Abstract
Introduction:
To determine the incidence and mortality of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) in a cohort of patients with risk factors admitted to the Surgical Intensive Care Unit (SICU).
Materials and Methods:
A prospective observational inception cohort study with no intervention was conducted over 12 months. All patients with at least one known risk factor for ALI/ARDS admitted to the SICU were included in the study. The APACHE II severity of disease classification system scoring was performed within 1 h of admission. The ventilatory parameters and chest radiographs were recorded every 24 h. The P/F ratio, PEEP and Lung Injury Score were calculated each day until the day of discharge from the Intensive Care Unit or for the first 7 days of admission, whichever was shorter.
Results:
The incidence of ARDS among those who were mechanically ventilated was 11.4%. Sepsis was the most common (34.6%) etiology. Among those with risk factors, the incidence of ARDS was 30% and that of ALI was 32.7%. The mortality in those with ARDS was 41.8%. Those who develop ARDS had higher APACHE II scores, lower pH and higher PaCO2 at admission compared with those who developed ALI or no lung injury.
Conclusion:
The incidence and mortality of ARDS was similar to other studies. Identifying those with risk factors for ARDS or mortality will enable appropriate interventional measures.
Keywords: Acute lung injury, acute respiratory distress syndrome, incidence, mortality
Introduction
Acute respiratory distress syndrome (ARDS) and acute lung injury (ALI) are life-threatening complications causing respiratory insufficiency in the critically ill patient. ALI is the term used to describe the pulmonary response to a broad range of injuries occurring either directly to the lung or as a consequence of injury or inflammation at other sites of the body. ARDS represents the more severe end of the spectrum of this condition. The syndromes are associated with a high mortality rate varying between 30% and 60%.
The definitions of ARDS have varied enormously from one clinical series to another. In most studies, the syndromes are defined either by the Lung Injury Score (LIS) proposed by Murray et al.[1] or by the North American European Consensus Conference (NAECC)[2] criteria. Although the NAECC criteria was considered the gold standard in the diagnosis of ALI/ARDS, recently, the Berlin definition has come into use.[3] It has been observed that both the LIS and the NAECC scoring systems identified similar populations when applied to patients with clearly defined risk factors.
Lung injury may be caused by direct or indirect mechanisms. Several prospective studies using the NAECC definition have identified risk factors that are independent predictors of mortality. Direct risk factors include sepsis, polytrauma, chest trauma, massive transfusions, pancreatitis, aspiration, pulmonary contusion, pneumonia and near drowning.[2]
The aims of this study were to determine the incidence of ALI/ARDS in a cohort of patients with risk factors admitted to the Surgical Intensive Care Unit (SICU) of a tertiary care hospital, to assess mortality in patients with ALI/ARDS, to evaluate correlation between the NAECC definition and LIS and to compare data with the literature. Surinder et al. have described a retrospective series on the etiology and outcomes of pulmonary and extrapulmonary ALI/ARDS in North India.[4,5] In a similar study from North India, Gupta et al. have identified that sepsis and malaria were important risk factors for the development of ARDS and APACHE III score >57 or SAPS II score >39, which were associated with increased risk of mortality.[5] To the best of our knowledge, there are very few data on the incidence of ALI/ARDS in the Indian subcontinent.
Materials and Methods
This study was a prospective observational inception cohort study with no intervention. It was approved by the institutional review board and an ethics committee, and the duration of the study was 12 months. All patients aged >14 years and admitted to the SICU of a large tertiary care hospital with at least one of the risk factors[2] for developing ALI/ARDs were included in the study. The risk factors include sepsis, severe pancreatitis, massive transfusion, aspiration, abdominal and chest trauma, major abdominal and abdominothoracic surgery or polytrauma. Patients were excluded from the study if there was any clinical evidence of cardiogenic pulmonary edema with left atrial enlargement, diagnosis of brain death made within 24 h of admission to the Intensive Care Unit (ICU) or history of restrictive lung disease or cardiopulmonary disease at admission.
