Abstract
The role of tumor stroma in progression to malignancy has become the subject of intense experimental and clinical interest. The stromal compartment of organs is composed of all the non-epithelial cell types and maintains the proper architecture and nutrient levels required for epithelial and, ultimately, organ function. The composition of the reactive stroma surrounding tumors is vastly different from normal stromal tissue. Stromal phenotype can be correlated with, and predictive of, disease recurrence. In addition, the stroma is now seen as a legitimate target for therapeutic intervention. Although much has been learned about the role of the stromal compartment in development and disease in recent years, a number of key questions remain. Here we review how some of these questions are beginning to be addressed using new models of stromal-epithelial interaction.
Background
Each bodily organ is broken down into distinct cell groups called tissues. Development is a process of self-assembly and growth in which cells comprising rudimentary tissues and structures exchange information, resulting in their rearrangement into a specific architecture and achievement of specific function (Grobstein, 1967). The breakdown of tissue architecture leads to loss of mutual regulation of growth and differentiation, malfunction, and disease. It is therefore important to understand how each cell type in the tumor microenvironment contributes to the disease process and to identify the molecular mechanisms and interactions responsible.
Most organs are compartmentalized into epithelial and mesenchymal tissues. The gross morphogenic influence of each tissue compartment began to be understood when it was discovered that epithelial and mesenchymal tissues could be separated from the host organ and recombined with tissues from different organs, which resulted in a directive (instructive) or permissive reprogramming of either tissue (Saxén, 1977). It was these historic discoveries that led to questions on the role of mesenchyme in disease.
The development of most organs occurs through epithelial-mesenchymal cross-talk (Thesleff et al., 1995). In adult organs, the mesenchymal compartment becomes known as stroma, which is composed of multiple cell types responsible, among many other roles, for matrix production and angiogenesis to support epithelial differentiation and function. This balanced, interdependent interaction between epithelium and stroma is called homeostasis. During the onset of disease, the cross-talk between these tissues and the influence of infiltrating immune and inflammatory cells function in maintaining homeostasis. In a disease like cancer, where most tumors are thought to initiate as a result of an epithelial mutation, it is believed that the stromal compartment (called the ‘tumor microenvironment’ in cancerous organs) initially reacts to inhibit tumor progression by maintaining differentiation and architecture, similar in some respects to its response to wounding or infection (Pierce et al., 1978). This initially protective response is subverted under the chronic wounding conditions in cancer so that in later-stage disease, a vicious cycle ensues where the cell types that compose the tumor microenvironment are co-opted to facilitate growth of the epithelial tumor. This incipient, tumor-promoting microenvironment is the subject of intense therapeutic interest as its phenotypic and molecular characterization has been correlated with disease-free recurrence in multiple tumor types (Ayala et al., 2003; Finak et al., 2008; Saadi et al., 2010).
Understanding the basic molecular processes by which tissues inform each other has progressed in parallel with the development of molecular biological techniques over the last 30 years. However, the lack of adequate in vivo models to study the underlying molecular changes associated with morphological breakdown and tumor progression impedes our understanding of the precise role of the tumor microenvironment, which is necessary for therapeutic targeting. Our laboratory has historically been interested in stromal-epithelial interactions in prostatic disease. The prostate is the second leading cause of cancer-related deaths in American men, and benign hyperplasia of the prostate, which is also a product of altered organ homeostasis and paracrine signaling, is a major cause of morbidity in aging men. The tumor microenvironment can either inhibit or enhance disease progression depending upon specific circumstances (Chung, 1991; Olumi et al., 1999). The expectation is that a clearer understanding of the tumor microenvironment will result in the identification of targets for therapeutic intervention. Accordingly, new models of tumor-stroma interactions are being developed by our group and others, which were the subject of our previous review (Strand et al., 2010) and will be covered here summarily.
