Abstract
Objective:
We tested the hypothesis that altering the pre-exercise muscle temperature would influence the magnitude of muscle damage induced by eccentric exercise.
Subjects:
Female students who had no experience in resistance training were placed into either a microwave treatment group (n = 10) or an icing treatment group (n = 10).
Design and Setting:
Subjects in each group performed 12 maximal eccentric actions of the forearm flexors of each arm on 2 separate occasions separated by 4 weeks. Before testing, the exercise arm was subjected to either passive warming (microwave) or control for the microwave treatment group or cooling (icing) or control for the icing treatment group. The control arm performed the same exercise protocol without treatment. Limbs were randomized for treatment or control and order of testing. Deep muscle temperature increased by approximately 3°C after the microwave treatment and decreased approximately 5°C after the icing treatment.
Measurements:
We evaluated changes in maximal isometric force and indirect markers of muscle damage, including range of motion, upper arm circumference, muscle soreness, and plasma creatine kinase activity, in the microwave and control and icing and control groups using a 2-way, repeated-measures analysis of variance.
Results:
All measures changed significantly (P < .01) after exercise, but neither of the treatments demonstrated significant effects on most of the variables compared with the control.
Conclusions:
These results suggest that pre-exercise cooling does not affect the magnitude of muscle damage in response to eccentric exercise. Similarly, pre-exercise passive muscle warming did not prove beneficial in attenuating indicators of muscle damage. Thus, any beneficial effects of warm-up exercise cannot be attributed to the effects of increased muscle temperature.
Keywords: warm-up, microwave diathermy, icing, maximal isometric force, range of motion, creatine kinase, muscle soreness
Performing unaccustomed eccentric exercise results in muscle damage that is characterized by a long-lasting deficit of muscle function and development of delayed-onset muscle soreness (DOMS).1,2 Although various preventive or treatment measures have been claimed to attenuate muscle damage and DOMS or to facilitate recovery from muscle damage, their efficacy is still largely unproven. In athletic training, warm-up exercises are routinely used to prevent injury, and evidence suggests that warm-up exercise might be effective in reducing the extent of eccentric, exercise-induced muscle damage and DOMS.3–7
Rodenburg et al5 showed that a combination of a warm-up exercise, stretching, and massage reduced some negative effects of eccentric exercise, whereas Nosaka and Clarkson4 found that 100 isokinetic concentric contractions of the elbow flexors before eccentric exercise of the same muscles resulted in less muscle damage than eccentric exercise alone. Some authors5,8 have speculated that increased muscle temperature induced by warm-up exercise could, through increasing muscle and connective tissue extensibility, render these structure less susceptible to eccentric, exercise-induced muscle damage. To isolate the effects of temperature from the other factors associated with warm-up exercise, it is necessary to increase muscle temperature passively by use of ultrasound or electromagnetic diathermy to simulate the thermal effects of warm-up exercise. In fact, it has been documented that passive warm-up is indeed effective in reducing muscle injury.9,10 Evans et al3 recently reported that passive warm-up before eccentric exercise using pulsed short-wave diathermy increased muscle temperature by approximately 1°C and attenuated swelling but not other clinical symptoms of muscle damage, including muscle soreness. They compared responses among 5 subject groups (low-heat passive warm-up, high-heat passive warm-up, active warm-up, no warm-up before eccentric exercise, and high-heat passive warm-up without eccentric exercise). Because the intersubject variability in responses to eccentric exercise was large,1,11 using a crossover design that allows for arm-to-arm comparisons between treatment and control conditions in the same subjects was preferable.
If the mechanism by which warm-up is effective in reducing the extent of muscle damage and DOMS involves an increase in muscle and tendon extensibility,6,12 then it can be postulated that lowering muscle temperature (and therefore compliance of the musculotendinous unit) would have the opposite effect on muscle damage (ie, the extent of muscle damage and DOMS would be exacerbated when eccentric exercise is performed at a lower muscle temperature). Shellock and Prentice7 and Safran et al8 noted that the occurrence of muscle injury increased when muscle temperature was low. The effects of icing after eccentric exercise on DOMS13,14 or recovery of muscle function15 have been previously reported; however, the effects of muscle cooling before eccentric exercise on the development of muscle damage and DOMS have not been studied to date.
Therefore, our purpose was to examine the effect of altering pre-exercise muscle temperature on the indicators of muscle damage after maximal eccentric exercise of the elbow flexors.