At admission, the clinical and surgical status, blood chemistry, blood gases and chest radiograph were documented for all patients who fulfilled the inclusion criteria. The measure of the severity of illness at admission to the ICU was done by the APACHE II (acute physiological and chronic health evaluation II) severity of disease classification system scoring within 1 h of admission to the ICU. The ventilatory parameters and the chest radiographs were recorded every 24 h thereafter using the lowest daily values for all variables of interest. Day 1 is the time interval from admission to SICU to 6:00 am the next day and, thereafter, the remaining days are calendar days from 6:00 am to 6:00 am on the next day. The P/F ratio, PEEP and LIS were calculated each day until the day of discharge from the ICU or for the first 7 days of admission to the surgical ICU if the duration of stay in the ICU was more than 7 days.
The infiltrates on chest X-ray were defined as opacities that cannot be explained completely by pleural effusions, mass, body habitus or collapse. Upper zone redistribution and pulmonary vascular congestion were not considered infiltrates. Daily chest radiographs were analyzed using the Picture Archival and Communications Systems (PACS) and were interpreted by two critical care physicians who were blinded to the clinical status of the patient and the presence of criteria for ARDS. All analyses were conducted using SPSS 11.5 (Statistical Package for Social Sciences). Continuous data were presented as mean ± SD. Mean values were compared by Student's t test. Pearson's chi square test was used for comparing the proportions. Logistic regression analysis was used to study the unadjusted and adjusted effect of risk factors on ARDS. A P value of < 0.05 was considered statistically significant.
Results
Incidence of ALI/ARDS
During the 12-month study period, 902 patients were admitted to the SICU among whom 223 had at least one risk factor for developing ARDS/ALI and 39 patients had multiple risk factors. Of these, 67 (7.4%) patients met the NAECC criteria for ARDS and 73 (8%) patients met the NAECC criteria for ALI. Five hundred and eighty-seven patients were mechanically ventilated during this period. The incidence of ARDS among them was 11.4%.
The incidence of ARDS by the NAECC criteria in patients with risk factors is 30% and that of ALI is 32.7%, and the in-hospital mortality for ARDS and ALI by the NAECC criteria was 41.8% and 11%, respectively [Table 1a and b]. Those patients with ALI, but not fulfilling the ARDS criteria, had a significantly lower mortality rate. The incidence of ARDS by Murray's criteria in patients with risk factors is 13% and that of severe lung injury is 35.7% [Table 2a]. However, using the Murray's criteria, the mortality associated with ARDS is about 60% and that with severe lung injury is 22% [Table 2b].
Table 1a.
Incidence of ALI/ARDS in patients with risk factors (by the NAECC criteria)

Table 1b.
Mortality in patients with ALI/ARDS (by the NAECC criteria)

Table 2a.
Incidence of ALI/ARDS in patients with risk factors (by Murray's criteria)
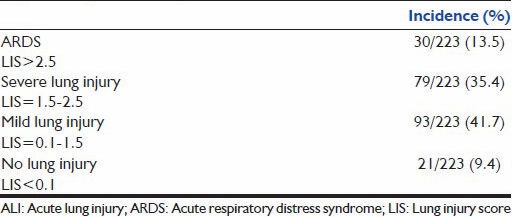
Table 2b.
Mortality in patients with ALI/ARDS (by Murray's criteria)

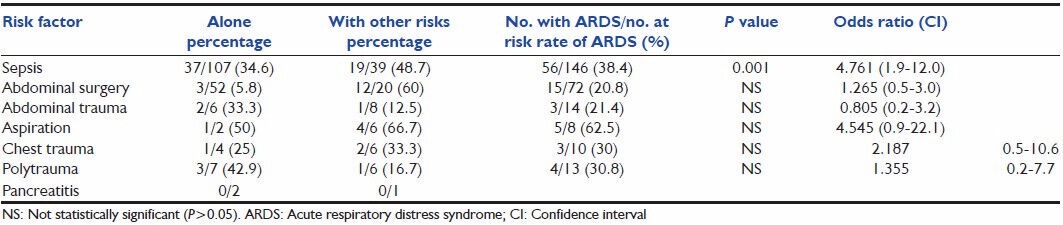
The predominant etiology of ARDS was sepsis (n = 56), primarily of an extrapulmonary source. In those who had sepsis alone, the incidence of those who developed ARDS was 34.6%. However, when sepsis was superimposed on any other risk factor, the incidence was higher (48.7%). Patients who underwent major thoraco-abdominal surgeries combined with sepsis had a very high incidence of 60%. Patients with gastric content aspiration had the highest incidence of ARDS (62.5%). The incidence of ARDS in those with polytrauma and chest trauma was about 30%.
