Abstract
The fundamental pathophysiology of sickle cell disease involves the polymerization of sickle hemoglobin in its T-state which develops under low oxygen saturation. One therapeutic strategy is to develop pharmacologic agents to stabilize the R-state of hemoglobin, which has higher oxygen affinity and would be expected to have slower kinetics of polymerization, potentially delaying the sickling of red cells during circulation. This therapeutic strategy has stimulated the laboratory investigation of aromatic aldehydes, aspirin derivatives, thiols and isothiocyanates that can stabilize the R-state of hemoglobin in vitro. One representative aromatic aldehyde agent, 5-hydoxymethyl-2-furfural (5-HMF, also known as Aes-103) increases oxygen affinity of sickle hemoglobin and reduces hypoxia-induced sickling in vitro and protects sickle cell mice from effects of hypoxia. It has completed pre-clinical testing and has entered clinical trials. The development of Hb allosteric modifiers as direct anti-sickling agents is an attractive investigational goal for the treatment of sickle cell disease.
Keywords: Sickle cell, 5-HMF, Anti-sickling, R-state, Hemoglobin allosteric effectors
1. Oxygen Affinity of Sickle Erythrocytes
The erythrocytes in sickle cell disease have long been known to demonstrate decreased oxygen affinity compared to those from healthy volunteers.1–4 This property is measured as an increase in the partial pressure of oxygen required to produce 50% oxygen saturation (P50), discussed in further detail below. This decreased oxygen affinity is caused at least in part by increased intracellular concentration of 2,3-diphosphoglycerate (2,3-DPG) in erythrocytes, observed generally in all forms of anemia and considered a compensatory adaption that facilitates oxygen release from red cells to the tissues. 2,3-DPG is a product of anaerobic glycolysis, which has been found in recent years to be regulated in erythrocytes by oxygen-regulated sequestration and inactivation of glycolytic enzymes by the cytoskeletal protein Band 3.5–7 Among patients with SCD, P50 and 2,3-DPG levels vary widely, and more elevated levels appear to decrease solubility of sickle hemoglobin (HbS),8–10 and to increase red cell sickling under hypoxia,7,11 although not confirmed by all investigators.12,13 In vitro manipulation of human sickle blood to reduce 2,3-DPG content in red cells also reduces hypoxia-induced sickling in vitro.14 Preliminary investigation suggests that decreased oxygen affinity of HbS may be associated with greater clinical symptoms,8,15 but more investigation is needed to confirm this association. Although decreased oxygen affinity may be adaptive in other anemias, it may be counter-adaptive in sickle cell disease due to its promotion of the T-state of HbS, which promotes sickling. These effects relate to alterations in the conformation of hemoglobin.
2. The Allosteric States of Hb and Sickle Cell Disease
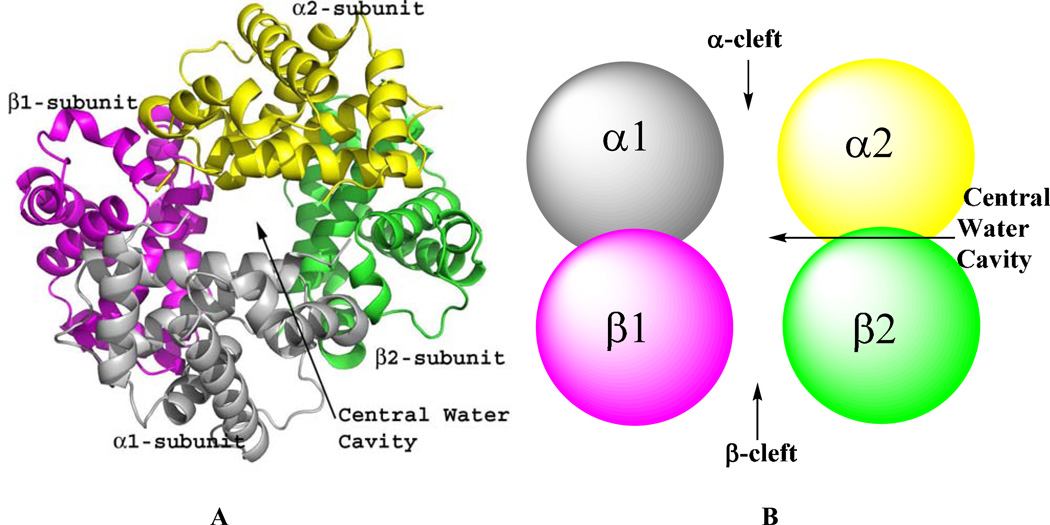
Hemoglobin historically has been shown to function in equilibrium between two classical states: the tense (T) state which exhibits low-affinity for ligand, and the relaxed (R) state which exhibits high affinity for ligand.16–19 The crystal structure of the T-state (unliganded or deoxygenated) or the R-state (liganded or oxygenated) Hb is each made up of two αβ heterodimers (α1β1 and α2β2) arranged around a 2-fold axis of symmetry to form a central water cavity with the α-cleft and β-cleft defining entries into the cavity (Fig. 1). The T→R allosteric transition is characterized by rotation of the α1β1 dimer relative to the α2β2 dimer, which significantly reshapes the central water cavity resulting in several differences between the quaternary T and R structures. Most notable is the formation of a larger central water cavity; including the α- and β-clefts in the quaternary T structure with respect to the quaternary R structure, as well as several different interdimer (α1β2 or α2β1, α1α2 and β1β2) hydrogen-bond and/or salt bridge interactions in the T or R structures that stabilize one state relative to the other. Despite the presence of βVal6 in HbS, normal and sickle Hb molecules have identical quaternary structures.
Figure 1.
Quaternary structure of human Hb. α1-subunit (grey), α2-subunit (yellow), β1-subunit (magenta) and β2-subunit (green). (A) Ribbon diagram showing the four Hb subunits arranged around a central water cavity. (B) Spherical illustration of the Hb subunits showing access to the central water cavity via the α-cleft and β-cleft.
The T and R structures were used to formulate the Monod-Wyman-Changeux (MWC) and the Koshland-Némethy-Filmer (KNF) allosteric models20,21 and later modified by Perutz with his stereochemical construct.16–19 Since then several R-like or T-like conformations within quaternary T- and R-states,22–28 as well as distinct quaternary relaxed states (R2, R3, RR2, RR3, etc) that extend beyond the classical T→R transition29,37 have been described and/or incorporated in modern allosteric models.38,39. Like the R and T structures, the relaxed structures also show significant differences in the geometry of the central water cavities.29,30,33,40 Unlike a quarter century ago, it is now widely accepted that Hb function involves an ensemble of relaxed Hb states in dynamic equilibrium.29,30,33–35,40 One such recent evidence is that aromatic aldehydes that increase the oxygen affinity of sickle Hb do so by binding to quaternary R2 structure and not the quaternary R structure to stabilize the relaxed state.41,42 An ongoing study also suggests thiols increase the oxygen affinity of Hb in part by forming a covalent adduct with βCys93 of both the quaternary R and R3 structures in a manner that should prevent formation of the characteristic T-state salt bridge interaction between βAsp94 and βHis146 when the Hb transitions to the T-state; and in so doing shift the allosteric equilibrium to the relaxed state (Safo, unpublished data). Unless noted otherwise, the R-state is used to represent the ensemble of relaxed states.