Stromal-Epithelial Interactions: A History
Grobstein’s seminal work in the 1950s on the role of mesenchyme in the development of the submandibular gland laid the foundation for a proper understanding of organogenesis (Grobstein, 1953). Soon it was discovered by Le Douarin that the endodermal germ layer was the source of inductive mesenchyme in multiple digestive tissues and that this tissue differentiated into organ-specific connective tissue at adulthood (Le Douarin et al., 1968). Work by Thesleff (Thesleff and Hurmerinta, 1981), Kollar (1970), and Cunha (1972) in tooth, skin, and urogenital development demonstrated that the interactions between epithelia and mesenchyme were reciprocal and sequential, eventually leading to the hypothesis that a chronological shift in the instructive potential of mesenchyme occurred during organogenesis (Yasugi, 1979).
Once the gross inductive properties of mesenchyme were realized, work began on dissecting the identity and function of each cell type comprising adult organ mesenchyme (stroma). The development of antibodies against proteins like smooth muscle alpha actin (Skalli et al., 1986) initiated the process of detailing stromal identity [and function, since actin is a cytoskeletal protein involved in contraction (Gabbiani, 2003)]. The stark differences in the composition of organ stroma are readily apparent when comparing breast with prostate (both are glandular organs) and are a result of evolved differences in function. Breast stroma is predominantly composed of adipose tissue to support milk production. Milk is excreted as a result of contraction of myoepithelial cells following stimulation by oxytocin. Prostate, in contrast, is predominantly composed of smooth muscle and fibroblasts and has basal epithelial cells with no muscular component. To achieve ejaculation of seminal fluid nervous stimulation of the smooth muscle of the prostate induces coordinated contractions of the organ. As cancer develops within the breast and prostate, a stromal reaction is observed, resulting in a modulation of indigenous and recruited (bone marrow, immune, inflammatory) cell types. Chronic reactive stroma produces growth factors and matrix proteins similar to those found in acute wound repair thereby altering (among other properties) the blood vessel and matrix density of the stromal compartment, which can contribute to malignant progression (Paszek et al., 2005).
The Schors were the first to demonstrate that the stroma surrounding tumors in adults was phenotypically similar to fetal stroma and that these changes were associated with disease progression (Schor and Schor, 1987; Schor et al., 1988) with further phenotypic characterizations of tumor stroma in different organs to follow (Ronnov-Jessen et al., 1996; Tuxhorn et al., 2002). Molecular signatures of the stroma of normal versus cancerous breast and prostate are revealing detailed insights into the identity of the stroma surrounding cancers (Dakhova et al., 2009; Ma et al., 2009); however, the experimental validation of the functional consequence (effect on morphology) of these molecular changes has been impeded by the lack of appropriate in vivo models of stromal-epithelial interactions. Our group has made some progress on this front by demonstrating the ability of patient-derived tumor stroma (called carcinoma-associated fibroblasts, or CAFs) to induce malignancy in benign human prostate epithelia using tissue recombination xenografting (Hayward et al., 1998; Hayward et al., 2001). The best model for a systemic picture of the morphological effects of changes in gene expression is transgenic animals; however, significant differences exist in stromal composition of murine versus human organs (notably, in prostate, differences in human versus murine stromal density are pronounced -- see Figure 1). In addition, because of the commonality of stromal sub-types in various organs, the search for organ-specific stromal promoters is still underway (Jackson et al., 2008). It is therefore important to understand the various tools available for the productive study of stromal-epithelial interactions and also to understand where these models fail to answer lingering questions.
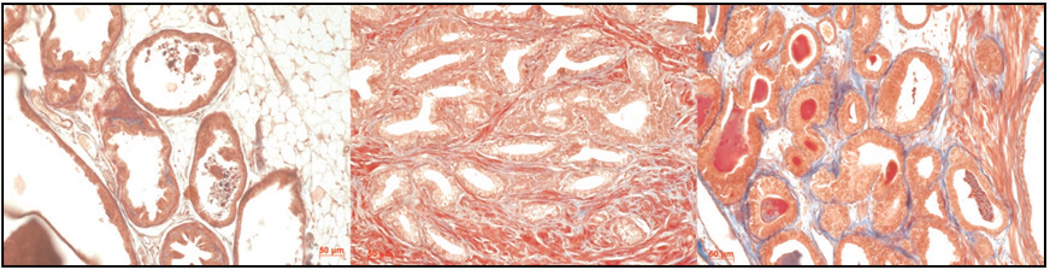
Figure 1.
