Abstract
Background:
Neonates and infants undergoing heart surgery on cardiopulmonary bypass (CPB) are at high risk for significant post-operative morbidity and mortality. Hence, there is a need to identify and quantify clinical factors during the early post-operative period that are indicative of short-term as well as long-term outcomes. Multiple inotrope scores have been used in practice to quantify the amount of cardiovascular support received by neonates.
Aims:
The goal of this study was to determine the association between inotropic/vasoactive support and clinical outcomes in children after open cardiac surgery.
Materials and Methods:
This is a retrospective analysis of the 208 patients who underwent cardiac surgery for congenital heart disease at a tertiary pediatric cardiac surgery Intensive Care Unit (ICU) from January 2012 to March 2013. Multiple demographic, intra-operative and post-operative variables were recorded, including the Vasoactive Inotrope Score (VIS).
Results:
A total of 208 patients underwent cardiac surgery for congenital heart disease in the study period. The mean age and weight in the study were 66.94 months and 16.31 kg, respectively. Statistically significant associations were found in the various variables and VIS, including infancy, weight < 10 kg, CPB time, pump failure and post-operative variables like sepsis, hematological complications, hepatic dysfunction, acute kidney injury during admission, mortality, prolonged ventilator requirement, CPB time (in min) and hospital stay.
Conclusions:
Inotrope score and its adaptations are an excellent tool to measure illness severity, deciding interventions and during parental counseling in the pediatric cardiac surgery ICUs.
Keywords: Cardiac surgery, cardiopulmonary bypass, infants, inotropes
Introduction
Infants who undergo heart surgery on cardiopulmonary bypass (CPB) are at high risk for significant post-operative morbidity and mortality.[1,2,3,4,5,6] Hence, there is a need to identify and quantify clinical factors during the early post-operative period that are indicative of short-term as well as long-term outcomes. Despite the large volume of clinical and physiological data obtained at the time of admission and on subsequent days in the Intensive Care Unit (ICU), a measure for predicting eventual morbidity and mortality is still lacking. A measure is required that could guide caregivers to choose the best diagnostic and therapeutic option for the betterment of the child who is recovering from the surgery. In addition, such data would prove useful for clinicians to prognosticate the child's recovery to the family on the likelihood of recovery from cardiac surgery. One such modality reported has been the Inotrope Score.
The Inotrope Score was initially described in a study by Wernovsky and colleagues. The purpose of this score was to quantify the amount of cardiovascular support received by neonates after arterial switch operation.[7] The purpose of this study was to evaluate the inotropic score (IS) as a predictor of morbidity and mortality in the early post-operative period among infants who underwent a cardiac surgery with CPB. The study was performed on 174 infants, of which 43% were neonates. The maximum IS for the first 24 h, subsequent 24 h and the mean IS were calculated. The mean IS was obtained by averaging the hourly scores during the first and second 24-h periods. They found that the maximum vasoactive-inotropic score level over the first 48 h was a good predictor of poor clinical outcome (death, cardiac arrest, prolonged mechanical circulatory support, renal replacement therapy and/or neurologic injury). High inotropic score was also associated with prolonged ICU stay, duration of mechanical ventilation and time to negative fluid balance.[8] However, there was a shorcoming to this score as it did not include the use of vasoactive medications like Nitroprusside, Nitroglycerine, Phosphodiesterase inhibitors and Vasopressin. This was then modified by Davidson et al.[9]
Davidson et al. performed a prospective observational study of 70 infants undergoing cardiothoracic surgery. They developed a modified inotropic score with inclusion of vasoactive medications known as Vasoactive Inotropic Score (VIS). Both VIS and IS were assessed at 24 (VIS24, IS24), 48 (VIS48, IS48), and 72 (VIS72, IS72) h after surgery. They found that a higher VIS at 48 h after cardiothoracic surgery was strongly associated with increased length of ventilation and prolonged ICU and total hospital stay, and that VIS was more predictive of poor short-term outcome than IS.[9]