3. Hemoglobin – A Target for Drug Design
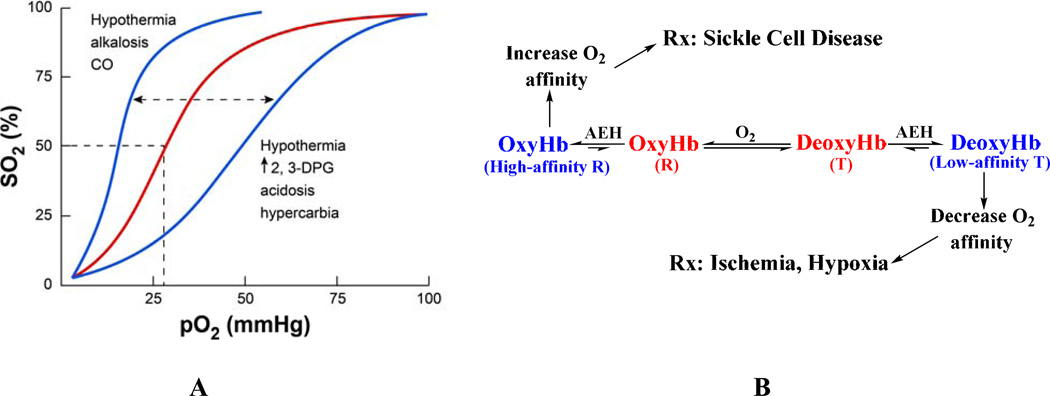
The allosteric equilibrium of Hb is modulated by several endogenous heterotopic effectors, e.g. 2,3-DPG, and hydrogen ions (H+); the former binds to the β-cleft and preferentially stabilize one quaternary state relative to the other.16,43,44 Stabilization of the R-state shifts the oxygen binding curve or oxygen equilibrium curve (OEC) of Hb to the left, producing a high-affinity Hb that more readily binds and holds oxygen (Fig. 2). A shift toward the T-state (right-shift of the OEC) produces a low-affinity Hb that readily releases oxygen. The degree of shift in the OEC is reported as an increase or decrease in P50, the oxygen tension at 50% Hb O2 saturation, while the degree of allosteric character is indicated by the slope of the oxygen binding curve (n50).
Figure 2.
The allosteric mechanism of Hb. (A) Oxygen equilibrium (or dissociation) curve of Hb. The normal P50 value is indicated by dashed lines. The left-shift and right-shift in the curves (blue color) are associated with various conditions, including AEHs. (B) Modulation of Hb allosteric transition by synthetic AEHs. Stabilization of either the R-state or T-state (colored blue) has potential therapeutic applications. The “R” represents all identified relaxed states including R2, R3, RR2, RR3, and the classical R.
A number of synthetic allosteric effectors of Hb (AEHs) also bind to the surface, α-cleft or β-cleft or the middle of the central water cavity to liganded Hb structure (in the R- or R2- or R3-state) and/or unliganded Hb structure (in the T-state) (Fig. 1) to either shift the OEC to the left or to the right (Figs. 2). The direction and magnitude of the shift dependent on preferential stabilization of one state over the other through additional hydrogen-bond and/or hydrophobic interactions that prevent the rotation associated with the allosteric transition and/or destabilization of a state by removing intersubunit interactions, which facilitates the allosteric movement.40,42,45–49 For example, RSR-13 (efaproxiral) and several other aromatic propionate analogs bind to the middle of the central water cavity of Hb;47,50–53 several angstroms away from the β-cleft where 2,3-DPG and other organic phosphates are known to bind.38,43,44 The binding ties the two dimers together, preferentially stabilizing the T-state relative to the R-state to lower the affinity of Hb for O2 and enhance its delivery to tissues in a manner physiologically similar to 2,3-DPG (Fig. 2B). These AEHs have potential therapeutic applications in treating ischemia-related cardiovascular diseases, such as angina, myocardial ischemia, stroke, trauma, where more O2 is needed to heal tissue or organs.54–59 A second class of AEHs, which includes several aromatic aldehydes, on the contrary, bind to Hb to increase its O2 affinity by preferentially stabilizing the R-state relative to the T-state (Fig. 2B). These compounds are potentially useful for the treatment of sickle cell disease.41,42,46,60–64
The availability of the crystal structures of T and the various relaxed states of Hb have contributed significantly to the design of quaternary state specific AEHs. Since these compounds bind to locations separate from the substrate (heme pocket) or endogenous 2,3-DPG (β-cleft) binding sites, they are not restricted by the need to generate molecules with higher affinities than the natural ligands; moreover these AEHs can elicit an effect regardless of the natural ligand concentration. Also, because the allosteric activity of Hb can be modulated by varying degrees, it allows the possibility to tailor drug activity to the severity of the disease state. It is interesting to note that some AEHs bind to the same allosteric sites of Hb, but produce very different magnitudes of OEC shift, and in some instances opposite shift.41,42,45,47–49 Based on these observation, a general hypothesis was proposed that the effectors’ ability to shift the allosteric equilibrium (i.e. the effectors potency and/or direction of shift) is due not only to where the molecule binds, but also how it interacts with the Hb dimer-dimer interface to stabilize or destabilize that allosteric state.48,49
4. Development of Allosteric Modifiers of Hb to treat Sickle Cell Disease
As atomic-level understanding of Hb allosteric property, and of the interactions between Hb molecules that contribute to Hb polymerization and formation of pathological fibers became clear, several classes of compounds, e.g. urea derivatives, amino acid derivatives, oligopeptides, carbohydrate derivatives, aromatic alcohols and acids were developed, most with the objective to disrupt HbS polymer formation.65–71 Unfortunately, most of these compounds exhibited weak if any significant antisickling activity, which is probably due to weak binding to shallow cavities on the surface of the Hb protein since such cavities do not exclude water and ions, and do not provide the necessary environment for strong hydrophobic interactions.
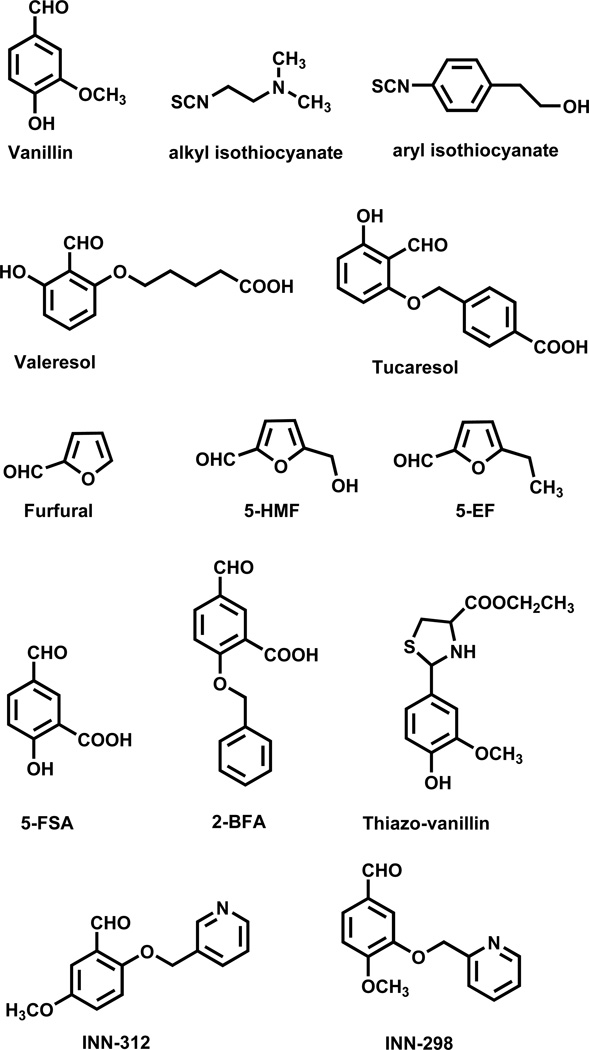
The realization that the polymerization process is exacerbated by the low O2 affinity of HbS as a result of unusually high concentration of 2,3-DPG in sickle RBC, led to another rational approach to treat SCD by increasing the affinity of HbS for oxygen - sufficiently to prevent premature release of oxygen, but not so extensively as to compromise tissue oxygenation. Sunshine et al. were among the first to suggest that increasing the O2 affinity of HbS by 4 mmHg could lead to therapeutically significant inhibition of intracellular polymerization.72 This approach was also bolstered by the milder clinical severity observed in sickle cell anemia patients in whom approximately 20% of the red cell Hb content is expressed as the high-O2-affinity fetal Hb (HbF), which is now known to inhibit HbS polymerization.73,74 The beginning of the 1970s saw the development of such AEHs, most notably, aromatic aldehydes, aspirin derivatives, thiols and isothiocyanates that form covalent adducts with Hb, modifying the protein allosteric property to increase its oxygen affinity.61,64,75–81 The Klotz group reported several benzaldehydes, including the food additive vanillin (Fig. 3) and showed that these compounds form a Schiff-base interaction with the amino terminus of α-globin.64 The interaction is sometimes described as transient covalent, lasting only for a short period of time, as the Schiff-base exists in equilibrium between the bound and the free aldehyde. Several isothiocyanates (Fig. 3) that form covalent adducts with Hb have also been reported for their antisickling activities, also by virtue of their ability to increase the oxygen affinity of Hb.78 The aliphatic isothiocyanates (Fig. 3) bind covalently to β-globin Cys93 to disrupt the native T-state salt-bridge interaction between βAsp94 and βHis146. This leads to T-state destabilization, explaining their left-shifting property.78 Binding to the βCys93 was also suggested to explain the significant increase in the solubility of the fully deoxygenated HbS by preventing direct polymer contacts.78 On the contrary, aromatic isothiocynates (Fig. 3) also react at the amino terminal amine on the α-chain of Hb and shows antisickling activities by increasing the oxygen affinity of HbS, which the authors suggested to be due to destabilization of the T-state.78 Although the isothiocynates seem quite promising since they can be administered at lower doses and less frequently, however, like other AEHs that form permanent covalent interactions with Hb, their lack of specificity could lead to toxicity.