Trichrome staining of mouse prostate (left), human prostate (center), and tissue recombination xenografting of human prostate epithelia and inductive fetal rat urogenital mesenchyme under the kidney capsule (right). The balance of smooth muscle (red fibers) and collagen (blue) in human prostate stroma is more closely modeled using human cells in tissue recombination xenografting than in mouse transgenic models.
Models of Stromal-Epithelial Interactions
A number of different models have been used to understand stromal-epithelial interactions. Every experimental model has strengths and weaknesses, and each should be acknowledged for its own contribution. Given the surging clinical relevance of the role of the stromal compartment in disease progression, an overview of in vitro, in vivo, and in silico models will be briefly covered.
In Vitro
In vitro studies of stromal-epithelial interactions can be accomplished using either primary or immortalized cell lines. Primary cells are separated from freshly dissected tissue and can be grown in culture for several passages before entering senescence. To overcome this replicative limitation, cells can be immortalized virally or spontaneously, among other methods. Both of these cell sources are valuable, but immortalized cell lines can easily be further manipulated to study the roles of ectopically over-expressed or knocked down genes. This applies to both epithelial and stromal cell lines. Of note, although previously existing for breast studies (Dawson et al., 1996), there have been no normal human prostate epithelial cell lines for the functional study of glandular remodeling. To meet this need, we have recently generated two spontaneously immortalized prostate epithelial cell lines, which recapitulate normal glandular morphogenesis when recombined with inductive mesenchyme or when grown in three-dimensional (3D) Matrigel cultures (Jiang et al., 2009). The value of an isogenic series of cell lines for dissecting the molecular mechanisms of glandular stability in cancer has been extensively demonstrated in breast studies (Miller et al., 2000). Our new cell lines therefore represent a significant addition to previous cell lines that we have generated (Hayward et al., 1995) for the study of prostate cancer progression and are currently being used to dissect the functional and morphological role of specific genes shown to be correlated with prostate cancer progression.
A few in vitro techniques have been shown to be very valuable for studying interactions between epithelia and stroma. Of course, individually, cell lines can be monitored for a number of parameters including proliferation, migration, and survival. However, co-culture is a technique where either primary or immortalized cells are mixed in a dish under various conditions. These can be admixed in the same well to study the effects of direct physical interaction or, alternatively, the cells can be placed in separate chambers that share the same conditioned medium to study the effects of paracrine signaling. This is a valuable reductionist model to study very specific situations, but is highly artificial since these cells are grown on 2D plastic with defined growth medium and oxygen.
Alternatively, cells can be grown in 3D conditions by adding Matrigel, a matrix that allows some normal cell lines to arrange into their appropriate architecture and undergo differentiation to achieve function. Importantly, it has been shown that matrix attachment is essential for setting up polarity and differentiation (Yamada and Cukierman, 2007) and also for regulating gene expression (Schafer et al., 2009). Recently, technological advances have been made in live cell imaging of the functional effects of co-culturing stroma and epithelia in 3D over time (4D) (Cukierman et al., 2001; Jedeszko et al., 2008). Even in 3D cultures, however, systemic effects are notably absent. Accordingly, results generated in 2D or 3D must be interpreted cautiously. Even so, significant advances have been made by correlating specific genetic changes with morphologic consequence, which have been correlated with disease progression (Bissell and Radisky, 2001).
In Vivo
Transgenic mouse models have provided a wealth of information on the role of gene loss or mutation in various organs. The advantage of an intact immune system, which clearly plays a vital role in impeding tumor progression (Coussens and Werb, 2002), is muted in part by differences in organ structure and immune response in human versus murine species. However, significant advances in the control of transgene expression have facilitated the contextual study of chronological and cell-specific gene regulation. The advent and subsequent refinements of Cre-Lox technology allow for the excision of genes at specific ages and locations thereby mitigating possible complications of confounding developmental phenotypes.
A persistent problem with transgenic models is the lack of promoters to express genes of interest in organ-specific stroma. This is made more difficult by the complexity of stromal cell types and the variation of their percentage within various organs (Jackson et al., 2008). Even so, the use of stromal promoters like FSP-1 to modulate gene expression has led to important insights into the role of the stroma in tumor initiation and progression (Bhowmick et al., 2004). It has also underscored the need for better promoters since problems with leaky or deficient expression patterns persist.