A number of studies came subsequently about the association of VIS with various post-operative issues. Most of these studies were conducted on neonates in view of their unique cardiovascular features and the complexity of cardiac procedures that are performed. Neonates undergoing cardiac surgery requiring CPB are at higher risk for morbidity and mortality compared with other age groups.[10] However, with advancements in operative techniques and perioperative management, mortality rates have significantly decreased in the last two decades. Presently, the focus of research has shifted from preventing mortality to decreasing morbidities.[9] Butts et al. conducted a study on 76 neonates undergoing corrective or palliative cardiac operations requiring CPB. They found that Low Cardiac Output Syndrome was not associated with duration of mechanical ventilation, ICU length of stay (LOS), hospital LOS and hospital cost. Higher VIS was found to be moderately associated with a longer duration of mechanical ventilation, longer ICU LOS and greater total hospital costs but not hospital LOS. LOS was not associated with early post-operative outcomes. Maximum VIS showed only modest correlation with duration of mechanical ventilation, ICU LOS and total hospital expenditure.[11]
Sanil and Aggarwal computed peak VIS within the initial 24 and 48 h after 51 consecutive open heart transplants (OHTs). Patients with peak VIS ≥ 15 constituted the high VIS group in their study. Children who persistently required a higher magnitude of pressor support for the first 48 h after OHT, as reflected by high peak VIS, had significantly longer ICU stay, inotropic requirement and ventilatory durations, and higher rates of short-term morbidities. Patients with longer CPB time had higher peak VIS. The authors found high peak VIS at 48 h to be an effective, yet simple clinical marker for adverse outcomes in pediatric OHT recipients.[12]
Gaies et al. conducted a study among 391 infants with 141 (36%) neonates and determined empirically high VIS to be maximum VIS > 15 in the first 24 h. High VIS was found to be significantly associated with 30-day mortality, cardiac arrest, mechanical circulatory support requirement, dialysis, neurologic injury, duration of mechanical ventilation and ICU LOS.[13]
With the increasing use of Levosimendan in pediatric patients with chronic heart failure and severe post-operative left ventricular dysfunction, it has been suggested it be included in the calculation of VIS, similar to Phosphodiesterase inhibitors (Milrinone/Amrinone).[14]
There is a need to have a validated, robust severity of illness score specifically designed for the infant pediatric cardiac surgery population, which shows a strong association with the clinical outcomes. Finding such an inotrope score could be a surrogate marker of illness severity and measure the vasoactive agent requirement as an intermediate predictor of eventual outcomes.
The goal of the present study was to determine the association between inotropic/vasoactive support and clinical outcomes in children after open cardiac surgery.
Materials and Methods
This was a retrospective analysis of the 208 patients who underwent cardiac surgery for congenital heart disease at a tertiary pediatric cardiac surgery ICU from January 2012 to March 2013. The findings of the prospective study on these patients have been presented and are under review.[15] The previously conducted prospective study on this cohort showed that, on multivariate logistic regression analysis, young age, CPB time, prolonged ventilator requirement, pump failure, sepsis and hematological complications were identified as independent variables for any degree for AKI.[15] The demographic variables obtained were age at surgery, sex, weight at surgery and cardiac diagnosis. Intra-operative information collected included CPB time, cross-clamp time, evidence of low-output syndrome or pump failure and Aristotle score for surgical complexity. Post-operative variables included prolonged ventilator requirement, evidence of sepsis, hematological dysfunction, hepatic dysfunction and requirement of renal replacement therapy.
Calculation of IS and VIS
The hourly doses of the following inotropic and vasoactive medications were recorded for the first 48 h after post-operative admission to the ICU: Dopamine, dobutamine, epinephrine, norepinephrine, milrinone and vasopressin. In our analysis, the IS was calculated as described by Wernovsky and the formula expanded to include other vasoactive agents commonly used in current practice to define a VIS as described by Davidson et al.[8,9]and Gaies et al.[13] The maximum VIS level over the first 48 h was recorded.