METHODS
Experimental Design
To examine whether altering pre-exercise muscle temperature affects the magnitude of eccentric, exercise-induced muscle damage, we performed 2 experiments. The first study investigated the effects of increased muscle temperature on changes in several indirect markers of muscle damage in comparison with the control condition by a crossover design. One arm performed a bout of eccentric exercise after a microwave treatment, and the other arm performed the same exercise without treatment. In the second study, we examined the effects of decreased muscle temperature on changes in the muscle-damage markers in another group of subjects. One arm had an icing treatment before exercise, and the other arm performed the same eccentric exercise without treatment. Use of the dominant or nondominant arm and the order of treatment and control condition were randomized for both experiments. Changes in the measures after eccentric exercise were compared between the treatment and control arms in each experiment and between the microwave and icing treatments.
Subjects
Twenty female students who were nonathletes and had not been involved in a resistance-training program participated in this study. Their mean (± SD) age, height, and mass were 19.6 ± 2.8 years, 158.2 ± 6.3 cm, and 53.4 ± 7.0 kg, respectively. Subjects were randomly placed into either the microwave (n = 10) or icing (n = 10) group. No significant differences were noted in the physical characteristics between groups. Subjects were free from any musculoskeletal disorders and were instructed not to take any medicine or dietary supplements or perform any sports activities or unaccustomed exercise during the experimental period. Each subject read and signed a written informed consent form consistent with the principles outlined in the Declaration of Helsinki.
Treatments
From among the various methods of increasing local muscle temperature,16,17 we chose microwave diathermy. Electromagnetic diathermy (short wave, microwave) produces heat when soft tissue resists the passage of electric energy and can increase muscle temperature 3°C to 4°C at depths of 30 to 50 mm.3,16–18 For the microwave group, 1 upper arm was randomly assigned to receive microwave treatment using a Microradar (model KTM-250; ITO Co, Osaka, Japan) before eccentric exercise. The probe of the Microradar (circular shaped, 150 mm in diameter) was placed approximately 5 cm above the upper arm, and microwave (100 W) was applied for 10 minutes while the subject sat in a chair with the arm relaxed on the armrest.
For the icing group, an ice-cold water bag (0°C) was applied for 15 minutes over the elbow flexors. This treatment has been demonstrated to decrease muscle temperature at least 5°C.15,19,20 The water bag containing crushed ice and water was 10 cm in width and 15 cm in length, enough to cover the upper arm. A towel was placed between the bag and the skin.
Muscle Temperature
After the microwave or icing treatment, we assessed changes in muscle temperature using a needle thermistor probe (model N451; Nikkiso-YSI Ltd, Tokyo, Japan) connected to a thermometer (model N550; Nikkiso-YSI). Because the temperature-measurement procedure was invasive and the monitoring itself might influence changes in the criterion measures, we measured muscle temperature on a separate occasion for all subjects after completing the exercises and measurements for both arms. The thermistor probe (22 gauge, 70 mm) was inserted into the belly of the biceps brachii to a depth of 15 to 20 mm, and the temperature of the muscle was recorded after stabilization. Measurements were taken before and 1, 5, and 10 minutes after treatment. Changes in muscle temperature before and after the 12 maximal eccentric actions described below were examined in a separate subject (male, age = 24 years, height = 171 cm, mass = 66 kg).
Exercise
Subjects performed 12 maximal eccentric actions of the elbow flexors of each arm on 2 separate occasions, separated by 4 weeks, using a modified arm-curl machine.11 One arm performed the exercise within 3 minutes after the treatment, and the other arm performed the same exercise without treatment. The order of conditions and the use of the treatment or control arm were counterbalanced among subjects.
During the exercises, the arm was positioned in front of the body on a padded support adjusted to 45° (0.79 radian) of shoulder flexion, and the forearm was kept supinated with the wrist placed against a lever arm of the arm-curl machine. The elbow joint was forcibly extended by the investigator after a 1-second maximal isometric contraction from a flexed (50°, 0.87 radian) to an extended (180°, 3.14 radian) position in 3 seconds. Subjects were verbally encouraged to generate maximal isometric force at the starting position and to maximally resist the action throughout the range of motion. This action was repeated every 15 seconds for a total of 12 actions; therefore, the total exercise time was 3 minutes. The peak force of each action was displayed on a digital indicator (model F360A; Unipulse, Saitama, Japan), which allowed us to monitor the force output and to motivate the subjects.