Figure 3.
Structures of synthetic allosteric effectors of hemoglobin
Peter Goodford’s group was the first to use the classical R structure to design left-shifting aromatic aldehyde-acid AEHs that were postulated to cross-link the two symmetry-related α-globin subunits via a Schiff-based interaction with the N-terminus of αVal1 of one α-subunit and a hydrogen-bond interaction with the opposite αVal1 of the second α-subunit, and stabilize the R-state relative to the T-state.82,83 The study resulted in clinical tested antisickling aromatic aldehydes that include valeresol (12C79; Fig. 3) and tucaresol (589C80; Fig. 3). A later study by Don Abraham suggested that the left-shifting properties of these agents were the result of two molecules (not one as proposed by Goodford), each forming a Schiff-base interaction with the N-terminus of the αVal1 nitrogen of the T structure (not the R structure as proposed by Goodford) in manner that destabilizes the T-state and left-shift the OEC to the R-state.60 Valeresol underwent human testing, and while very potent, was not orally bioavailable, and had a very short duration of action of 3–4 hrs following IV administration.84,85 Tucaresol, although orally bioavailable with more favorable in-vivo human pharmacokinetics than valeresol.84–88 was found to cause immune-mediated toxicity in longer-term phase II studies.89 While shown not to bind as designed, the discovery of tucaresol and valeresol clearly indicated proof of principle that the allosteric property of Hb could be altered pharmacologically.
Although Goodford and Abraham had proposed seemingly opposing views of the antisickling mechanism of left-shifting aromatic aldehydes, it wasn’t until several years later that the exact mechanism underlying the antisickling effect of these compounds was unraveled.42 Our group, working with aromatic aldehydes, e.g., vanillin, furfural, 5-ethyl-2-furfural (5-EF), and 5-hydoxymethyl-2-furfural (5-HMF)(Fig. 3), showed that these compounds bind to the α-cleft of both quaternary R2 structure (and not the classical R structure) and quaternary T structure. Although binding adds to the stability of the R2 structure, they additionally destabilize the T structure, which in effect shifts the allosteric equilibrium to the R-state and increases the oxygen affinity of Hb.42 In contrast to the R2 structure, the α-cleft of the R structure is sterically crowded due to the presence of the C-terminal residues αTyr140 and αArg141, thus precluding binding to these effectors.42 It should be noted that when Peter Goodford proposed his design model, only the classical R and T structures were known. Interestingly, there are several aromatic aldehyde AEHs that also bind to the α-cleft to form Schiff-base with αVal1 nitrogen, but instead of left-shifting the OEC, they rather decrease Hb affinity for oxygen.40,48,49,90 An ortho or para substituted carboxylate moiety (relative to the aldehyde functional group) in these right-shifters, e.g., 5-formylsalicylic acid (5-FSA; Fig. 3) and 2-(benzyloxy)-5-formylbenzoic acid (2-BFA; Fig. 3), make intersubunit salt-bridge interactions with the guanidinium group of αArg141 on the opposite α-subunit in the T structure that stabilizes the T-state. The left-shifting aromatic aldehydes lack these carboxylate moieties.
With the important lesson learned about potential toxicity issues with covalent AEHs, it was realized that for any antisickling agent to become a successful drug candidate, one has to start with a non-toxic or very low toxicity scaffold in designing new agents. Abraham revisited the food-additive vanillin that had previously been shown by Klotz to have antisickling activity,64 which he translated into a phase I clinical trial. Although non-toxic, like valeresol, vanillin was not orally bioavailable, and the phase I clinical study was terminated. Aldehydes are subject to aldehyde dehydrogenase (ALDH)-mediated oxidative metabolism in human RBC and liver, which may have been very rapid for both vanillin and valeresol, explaining their non-oral bioavailability. A decade later, a study with the prodrug of vanillin, where the aldehyde group was protected by L-cysteine to form a thiazolidine complex (thiazo-vanillin; Fig. 3) was shown to have significantly improved oral pharmacokinetic and pharmacodynamic properties when compared to the corresponding free aldehyde vanillin, suggesting a viable strategy to improve oral bioavailability, as well as the efficacy of similar antisickling aldehydes.91
In a collaborative effort between Don Abraham, Martin Safo, Osheiza Abdulmalik, and Toshio Asakura, 5-HMF (Fig. 3) was shown to have remarkable antisickling activity.42,63 A single oral dose of 100 mg/kg of 5-HMF was sufficient to protect transgenic sickle mice from death from acute pulmonary sequestration of sickle cells after a hypoxic challenge,63 while chronic administration of up to 375 mg/kg/day of 5-HMF for two years was nontoxic to rats or mice.92 Acute oral median lethal dose (LD50) values for rats were 2.5–5.0 g/kg for 5-HMF (US EPA, 1992). 5-HMF also shows no in vitro cytotoxic effects on RBCs, and plasma proteins do not inhibit its binding to intracellular Hb.42,63
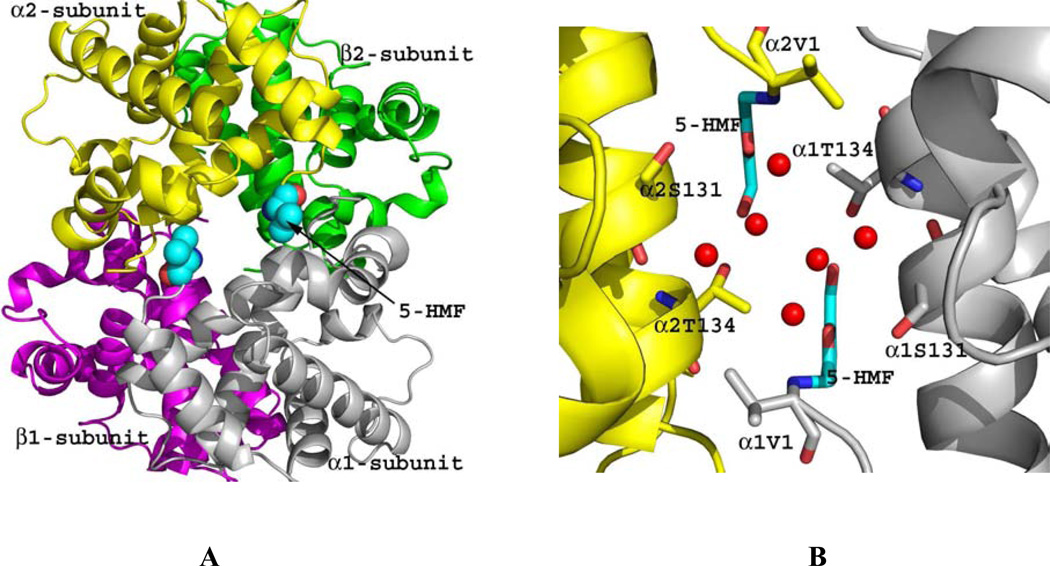
Crystal structures of 5-HMF and similar furfural analogs (Fig. 4) in complex with the quaternary T or R2 structure show the compounds to form Schiff-base interaction with the αVal1 nitrogen in a symmetry-related fashion. The binding of 5-HMF to the R2 structure adds additional inter-subunit interaction through a series of direct and intricate water-mediated interactions that tie the two α-subunits together and restrict the transition to the T-state (Fig. 4). The high-specificity of 5-HMF for Hb is most likely due to this intricate and strong hydrogen-bond network. Other less potent antisickling aldehydes, e.g. vanillin and furfural lack this intricate sheath of water molecules, explaining their reduced allosteric activity.42 In contrast to binding to the R2 structure, binding of 5-HMF, as well as other aldehydes, to the wider T structure α-cleft always result in weak binding of the compounds, which does not seem to add additional intersubunit interaction across the dimer interface.42 Binding rather disrupts a T-state water-mediated bridge between α1Val1 and the opposite subunit residue α1Arg141; effectively destabilizing the T structure and shifting the equilibrium to the R-state.42
Figure 4.