Wernowsky IS = Dopamine dose (μg/kg/min) + Dobutamine dose (μg/kg/min) +100 × epinephrine dose (μg/kg/min)
VIS = IS + 10 X Milrinone dose (μg/kg/min) +10,000 × Vasopressin dose (U/kg/min) + 100 × Norepinephrine dose (μg/kg/min)
Gaies et al. sought to dichotomize maximum VIS into a “high” and “low” variable based on the sensitivity and specificity of this measurement as a predictor of poor outcome at various cut-off points. They found a cut-off point of maximum VIS 20-24 in the first 24 h and 15-19 in the subsequent 24 h as the optimal for predicting a poor outcome.[8]
In our study, a score of less than 10 was empirically designated as low VIS, while that of more than 10 was called high VIS. All the patients were divided into two categories based on their requirement of inotropes following cardiac surgery as calculated by maximum VIS at 48 h: Those with a score less than 10 (n = 154) and those who had a score of more than 10 (n = 54).
Definitions of outcomes
The diagnosis of pump failure or low output syndrome, as defined by Hoffman, includes a combination of clinical signs of poor perfusion, an increase in existing pharmacologic agent to treat low cardiac output, an increase in lactate of 0.22 mmol/L on two successive arterial blood gases or a metabolic acidosis with an increase in base deficit of >4, with or without a >30% difference in arterial mixed venous oxygen saturation.[16]
Hematological dysfunction was defined as platelet count <80,000/mm[3] or a decline of 50% in the platelet count from the highest value recorded over the last 48 h.[17]
Hepatic dysfunction was defined as alanine transaminase level 2 × upper limit of the normal. Prolonged ventilatory requirement was defined as need for invasive ventilation for more than 48 h.[17]
Data management
Demographic data and study variables were expressed as mean ± SD. Linear and step-wise multiple logistic regression analyses to find the association of VIS with pre-operative, intra-operative and post-operative variables in order to determine the association between inotropic/vasoactive support and clinical outcome in children after cardiac surgery.
Results
Baseline characteristics
A total number 208 patients underwent cardiac surgery for congenital heart disease in the 15-month study period from January 2012 to March 2013. The mean age and weight in the study were 66.94 months and 16.31 kg, respectively. The mean CPB time and the mean hospital stay was 71.79 min and 11.51 days, respectively. One hundred and fifty patients were male. Prolonged ventilator support was required in 33 patients (15.87%). Eight patients in our study developed pump failure during the surgery. Sepsis developed in 19.71% of the patients. Sepsis and prolonged ventilator requirement were the most common complications. Hematological complications developed in 10.58% of the patients. Five patients (2.4%) died following cardiac surgery.
Spectrum of the surgeries included ventricular septal defect (VSD) closure, total anomalous pulmonary venous connection (TAPVC) repair, complete AV canal repair, Tetralogy of Fallot (TOF) correction, arterial switch operation for d-TGA (transposition of great arteries with or without VSD), coarctation of aorta repair, Truncus arteriosus repair and Fontan procedure.
Linear regression analysis
Linear regression analysis was performed to analyze the association of VIS and baseline and operative variables. One hundred and ten male patients had a low VIS (VIS < 10) at 48 h, and 44 males had a high VIS score. Forty-four females had low VIS and 14 females had high VIS. On statistical analysis, no association was found between VIS and gender of the patients [Table 1].
Table 1.
Linear regression analysis showing the association of VIS with baseline characteristics, intraoperative and post-operative variables
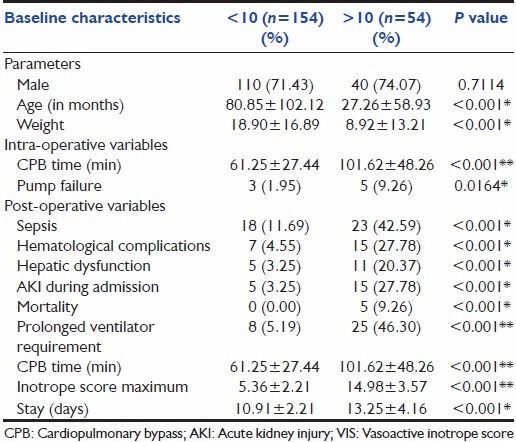
The mean age in months of the children with VIS less than 10 was approximately 81 months. The mean age in months of the children with VIS more than 10 was approximately 26 months.