Criterion Measures
The reliability of the measurements used to evaluate muscle damage has been established,1,11,21 and we were familiar with the testing protocols. All measurements, except blood samples and muscle-soreness assessments, were taken immediately before and after exercise and every 24 hours for 5 days post-exercise. Blood samples and muscle-soreness assessments were taken at the same time points except for immediately after exercise.
Maximal Voluntary Isometric Force
Maximal isometric force was measured as the peak force attained during a 3-second maximally voluntary effort at an elbow angle of 90° (1.57 radian), and 90° (1.57 radian) of shoulder flexion. The measurement was recorded twice (1 minute between measurements) using a load cell (model 1269; Takei Scientific Instruments Co Ltd, Nigata, Japan) positioned between 2 cables, 1 connected to the wrist and the other to the frame of the measurement device. Analog force data from the load cell was captured by a software program (LabVIEW, National Instruments Corp, Austin, TX) and converted to digital data on a computer (Macintosh Performer 5410; Apple Computer, Inc, Cupertino, CA). The mean value of the resulting measurements was used for later analysis.
Range of Motion
Relaxed and flexed elbow angles were each measured twice by a goniometer. The subject held the arm by her side in a relaxed and resting manner for the relaxed angle measurement, and flexed angle was determined when the subject maximally and voluntarily flexed the elbow joint. We subtracted the flexed angle from the relaxed angle and used this value as the measure of range of motion of the elbow joint.
Upper Arm Circumference
Circumference was assessed at 3, 5, 7, 9, and 11 cm from the elbow joint by a tape measure while the subject let the arm hang down by the side. Circumference at each site and the mean value of the 5 measurement sites were used for the analysis.
Delayed-Onset Muscle Soreness
We evaluated DOMS and maximal flexion and extension of the elbow joint using a visual analog scale that had a 50-mm line with “no pain” on one end and “extremely sore” on the other. In palpating muscle soreness, an investigator placed 4 fingers against the biceps brachii (proximal, middle, and distal) and applied digital pressure with the tips of the fingers toward the deeper tissues for approximately 3 seconds. The pressure to the muscles was consistent each day, and the same experienced investigator evaluated the muscle soreness everyday. In our previous study,21 the pressure was shown to be approximately 1 kg/cm2 for the palpation assessment.
Plasma Creatine Kinase Activity
Approximately 5 mL of blood was drawn from the antecubital vein at each measurement time point, except immediately after exercise, and centrifuged for 10 minutes to obtain plasma and stored at −20°C. Plasma creatine kinase (CK) activity was determined spectrophotometrically by the VP-Super (Dinabott Co Ltd, Tokyo, Japan) using test kits. The normal reference range of plasma CK activity for male adults is 45 to135 IU/L.
Statistical Analysis
Results are expressed as mean ± SEM. Changes in the criterion measures over time were initially compared between treatment and control arms for each group by a 2-way analysis of variance with repeated measures. Comparisons between the treatments (microwave and icing) for the changes in the measures were also made by a 2-way analysis of variance with repeated measures. A 2 × 7 (6 for DOMS and CK) factorial analysis of variance was used for the comparison between conditions (microwave and control, icing and control, microwave and icing), and the within-groups factor was time (pre-exercise, postexercise, 1–5 days postexercise). When the analysis of variance produced a significant main effect, we computed a Tukey post hoc test to detect differences in the measures between the bouts as well as between the groups at different time points. Statistical significance was set at P < .05 for all analyses. Because changes in the measures over time for the control arm were not significantly different between the microwave and icing treatment groups, the data for the control arms from both groups were combined and expressed as one “control condition” in the results.
RESULTS
Changes in Muscle Temperature by Treatment
Muscle temperature increased from 33.9 ± 0.8°C to 37.5 ± 1.4°C after the microwave treatment and remained elevated by approximately 3°C for at least 10 minutes. Therefore, muscle temperature during 12 maximal eccentric actions of the forearm flexors in the microwave treatment was at least 3°C higher than the control condition. Icing reduced muscle temperature approximately 8°C from 34.1 ± 0.9°C to 26.4 ± 1.0°C, and the temperature was still 5 to 8°C lower for at least 10 minutes after icing. The effect of the 12 maximal eccentric contractions of the forearm flexors on muscle temperature was evaluated in a single subject, and the exercise protocol caused muscle temperature to rise less than 1°C.
Muscle Force During Exercise
Peak forces and the total amount of work performed during the 12 maximal eccentric contractions of the forearm flexors were not significantly different between treatment and control arms for both groups or between the treatment arms (microwave and icing).
