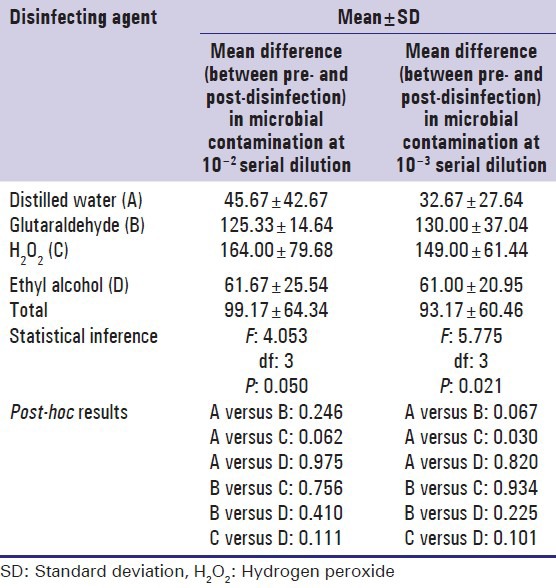
A statistically significant difference [P: 0.021, Table 5] was observed in the mean reduction in total viable count between the various groups at 10−3 serial dilution. However, the post-hoc test revealed a significant difference (P: 0.030) between distilled water and H2O2 groups, but not between other groups [Table 5].
Inference
The Standard plane mirror and disposable mirrors had less microbial contamination compared with other mirror types. Maximum reduction in microbial load was observed following disinfection with H2O2, Glutaraldehyde, Ethyl Alcohol, and Distilled water in descending order.
Discussion
Cross infection control is the most important and pertinent topic among health care workers today. It has successfully gained the international concern and is taking the shape of a global problem. Cross infection in its simplest form can be defined as the transfer of an infectious agent from one individual to another in a clinical environment.[12] New infectious diseases have been found at a rate of one disease per year over the past 22 years.[13]
Most of the time the dental practitioners are exposed to an environment where exists a real danger of infection not only to themselves, but also to the patients. Dentists, patients, and other dental staff are at high risk to infectious diseases such as AIDS, hepatitis, herpes simplex and cytomegalovirus.[12]
Worldwide approximately 300-400 million people are chronic hepatitis B carriers.[12] Hepatitis virus transmission is the major occupational hazards for dental personnel. Furthermore, HIV can be transmitted by transfusions, needle stick injury or contact of mucous membrane with the blood or body fluids of a carrier.[14] Dentists in particular due to the nature of their work are very prone to such detriments. Keeping this in mind, the Centre for Disease Control issued guidelines that emphasize that every patient should be considered as potentially infectious, and the principles of infection control should be strictly followed.[12]
Based on the ability to transmit infection, the dental instruments can be classified into critical, semi-critical and noncritical. Instruments such as mouth mirrors, mouth props and suction tips are categorized under semi-critical items as they come in contact with the epithelial surface, but do not penetrate the epithelial barrier.[13] To ensure protection against cross infection from the instruments contaminated with saliva and blood, the most effective procedure is sterilization. It is a procedure that ensures total destruction of all living organisms, including viruses and spores. Disinfection, on the other hand is an intermediate method used to reduce the number of pathogens through chemical agents.[13] The present study was undertaken to assess the disinfecting efficacy of three chemical disinfectants on contaminated diagnostic instruments.
This study was carried out among 25-30 years old adults working at a research laboratory, Bhopal, Madhya Pradesh. Individuals were selected from the research laboratory to avoid difficulties associated with transportation of the sample if were to be collected from their individual houses.
In the present study, the level of microbial contamination was assessed using the diagnostic instruments of different make. This was undertaken to explore the diagnostic instruments with the least possibility of microbial contamination amongst the ones that are marketed in the recent times. The Standard plane mirrors and Disposable mirrors were found to have less microbial contamination compared to other mirror types. The difference in the adherence of micro-organisms between the instruments of different make may be attributed to the following factors:
Difference in the design of the instruments
Difference in the surface characteristic of the instruments
Difference in the type and quality of the material used.