Sepsis is an important risk factor for developing ARDS, with a P < 0.05. Major abdominal surgery when combined with sepsis is also a significant risk factor (P < 0.05), although by itself not significant. The other presumed risk factors do not significantly contribute to the development of ARDS. However, the numbers in these categories are less.
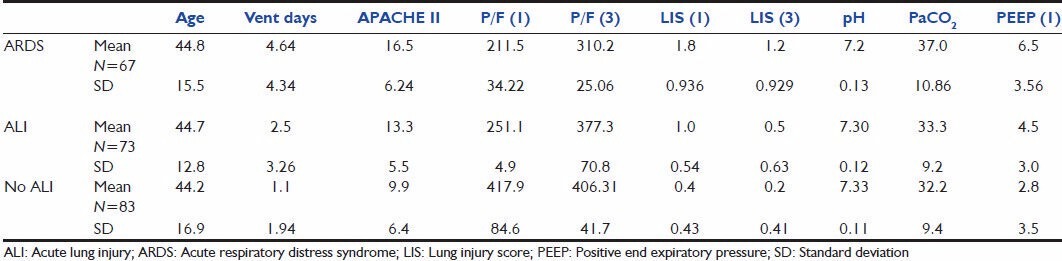
On comparison among patients with ARDS, ALI and no lung injury [Table 4], there was no difference in the mean age. In those who developed ARDS, the APACHE 2 score at admission was higher and the pH was lower compared with those with ALI/no lung injury. The mean duration of ventilated days in the ARDS group was 4 as against 2.5 in those with ALI and 1.1 in those with no lung injury. The P/F ratio was significantly low on the day of admission in those with ARDS, and continued to be so on the third day. However, the mean P/F ratio was higher than 200, suggesting that all patients who developed ARDS did not have significant lung injury on the first day. The LIS on the first day was higher in those with ARDS and, although it declined on Day 3, it still continued to remain higher than those with ALI or no lung injury. Tests of significance performed showed a significant difference among all the observed parameters among those who developed ARDS, ALI and no lung injury, except age.
Table 4.
Clinical characteristics and physiological variables of patients with ARDS, ALI and no lung injury
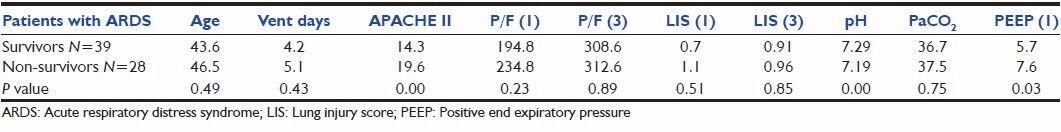
Clinical characteristics of all the patients with ARDS and the comparison of survivors and non-survivors showed [Table 5] that both survivors and non-survivors exhibited almost similar age distribution. However, non-survivors showed a greater incidence of severe underlying disease as evidenced by significantly higher APACHE II scores and lower pH. Tests of significance performed showed no significant differences between survivors and non-survivors in oxygenation variables and LIS.
Table 5.
Clinical characteristics and physiological variables of patients with ARDS: Comparison between survivors and non-survivors
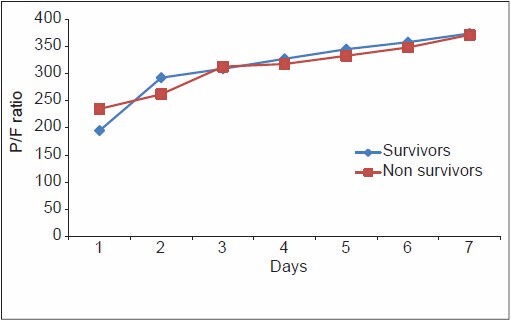
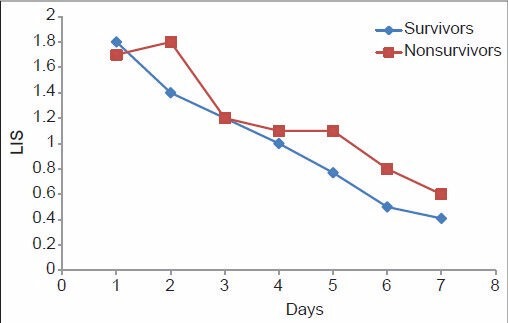
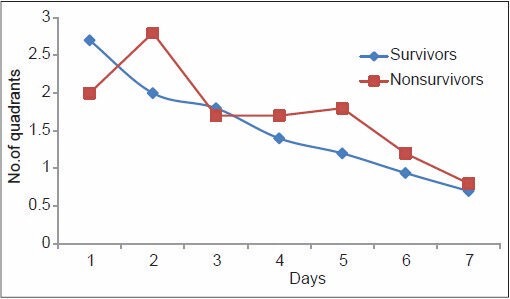
From Table 6, it can be noted that although the PaO2/FiO2 ratio of the non-survivors of ARDS was higher compared with the survivors on Day one, the trend changed on Day 4, with non-survivors showing a consistently lower PaO2/FiO2 ratio thereafter. The mean LIS of the non-survivors of ARDS was higher on Day 1, and continued to be so until death. The radiological injury did not show any significant difference until Day 4, from when the non-survivor group showed a consistently higher injury. The mean PEEP in the non-survivor group remained consistently high since the onset of the inciting insult, which implies that those who required higher PEEP to maintain oxygenation were at higher risk of death due to ARDS. This is demonstrated in Figures 1–4. The lung injury, as evidenced by low P/F ratio, higher PEEP and more number of radiological quadrants involved, are more pronounced on Day 4 than on the day of onset of illness. This may be because of the large number of patients with extrapulmonary causes of ARDS.