Maximal Isometric Force
Maximal isometric-force values were similar among the microwave (112.2 ± 4.9 N), icing (108.8 ± 5.2 N), and control (110.6 ± 4.0 N) arms. Maximal isometric force decreased significantly to approximately 60% of the pre-exercise value immediately after exercise, reached nadir at 1 day after exercise (50% to 55% of the pre-exercise value), and recovered to 70% to 75% of the pre-exercise value by 5 days postexercise (Figure 1). The extent of strength loss and rates of recovery were very similar for both treatment groups and showed no significant differences from the control data.
Figure 1.
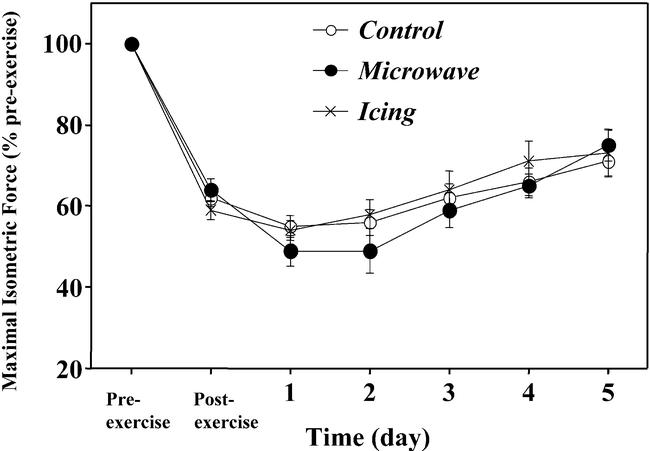
Changes in maximal isometric force before (pre-exercise), immediately after (postexercise), and 1 to 5 days after eccentric exercise in the treatment (microwave, icing) and control conditions. No significant differences among conditions were noted.
Range of Motion
No significant differences were seen in the pre-exercise values of the relaxed and flexed elbow angles among any of the groups. Relaxed elbow angle decreased significantly after exercise, with the maximal decrease being observed after 2 to 3 days. Compared with the control (10.3°) and icing (8.9°) arms, the microwave treatment arm showed a significantly larger decrease in relaxed angle (15.5°) 2 to 3 days after exercise. Flexed elbow angle was significantly greater immediately after exercise, increased further by 1-day postexercise, and gradually decreased over the next 4 days. Changes in flexed angle were significantly larger in the microwave treatment arm (14.2°), compared with the control (11.9°) or icing (9.1°) arm. Pre-exercise range of motion for the microwave, icing, and control conditions were 127.6 ± 1.2°, 126.2 ± 1.5°, and 125.9 ± 1.7°, respectively, values that were not significantly different. Figure 2 shows the relative changes in range of motion from the pre-exercise value after exercise. Range of motion decreased approximately 15° immediately after exercise and decreased further 1 to 3 days after exercise. Changes in range of motion over time were significantly larger for the microwave treatment, compared with the icing and control conditions, with the significant differences observed 2 to 5 days after exercise.
Figure 2.
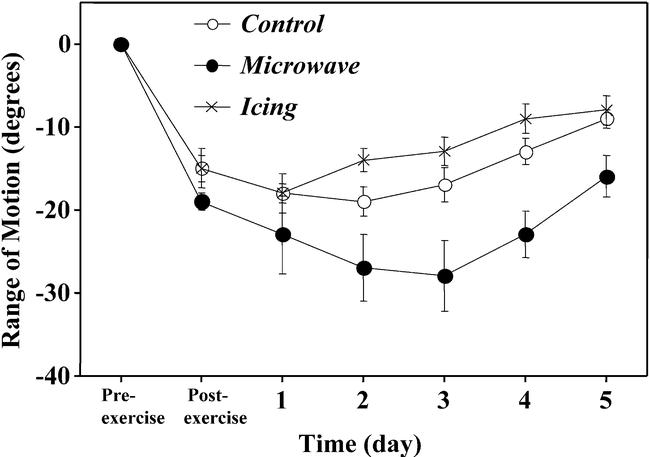
Changes in range of motion of the elbow joint before (pre-exercise), immediately after (postexercise), and 1 to 5 days after eccentric exercise in the treatment (microwave, icing) and control conditions. Significant (P < .01) differences were seen between the control and microwave and microwave and icing groups but not between the control and icing groups.
Upper Arm Circumference
Pre-exercise circumferences were similar among groups before exercise and increased in a similar fashion after exercise in all groups (Figure 3). Circumference peaked 4 to 5 days after exercise (increasing approximately 8 to 10 mm from pre-exercise values), and the amount of increase was not significantly different among the 5 sites.