Crystal structure of quaternary R2 in complex with two molecules of 5-HMF (cyan spheres). Hb subunits are in ribbons (α1-subunit in grey, α2-subunit in yellow, β1-subunit in magenta and β2-subunit in green). (A) Two molecules of 5-HMF bound at the α-cleft in a symmetry-related fashion to the N-terminal αVal1. (B) Close-up view of the bound 5-HMF molecules. Each molecule forms a Schiff-base interaction with the αVal1 nitrogen. The sheath of water molecules (red spheres) form an intricate hydrogen-bond interactions with the bound compounds and the protein to tie the two α-subunits together.
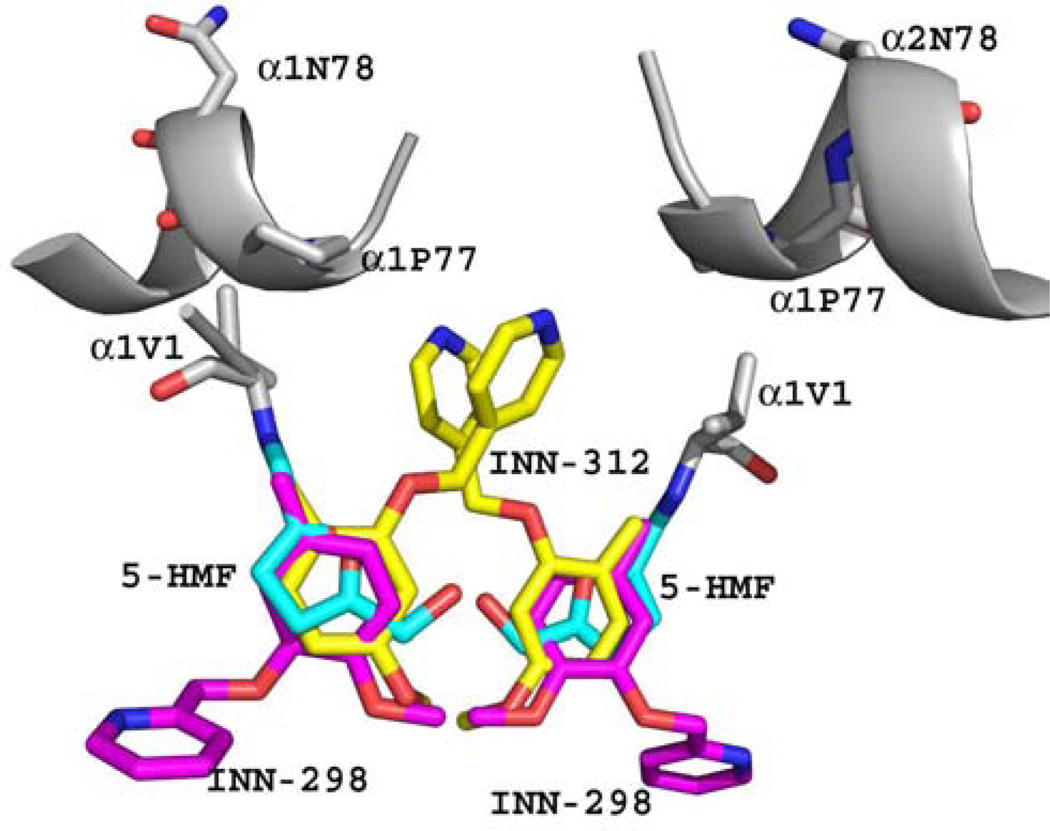
Based on the atomic interactions between Hb and 5-HMF or vanillin, several pyridyl derivatives of vanillin were developed by our group.41,46 These compounds (INN) bind to the same Hb site as 5-HMF and exhibit superior left-shifting ability and antisickling activities, some exhibiting as much as 90-fold and 2.5-fold potency than vanillin and 5-HMF, respectively.41,46 Similar to 5-HMF, the INN compounds also bind to the α-cleft to form Schiff-base adducts with the N-terminal αVal1 nitrogen (Fig. 5), and through a series of direct and intricate water-mediated interactions (via the hydroxyl moiety) tie the two α-subunits together to stabilize the relaxed state in the R2 form.41 A most interesting finding was that one of the studied compounds, INN-312 in addition to its left-shifting property, also stereospecifically inhibited polymer formation.41 Binding of INN-312 at the α-cleft disposes the methoxypyridine substituent (which is ortho to the aldehyde group) toward the surface of the Hb tetramer to make contact with residues of the αF-helix (Fig. 5), which was suggested to lead to destabilization of polymer contact.41 Asn78α on the F-helix has been implicated in stabilizing the HbS fiber; consistently, a variant Hb, Stanleyville (Asn78α ↔ Lsy78α) inhibits polymerization by destabilizing ontacts between deoxy-HbS molecules.93 This finding suggests a novel therapeutic approach involving the design of compounds that stereospecifically inhibit deoxy-HbS polymer formation while increasing the oxygen affinity of Hb.
Figure 5.
Superposition of bound 5-HMF (cyan), INN-312 (yellow) and INN-298 (magenta) molecules in the quaternary R2 structures. The Hb molecule (grey) is shown for that of the 5-HMF complex. All three AEHs form Schiff-base interaction with the αVal1 nitrogen. The meta-positioned methoxypyridine substituent (relative to the aldehyde moiety) in INN-298 is directed toward the center of the water cavity, while the ortho-positioned methoxypyridine substituent in INN-312 is directed toward the surface of the Hb to make contact with F helix, which has been implicated in polymer formation.
5. Clinical Development
Aes-103 (5-HMF) has undergone pre-clinical testing for potential treatment for SCD as described in the preceding section, including results from the National Toxicology Program.92 It is an organic compound derived from decomposition of certain sugars, found commonly in small amounts in foods such as coffee and prunes. It increases oxygen affinity of human sickle blood and inhibits hypoxia-induced sickling in vitro.63,94,95 Short-term administration of high doses in healthy volunteers and lung surgery patients has been well tolerated.96,97 Aes-103 has progressed through Phase 1 double blind, placebo controlled, dose escalation safety and tolerability trials in healthy volunteers and adults with sickle cell disease (ClinicalTrials.gov Identifier NCT01597401),98 and is expected to enter Phase 2 clinical trials.
Although the development of 5-HMF is very encouraging, there are several challenges facing the use of covalent Hb modifiers as antisickling agents; some enumerated above, include non-specific binding, and for aldehydes, rapid metabolism in vivo that may compromise their effectiveness. However, a large body of data on vanillin and 5-HMF suggests that these aldehydes have little toxicity at relatively high doses, and they also provide a structural scaffold to design additional non-toxic AEHs. We also note that some antisickling aldehydes, such as 5-HMF and tucaresol, are less susceptible to rapid metabolism, and moreover there are proven ways to derivatize aldehydes into prodrugs that would protect the aldehydes if needed. Another challenging problem for the use of AEHs pertains to efficacy, i.e., difficulty with designing pharmaceutically useful agents capable of sustained modification of the large amounts of intracellular Hb (approximately 5 mM). While this may seem pharmacologically challenging, we note that therapeutic efficacy might require modification of only a fraction of HbS as suggested by the mild clinical course of patients with about 20% HbF.74,99,100 Modification of 10–24% of HbS was obtained in an early phase trial with tucaresol, showing the feasibility of modifying this percentage of hemoglobin in vivo.86 As discussed earlier, as little as a 4 mmHg decrease in the P50 has been predicted to provide clinical benefit,72 and this magnitude of change may be achievable in vivo.