Table 6.
Pattern of P/F ratio, PEEP, LIS and radiological injury among survivors and non-survivors of ARDS
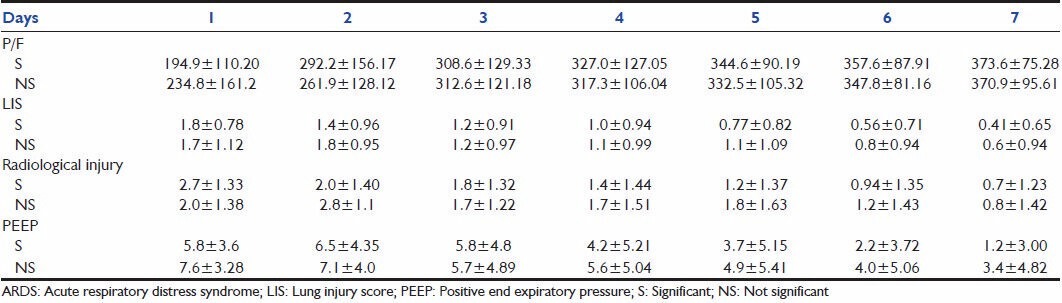
Figure 1.
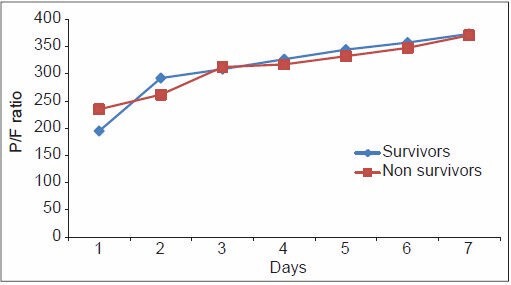
Pattern of P/F ratio among survivors and non-survivors of acute respiratory distress syndrome
Figure 4.
Pattern of PEEP among survivors and non-survivors of acute respiratory distress syndrome
Figure 2.
Pattern of radiological injury among survivors and non-survivors of acute respiratory distress syndrome
Figure 3.
Pattern of lung injury score among survivors and non-survivors of acute respiratory distress syndrome
Discussion
In this study, patients with factors associated with increased risk of ALI/ARDS were followed-up prospectively to determine the incidence of ALI/ARDS. The factors studied were selected from other studies and our pilot study to select the population at high risk for developing ALI/ARDS.
Incidence of ALI/ARDS
During the 12-month study period, 902 patients were admitted to the SICU. Sixty-seven (7.4%) patients met the NAECC criteria for ARDS and 73 (8%) patients met the criteria for ALI. Five hundred and eighty-seven patients were mechanically ventilated during this period, and the incidence of ARDS among them was 11.4%. The incidence of ARDS among those with risk factors for developing ARDS was 30% and that of ALI was 32.7%.