Figure 3.
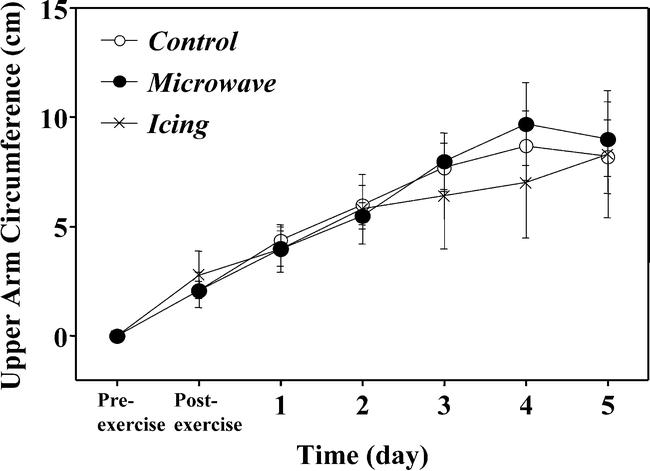
Changes in upper arm circumference before (pre-exercise), immediately after (postexercise), and 1 to 5 days after eccentric exercise in the treatment (microwave, icing) and control conditions. No significant differences were demonstrated among conditions.
Delayed-Onset Muscle Soreness
Before exercise, no subject reported any soreness during palpation or extension assessments. The DOMS developed after exercise, with both palpation and extension soreness peaking 1 to 3 days postexercise (Figure 4). The severity of DOMS was not significantly different between the treatment and control arms for both groups or between the microwave and icing conditions.
Figure 4.
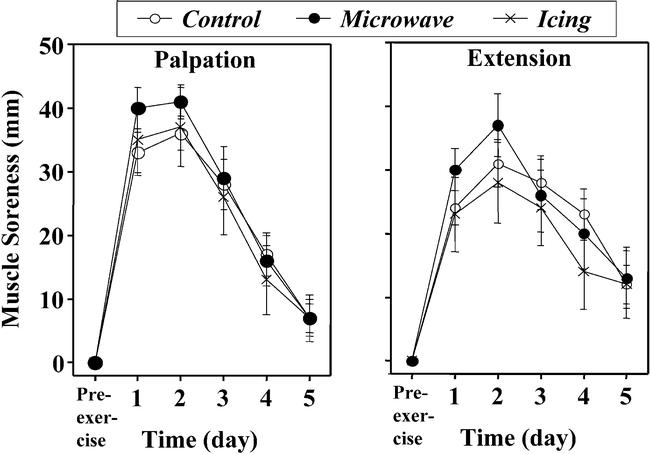
Changes in muscle soreness with palpation (A) and extension (B) before (pre-exercise) and 1 to 5 days after eccentric exercise in the treatment (microwave, icing) and control conditions. No significant differences were noted among conditions.
Plasma Creatine Kinase Activity
Pre-exercise plasma CK values were in the normal reference ranges for all subjects. Large individual variations in the response of plasma CK activity to exercise were noted (Figure 5); however, the means and ranges of peak CK activity (microwave range: 309–13 086 IU/L; icing: 410–11 493 IU/L; control range: 458–14 664 IU/L) were similar among conditions. The CK increased significantly after exercise, peaking at 4 to 5 days, but no significant difference between treatment and control arms or between microwave and icing conditions was observed.
Figure 5.
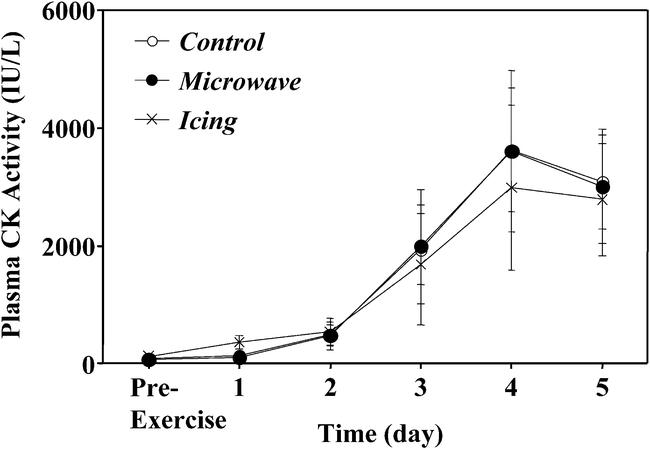
Changes in plasma creatine kinase (CK) activity before (pre-exercise) and 1 to 5 days after eccentric exercise in the treatment (microwave, icing) and control conditions. No significant differences were seen among conditions.