A few refinements to transgenic mouse technology are available for studying stromal-epithelial interactions in vivo. For those animals where the transgene disrupts normal development, tissue rescue is a method where under-developed tissues can be removed and re-grafted into suitable hosts to allow further differentiation, which has been shown useful for studying stromal-epithelial interactions in prostate (Placencio et al., 2008).
Tissue recombination is a technique that has been used for decades to determine the morphological influence of various tissues. This technique has some advantages over transgenic models including abbreviated time courses (usually 1–3 months) and lower costs in addition to ease of manipulation of genes in human cells. Our laboratory has used tissue recombination to determine the effects of tumor stroma on prostate epithelia (Hayward et al., 2001) and mutated epithelia on stroma (Ao et al., 2006; Ao et al., 2007; He et al., 2007), and now to recapitulate functional remodeling of normal human prostate (Jiang et al., 2009). Besides the lack of functionally normal human prostate epithelial cell lines, a historic deficiency in tissue recombination has been the lack of an inductive stromal cell line capable of eliciting normal prostate epithelial morphogenesis. Currently, inductive fetal urogenital mesenchyme has to be freshly harvested and recombined with epithelial cell lines. Another limitation is the necessity for use of immunocompromised mouse hosts to accommodate grafting of human cell lines. The absence of immune cells in immunocompromised mice can be overcome with the use of syngeneic mouse epithelial and stromal cells, but, of course, loses the advantage of studying human cells.
Another facet of stromal biology that has not been properly modeled is the acknowledged heterogeneity in stroma. Both transgenic and tissue recombination techniques have failed to address the morphologic and possible protumorigenic effects of distinct stromal sub-populations that have been correlated with disease progression.
To meet this need, we have optimized the tissue recombination technique by generating a benign immortalized human stromal cell line that can be mixed in various proportions with inductive fetal mesenchyme without affecting normal glandular morphogenesis (Figure 2). These cells can be tracked with fluorophores in tissue recombination grafts and genetic modifications can be made to study the effect of a distinct stromal cell subpopulation on normal glandular architecture and function as well as on the transformation of our iso-genic series of epithelia with specific genetic mutations (Figure 3). Unpublished results suggest that this will be a useful approach for studying the effect of stromal heterogeneity on tumor initiation and progression in addition to benign disease.
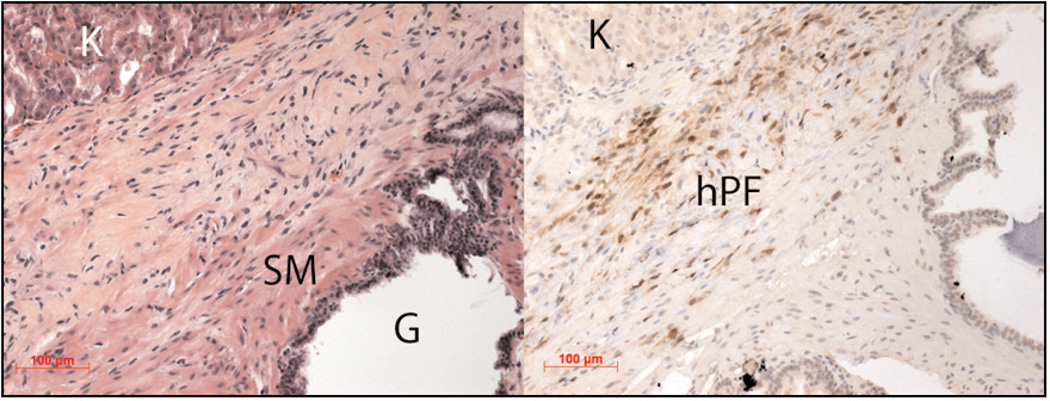
Figure 2.
Modeling stromal heterogeneity in vivo. Left: H&E immunostaining of a three month tissue recombination graft under the kidney (K) capsule of a SCID mouse shows glandular formation of human prostate epithelia (G), smooth muscle differentiation of rat urogenital mesenchyme (SM), and human prostate fibroblasts (hPF) expressing GFP, which can be identified in a serial section using immunohistochemistry (right panel).