The incidence of ARDS is similar to the 6.9% reported in a multiple institution study in France[6] and 7.7% reported from four ICUs in Argentina.[7] A lower incidence of 2-3% has been reported in retrospective studies.[8] ARDS accounted for 11.4% of ventilated patients in our study. Some have reported higher incidences [Table 7] among those who are ventilated, 19.7% in a study from Argentina.[9] Luhr et al.[10] and Roupie et al.[7] have reported an incidence of 18% and 15.8%, respectively. Villar et al.,[11] in the recent ALIEN study in Spain, have reported an incidence of 7.2%, as did Sigurdsson et al. in Iceland.[12] Others have reported lower incidences, such as the 3.6% of Lewandowski et al.[12] in Berlin. Our high incidence might be due to the fact that our ICU acts as a referral center.
Table 7.
Comparative incidence and mortality in ARDS
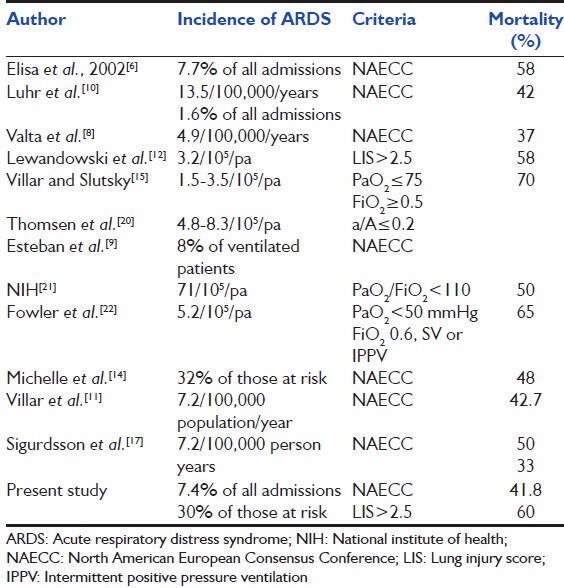
In our study, the incidence of ALI/ARDS using the NAECC criteria in those with risk factors is 32.7% and 30%, respectively. Leonard et al.[13] have reported an incidence of 25% in a similar study using Murray's criteria. However, the risk factor definition is different in that we have included major abdominal/thoracoabdominal surgery as a risk factor, which explains the higher incidence of ARDS in our study. Using LIS more than 2.5 as a criterion for defining ARDS, the incidence is 13.5%. It may be noted that using the above-mentioned criterion to define ARDS picks up only the severe end of the spectrum and is thereby less sensitive as a criterion for defining ARDS. ALI defined by LIS between 0.1 and 2.5 is more sensitive for diagnosing ALI compared with the NAECC criteria. In a similar study, Michelle et al.[14] have reported an incidence of 32% of ARDS in those at risk using the NAECC criteria.
In our study, the mortality in those with well-established ARDS is 41.8%. This figure is similar to the results of the recent studies describing and improving the outcome in ARDS with mortalities between 30% and 46%. Luhr et al.,[10] Valta et al.[8] and Villar et al.[15] have reported 42%, 37% and 43% rates, respectively. Michelle et al.[14] have reported a mortality of 46%. Elisa et al.[6] have reported 58% mortality in those with ARDS diagnosed by the NAECC criteria, which is much higher compared with that of the recent studies. Doyle et al.[16] and Lewandowski et al.[12] reported mortalities of 57% and 58.8%, defining ARDS as LIS >2.5, which is comparable to the 60% in our study using the same criterion. In recent studies, Villar et al.[11] from the ALIEN study had an ICU and hospital mortality of 42.7% and 47.8%, respectively, and Sigurdsson et al.[17] reported a hospital mortality of 33%.
Risk factors for ARDS were similar to previous studies in which sepsis was identified as the main cause of ARDS. Intra-abdominal sepsis was the most common cause. We did not have many cases of pneumonia as the primary risk factor because the study was performed in the SICU. Table 3 shows the proportions of patients who developed ARDS with each precipitating condition. Patients with sepsis are more likely to develop ARDS than those with other risk factors (OR 4.76; CI 1.9-12). Villar et al.[11] and Sigurdsson et al.[17] had similar findings of sepsis and pneumonia as the most common causes of ARDS. Sepsis also significant increases the risk of ARDS in patients who had undergone major abdominal surgery (60% vs 5.8%). The incidence of ARDS in those who had polytrauma or chest trauma is 30% each, comparable to the 23% and 29.7% reported by Hudson et al.,[13] Gastric content aspiration is also a significant risk factor, with the highest incidence of 62.5% in those at risk (OR 4.545; CI 0.9-22.1). This is unlike the 26.3% reported by Hudson et al.[13] Gastric content aspiration accounted for 7.5% of all the cases of ARDS, which is marginally lower compared with the 9-19% reported earlier by Zilberberg et al.[18] and Valta et al.[8] Only 6% of the ARDS was due to trauma, as the total number of trauma cases was small. This may be explained by the fact that most of our polytrauma patients do not have access to emergency ambulance services and are lost at the site of the accident itself.