DISCUSSION
Our main findings were that altering muscle temperature using microwave or icing before exercise had no effect on the responses to a bout of eccentric exercise in terms of changes in maximal isometric force, upper arm circumference, DOMS, or plasma CK activity. Furthermore, the findings that the microwave treatment resulted in significantly larger changes in relaxed and flexed elbow angles and range of motion, compared with the icing and control conditions, are contrary to the hypothesis that the microwave treatment would attenuate the responses to eccentric exercise.
It has previously been reported that muscle function depends on muscle temperature.6,22–26 In our study, pre-exercise maximal isometric force, relaxed and flexed elbow angles, and range of motion were similar for both treatment and control arms. Peak eccentric force during eccentric exercise was not affected by either treatment, indicating that the thermal interventions did not alter the extent of the damaging stimulus. Thus, we can assume that the changes in muscle temperature because of the treatment were not large enough to affect muscle function or that the volume of muscle affected by the treatments was not sufficient to demonstrate a change.
The pretreatment muscle temperature (approximately 34°C) was similar to the level reported in previous studies,6,22–24 and similar changes in muscle temperature were shown after microwave diathermy3,16,18 or icing.13,19,20,27 Evans et al3 showed that a 3.5°C increase in muscle temperature of the biceps brachii from pulsed short-wave diathermy did not result in muscle damage. We found muscle temperature was at least 3°C higher for the microwave treatment arm and at least 2°C lower for the icing arm compared with the control arm when the eccentric exercise was performed. Because muscle temperature of the icing arm remained 5°C to 8°C lower than pretreatment level for 10 minutes and the increase in muscle temperature after the 12 maximal eccentric contractions of the forearm flexors was small (less than 1°C), the icing-condition muscle temperature was considered to be lower than the control condition. During prolonged exposure to cold environments, muscle temperature can fall by more than 2°C, and prolonged exercise can increase muscle temperature more than 3°C.28,29 Therefore, the muscle-temperature alterations in our study were considered to be within the physiologic range that might be expected after warm-up exercise.
If pre-exercise muscle temperature affected the magnitude of muscle damage induced by eccentric exercise, differences between microwave treatment and control or between icing and control or microwave and icing would have been evident. However, no such differences between the conditions were found for any of the criterion measures except elbow angles and range of motion (see Figures 1–5). Although the mechanism responsible for the muscle shortening that leads to the reduced joint angle after damaging eccentric exercise is not clear, it is believed to be related to muscle swelling and increased intramuscular pressure.2 Further studies are required to confirm this finding before additional speculation can be made regarding this unexpected result. Despite the changes seen in angles and range of motion, the lack of difference in other markers of muscle damage suggests no major influence of pre-exercise temperature on the responses of the elbow flexors to eccentric exercise.
Safran et al9 stated that warm-up leads to more relaxed muscles, increased extensibility of connective tissue within muscle, decreased muscle viscosity, and smoother contractions. It has been suggested that muscle temperature increased by warm-up exercise would render the environment of the muscle and connective tissue less susceptible to eccentric, exercise-induced muscle damage.7,8 Nosaka and Clarkson4 found that 100 repetitions of isokinetic movements of the forearm before eccentric exercise of the elbow flexors resulted in less apparent muscle damage compared with eccentric exercise without previous isokinetic exercise. Although the role of warm-up exercise in reducing the magnitude of muscle damage is unknown, increased muscle temperature was deemed to be one of the likely factors. However, we found no positive effects for microwave treatment (see Figures 1, 3–5), and the effect on range of motion was negative (see Figure 2). Furthermore, there was no evidence of negative effects for the icing condition (see Figures 1–5). Therefore, it appears that warm-up exercise may lessen the chance to develop eccentric, exercise-induced muscle damage by mechanisms other than increased muscle temperature.