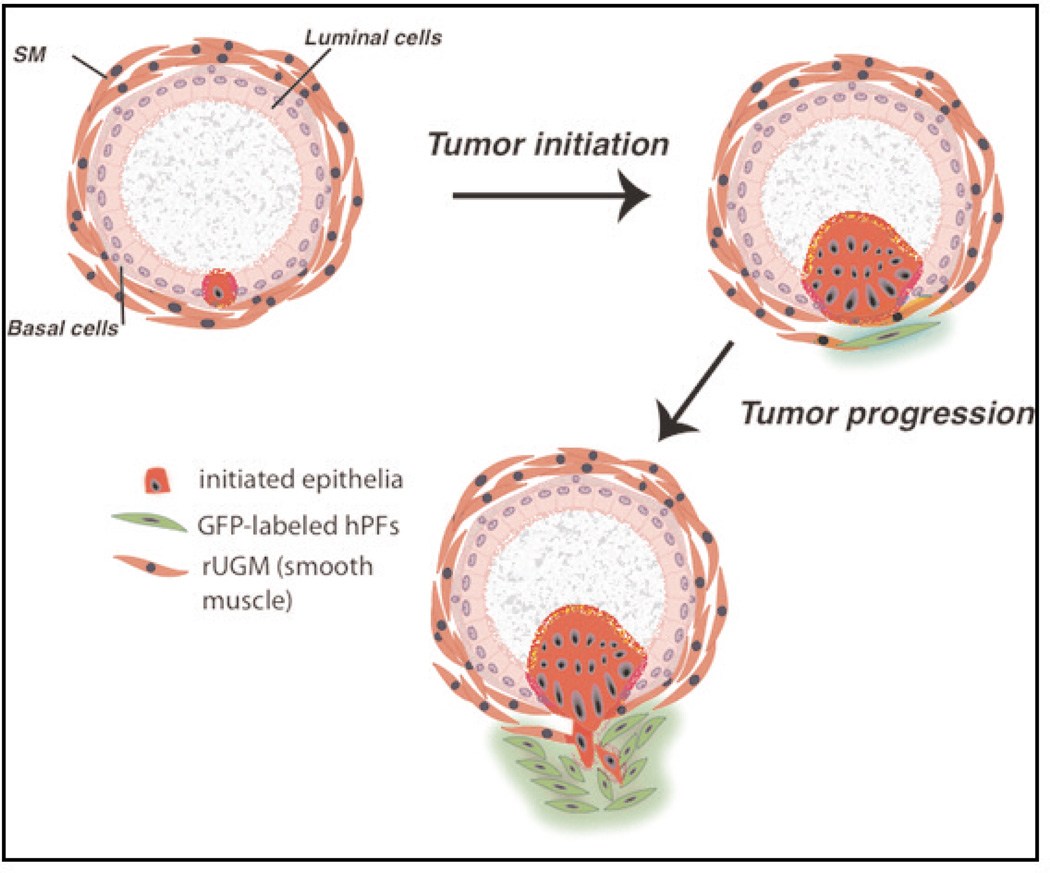
Figure 3.
Schematic sequence of proposed events in prostate cancer progression modeled with normal human prostate epithelia and stromal cell lines and inductive mesenchyme (rUGM).
In Silico
Another method that is being used to explore the relationship between epithelia and stroma is mathematical modeling. Designing a useful model in silico requires proper definition of the complex behavior and relationships of the multiple cell types within a tumor. This methodology has been used in other disciplines such as ecology and economics for some time and is currently being extracted for use in tumor biology (Basanta and Deutsch, 2008). We have begun to apply ecological terminology to cellular behavior and interactions within a tumor microenvironment in order to use the powerful computational tools that have been used by ecologists to describe evolutionary dynamics (Strand et al., 2010).
The advantage of mathematical modeling is the ability to integrate multiple dynamic (space and time) variables in a mechanistic and modular fashion. Changing individual parameters in a dynamical system often leads to the emergence of a behavior that would not have been observable if the component variables were examined in isolation. Once predictions are generated in silico, they are validated in vitro and in vivo using the various models described above.