CONCLUSIONS
Pharmacological stabilization of the R-state of sickle hemoglobin offers a therapeutic strategy that directly inhibits the fundamental pathological mechanism of sickle cell disease, the polymerization of sickle hemoglobin. Although challenges remain in the implementation of this strategy, one agent, 5-HMF (Aes-103) currently is in active clinical trial to pursue the goal of directly inhibiting erythrocyte sickling in vivo. This is one of the first pharmaceutical agents designed expressly for sickle cell disease to enter clinical trial. Additional investigation of the potential utility of sickle erythrocyte P50 as a potential biomarker of clinical subphenotype is warranted, now that such subphenotypes of sickle cell disease are becoming better defined.
KEY POINTS.
The T-state of sickle Hb is prone to polymerize, promoting red cell sickling.
Stabilizers of the R-state of sickle Hb have the potential to directly inhibit sickling.
R-state stabilizers also increase the affinity of sickle Hb for oxygen.
An R-state stabilizer, 5-hydoxymethyl-2-furfural (5-HMF, also known as Aes-103) is currently in clinical trials.
ACKNOWLEDGMENTS
M.K.S. gratefully acknowledges research support from the Virginia Commonwealth University Presidential Research Initiative Program Award. The structural biology resources were provided in part by the National Cancer Institute to the VCU Massey Cancer Center (CA 16059-28). During the phase one clinical trials of Aes-103, G.J.K. received research support from the National Heart, Lung and Blood Institute Division of Intramural Research (1-ZIA-HL006149-01) with additional project support from the National Center for Advancing Translational Sciences Therapeutics for Rare and Neglected Diseases Program (1-ZIB-TR000002-01).
DISCLOSURE: Dr. Safo is a co-owner of a patent for the use of 5-HMF in sickle cell disease, and he receives research funding from AesRx, LLC, a licensee for Aes-103 (5-HMF). Dr. Kato has collaborated with AesRx, LLC through a Clinical Trials Agreement between AesRx, LLC and the National Heart, Lung and Blood Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Riggs SA, Wells M. The oxygen equilibrium of sickle-cell hemoglobin. Biochim. Biophys. Acta. 1961;50:243–248. doi: 10.1016/0006-3002(61)90322-5. [DOI] [PubMed] [Google Scholar]
- 2.Charache S, Grisolia S, Fiedler AJ, Hellegers AE. Effect of 2,3-diphosphoglycerate on oxygen affinity of blood in sickle cell anemia. J. Clin. Invest. 1970;49:806–812. doi: 10.1172/JCI106294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seakins M, Gibbs WN, Milner PF, Bertles JF. Erythrocyte hb-S concentration. an important factor in the low oxygen affinity of blood in sickle cell anemia. J. Clin. Invest. 1973;52:422–432. doi: 10.1172/JCI107199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milner PF. Oxygen transport in sickle cell anemia. Arch. Intern. Med. 1974;133:565–572. [PubMed] [Google Scholar]
- 5.Chu H, Breite A, Ciraolo P, Franco RS, Low PS. Characterization of the deoxyhemoglobin binding site on human erythrocyte band 3: Implications for O2 regulation of erythrocyte properties. Blood. 2008;111:932–938. doi: 10.1182/blood-2007-07-100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber RE, Voelter W, Fago A, Echner H, Campanella E, Low PS. Modulation of red cell glycolysis: Interactions between vertebrate hemoglobins and cytoplasmic domains of band 3 red cell membrane proteins. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287:R454–R464. doi: 10.1152/ajpregu.00060.2004. [DOI] [PubMed] [Google Scholar]
- 7.Rogers SC, Ross JG, d'Avignon A, Gibbons LB, Gazit V, Hassan MN, McLaughlin D, Griffin S, Neumayr T, Debaun M, DeBaun MR, Doctor A. Sickle hemoglobin disturbs normal coupling among erythrocyte O2 content, glycolysis, and antioxidant capacity. Blood. 2013;121:1651–1662. doi: 10.1182/blood-2012-02-414037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poillon WN, Kim BC, Welty EV, Walder JA. The effect of 2,3-diphosphoglycerate on the solubility of deoxyhemoglobin S. Arch. Biochem. Biophys. 1986;249:301–305. doi: 10.1016/0003-9861(86)90006-8. [DOI] [PubMed] [Google Scholar]
- 9.Poillon WN, Kim BC. 2,3-diphosphoglycerate and intracellular pH as interdependent determinants of the physiologic solubility of deoxyhemoglobin S. Blood. 1990;76:1028–1036. [PubMed] [Google Scholar]
- 10.Poillon WN, Robinson MD, Kim BC. Deoxygenated sickle hemoglobin. modulation of its solubility by 2,3-diphosphoglycerate and other allosteric polyanions. J. Biol. Chem. 1985;260:13897–13900. [PubMed] [Google Scholar]
- 11.Jensen FB. The dual roles of red blood cells in tissue oxygen delivery: Oxygen carriers and regulators of local blood flow. J. Exp. Biol. 2009;212:3387–3393. doi: 10.1242/jeb.023697. [DOI] [PubMed] [Google Scholar]
- 12.Beutler E, Paniker NV, West C. The effect of 2,3-DPG on the sickling phenomenon. Blood. 1971;37:184–186. [PubMed] [Google Scholar]
- 13.Swerdlow PH, Bryan RA, Bertles JF, Poillon WN, Magdoff-Fairchild B, Milner PF. Effect of 2, 3-diphosphoglycerate on the solubility of deoxy-sickle hemoglobin. Hemoglobin. 1977;1:527–537. doi: 10.3109/03630267709003417. [DOI] [PubMed] [Google Scholar]
- 14.Poillon WN, Kim BC, Labotka RJ, Hicks CU, Kark JA. Antisickling effects of 2,3-diphosphoglycerate depletion. Blood. 1995;85:3289–3296. [PubMed] [Google Scholar]
- 15.Adhikary PK, Hara S, Dwivedi C, Davis JW, Weaver C, Pavuluri SR. Vaso-occlusive crisis episodes in sickle cell disease. J. Med. 1986;17:227–240. [PubMed] [Google Scholar]
- 16.Perutz MF, Wilkinson AJ, Paoli M, Dodson GG. The stereochemical mechanism of the cooperative effects in hemoglobin revisited. Annu. Rev. Biophys. Biomol. Struct. 1998;27:1–34. doi: 10.1146/annurev.biophys.27.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Perutz MF, Fermi G, Luisi B, Shaanan B, Liddington RC. Stereochemistry of cooperative mechanisms in hemoglobin. Cold Spring Harb. Symp. Quant. Biol. 1987;52:555–565. doi: 10.1101/sqb.1987.052.01.063. [DOI] [PubMed] [Google Scholar]
- 18.Perutz MF. Nature of haem-haem interaction. Nature. 1972;237:495–499. doi: 10.1038/237495a0. [DOI] [PubMed] [Google Scholar]
- 19.Perutz MF. Stereochemical mechanism of cooperative effects in haemoglobin. Biochimie. 1972;54:587–588. doi: 10.1016/s0300-9084(72)80142-1. [DOI] [PubMed] [Google Scholar]
- 20.Monod J, Wyman J, Changeux JP. On the nature of allosteric transitions: A plausible model. J. Mol. Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 21.Koshland DE, Jr, Nemethy G, Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966;5:365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- 22.Sawicki CA, Gibson QH. Quaternary conformational changes in human hemoglobin studied by laser photolysis of carboxyhemoglobin. J. Biol. Chem. 1976;251:1533–1542. [PubMed] [Google Scholar]