Although the increase in muscle temperature observed through active warm-up will likely raise muscle temperature enough for muscle compliance to increase, other factors also influence the viscoelastic properties of the musculotendinous unit. These may include the modification of stretch reflexes during warm-up, which may serve to protect the contractile apparatus from muscle damage through reduced stiffness.30 Furthermore, there is evidence that the increased compliance observed with passive warming of a muscle does not result in an increase in the length at which damaging strain occurs during eccentric stretching.31
The fact that our results did not support the hypothesis that increasing muscle temperature by passive warm-up exercise renders the muscle less susceptible to muscle damage does not detract from the potential importance of active warm-up exercise in reducing the occurrence or severity of muscle damage and DOMS induced by eccentric exercise. Performing general and specific warm-up exercise along with dynamic flexibility exercises may be effective in reducing eccentric, exercise-induced muscle damage and DOMS.12,32 However, further study is needed to investigate how and why warm-up exercise works and what is the most effective warm-up procedure to prevent muscle damage and DOMS. The implications of our findings are first that increased muscle temperature had no attenuating effect on the severity of muscle damage or soreness associated with eccentric exercise. Second, performing eccentric exercise when muscles were colder than normal did not increase the severity of the muscle-damage response.
REFERENCES
- 1.Clarkson PM, Nosaka K, Braun B. Muscle function after exercise-induced muscle damage and rapid adaptation. Med Sci Sports Exerc. 1992;24:512–520. [PubMed] [Google Scholar]
- 2.Howell JN, Chleboun G, Conatser R. Muscle stiffness, strength loss, swelling and soreness following exercise-induced injury in humans. J Physiol. 1993;464:183–196. doi: 10.1113/jphysiol.1993.sp019629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans RK, Knight KL, Draper DO, Parcell AC. Effects of warm-up before eccentric exercise on indirect markers of muscle damage. Med Sci Sports Exerc. 2002;34:1892–1899. doi: 10.1097/00005768-200212000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Nosaka K, Clarkson PM. Influence of previous concentric exercise on eccentric exercise-induced muscle damage. J Sports Sci. 1997;15:477–483. doi: 10.1080/026404197367119. [DOI] [PubMed] [Google Scholar]
- 5.Rodenburg JB, Steenbeek D, Schiereck P, Bär PR. Warm-up, stretching and massage diminish harmful effects of eccentric exercise. Int J Sports Med. 1994;15:414–419. doi: 10.1055/s-2007-1021080. [DOI] [PubMed] [Google Scholar]
- 6.Sargeant AJ. Effect of muscle temperature on leg extension force and short-term power output in humans. Eur J Appl Physiol Occup Physiol. 1987;56:693–698. doi: 10.1007/BF00424812. [DOI] [PubMed] [Google Scholar]
- 7.Shellock FG, Prentice WE. Warming-up and stretching for improved physical performance and prevention of sports-related injuries. Sports Med. 1985;2:267–278. doi: 10.2165/00007256-198502040-00004. [DOI] [PubMed] [Google Scholar]
- 8.Safran MR, Garrett WE, Jr, Seaber AV, Glisson RR, Ribbeck BM. The role of warmup in muscular injury prevention. Am J Sports Med. 1988;16:123–129. doi: 10.1177/036354658801600206. [DOI] [PubMed] [Google Scholar]
- 9.Safran MR, Seaber AV, Garrett WE., Jr Warm-up and muscle injury prevention: an update. Sports Med. 1989;8:239–249. doi: 10.2165/00007256-198908040-00004. [DOI] [PubMed] [Google Scholar]
- 10.Strickler T, Malone T, Garrett WE. The effects of passive warming on muscle injury. Am J Sports Med. 1990;18:141–145. doi: 10.1177/036354659001800206. [DOI] [PubMed] [Google Scholar]
- 11.Nosaka K, Clarkson PM. Changes in indicators of inflammation after eccentric exercise of the elbow flexors. Med Sci Sports Exerc. 1996;28:953–961. doi: 10.1097/00005768-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Szymanski DJ. Recommendations for the avoidance of delayed-onset muscle soreness. Strength Cond J. 2001;23:7–13. [Google Scholar]
