Abstract
Numerous signaling pathways have been shown to mediate cardioprotection, but the end effectors that mediate protection are only beginning to be elucidated. Numerous cardioprotective drugs are shown to converge on glycogen synthase kinase-3β (GSK-3β) . The phosphorylation and inhibition of GSK-3β lead to inhibition or delayed activation of the mitochondrial permeability transition, a key regulator of apoptosis.
The ability to treat patients with a drug to reduce cell damage or death following ischemia/reperfusion or myocardial infarction has been an elusive goal. A number of drugs that have been shown to be protective in animal models have failed to provide protection in clinical trials. A major reason for the lack of success is that most cardioprotective drugs need to be present prior to ischemia to afford measurable protection. Unfortunately, patients do not arrive at an emergency room prior to a heart attack. In recent years, with improved understanding of cardioprotection and mechanisms regulating cell death and apoptosis, there have been several exciting and promising studies showing that activation of signaling pathways that involve mitochondria-regulated cell death pathways at the start of reperfusion can reduce ischemia/reperfusion–related death (1, 2). An improved understanding of cardioprotective mechanisms has allowed intervention downstream in the pathway where drugs will have fewer nonspecific side effects and are more targeted to the end effectors of the cell death pathway. Our understanding of cardioprotection has been enhanced by studies of preconditioning (PC), the phenomenon in which brief intermittent periods of ischemia protect against a subsequent prolonged period of ischemia. PC has been consistently shown to reduce cell death (infarct size) in every species examined. Studies have documented a number of signaling pathways involved in cardioprotection, but many important questions remain unanswered. Although many kinase pathways have been shown to be necessary for protection, the targets by which these signaling pathways reduce cell death, and which pathways can be targeted upon reperfusion, are poorly understood (1). Recent studies have suggested that mitochondrial channels and proteins, which regulate apoptosis, are key targets of cardioprotective signaling pathways (1, 3–8). If significant apoptosis occurs during reperfusion (reviewed in ref. 2), then these mitochondrial death pathways are an attractive target.
Signaling pathways that mediate cardioprotection
The mitochondrial ATP-dependent K+ (mitoKATP) channel has been proposed as an end target of PC signaling pathways, although some recent studies suggest that the mitoKATP channel can also initiate protective signaling pathways. The precise mechanism by which activation of the mitoKATP channel results in attenuation of cell death is not clear, but there are data suggesting that its activation can reduce apoptosis (3, 6). There are also data suggesting that PC may involve inhibition of the mitochondrial permeability transition (MPT) (4, 5). Additionally, recent data show that PC can lead to targeting of PKC and MAPKs to the mitochondria, where they appear to associate with proteins reported to be part of the MPT (adenine nucleotide translocator [ANT] and voltage-dependent anion channel [VDAC]) (7, 8). One of the major unresolved issues in cardioprotection is how the kinase signaling cascades interact with mitochondrial components of apoptosis and cell death. Do multiple kinases act on multiple targets, or do the kinases form a linear cascade that converges on a few kinases and targets? New data on this important question are provided by an article in this issue of the JCI (9). Juhaszova and colleagues show that PC and many other cardioprotective agents result in phosphorylation of mitochondrial glycogen synthase kinase-3β (GSK-3β). GSK-3β is also shown to cosegregate with VDAC and ANT, components of the MPT.
Juhaszova et al. (9) have performed a comprehensive study of cardioprotective agents and divided them into those with memory (those that still provided protection an hour after addition) and those without memory. They present data suggesting that cardioprotective drugs with memory cause mitochondrial swelling while those without memory do not. They hypothesize that memory is due to a swelling-induced increase in electron transport leading to increased reactive oxygen species (ROS), which in turn activates PKC, which phosphorylates GSK-3β and leads to protection. Cardioprotection without memory is due to direct activation of kinases. Memory is conferred because of a feed-forward mechanism in which mitochondrial swelling leads to activation of PKC, which results in further activation of the mitoKATP channel and continued mitochondrial swelling. It is not clear how this feed-forward mechanism is turned off, and it is also not clear why activation of PKC by agents that do not confer memory does not initiate such a feed-forward cascade. In fact the distinction between agents with memory and those without memory can differ in different models.