- 23.Samuni U, Dantsker D, Juszczak LJ, Bettati S, Ronda L, Mozzarelli A, Friedman JM. Spectroscopic and functional characterization of T state hemoglobin conformations encapsulated in silica gels. Biochemistry. 2004;43:13674–13682. doi: 10.1021/bi048531d. [DOI] [PubMed] [Google Scholar]
- 24.Song XJ, Simplaceanu V, Ho NT, Ho C. Effector-induced structural fluctuation regulates the ligand affinity of an allosteric protein: Binding of inositol hexaphosphate has distinct dynamic consequences for the T and R states of hemoglobin. Biochemistry. 2008;47:4907–4915. doi: 10.1021/bi7023699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson J, Phillips K, Luisi B. The crystal structure of horse deoxyhaemoglobin trapped in the high-affinity (R) state. J. Mol. Biol. 1996;264:743–756. doi: 10.1006/jmbi.1996.0674. [DOI] [PubMed] [Google Scholar]
- 26.Schumacher MA, Dixon MM, Kluger R, Jones RT, Brennan RG. Allosteric transition intermediates modelled by crosslinked haemoglobins. Nature. 1995;375:84–87. doi: 10.1038/375084a0. [DOI] [PubMed] [Google Scholar]
- 27.Abraham DJ, Peascoe RA, Randad RS, Panikker J. X-ray diffraction study of di and tetra-ligated T-state hemoglobin from high salt crystals. J. Mol. Biol. 1992;227:480–492. doi: 10.1016/0022-2836(92)90902-v. [DOI] [PubMed] [Google Scholar]
- 28.Kavanaugh JS, Rogers PH, Arnone A. Crystallographic evidence for a new ensemble of ligand-induced allosteric transitions in hemoglobin: The T-to-T(high) quaternary transitions. Biochemistry. 2005;44:6101–6121. doi: 10.1021/bi047813a. [DOI] [PubMed] [Google Scholar]
- 29.Jenkins JD, Musayev FN, Danso-Danquah R, Abraham DJ, Safo MK. Structure of relaxed-state human hemoglobin: Insight into ligand uptake, transport and release. Acta Crystallogr. D Biol. Crystallogr. 2009;65:41–48. doi: 10.1107/S0907444908037256. [DOI] [PubMed] [Google Scholar]
- 30.Safo MK, Abraham DJ. The enigma of the liganded hemoglobin end state: A novel quaternary structure of human carbonmonoxy hemoglobin. Biochemistry. 2005;44:8347–8359. doi: 10.1021/bi050412q. [DOI] [PubMed] [Google Scholar]
- 31.Safo MK, Burnett JC, Musayev FN, Nokuri S, Abraham DJ. Structure of human carbonmonoxyhemoglobin at 2.16 A: A snapshot of the allosteric transition. Acta Crystallogr. D Biol. Crystallogr. 2002;58:2031–2037. doi: 10.1107/s0907444902015809. [DOI] [PubMed] [Google Scholar]
- 32.Mueser TC, Rogers PH, Arnone A. Interface sliding as illustrated by the multiple quaternary structures of liganded hemoglobin. Biochemistry. 2000;39:15353–15364. doi: 10.1021/bi0012944. [DOI] [PubMed] [Google Scholar]
- 33.Silva MM, Rogers PH, Arnone A. A third quaternary structure of human hemoglobin A at 1.7-A resolution. J. Biol. Chem. 1992;267:17248–17256. [PubMed] [Google Scholar]
- 34.Gong Q, Simplaceanu V, Lukin JA, Giovannelli JL, Ho NT, Ho C. Quaternary structure of carbonmonoxyhemoglobins in solution: Structural changes induced by the allosteric effector inositol hexaphosphate. Biochemistry. 2006;45:5140–5148. doi: 10.1021/bi052424h. [DOI] [PubMed] [Google Scholar]
- 35.Lukin JA, Kontaxis G, Simplaceanu V, Yuan Y, Bax A, Ho C. Quaternary structure of hemoglobin in solution. Proc. Natl. Acad. Sci. U. S. A. 2003;100:517–520. doi: 10.1073/pnas.232715799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schumacher MA, Zheleznova EE, Poundstone KS, Kluger R, Jones RT, Brennan RG. Allosteric intermediates indicate R2 is the liganded hemoglobin end state. Proc. Natl. Acad. Sci. U. S. A. 1997;94:7841–7844. doi: 10.1073/pnas.94.15.7841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janin J, Wodak SJ. The quaternary structure of carbonmonoxy hemoglobin ypsilanti. Proteins. 1993;15:1–4. doi: 10.1002/prot.340150102. [DOI] [PubMed] [Google Scholar]
- 38.Yonetani T, Tsuneshige A. The global allostery model of hemoglobin: An allosteric mechanism involving homotropic and heterotropic interactions. C. R. Biol. 2003;326:523–532. doi: 10.1016/s1631-0691(03)00150-1. [DOI] [PubMed] [Google Scholar]
- 39.Henry ER, Bettati S, Hofrichter J, Eaton WA. A tertiary two-state allosteric model for hemoglobin. Biophys. Chem. 2002;98:149–164. doi: 10.1016/s0301-4622(02)00091-1. [DOI] [PubMed] [Google Scholar]
- 40.Safo MK, Ahmed MH, Ghatge MS, Boyiri T. Hemoglobin-ligand binding: Understanding hb function and allostery on atomic level. Biochim. Biophys. Acta. 2011;1814:797–809. doi: 10.1016/j.bbapap.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 41.Abdulmalik O, Ghatge MS, Musayev FN, Parikh A, Chen Q, Yang J, Nnamani IN, Danso-Danquah R, Eseonu DN, Asakura K, Abraham DJ, Venitz J, Safo MK. Crystallographic analysis of human hemoglobin elucidates the structural basis of the potent and dual antisickling activity of pyridyl derivatives of vanillin. 2011;D67:920–928. doi: 10.1107/S0907444911036353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Safo MK, Abdulmalik O, Danso-Danquah R, Burnett JC, Nokuri S, Joshi GS, Musayev FN, Asakura T, Abraham DJ. Structural basis for the potent antisickling effect of a novel class of five-membered heterocyclic aldehydic compounds. J. Med. Chem. 2004;47:4665–4676. doi: 10.1021/jm0498001. [DOI] [PubMed] [Google Scholar]
- 43.Arnone A. X-ray diffraction study of binding of 2,3-diphosphoglycerate to human deoxyhaemoglobin. Nature. 1972;237:146–149. doi: 10.1038/237146a0. [DOI] [PubMed] [Google Scholar]
- 44.Richard V, Dodson GG, Mauguen Y. Human deoxyhaemoglobin-2,3-diphosphoglycerate complex low-salt structure at 2.5 A resolution. J. Mol. Biol. 1993;233:270–274. doi: 10.1006/jmbi.1993.1505. [DOI] [PubMed] [Google Scholar]
- 45.Safo MK, Bruno S. Allosteric Effectors of Hemoglobin: Past, Present and Future. In: Mozzarelli A, Bettati S, editors. Chemistry and Biochemistry of Oxygen Therapeutics: From Transfusion to Artificial Blood. John Wiley & Sons, Ltd.; 2011. pp. 285–300. [Google Scholar]
- 46.Nnamani IN, Joshi GS, Danso-Danquah R, Abdulmalik O, Asakura T, Abraham DJ, Safo MK. Pyridyl derivatives of benzaldehyde as potential antisickling agents. Chem. Biodivers. 2008;5:1762–1769. doi: 10.1002/cbdv.200890165. [DOI] [PubMed] [Google Scholar]
- 47.Safo MK, Moure CM, Burnett JC, Joshi GS, Abraham DJ. High-resolution crystal structure of deoxy hemoglobin complexed with a potent allosteric effector. Protein Sci. 2001;10:951–957. doi: 10.1110/ps.50601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abraham DJ, Safo MK, Boyiri T, Danso-Danquah RE, Kister J, Poyart C. How allosteric effectors can bind to the same protein residue and produce opposite shifts in the allosteric equilibrium. Biochemistry. 1995;34:15006–15020. doi: 10.1021/bi00046a007. [DOI] [PubMed] [Google Scholar]
- 49.Boyiri T, Safo MK, Danso-Danquah RE, Kister J, Poyart C, Abraham DJ. Bisaldehyde allosteric effectors as molecular ratchets and probes. Biochemistry. 1995;34:15021–15036. doi: 10.1021/bi00046a008. [DOI] [PubMed] [Google Scholar]