Although significant differences between tumor and organismal ecosystems are acknowledged, several groups including our own have demonstrated the potential for mathematical models to successfully predict the effects of specific stromal microenvironments on tumor progression (Anderson et al., 2000; Anderson et al., 2006; Anderson et al., 2008; Basanta et al., 2009). Given the current deficiency in the parameterization of the component parts of a given tumor, our goal is to more accurately define the individual cellular behaviors that cause tumors to either remain indolent or progress to malignancy, which will hopefully be a prognostic tool for physicians to use clinically.
Future Directions -- How Do We Move From Bench to Bedside?
As we have described, there are a number of powerful tools available to examine the role of microenvironmental influences on tumor progression. The essential problem in translating work in this area to the clinic is the complex nature of the interactions involved and our currently limited ability to discern key functional nodes in intracellular signaling pathways that can be disabled to allow us to inhibit tumor progression without killing normal tissue. Generally, the changes in intercellular signaling are relatively subtle. Thus, by identifying combinations of pathways that can be partially suppressed or moderately up-regulated, we can apply pressure to tumors with minimal side effects on the overall health of the patient. It seems likely that clinicians will have to become accustomed to measures that do not necessarily set out to destroy a cancer but rather to bring it under control and allow it to be treated like other chronic conditions, coordinate with a long-term treatment regimen and monitoring protocol for the patient and attending physician.
Acknowledgements
The project described was supported by the Department of Defense Prostate Cancer Training Award W81XWH-07-1-0479 to DWS, the National Institute of Diabetes and Digestive and Kidney Diseases Award Number 2R01 DK67049-05 to SWH, and the National Cancer Institute Tumor Microenvironment Network Award Number CA126505 to Lynn Matrisian. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Defense, the National Institute of Diabetes and Digestive and Kidney Diseases, or the National Institutes of Health.
References
- Anderson AR, Rejniak KA, Gerlee P, Quaranta V. Microenvironment driven invasion: a multiscale multimodel investigation. J Math Biol. 2008 Oct 7; doi: 10.1007/s00285-008-0210-2. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AR, Weaver AM, Cummings PT, Quaranta V. Tumor morphology and phenotypic evolution driven by selective pressure from the microenvironment. Cell. 2006;127(5):905–915. doi: 10.1016/j.cell.2006.09.042. [DOI] [PubMed] [Google Scholar]
- Anderson ARA, Chaplain MAJ, Newman EL, Steele RJC, Thompson AM. Mathematical modelling of tumour invasion and metastasis. Comput Math Methods Med. 2000;2(2):129–154. [Google Scholar]
- Ao M, Franco OE, Park D, Raman D, Williams K, Hayward SW. Cross-talk between paracrineacting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium. Cancer Res. 2007;67(9):4244–4253. doi: 10.1158/0008-5472.CAN-06-3946. [DOI] [PubMed] [Google Scholar]
- Ao M, Williams K, Bhowmick NA, Hayward SW. Transforming growth factor-β promotes invasion in tumorigenic but not in nontumorigenic human prostatic epithelial cells. Cancer Res. 2006;66(16):8007–8016. doi: 10.1158/0008-5472.CAN-05-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala G, Tuxhorn JA, Wheeler TM, Frolov A, Scardino PT, Ohori M, Wheeler M, Spitler J, Rowley DR. Reactive stroma as a predictor of biochemical-free recurrence in prostate cancer. Clin Cancer Res. 2003;9(13):4792–4801. [PubMed] [Google Scholar]
- Basanta D, Deutsch A. A game theoretical perspective on the somatic evolution of cancer. In: Bellomo N, Chaplain M, de Angelis E, editors. Selected Topics in Cancer Modeling. Boston, Massachusetts, USA: Birkhauser; 2008. [Google Scholar]