Table 3.
Incidence of ARDS by clinical risk factors and etiology of ARDS in a cohort
With reference to clinical characteristics and physiological data in patients with ALI/ARDS and no lung injury, it is seen that the patients who develop ARDS were sicker with higher APACHE II scores, lower pH and higher PaCO2 at admission compared with those who developed ALI or no lung injury. Sigurdsson et al.[17] similarly found that higher age and APACHE II score increased the odds of hospital mortality. We observed that the P/F ratio and LIS on Days 1 and 3 were higher in the ARDS group compared with the other groups.
In those who developed ARDS, there are conflicting data about the prognostic role of oxygenation variables among the survivors and the non-survivors. Doyle et al.[16] compared patients by PaO2/FiO2 ratio and did not reveal any significant difference between those patients with PaO2/FiO2 ratio <150 and that between 150 and 299, and there was no difference in mortality between them. Bone et al.[19] have found that the PaO2/FiO2 at ARDS onset was similar in survivors and non-survivors. But, 24 h later, it was significantly higher in survivors, which is similar to our observation.
The LIS did not predict mortality at 24, 48 or 72 h, as reported by Doyle et al.[16] and Zilberberg et al.[18] In our study, it was found that the LIS on Day 2 was higher than that on Day 1 in those who succumbed to ARDS, but was not useful in predicting death in these patients. Among the observed respiratory variables such as the P/F ratio, LIS and radiological scoring, none significantly predicted mortality in those with ARDS. However, higher APACHE II scores, lower pH and higher PaCO2 at admission were observed in those who developed ARDS. This emphasizes the importance of non-pulmonary factors in determining the outcome in patients with ALI. This is consistent with most of the other studies in patients with ARDS that have reported that survival was less often related to initial lung function and more frequently with sepsis[23] and the development of multiorgan system failure.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138:720–3. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 2.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–24. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 3.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal R, Aggarwal AN, Gupta D, Behera D, Jindal SK. Etiology and Outcomes of Pulmonary and Extrapulmonary Acute Lung Injury/ARDS in a Respiratory ICU in North India. Chest. 2006;130:724–9. doi: 10.1378/chest.130.3.724. [DOI] [PubMed] [Google Scholar]
- 5.Gupta D, Ramanathan RP, Aggarwal AN, Jindal SK. Assessment of factors predicting outcome of acute respiratory distress syndrome in North India. Respirology. 2001;6:125–30. doi: 10.1046/j.1440-1843.2001.00324.x. [DOI] [PubMed] [Google Scholar]
- 6.Estenssoro EE, Dubin AA, Laffaire EE, Canales HH, Sáenz GG, Moseinco MM, et al. Incidence, clinical course, and outcome in 217 patients with acute respiratory distress syndrome. Crit Care Med. 2002;30:2450–6. doi: 10.1097/00003246-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Roupie E, Lepage E, Wysocki M, Fagon JY, Chastre J, Dreyfuss D, et al. Prevalence, etiologies and outcome of the acute respiratory distress syndrome among hypoxemic ventilated patients SRLF Collaborative Group on Mechanical Ventilation. Société de Réanimation de Langue Française. Intensive Care Med. 1999;25:920–9. doi: 10.1007/s001340050983. [DOI] [PubMed] [Google Scholar]