- 50.Abraham DJ, Wireko FC, Randad RS, Poyart C, Kister J, Bohn B, Liard JF, Kunert MP. Allosteric modifiers of hemoglobin: 2-[4-[[(3,5-disubstituted anilino)carbonyl]methyl]phenoxy]-2-methylpropionic acid derivatives that lower the oxygen affinity of hemoglobin in red cell suspensions, in whole blood, and in vivo in rats. Biochemistry. 1992;31:9141–9149. doi: 10.1021/bi00153a005. [DOI] [PubMed] [Google Scholar]
- 51.Randad RS, Mahran MA, Mehanna AS, Abraham DJ. Allosteric modifiers of hemoglobin. 1. design, synthesis, testing, and structure-allosteric activity relationship of novel hemoglobin oxygen affinity decreasing agents. J. Med. Chem. 1991;34:752–757. doi: 10.1021/jm00106a041. [DOI] [PubMed] [Google Scholar]
- 52.Chen Q, Lalezari I, Nagel RL, Hirsch RE. Liganded hemoglobin structural perturbations by the allosteric effector L35. Biophys. J. 2005;88:2057–2067. doi: 10.1529/biophysj.104.046136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lalezari I, Lalezari P, Poyart C, Marden M, Kister J, Bohn B, Fermi G, Perutz MF. New effectors of human hemoglobin: Structure and function. Biochemistry. 1990;29:1515–1523. doi: 10.1021/bi00458a024. [DOI] [PubMed] [Google Scholar]
- 54.Hou H, Khan N, Grinberg OY, Yu H, Grinberg SA, Lu S, Demidenko E, Steffen RP, Swartz HM. The effects of efaproxyn (efaproxiral) on subcutaneous RIF-1 tumor oxygenation and enhancement of radiotherapy-mediated inhibition of tumor growth in mice. Radiat. Res. 2007;168:218–225. doi: 10.1667/RR0962.1. [DOI] [PubMed] [Google Scholar]
- 55.Grinberg OY, Miyake M, Hou H, Steffen RP, Swartz HM. The dose-dependent effect of RSR13, a synthetic allosteric modifier of hemoglobin, on physiological parameters and brain tissue oxygenation in rats. Adv. Exp. Med. Biol. 2003;530:287–296. doi: 10.1007/978-1-4615-0075-9_27. [DOI] [PubMed] [Google Scholar]
- 56.Khandelwal SR, Randad RS, Lin PS, Meng H, Pittman RN, Kontos HA, Choi SC, Abraham DJ, Schmidt-Ullrich R. Enhanced oxygenation in vivo by allosteric inhibitors of hemoglobin saturation. Am. J. Physiol. 1993;265:H1450–H1453. doi: 10.1152/ajpheart.1993.265.4.H1450. [DOI] [PubMed] [Google Scholar]
- 57.Kunert MP, Liard JF, Abraham DJ. RSR-13, an allosteric effector of hemoglobin, increases systemic and iliac vascular resistance in rats. Am. J. Physiol. 1996;271:H602–H613. doi: 10.1152/ajpheart.1996.271.2.H602. [DOI] [PubMed] [Google Scholar]
- 58.Suh JH, Stea B, Nabid A, Kresl JJ, Fortin A, Mercier JP, Senzer N, Chang EL, Boyd AP, Cagnoni PJ, Shaw E. Phase III study of efaproxiral as an adjunct to whole-brain radiation therapy for brain metastases. J. Clin. Oncol. 2006;24:106–114. doi: 10.1200/JCO.2004.00.1768. [DOI] [PubMed] [Google Scholar]
- 59.Scott C, Suh J, Stea B, Nabid A, Hackman J. Improved survival, quality of life, and quality-adjusted survival in breast cancer patients treated with efaproxiral (efaproxyn) plus whole-brain radiation therapy for brain metastases. Am. J. Clin. Oncol. 2007;30:580–587. doi: 10.1097/COC.0b013e3180653c0d. [DOI] [PubMed] [Google Scholar]
- 60.Wireko FC, Abraham DJ. X-ray diffraction study of the binding of the antisickling agent 12C79 to human hemoglobin. Proc. Natl. Acad. Sci. U. S. A. 1991;88:2209–2211. doi: 10.1073/pnas.88.6.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abraham DJ, Mehanna AS, Wireko FC, Whitney J, Thomas RP, Orringer EP. Vanillin, a potential agent for the treatment of sickle cell anemia. Blood. 1991;77:1334–1341. [PubMed] [Google Scholar]
- 62.Swerdlow PS, Orringer EP, Abraham DJ. Dietary management of sickle cell anaemia with vanillin. Free Rad. Res. Comms. 1992;17:351–352. doi: 10.3109/10715769209079527. [DOI] [PubMed] [Google Scholar]
- 63.Abdulmalik O, Safo MK, Chen Q, Yang J, Brugnara C, Ohene-Frempong K, Abraham DJ, Asakura T. 5-hydroxymethyl-2-furfural modifies intracellular sickle haemoglobin and inhibits sickling of red blood cells. Br. J. Haematol. 2005;128:552–561. doi: 10.1111/j.1365-2141.2004.05332.x. [DOI] [PubMed] [Google Scholar]
- 64.Zaugg RH, Walder JA, Klotz IM. Schiff base adducts of hemoglobin. modifications that inhibit erythrocyte sickling. J. Biol. Chem. 1977;252:8542–8548. [PubMed] [Google Scholar]
- 65.Mehanna AS. Sickle cell anemia and antisickling agents then and now. Curr. Med. Chem. 2001;8:79–88. doi: 10.2174/0929867013373778. [DOI] [PubMed] [Google Scholar]
- 66.Sheh L, Mokotoff M, Abraham DJ. Design, synthesis, and testing of potential antisickling agents. 9. cyclic tetrapeptide homologs as mimics of the mutation site of hemoglobin S. Int. J. Pept. Protein Res. 1987;29:509–520. doi: 10.1111/j.1399-3011.1987.tb02278.x. [DOI] [PubMed] [Google Scholar]
- 67.Patwa DC, Abraham DJ, Hung TC. Design, synthesis, and testing of potential antisickling agents. 6. rheologic studies with active phenoxy and benzyloxy acids. Blood Cells. 1987;12:589–601. [PubMed] [Google Scholar]
- 68.Abraham DJ, Perutz MF, Phillips SE. Physiological and x-ray studies of potential antisickling agents. Proc. Natl. Acad. Sci. U. S. A. 1983;80:324–328. doi: 10.1073/pnas.80.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Perutz MF, Fermi G, Abraham DJ, Poyart C, Bursaux E. Hemoglobin as a receptor of drugs and peptides: X-ray studies of the stereochemistry of binding. J. Am. Chem. Soc. 1986;108:1064–1078. [Google Scholar]
- 70.Abraham DJ, Gazze DM, Kennedy PE, Mokotoff M. Design, synthesis, and testing of potential antisickling agents. 5. disubstituted benzoic acids designed for the donor site and proline salicylates designed for the acceptor site. J. Med. Chem. 1984;27:1549–1559. doi: 10.1021/jm00378a005. [DOI] [PubMed] [Google Scholar]
- 71.Abraham DJ, Mokotoff M, Sheh L, Simmons JE. Design, synthesis, and testing of antisickling agents. 2. proline derivatives designed for the donor site. J. Med. Chem. 1983;26:549–554. doi: 10.1021/jm00358a017. [DOI] [PubMed] [Google Scholar]
- 72.Sunshine HR, Hofrichter J, Eaton WA. Requirement for therapeutic inhibition of sickle haemoglobin gelation. Nature. 1978;275:238–240. doi: 10.1038/275238a0. [DOI] [PubMed] [Google Scholar]
- 73.Wood WG, Pembrey ME, Serjeant GR, Perrine RP, Weatherall DJ. Hb F synthesis in sickle cell anaemia: A comparison of saudi arab cases with those of african origin. Br. J. Haematol. 1980;45:431–445. doi: 10.1111/j.1365-2141.1980.tb07163.x. [DOI] [PubMed] [Google Scholar]
- 74.Pembrey ME, Wood WG, Weatherall DJ, Perrine RP. Fetal haemoglobin production and the sickle gene in the oases of eastern saudi arabia. Br. J. Haematol. 1978;40:415–429. doi: 10.1111/j.1365-2141.1978.tb05813.x. [DOI] [PubMed] [Google Scholar]
- 75.Garel MC, Domenget C, Caburi-Martin J, Prehu C, Galacteros F, Beuzard Y. Covalent binding of glutathione to hemoglobin. I. inhibition of hemoglobin S polymerization. J. Biol. Chem. 1986;261:14704–14709. [PubMed] [Google Scholar]