- Basanta D, Strand DW, Lukner RB, Franco OE, Cliffel DE, Ayala GE, Hayward SW, Anderson AR. The role of transforming growth factor-beta-mediated tumor-stroma interactions in prostate cancer progression: an integrative approach. Cancer Res. 2009;69(17):7111–7120. doi: 10.1158/0008-5472.CAN-08-3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303(5659):848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1(1):46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung LW. Fibroblasts are critical determinants in prostatic cancer growth and dissemination. Cancer Metastasis Rev. 1991;10(3):263–274. doi: 10.1007/BF00050797. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294(5547):1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- Cunha GR. Tissue interactions between epithelium and mesenchyme of urogenital and integumental origin. Anat Rec. 1972;172:529–542. doi: 10.1002/ar.1091720307. [DOI] [PubMed] [Google Scholar]
- Dakhova O, Ozen M, Creighton CJ, Li R, Ayala G, Rowley D, Ittmann M. Global gene expression analysis of reactive stroma in prostate cancer. Clin Cancer Res. 2009;15(12):3979–3989. doi: 10.1158/1078-0432.CCR-08-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson PJ, Wolman SR, Tait L, Heppner GH, Miller FR. MCF10AT: a model for the evolution of cancer from prolif-erative breast disease. Am J Pathol. 1996;148(1):313–319. [PMC free article] [PubMed] [Google Scholar]
- Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, Chen H, Omeroglu G, Meterissian S, Omeroglu A, Hallett M, Park M. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14(5):518–527. doi: 10.1038/nm1764. [DOI] [PubMed] [Google Scholar]
- Gabbiani G. The myofibroblast in wound healing and fibro-contractive diseases. J Pathol. 2003;200(4):500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- Grobstein C. Epithelio-mesenchymal specificity in the morphogenesis of mouse submandibular rudiments in vitro. J Exp Zool. 1953;124:383–444. [Google Scholar]
- Grobstein C. Mechanisms of organogenetic tissue interaction. J Natl Cancer Inst Monogr. 1967;26:279–299. [PubMed] [Google Scholar]
- Hayward SW, Dahiya R, Cunha GR, Bartek J, Despande N, Narayan P. Establishment and characterization of an immortalized but non-tumorigenic human prostate epithelial cell line: BPH-1. In Vitro. 1995;31A:14–24. doi: 10.1007/BF02631333. [DOI] [PubMed] [Google Scholar]
- Hayward SW, Haughney PC, Rosen MA, Greulich KM, Weier HU, Dahiya R, Cunha GR. Interactions between adult human prostatic epithelium and rat urogenital sinus mesenchyme in a tissue recombination model. Differentiation. 1998;63(3):131–140. doi: 10.1046/j.1432-0436.1998.6330131.x. [DOI] [PubMed] [Google Scholar]
- Hayward SW, Wang Y, Cao M, Hom YK, Zhang B, Grossfeld GD, Sudilovsky D, Cunha GR. Malignant transformation in a nontumorigenic human prostatic epithelial cell line. Cancer Res. 2001;61(22):8135–8142. [PubMed] [Google Scholar]
- He Y, Franco OE, Jiang M, Williams K, Love HD, Coleman IM, Nelson PS, Hayward SW. Tissue-specific consequences of cyclin D1 overexpression in prostate cancer progression. Cancer Res. 2007;67(17):8188–8197. doi: 10.1158/0008-5472.CAN-07-0418. [DOI] [PubMed] [Google Scholar]
- Jackson RS, Franco OE, 2nd, Bhowmick NA. Gene targeting to the stroma of the prostate and bone. Differentiation. 2008;76(6):606–623. doi: 10.1111/j.1432-0436.2008.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedeszko C, Sameni M, Olive MB, Moin K, Sloane BF. Visualizing protease activity in living cells: from two dimensions to four dimensions. Curr Protoc Cell Biol. 2008;39:4.20.1–4.20.15. doi: 10.1002/0471143030.cb0420s39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Strand DW, Fernandez S, He Y, Yi Y, Birbach A, Qiu Q, Schmid J, Tang DG, Hayward SW. Functional remodeling of benign human prostatic tissues in vivo by spontaneously immortalized progenitor and intermediate cells. Stem Cells. 2010;28(2):344–356. doi: 10.1002/stem.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollar EJ. Induction of hair follicles by embryonic dermal papillae. J Invest Dermatol. 1970;55:374–378. doi: 10.1111/1523-1747.ep12260492. [DOI] [PubMed] [Google Scholar]
- Le Douarin N, Bussonnet C, Chaumont F. Étude des capac-ités de différenciation et du rôle morphogène de l’endoderme pharyngien chez l’embryon d’oiseau.”. Ann Embryol Morphol. 1968;1:29–39. [Google Scholar]