- 8.Valta P, Uusaro A, Nunes S, Ruokonen E, Takala J. Acute respiratory distress syndrome: Frequency, clinical course, and costs of care. Crit Care Med. 1999;27:2367–74. doi: 10.1097/00003246-199911000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Esteban A, Anzueto A, Alía I, Gordo F, Apezteguía C, Pálizas F, et al. How is mechanical ventilation employed in the intensive care unit. An international utilization review? Am J Respir Crit Care Med. 2000;161:1450–8. doi: 10.1164/ajrccm.161.5.9902018. [DOI] [PubMed] [Google Scholar]
- 10.Luhr OR, Antonsen K, Karlsson M, Aardal S, Thorsteinsson A, Frostell CG, et al. Incidence and mortality after acute respiratory failure and acute respiratory distress syndrome in Sweden, Denmark, and Iceland. The ARF Study Group. Am J Respir Crit Care Med. 1999;159:1849–61. doi: 10.1164/ajrccm.159.6.9808136. [DOI] [PubMed] [Google Scholar]
- 11.Villar JJ, Blanco JJ, Añón JM, Santos-Bouza AA, Blanch LL, Ambrós AA, et al. The ALIEN study: Incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med. 2011;37:1932–41. doi: 10.1007/s00134-011-2380-4. [DOI] [PubMed] [Google Scholar]
- 12.Lewandowski K, Metz J, Deutschmann C, Preiss H, Kuhlen R, Artigas A, et al. Incidence, severity, and mortality of acute respiratory failure in Berlin, Germany. Am J Respir Crit Care Med. 1995;151:1121–5. doi: 10.1164/ajrccm.151.4.7697241. [DOI] [PubMed] [Google Scholar]
- 13.Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151:293–301. doi: 10.1164/ajrccm.151.2.7842182. [DOI] [PubMed] [Google Scholar]
- 14.Meade MO, Cook RJ, Guyatt GH, Groll R, Kachura JR, Bedard M, et al. Interobserver Variation in Interpreting Chest Radiographs for the Diagnosis of Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2000;161:85–90. doi: 10.1164/ajrccm.161.1.9809003. [DOI] [PubMed] [Google Scholar]
- 15.Villar J, Slutsky AS. The incidence of the adult respiratory distress syndrome. Am Rev Respir Dis. 1989;140:814–6. doi: 10.1164/ajrccm/140.3.814. [DOI] [PubMed] [Google Scholar]
- 16.Doyle RL, Szaflarski N, Modin GW, Wiener-Kronish JP, Matthay MA. Identification of patients with acute lung injury. Predictors of mortality. Am J Respir Crit Care Med. 1995;152:1818–24. doi: 10.1164/ajrccm.152.6.8520742. [DOI] [PubMed] [Google Scholar]
- 17.Sigurdsson MI, Sigvaldason K, Gunnarsson TS, Moller A, Sigurdsson GH. Acute respiratory distress syndrome: Nationwide changes in incidence, treatment and mortality over 23 years. Acta Anaesthesiol Scand. 2012;57:37–45. doi: 10.1111/aas.12001. [DOI] [PubMed] [Google Scholar]
- 18.Zilberberg MD, Epstein SK. Acute lung injury in the medical ICU: Comorbid conditions, age, etiology, and hospital outcome. Am J Respir Crit Care Med. 1998;157:1159–64. doi: 10.1164/ajrccm.157.4.9704088. [DOI] [PubMed] [Google Scholar]
- 19.Bone RC, Fisher CJ, Clemmer TP, Slotman GJ, Metz CA, Balk RA. A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med. 1987;317:653–8. doi: 10.1056/NEJM198709103171101. [DOI] [PubMed] [Google Scholar]
- 20.Thomsen GE, Morris AH. Incidence of the adult respiratory distress syndrome in the state of Utah. Am J Respir Crit Care Med. 1995;152:965–71. doi: 10.1164/ajrccm.152.3.7663811. [DOI] [PubMed] [Google Scholar]
- 21.Respiratory diseases; Task Force report on problems, research approaches and needs. Bethesda (MD): The Association; 1972. Task Force on Research in Respiratory Diseases, National Heart and Lung Institute. Lung Program; p. 201. [Google Scholar]
- 22.Fowler AA, Hamman RF, Good JT, Benson KN, Baird M, Eberle DJ, et al. Adult respiratory distress syndrome: Risk with common predispositions. Ann Intern Med. 1983;98:593–7. doi: 10.7326/0003-4819-98-5-593. [DOI] [PubMed] [Google Scholar]
- 23.Siegel M. Acute respiratory distress syndrome: Prognosis and outcome in adults. Waltham: Woters Kluwer Health; 2014. [Last accessed on 2014 Jun 3]. Available from: http://www.uptodate.com/contents/acute-respiratory-distresssyndrome-prognosis-and-outcomes-in-adults?source=search_resultandsearch=ARDSandselectedTitle=6 ~ 150 . [Google Scholar]