There was a significant association between younger patient age at the time of surgery for congenital cardiac disease and maximum VIS at 48 h (P < 0.001). The mean weight in kilograms of the children with VIS less than 10 was around 18 kg. The mean weight in kilograms of the children with VIS more than 10 was around 26 kg. There was a significant association between lesser child weight at the time of surgery for congenital cardiac disease and maximum VIS at 48 h (P < 0.001) [Table 1].
Among the intra-operative variables, children with low VIS had mean CPB time of 61 min and children with high VIS had mean CPB time of 102 min. There was also a significant association between increased CPB time and VIS at 48 h (P < 0.001).
Three children who developed pump failure during the surgery had low VIS and five had high VIS. An association was observed between VIS and pump failure, with the P value being 0.0164 [Table 1].
Similarly significant associations were found in the various post-operative variables and VIS, like sepsis, hematological complications, hepatic dysfunction, AKI during admission, mortality, prolonged ventilator requirement, CPB time (in min) and hospital stay.
Multiple regression analysis
On multiple regression analysis to determine whether VIS was an independent predictor of hospital stay, AKI and mortality, it was not found to be statistical significant.
Discussion
Cardiac surgery for congenital heart disease often results in a decrease in cardiac output during the immediate post-operative period, which is the most critical period (the first 12-24 h after surgery). In approximately 25% of infants and young children, low cardiac output develops and these patients are at higher risk of death in the post-operative period. The management of these patients relies on multiple strategies intended to mitigate the potential threat of low cardiac output. As part of this management, inotropic and vasoactive agents are routinely employed after cardiac surgery in infants to decrease the risk of low cardiac output.[17]
Vasoactive medications are typically started in the operating room at the discretion of the attending cardiac surgeon and anesthesiologist. Patients with hypotension typically receive dobutamine and epinephrine initially. Vasopressin is used when patients have refractory tachyarrhythmias or have sinus rates that prohibitively limit the length of diastole. There are no protocols for the initiation or titration of particular medications.[8]
Gaies et al. compared the maximum IS and VIS for both the first 24 h and the subsequent 24 h and also the mean IS and VIS, and found that the maximum vasoactive-inotropic score level over the first 48 h was the best predictor among these of poor clinical outcome (death, cardiac arrest, mechanical circulatory support, renal replacement therapy and/or neurologic injury).[8]
Davidson et al. found that a higher VIS at 48 h after cardiothoracic surgery was most significantly associated with poorer outcomes among VIS and IS at 24 (VIS24, IS24), 48 (VIS48, IS48) and 72 (VIS72, IS72) h after surgery and maximum VIS and IS scores in the first 48 h (VIS48 max and IS48 max). The findings in this study suggested that duration of intensive cardiovascular support may be more important than maximal intensity of therapy as a predictor of these short-term outcomes. The authors discussed that VIS48 may place less emphasis on patients with transient poor cardiac function or vasoplegia whose outcomes are likely to be good, while potentially identifying patients with persistent issues such as myocardial injury, poor surgical repair or capillary leak. VIS48 has the added clinical benefit of ease of calculation, requiring a simple bedside check without review of prior records.[9] In our study, we computed maximum VIS in 48 h and found it to be significantly associated with poorer post-operative outcomes, morbidity and mortality.
In our study, a strong correlation was found between requirement of prolonged respiratory support and a high maximum VIS. There were 25 infants in our study group who required prolonged respiratory support and had high VIS score at 48 h. Of the eight children in our study who had low VIS score but required longer respiratory support, other causes of respiratory failure were present. These included airway anomalies in two patients (bronchomalacia), severe post-extubation stridor requiring re-intubation and respiratory support in four patients and post-surgery diaphragmatic palsy (phrenic nerve injury) in two patients.