- 13.Isabell WK, Durrant E, Myrer W, Anderson S. The effects of ice massage, ice massage with exercise, and exercise on the prevention and treatment of delayed onset muscle soreness. J Athl Train. 1992;27:208–217. [PMC free article] [PubMed] [Google Scholar]
- 14.Yackzan L, Adams C, Francis KT. The effects of ice massage on delayed muscle soreness. Am J Sports Med. 1984;12:159–165. doi: 10.1177/036354658401200214. [DOI] [PubMed] [Google Scholar]
- 15.Paddon-Jones DJ, Quigley BM. Effect of cryotherapy on muscle soreness and strength following eccentric exercise. Int J Sports Med. 1997;18:588–593. doi: 10.1055/s-2007-972686. [DOI] [PubMed] [Google Scholar]
- 16.Donley PB. Shortwave and microwave diathermy. In: Prentice WE, editor. Therapeutic Modalities in Sports Medicine. 2nd ed. St Louis, MO: Time Mirror/Mosby College Publishing; 1990. pp. 149–167. [Google Scholar]
- 17.Goats GC. Microwave diathermy. Br J Sports Med. 1990;24:212–218. doi: 10.1136/bjsm.24.4.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Draper DO, Knight K, Fujiwara T, Castel JC. Temperature change in human muscle during and after pulsed short-wave diathermy. J Orthop Sports Phys Ther. 1999;29:13–22. doi: 10.2519/jospt.1999.29.1.13. [DOI] [PubMed] [Google Scholar]
- 19.Lowdon BJ, Moore RJ. Determinants and nature of intramuscular temperature changes during cold therapy. Am J Phys Med. 1975;54:223–233. [PubMed] [Google Scholar]
- 20.Zemke JE, Andersen JC, Guion KW, McMillan J, Joyner AB. Intramuscular temperature responses in the human leg to two forms of cryotherapy: ice massage and ice bag. J Orthop Sports Phys Ther. 1998;27:301–307. doi: 10.2519/jospt.1998.27.4.301. [DOI] [PubMed] [Google Scholar]
- 21.Nosaka K, Newton M, Sacco P. Delayed-onset muscle soreness does not reflect the magnitude of eccentric exercise-induced muscle damage. Scand J Med Sci Sports. 2002;12:337–346. doi: 10.1034/j.1600-0838.2002.10178.x. [DOI] [PubMed] [Google Scholar]
- 22.Bergh U, Ekblom B. Influence of muscle temperature on maximal muscle strength and power output in human skeletal muscles. Acta Physiol Scand. 1979;107:33–37. doi: 10.1111/j.1748-1716.1979.tb06439.x. [DOI] [PubMed] [Google Scholar]
- 23.Bigland-Ritchie B, Thomas CK, Rice CL, Howarth JV, Woods JJ. Muscle temperature, contractile speed, and motoneuron firing rates during human voluntary contractions. J Appl Physiol. 1992;73:2457–2461. doi: 10.1152/jappl.1992.73.6.2457. [DOI] [PubMed] [Google Scholar]
- 24.Davies CTM, Young K. Effect of temperature on the contractile properties and muscle power of triceps surae in humans. J Appl Physiol. 1983;55:191–195. doi: 10.1152/jappl.1983.55.1.191. [DOI] [PubMed] [Google Scholar]
- 25.Meigal AY, Lupandin YV, Hänninen O. Influence of cold and hot conditions on postactivation in human skeletal muscles. Pflugers Arch. 1996;432:121–125. doi: 10.1007/s004240050113. [DOI] [PubMed] [Google Scholar]
- 26.Ranatunga KW, Sharpe B, Turnbull B. Contractions of a human skeletal muscle at different temperatures. J Physiol. 1987;390:383–395. doi: 10.1113/jphysiol.1987.sp016707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myrer JW, Draper DO, Durrant E. Contrast therapy and intramuscular temperature in the human leg. J Athl Train. 1994;29:318–322. [PMC free article] [PubMed] [Google Scholar]
- 28.Guyton AC. Textbook of Medical Physiology. 8th ed. Philadelphia, PA: WB Saunders Co; 1991. pp. 797–808. [Google Scholar]
- 29.Wirth VJ, Van Lunen BL, Mistry D, Saliba E, McCue FC. Temperature changes in deep muscles of humans during upper and lower extremity exercise. J Athl Train. 1998;33:211–215. [PMC free article] [PubMed] [Google Scholar]
- 30.Kuitunen S, Avela J, Kyröläinen H, Nicol C, Komi PV. Acute and prolonged reduction in joint stiffness in humans after exhausting stretch-shortening cycle exercise. Eur J Appl Physiol. 2002;88:107–116. doi: 10.1007/s00421-002-0669-2. [DOI] [PubMed] [Google Scholar]
- 31.Noonan TJ, Best TM, Seaber AV, Garrett WE., Jr Thermal effects on skeletal muscle tensile behavior. Am J Sports Med. 1993;21:517–522. doi: 10.1177/036354659302100407. [DOI] [PubMed] [Google Scholar]
- 32.Cross KM, Worrell TW. Effects of a static stretching program on the incidence of lower extremity musculotendinous strains. J Athl Train. 1999;34:11–14. [PMC free article] [PubMed] [Google Scholar]