- Ma XJ, Dahiya S, Richardson E, Erlander M, Sgroi DC. Gene expression profiling of the tumor microenvironment during breast cancer progression. Breast Cancer Res. 11R7(1):2009. doi: 10.1186/bcr2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller FR, Santner SJ, Tait L, Dawson PJ. MCF10DCIS.com xenograft model of human comedo ductal carcinoma in situ. J Natl Cancer Inst. 2000;92(14):1185–1186. doi: 10.1093/jnci/92.14.1185a. [DOI] [PubMed] [Google Scholar]
- Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59(19):5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeosta-sis and the malignant phenotype. Cancer Cell. 2005;8(3):241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Pierce G, Shikes R, Fink L. Cancer: A Problem of Developmental Biology. New Jersey, USA: Prentice Hall, Englewood Cliffs; 1978. [Google Scholar]
- Placencio VR, Sharif-Afshar AR, Li X, Huang H, Uwamariya C, Neilson EG, Shen MM, Matusik RJ, Hayward SW, Bhowmick NA. Stromal transforming growth factor-beta signaling mediates prostatic response to androgen ablation by paracrine Wnt activity. Cancer Res. 2008;68(12):4709–4718. doi: 10.1158/0008-5472.CAN-07-6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnov-Jessen L, Petersen OW, Bissell MJ. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev. 1996;76(1):69–125. doi: 10.1152/physrev.1996.76.1.69. [DOI] [PubMed] [Google Scholar]
- Saadi A, Shannon NB, Lao-Sirieix P, O’Donovan M, Walker E, Clemons NJ, Hardwick JS, Zhang C, Das M, Save V, Novelli M, Balkwill F, Fitzgerald RC. Stromal genes discriminate preinvasive from invasive disease, predict outcome, and highlight inflammatory pathways in digestive cancers. Proc Natl Acad Sci U S A. 2010;107(5):2177–2182. doi: 10.1073/pnas.0909797107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxén L. Directive versus permissive induction: a working hypothesis. In: Burger JW, Lash MM, editors. Cell and Tissue Interactions. New York, USA: Raven Press, New York; 1977. pp. 1–10. [PubMed] [Google Scholar]
- Schafer ZT, Grassian AR, Song L, Jiang Z, Gerhart-Hines Z, Irie HY, Gao S, Puigserver P, Brugge JS. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature. 2009;461(7260):109–113. doi: 10.1038/nature08268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schor SL, Schor AM. Hypothesis: Persistent expression of fetal phenotypic charicteristics by fibroblasts is associated with an increased susceptability to neoplastic disease. Expl Cell Biol. 1987;55:11–17. doi: 10.1159/000163389. [DOI] [PubMed] [Google Scholar]
- Schor SL, Schor AM, Rushton G. Fibroblasts from cancer patients display a mixture of both foetal and adult-like phe-notypic characteristics. J Cell Sci. 1988;90:401–407. doi: 10.1242/jcs.90.3.401. [DOI] [PubMed] [Google Scholar]
- Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gillessen D, Gabbiani G. A monoclonal antibody against a smooth muscle actin A new probe for smooth muscle differentiation. J Cell Biol. 1986;103:2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand DW, Franco OE, Basanta D, Anderson AR, Hayward SW. Perspectives on tissue interactions in development and disease. Curr Mol Med. 2010;10(1):95–112. doi: 10.2174/156652410791065363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thesleff I, Hurmerinta K. Tissue interactions in tooth development. Differentiation. 1981;18(j):75–88. doi: 10.1111/j.1432-0436.1981.tb01107.x. [DOI] [PubMed] [Google Scholar]
- Thesleff I, Vaahtokari A, Partanen AM. Regulation of organogenesis Common molecular mechanisms regulating the development of teeth and other organs. Int J Dev Biol. 1995;39(1):35–50. [PubMed] [Google Scholar]
- Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res. 2002;8(9):2912–2923. [PubMed] [Google Scholar]
- Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130(4):601–610. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Yasugi S. Chronological changes in the inductive ability of the mesenchyme of the digestive organs in avian embryos. Dev Growth Differ. 1979;21:343–348. doi: 10.1111/j.1440-169X.1979.00343.x. [DOI] [PubMed] [Google Scholar]