- 76.Caburi-Martin J, Garel MC, Domenget C, Prehu C, Beuzard Y. Contact inhibition within hemoglobin S polymer by thiol reagents. Biochim. Biophys. Acta. 1986;874:82–89. doi: 10.1016/0167-4838(86)90105-6. [DOI] [PubMed] [Google Scholar]
- 77.Garel MC, Domenget C, Galacteros F, Martin-Caburi J, Beuzard Y. Inhibition of erythrocyte sickling by thiol reagents. Mol. Pharmacol. 1984;26:559–565. [PubMed] [Google Scholar]
- 78.Park S, Hayes BL, Marankan F, Mulhearn DC, Wanna L, Mesecar AD, Santarsiero BD, Johnson ME, Venton DL. Regioselective covalent modification of hemoglobin in search of antisickling agents. J. Med. Chem. 2003;46:936–953. doi: 10.1021/jm020361k. [DOI] [PubMed] [Google Scholar]
- 79.Zaugg RH, Walder JA, Walder RY, Steele JM, Klotz IM. Modification of hemoglobin with analogs of aspirin. J. Biol. Chem. 1980;255:2816–2821. [PubMed] [Google Scholar]
- 80.Walder JA, Zaugg RH, Iwaoka RS, Watkin WG, Klotz IM. Alternative aspirins as antisickling agents: Acetyl-3,5-dibromosalicylic acid. Proc. Natl. Acad. Sci. U. S. A. 1977;74:5499–5503. doi: 10.1073/pnas.74.12.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Walder JA, Zaugg RH, Walder RY, Steele JM, Klotz IM. Diaspirins that cross-link beta chains of hemoglobin: Bis(3,5-dibromosalicyl) succinate and bis(3,5-dibromosalicyl) fumarate. Biochemistry. 1979;18:4265–4270. doi: 10.1021/bi00587a001. [DOI] [PubMed] [Google Scholar]
- 82.Beddell CR, Goodford PJ, Kneen G, White RD, Wilkinson S, Wootton R. Substituted benzaldehydes designed to increase the oxygen affinity of human haemoglobin and inhibit the sickling of sickle erythrocytes. Br. J. Pharmacol. 1984;82:397–407. doi: 10.1111/j.1476-5381.1984.tb10775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Merrett M, Stammers DK, White RD, Wootton R, Kneen G. Characterization of the binding of the anti-sickling compound, BW12C, to haemoglobin. Biochem. J. 1986;239:387–392. doi: 10.1042/bj2390387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Keidan AJ, Franklin IM, White RD, Joy M, Huehns ER, Stuart J. Effect of BW12C on oxygen affinity of haemoglobin in sickle-cell disease. Lancet. 1986;1:831–834. doi: 10.1016/s0140-6736(86)90941-4. [DOI] [PubMed] [Google Scholar]
- 85.Fitzharris P, McLean AE, Sparks RG, Weatherley BC, White RD, Wootton R. The effects in volunteers of BW12C, a compound designed to left-shift the blood-oxygen saturation curve. Br. J. Clin. Pharmacol. 1985;19:471–481. doi: 10.1111/j.1365-2125.1985.tb02672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arya R, Rolan PE, Wootton R, Posner J, Bellingham AJ. Tucaresol increases oxygen affinity and reduces haemolysis in subjects with sickle cell anaemia. Br. J. Haematol. 1996;93:817–821. doi: 10.1046/j.1365-2141.1996.d01-1744.x. [DOI] [PubMed] [Google Scholar]
- 87.Rolan PE, Mercer AJ, Wootton R, Posner J. Pharmacokinetics and pharmacodynamics of tucaresol, an antisickling agent, in healthy volunteers. Br. J. Clin. Pharmacol. 1995;39:375–380. doi: 10.1111/j.1365-2125.1995.tb04465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rolan PE, Parker JE, Gray SJ, Weatherley BC, Ingram J, Leavens W, Wootton R, Posner J. The pharmacokinetics, tolerability and pharmacodynamics of tucaresol (589C80; 4[2-formyl-3-hydroxyphenoxymethyl] benzoic acid), a potential anti-sickling agent, following oral administration to healthy subjects. Br. J. Clin. Pharmacol. 1993;35:419–425. doi: 10.1111/j.1365-2125.1993.tb04160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rhodes J. Discovery of immunopotentiatory drugs: Current and future strategies. Clin. Exp. Immunol. 2002;130:363–369. doi: 10.1046/j.1365-2249.2002.02016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Safo MK, Boyiri T, Burnett JC, Danso-Danquah R, Moure CM, Joshi GS, Abraham DJ. X-ray crystallographic analyses of symmetrical allosteric effectors of hemoglobin: Compounds designed to link primary and secondary binding sites. Acta Crystallogr. D Biol. Crystallogr. 2002;58:634–644. doi: 10.1107/s0907444902002627. [DOI] [PubMed] [Google Scholar]
- 91.Zhang C, Li X, Lian L, Chen Q, Abdulmalik O, Vassilev V, Lai CS, Asakura T. Anti-sickling effect of MX-1520, a prodrug of vanillin: An in vivo study using rodents. Br. J. Haematol. 2004;125:788–795. doi: 10.1111/j.1365-2141.2004.04892.x. [DOI] [PubMed] [Google Scholar]
- 92. http://ntp.niehs.nih.gov/go/TS-M950006.
- 93.Rhoda MD, Martin J, Blouquit Y, Garel MC, Edelstein SJ, Rosa J. Sickle cell hemoglobin fiber formation strongly inhibited by the stanleyville II mutation (alpha 78 asn leads to lys) Biochem. Biophys. Res. Commun. 1983;111:8–13. doi: 10.1016/s0006-291x(83)80109-0. [DOI] [PubMed] [Google Scholar]
- 94.Mendelsohn LG, Pedoeim L, van Beers EJ, Saiyed R, Nichols JS, Wang X, Kato GJ. Effect of aes-103 anti-sickling agent on oxygen affinity and stability of red blood cells from patients with sickle cell anemia. ASH Annual Meeting Abstracts. 2012;120:85. [Google Scholar]
- 95.van Beers EJ, Samsel L, Mendelsohn LG, Saiyed R, Nichols JS, McCoy JP, Kato GJ. Imaging flow cytometry for fully automated quantification of percentage of sickled cells in sickle cell anemia. ASH Annual Meeting Abstracts. 2012;120:2105. [Google Scholar]
- 96.Gatterer H, Greilberger J, Philippe M, Faulhaber M, Djukic R, Burtscher M. Short-term supplementation with alpha-ketoglutaric acid and 5-hydroxymethyl furfural does not prevent the hypoxia induced decrease of exercise performance despite attenuation of oxidative stress. Int. J. Sports Med. 2013;34:1–7. doi: 10.1055/s-0032-1312584. [DOI] [PubMed] [Google Scholar]
- 97.Matzi V, Lindenmann J, Muench A, Greilberger J, Juan H, Wintersteiger R, Maier A, Smolle-Juettner FM. The impact of preoperative micronutrient supplementation in lung surgery. A prospective randomized trial of oral supplementation of combined alpha-ketoglutaric acid and 5-hydroxymethylfurfural. Eur. J. Cardiothorac. Surg. 2007;32:776–782. doi: 10.1016/j.ejcts.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 98.Stern W, Mathews D, McKew J, Shen X, Kato GJ. A phase 1, first-in-man, dose-response study of aes-103 (5-HMF), an anti-sickling, allosteric modifier of hemoglobin oxygen affinity in healthy norman volunteers. ASH Annual Meeting Abstracts. 2012;120:3210. [Google Scholar]
- 99.Padmos MA, Roberts GT, Sackey K, Kulozik A, Bail S, Morris JS, Serjeant BE, Serjeant GR. Two different forms of homozygous sickle cell disease occur in saudi arabia. Br. J. Haematol. 1991;79:93–98. doi: 10.1111/j.1365-2141.1991.tb08013.x. [DOI] [PubMed] [Google Scholar]
- 100.el-Hazmi MA. Heterogeneity and variation of clinical and haematological expression of haemoglobin S in saudi arabs. Acta Haematol. 1992;88:67–71. doi: 10.1159/000204654. [DOI] [PubMed] [Google Scholar]