The variations in ICU and hospital stays, Davidson et al. discussed, might be due to factors not immediately associated with VIS, such as poor feeding, vocal cord paralysis/paresis, phrenic nerve injury and chylothorax.[9] Even in the presence of these other factors, VIS48 showed a strong, independent association with length of ICU and hospital stay. Butts et al. explained the absence of correlation between maximum VIS and hospital LOS in their study among neonatal population by the complexity of other issues affecting hospital LOS in the neonatal population (i.e. feeding difficulties).[11] In our study, there was a strong independent correlation between hospital LOS and VIS score.
We opine from our study that high VIS was likely to be a marker for poor cardiac physiology (e.g. ventricular dysfunction) in the immediate post-operative period. This poor physiology might lead to prolonged therapies, more frequent complications and borderline pulmonary function that impaired convalescence, particularly feeding.[9]
In the original prospective study, we found that young age, CPB time, prolonged ventilator requirement, pump failure, sepsis and hematological complications were identified as independent variables for any degree for AKI.[15] We found that VIS was strongly associated with infancy, increasing CPB time, pump failure, ventilatory requirement, AKI and multiple organ dysfunctions and prolonged hospital stay.
The inotrope requirement of a child post cardiac surgery is an interplay between multiple physiolgical parameters, including pump failure, sepsis, duration of the cardiac surgery and multiple organ dysfunctions. Thus, it is not a surprise to find that VIS could not be an independent variable in the multivariate analysis. Moreover, our study was a single-center study with a small sample size retrospective analysis.
IS and its adaptations are an excellent tool to measure illness severity in patients undergoing cardiac surgery. The results of this study further provide evidence that children who undergo open heart surgery on CPB, and who require high levels of vasoactive support during the early post-operative period, have an increased likelihood of morbidity and mortality. The score can be hence used as a good tool for the ICU caregivers to decide various diagnostic and therapeutic options for patients recovering cardiac surgery and during parental counseling for telling the child's likelihood of recovery.
Further studies are required to determine whether our results are reproducible in multiple centers and whether it can independently predict the clinical outcomes. We believe that this tool might improve the evidenced-based practice and improve the quality of designs in the pediatric cardiac ICUs.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Curzon CL, Milford-Beland S, Li JS, O'Brien SM, Jacobs JP, Jacobs ML, et al. Cardiac surgery in infants with low birth weight is associated with increased mortality: Analysis of the Society of Thoracic Surgeons Congenital Heart Database. J Thorac Cardiovasc Surg. 2008;135:546–51. doi: 10.1016/j.jtcvs.2007.09.068. [DOI] [PubMed] [Google Scholar]
- 2.Dorfman AT, Marino BS, Wernovsky G, Tabbutt S, Ravishankar C, Godinez RI, et al. Critical heart disease in the neonate: Presentation and outcome at a tertiary care center. Pediatr Crit Care Med. 2008;9:193–202. doi: 10.1097/PCC.0b013e318166eda5. [DOI] [PubMed] [Google Scholar]
- 3.Gaynor JW, Mahle WT, Cohen MI, Ittenbach RF, DeCampli WM, Steven JM, et al. Risk factors for mortality after the Norwood procedure. Eur J Cardiothorac Surg. 2002;22:82–9. doi: 10.1016/s1010-7940(02)00198-7. [DOI] [PubMed] [Google Scholar]
- 4.Mahle WT, Tavani F, Zimmerman RA, Nicolson SC, Galli KK, Gaynor JW, et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation. 2002;106:I109–14. [PubMed] [Google Scholar]