We could not compare this finding with previously published literature as the literature comparing the level of microbial contamination using the diagnostic dental instruments of different make are nonexistent. These results need validation with studies on a larger sample.
We compared efficacy of three chemical disinfectants (2.0% glutaraldehyde, 6.0% H2O2 and 99.9% ethyl alcohol) with distilled water as a control. The disinfecting efficacy was assessed on the basis of reduction in the total viable count.
Jokar et al. (2011)[15] in their study compared the efficacy of alcohol isopropyl and ethanol in reducing contamination of medical diagnostic devices. Our study followed the same protocol, except that we estimated the total viable count instead of qualitatively assessing the positive cultures.
In our study, the maximum reduction in microbial load was observed following disinfection with H2O2 and Glutaraldehyde, Ethyl Alcohol, Distilled water in descending order.
Taha et al. (2010)[16] in their study compared the effectiveness of four different disinfectant solutions in rapid decontamination of Gutta-percha cones. In their study, 320 Gutta-percha cones were placed in the bacterial suspensions for 30 min, and then immersed in disinfectant solutions. The results of their study were consistent with the results of our study indicating H2O2 to be the most effective disinfecting agent.
Badrian et al. (2012)[17] in their study investigated the effect of three different types of disinfecting agents on circular samples of alginate impression material which were deliberately contaminated. Among all the three disinfectants used, Epimax (H2O2 based) showed the highest reduction and was successful in completely eradicating all the tested micro-organisms within 10 min. These results were in contrast to the results of our study where although H2O2 showed maximum reduction, it could not completely eliminate viable micro-organisms. These variations in the results could be due to product differences. Furthermore, only selected micro-organisms were tested in this study while we evaluated the total viable count without emphasizing on specific strains.
Eralp et al. (2006)[8] evaluated the effectiveness of various disinfectants on different types of contaminated dental instruments. They found 2% glutaraldehyde to be more effective than other disinfectants, although none of the disinfectants showed complete elimination similar to our findings.
Pradeep et al. (2013)[18] compared sterilizing effect of five disinfectant solutions including ethyl alcohol (95%) and H2O2 (3%) on Gutta-percha points. They found both disinfectants to accomplish sterilization within 5 min of immersion. Our results were contradictory to these findings. We observed only a partial reduction in total viable count rather than complete sterilization. We used diagnostic instruments while this study assessed disinfecting efficacy on Gutta-percha points. The variations in the results could be attributed this difference.
Summary and Conclusion
We found a significant reduction in total viable count with all the disinfectants. However, none of the disinfectants succeeded in completely eliminating the microbial contamination following disinfection. This finding challenges the reliability of chemical disinfectants in preventing cross infection as the presence of even smaller percentage of pathogenic micro-organisms can result in cross contamination. The result so obtained favors the recommendation of sterilization using autoclaved instruments rather than relying on chemical disinfection methods.
The study had some inherent drawbacks:
The study was carried out on a small sample size
The participants had relatively good oral hygiene and were free from any major oral disease; hence, they cannot be considered as true representatives in a field setting
The confined laboratory environment wherein the decontamination procedures are meticulously followed might have reduced the contamination of diagnostic instruments, which may not be the case in field setting
We used only diagnostic instruments to assess the disinfecting efficacy. A better evaluation of the disinfecting efficacy could be done using a variety of operative instruments as well
Effect of mechanical cleaning by wiping the instruments prior to disinfection was not considered in our study.