Cardioprotective signaling pathways converge on GSK-3β
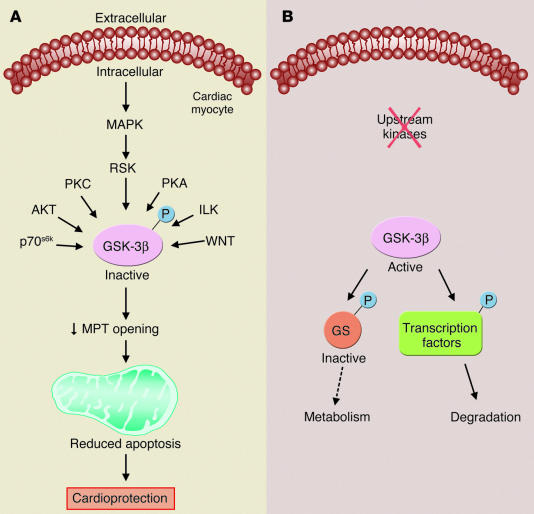
One of the key findings of the paper is that both classes of cardioprotective drugs ultimately mediate protection by phosphorylation of GSK-3β (9). Previous studies have suggested that PC results in phosphorylation and inhibition of GSK-3β (10), and that drugs that inhibit GSK-3β are cardioprotective (10, 11). As shown in Figure 1, GSK-3β is regulated by a number of signaling pathways (see ref. 12). Data presented by Juhaszova et al. suggest a central role for GSK-3β in the signaling pathways of a wide variety of cardioprotective drugs (9). The data show that insulin, diazoxide, and the Na+-H+ exchange (NHE) inhibitor HOE694 all increase phosphorylation of GSK-3β. Additional elegant studies are provided showing that mice that possess a constitutively active mutant form of GSK-3β, in which serine 9 is replaced by alanine, do not exhibit cardioprotection when treated with PC, diazoxide, NHE inhibitors, insulin, or GSK inhibitors. Whereas either PC or these drugs all increase the time to laser-induced mitochondrial depolarization in myocytes from wild-type hearts, these drugs did not show this protection in myocytes from hearts with constitutively active mutant GSK-3β. It may appear surprising that the GSK-3β inhibitor Li+ is not protective in myocytes with constitutively activated GSK-3β, since one would expect that Li+ would still inhibit GSK-3β, even if inhibition can no longer be achieved by phosphorylation. However, it has been reported that Li+ can inhibit GSK activity directly as well as by inhibiting phosphorylation, and the relative importance of these modes of inhibition is not clear (13). In further support of a role for GSK-3β in cardioprotection, studies were performed in which GSK-3β was decreased using short interfering RNA targeted against GSK-3β. Reduction of GSK-3β levels would be expected to be protective, since inhibition of GSK-3β with phosphorylation is protective. Reduction of GSK-3β by 75% resulted in protection similar to that observed with insulin. Based on the observation that inhibition of GSK-3β, as occurs with phosphorylation of GSK-3β, delays the time to laser-induced depolarization, it is suggested that GSK-3β regulates the MPT. In agreement with Juhaszova et al. (9), another recent study using a similar model of laser-induced ROS-mediated opening of the MPT showed that a variety of cardioprotective drugs such as diazoxide, nicorandil, and cyclosporin increase the time between ischemic insult and ROS-induced loss of mitochondrial membrane potential (14).
Figure 1.
GSK-3β is phosphorylated and inhibited by a number of kinases. In the absence of upstream kinases, unphosphorylated GSK-3β is active, and it phosphorylates and inactivates downstream targets such as glycogen synthase to modulate metabolism or phosphorylates transcription factors and targets them for degradation. Juhaszova et al. (9) suggest that phosphorylation and inactivation of GSK-3β also reduce activity of the MPT. Most GSK-3β substrates are phosphorylated by another kinase prior to phosphorylation by GSK-3β (12). GS, glycogen synthase; p70s6k, p70 ribosomal S6 kinase; RSK, p90 ribosomal S6 kinase; ILK, integrin-linked kinase; WNT, homologues of Wingless; P, phosphorylation.
Unresolved issues
The mechanism responsible for targeting proteins to the proper cellular location is an emerging unresolved issue. How is GSK-3β targeted to the mitochondria? It is suggested that the mitoKATP channel might serve as a scaffolding protein or receptor for PKC, but it is unclear how GSK-3β is targeted to the mitochondria. It is important to determine which PKC isoform is involved in phosphorylating GSK-3β, since different PKC isoforms have been shown to have opposing effects on cardioprotection. Inhibition of PKCδ at the start of reperfusion is protective (15). Since multiple kinases can phosphorylate GSK-3β, why does inhibition of a single kinase (such as PKC or MAPK) inhibit protection? The hypothesis that most cardioprotective agents converge on GSK-3β would suggest that phosphorylation of GSK-3β is sufficient to inhibit the MPT and provide protection. It will also be important to define the precise target of GSK-3β. Data are presented showing that GSK-3β cosegregates with VDAC and ANT, but, given the abundance of these proteins in the mitochondria, this may not be surprising (9). Based on data showing that phosphorylation of GSK-3β increases the time between laser-induced generation of ROS and depolarization of mitochondria, Juhaszova et al. conclude that GSK-3β regulates the MPT (9). The MPT is poorly understood, and the precise molecular composition of the MPT needs to be defined (16). Advancement in cardioprotection and apoptosis will require a better understanding of the composition and regulation of the MPT and its role in apoptosis. Perhaps the most important issue is whether inhibition of GSK-3β is protective if it is initiated upon reperfusion. Inhibition of several kinases upon reperfusion has been reported to block protection afforded by insulin or other protective drugs administered upon reperfusion. Since these kinases phosphorylate GSK-3β, it is plausible that GSK-3β is a target during reperfusion. Consistent with this, a recent study by Gross et al. (11) showed that addition of GSK-3β inhibitors 5 minutes before the start of reperfusion resulted in a significant reduction in infarct size.