- 5.McQuillen PS, Barkovich AJ, Hamrick SE, Perez M, Ward P, Glidden DV, et al. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke. 2007;38:736–41. doi: 10.1161/01.STR.0000247941.41234.90. [DOI] [PubMed] [Google Scholar]
- 6.Stasik CN, Gelehrter S, Goldberg CS, Bove EL, Devaney EJ, Ohye RG. Current outcomes and risk factors for the Norwood procedure. J Thorac Cardiovasc Surg. 2006;131:412–7. doi: 10.1016/j.jtcvs.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 7.Wernovsky G, Wypij D, Jonas RA, Mayer JE, Jr, Hanley FL, Hickey PR, et al. Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants. A comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation. 1995;92:2226–35. doi: 10.1161/01.cir.92.8.2226. [DOI] [PubMed] [Google Scholar]
- 8.Gaies MG, Gurney JG, Yen AH, Napoli ML, Gajarski RJ, Ohye RG, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med. 2010;11:234–8. doi: 10.1097/PCC.0b013e3181b806fc. [DOI] [PubMed] [Google Scholar]
- 9.Davidson J, Tong S, Hancock H, Hauck A, Cruz E, Kaufman J. Prospective validation of the vasoactive inotropic score and correlation to short-term outcomes in neonates and infants after cardiothoracic surgery. Intensive Care Med. 2012;38:1184–90. doi: 10.1007/s00134-012-2544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balaguru D, Haddock PS, Puglisi JL, Bers DM, Coetzee WA, Artman M. Role of the sarcoplasmic reticulum in contraction and relaxation of immature rabbit ventricular myocytes. J Mol Cell Cardiol. 1997;29:2747–57. doi: 10.1006/jmcc.1997.0509. [DOI] [PubMed] [Google Scholar]
- 11.Butts RJ, Scheurer MA, Atz AM, Zyblewski SC, Hulsey TC, Bradley SM, et al. Comparison of maximum vasoactive inotropic score and low cardiac output syndrome as markers of early postoperative outcomes after neonatal cardiac surgery. Pediatr Cardiol. 2012;33:633–8. doi: 10.1007/s00246-012-0193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanil Y, Aggarwal S. Vasoactive-inotropic score after pediatric heart transplant: A marker of adverse outcome. Pediatr Transplant. 2013;17:567–72. doi: 10.1111/petr.12112. [DOI] [PubMed] [Google Scholar]
- 13.Gaies MG, Jeffries HE, Niebler RA, Pasquali SK, Donohue JE, Yu S, et al. Vasoactive-Inotropic Score Is Associated With Outcome After Infant Cardiac Surgery: An Analysis From the Pediatric Cardiac Critical Care Consortium and Virtual PICU System Registries. Pediatr Crit Care Med. 2014;15:529–37. doi: 10.1097/PCC.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Favia I, Vitale V, Ricci Z. The vasoactive-inotropic score and levosimendan: Time for LVIS? J Cardiothorac Vasc Anesth. 2013;27:e15–6. doi: 10.1053/j.jvca.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Sethi SK, Sharma R, Kumar M, Bazaz S, Bhan A, Kher V. Incidence and Risk Factors of Acute Kidney Injury and Mortality in Pediatric Cardiothoracic ICU: First Prospective Study from India. Proceedings of the 1st international Symposium on AKI in Children at the 7th International Conference on Pediatric Continuous Renal Replacement therapy; 2012 September 27-30; Ohio; USA. Pediatr Nephrol. 2013;28:1379–532. [Google Scholar]
- 16.Hoffman TM, Wernovsky G, Atz AM, Bailey JM, Akbary A, Kocsis JF, et al. Prophylactic intravenous use of milrinone after cardiac operation in pediatrics (PRIMACORP) study. Prophylactic intravenous use of milrinone after cardiac operation in pediatrics. Am Heart J. 2002;143:15–21. doi: 10.1067/mhj.2002.120305. [DOI] [PubMed] [Google Scholar]
- 17.Sethi SK, Goyal D, Yadav DK, Shukla U, Kajala PL, Gupta VK, et al. Predictors of acute kidney injury post-cardiopulmonary bypass in children. Clin Exp Nephrol. 2011;15:529–34. doi: 10.1007/s10157-011-0440-2. [DOI] [PubMed] [Google Scholar]