Acknowledgments
First and foremost I offer my sincerest gratitude to my supervisor, Resp. Dr Chandrashekar BR, who has supported me throughout my work with his patience and knowledge. The good advice and support of Resp. Dr Vrinda Saxena, Professor and Head of the Department of Public health dentistry, has been invaluable on both academic and personal level, for which I am extremely grateful. I would also thank my colleague Dr Poonam Tomar, Dr Ruchika Gupta and Dr Garima Khandelwal for their kind support.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Beck JD, Harald L. Epidemiological principles in studying periodontal diseases. Periodontology. 2000;1993:34–45. doi: 10.1111/j.1600-0757.1993.tb00218.x. [DOI] [PubMed] [Google Scholar]
- 2.Burt BA. The role of epidemiology in the study of periodontal diseases. Periodontology. 2000;1993:26–33. doi: 10.1111/j.1600-0757.1993.tb00217.x. [DOI] [PubMed] [Google Scholar]
- 3.Abichandani SJ, Nadiger R. Cross contamination in dentistry: A comprehensive overview. Chron Young Sci. 2013;4:51. [Google Scholar]
- 4.Hogg NJV, Morrison AD. Resterilization of instruments used in a hospital-based oral and maxillofacial surgery clinic. J Can Dent Assoc. 2005;71:179–82. [PubMed] [Google Scholar]
- 5.Angelillo IF, Bianco A, Nobile CGA, Pavia M. Evaluation of the efficacy of glutaraldehyde and peroxygen for disinfection of dental instruments. Letters in Applied Microbiology. 1998;27:292–96. [PubMed] [Google Scholar]
- 6.Cuny E, Bednarsh H. Instrument sterilization in dentistry. [Last cited 2013 Dec 13]. Available from: http://www.ineedce.com/courses/1453/pdf/instrumentsterilization.pdf .
- 7.Hopper WR, Moss R. Common breaks in sterile technique: Clinical perspectives and perioperative implications. AORN Journal. 2010;91:350–64. doi: 10.1016/j.aorn.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 8.Eralp A, Gulcin, Sultan N, Ozdemir A. An In vitro evaluation of various disinfectants on different types of contaminated dental materials. Arastirma. 2006;30:25–30. [Google Scholar]
- 9.Russell AD. Glutaraldehyde: Current status and uses. Infect Control Hosp Epidemiol Nov. 1994;15:724–33. doi: 10.1086/646845. [DOI] [PubMed] [Google Scholar]
- 10.McDonnell G, Russell AD. Antiseptics and disinfectants: Activity, action and resistance. Clin. Microbiol. Rev. 1999;12:147–79. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vinay P, Reddy GY, Hegde N, Priyadarshini Sterilization methods in orthodontics- a review. International Journal of Dental Clinics. 2011;3:44. [Google Scholar]
- 12.Khan AA, Javed O, Khan M, Mehboob B, Baig S. Cross infection control. Pakistan Oral and Dental Journal. 2012 Apr;32:31. [Google Scholar]
- 13.Shah AH, Wyne AH. Cross-infection control in dentistry: A review. Pakistan Oral and Dental Journal. 2010 Jun;30:168–74. [Google Scholar]
- 14.Younai F, Murphy D, Kotelchuck D. Occupational exposures to blood in a dental teaching environment: Results of a ten-year surveillance study. J Dent Educ. 2001;65:436–48. [PubMed] [Google Scholar]
- 15.Jokar A, Mohebbi Z. Comparing the efficacy of alcohol isopropyl and ethanol on the reduction of contamination of medical check-up devices in children ward and neonatal intensive care unit (NICU) Int. Res. J. Pharm. Pharmacol. 2011 Aug;1:75. [Google Scholar]
- 16.Taha MY, Al-Sabawi NA, Shehab EY. Rapid decontamination of gutta percha cones using different chemical agents. Al–Rafidain Dent J. 2010;10:30. [Google Scholar]
- 17.Badrian H, Ghasemi E, Khalighinejad N, Hosseini N. The effect of three different disinfection materials on alginate impression by spray method. ISRN Dentistry, 2012. 2012 doi: 10.5402/2012/695151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pradeep K, Kidiyoor KH, Jain P, Rao N. Chair side disinfection of gutta-percha points-An in vitro comparative study between 5 different agents at different concentrations. Endodontology. 2013 Jun;25:73. [Google Scholar]