Footnotes
See the related article beginning on page 1535.
Nonstandard abbreviations used: adenine nucleotide translocator (ANT); glycogen synthase kinase (GSK); mitochondrial ATP-dependent K+ [channel] (mitoKATP); mitochondrial permeability transition (MPT); Na+-H+ exchange (NHE); preconditioning (PC); reactive oxygen species (ROS); voltage-dependent anion channel (VDAC).
Conflict of interest: The author has declared that no conflict of interest exists.
References
- 1.Murphy E. Primary and secondary signaling pathways in early preconditioning that converge on the mitochondria to produce cardioprotection. Circ. Res. 2004;94:7–17. doi: 10.1161/01.RES.0000108082.76667.F4. [DOI] [PubMed] [Google Scholar]
- 2.Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK) pathway. Cardiovasc. Res. 2004;61:448–460. doi: 10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 3.Akao M, Teshima Y, Marban E. Antiapoptotic effect of nicorandil mediated by mitochondrial ATP-sensitive potassium channels in cultured cardiac myocytes. J. Am. Coll. Cardiol. 2002;40:803–810. doi: 10.1016/s0735-1097(02)02007-7. [DOI] [PubMed] [Google Scholar]
- 4.Javadov SA, et al. Ischemic preconditioning inhibits opening of mitochondrial permeability transition pores in the reperfused rat heart. J. Physiol. 2003;549:513–524. doi: 10.1113/jphysiol.2003.034231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hausenloy DJ, Maddock HL, Baxter GF, Yellon DM. Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? Cardiovasc. Res. 2002;55:534–543. doi: 10.1016/s0008-6363(02)00455-8. [DOI] [PubMed] [Google Scholar]
- 6.Korge P, Honda HM, Weiss JN. Protection of cardiac mitochondria by diazoxide and protein kinase C: implications for ischemic preconditioning. Proc. Natl. Acad. Sci. U. S. A. 2002;99:3312–3317. doi: 10.1073/pnas.052713199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baines CP, et al. Protein kinase Cε interacts with and inhibits the mitochondrial transition pore in cardiac mitochondria. Circ. Res. 2003;92:873–880. doi: 10.1161/01.RES.0000069215.36389.8D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baines CP, et al. Mitochondrial PKCε and MAPK form signaling modules in the murine heart: enhanced mitochondrial PKCε-MAPK interactions and differential MAPK activation in PKCε-induced cardioprotection. Circ. Res. 2002;90:390–397. doi: 10.1161/01.res.0000012702.90501.8d. [DOI] [PubMed] [Google Scholar]
- 9.Juhaszova M, et al. Glycogen synthase kinase-3β mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J. Clin. Invest. 2004;113:1535–1549. doi:10.1172/JCI200419906. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tong H, Imahashi K, Steenbergen C, Murphy E. Phosphorylation of glycogen synthase kinase-3β during preconditioning through a phosphatidylinositol-3-kinase dependent pathway is cardioprotective. Circ. Res. 2002;90:377–379. doi: 10.1161/01.res.0000012567.95445.55. [DOI] [PubMed] [Google Scholar]
- 11.Gross ER, Hsu AK, Gross GJ. Opioid-induced cardioprotection occurs via glycogen synthase kinase β inhibition during reperfusion in intact rat hearts. Circ. Res. 2004;94:960–966. doi: 10.1161/01.RES.0000122392.33172.09. [DOI] [PubMed] [Google Scholar]
- 12.Cohen P, Frame S. The renaissance of GSK-3. Nat. Rev. Mol. Cell Biol. 2001;2:769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 13.Jope RS. Lithium and GSK-3: one inhibitor, two inhibitory actions, multiple outcomes. Trends Pharmacol. Sci. 2003;24:441–443. doi: 10.1016/S0165-6147(03)00206-2. [DOI] [PubMed] [Google Scholar]
- 14.Hausenloy, D.J., Yellon, D.M., Mani-Babu, S., and Duchen, M.R. 2004. Preconditioning protects by inhibiting the mitochondrial permeability transition. Am. J. Physiol. doi:10.1152/1jpheart.00678.2003. [DOI] [PubMed]
- 15.Inagaki K, et al. Inhibition of δ-protein kinase C protects against reperfusion injury of the ischemic heart in vivo. Circulation. 2003;108:2304–2307. doi: 10.1161/01.CIR.0000101682.24138.36. [DOI] [PubMed] [Google Scholar]
- 16.Kakoszka JE, et al. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]