Abstract
Background & Aims
Hepatic stellate cells (HSCs) that express glial fibrillary acidic protein (GFAP) are located between the sinusoidal endothelial cells and hepatocytes. HSCs are activated during liver injury and cause hepatic fibrosis by producing excessive extracellular matrix. HSCs also produce many growth factors, chemokines and cytokines, and thus may play an important role in acute liver injury. However, this function has not been clarified due to unavailability of a model in which HSCs are depleted from the normal liver.
Methods
We treated mice expressing HSV-thymidine kinase under the GFAP promoter (GFAP-Tg) with 3 consecutive (3 days apart) CCl4 (0.16 μl/g; ip) injections to stimulate HSCs to enter the cell cycle and proliferate. This was followed by 10-day ganciclovir (40 μg/g/day; ip) treatment, which is expected to eliminate actively proliferating HSCs. Mice were then subjected to hepatic ischemia/reperfusion (I/R) or endotoxin treatment.
Results
CCl4/ganciclovir treatment caused depletion of the majority of HSCs (about 64-72%), while the liver recovered from the initial CCl4-induced injury (confirmed by histology, serum ALT and neutrophil infiltration). The magnitude of hepatic injury due to I/R or endotoxemia (determined by histopathology and serum ALT) was lower in HSC-depleted mice. Their hepatic expression of TNF-α, neutrophil chemoattractant CXCL1 and endothelin-A receptor also was significantly lower than the control mice.
Conclusions
HSCs play an important role both in I/R- and endotoxin-induced acute hepatocyte injury, with TNF-α and endothelin-1 as important mediators of these effects.
Keywords: Hepatic stellate cells, Depletion, Ischemia/Reperfusion, Endotoxemia, Lipopolysaccharide, Hepatocytes, Liver, Injury, Necrosis, Apoptosis
Introduction
The perisinusoidal hepatic stellate cells (HSCs) constitute 8-12% of the liver cell population, express glial fibrillary acidic protein (GFAP) and/or desmin, and are the major storage site of retinoids [1]. During liver injury, HSCs undergo activation characterized by the loss of retinoids, expression of α-smooth muscle actin and differentiation into proliferating myofibroblast-like cells. Activated HSCs produce excessive extracellular matrix (ECM) and exhibit increased expression of tissue inhibitors of metalloproteinases and reduced or unchanged expression of matrix metalloproteinases [2-4], thus becoming the major cell type responsible for hepatic fibrosis [5]. Activated HSCs are postulated to contribute to portal hypertension by their high contractility and up-regulation of the powerful vasoconstrictor endothelin-1 (ET-1) and its receptors [6,7].
HSCs express intercellular adhesion molecule-1 [8], produce various cytokines and chemokines [8-10], and thus can play important role in hepatic inflammation. Gram-negative bacterial endotoxin (lipopolysaccharide:LPS) stimulates the synthesis of nitric oxide (NO), ET-1, tumor necrosis factor (TNF)-α and interleukin (IL)-6 in both quiescent and activated HSCs; LPS-challenged HSCs stimulate NO synthesis, inhibit DNA synthesis and cause apoptosis of cultured hepatocytes [11-14].
Recent work demonstrates that HSCs also influence hepatic immunological functions. HSCs induce apoptosis of allogeneic CD4+ and CD8+ T cells [8,15], present bacterial lipid antigens to NKT cells [16], expand immunosuppressive regulatory T cells [8], and render dendritic cells immunosuppressive [10]. Furthermore, HSCs secrete powerful antioxidant protein(s) that protect hepatocytes from ischemia/reperfusion (I/R) injury [17].
Thus, HSCs are highly versatile cells that can profoundly influence hepatic structure and functions in physiology and pathology. Most of the in vivo work confirming their role in hepatic pathology has been focused on fibrosis. A fungal metabolite gliotoxin was found to cause apoptosis of activated rat and human HSCs in vitro, and of rat HSCs in vivo resulting in resolution of fibrosis [18,19]. However, gliotoxin also induces apoptosis of KCs and endothelial cells in the fibrotic liver [20,21]. Ebrahimkhani et al [22] administered gliotoxin into bile duct-ligated mice in conjugation with single-chain antibody C1-3, which recognizes synaptophysin expressed by activated HSCs [23]; C1-3-gliotoxin caused resolution of fibrosis by selectively depleting HSCs. Using a similar mouse model described in the present study, it was reported recently that concomitant treatment of B6.Cg-Tg(Gfap-Tk)7.1Mvs/J transgenic mice with ganciclovir promoted depletion of HSCs, and caused amelioration of CCl4-induced fibrosis and hepatic injury [24]. However, the role of HSCs in acute injury to the normal liver has not been evaluated. Here, we show amelioration of I/R- and endotoxin-induced acute injury to otherwise normal HSC-depleted liver, suggesting HSCs’ critical role in pathologies unrelated to activation-dependence.
Materials and methods
Animals
The protocols were approved by the IACUC according to NIH guidelines. Wild-type male C57BL/6 (WT-B6) and B6.Cg-Tg(Gfap-Tk)7.1Mvs/J (GFAP-Tg) mice were from The Jackson laboratory. GFAP-Tg mice express the herpes simplex virus thymidine kinase (HSV-TK) transgene under the GFAP promoter [25]. HSV-TK phosphorylates nontoxic ganciclovir (GCV) to GCV-monophosphate, which is converted to GCV-triphosphate by cellular guanylate kinase; phosphorylated GCV incorporates into the DNA causing death of replicating cells [25,26]. GFAP is expressed exclusively by HSCs in the liver, which are quiescent physiologically [1]. We treated the GFAP-Tg mice or WT-B6 mice with three CCl4 injections (0.16 μl/g in 50 μl peanut oil; ip), 3 days apart; control mice received peanut oil. After the last CCl4 injection, each group was divided into two subgroups, one receiving GCV (40 μg/g/day; ip) and the other vehicle (PBS) for 10 days. CCl4 treatment activates HSCs, which enter the cell cycle thus making them susceptible to phosphorylated GCV-induced death. Mice were subjected to I/R or endotoxin the day after completion of GCV treatment (Supplementary Fig. 1).
Ischemia/Reperfusion (I/R) or endotoxemia
For I/R, all structures (portal vein, hepatic artery and bile duct) to the left liver lobes were occluded with a microvascular clamp for 60 min. The right lobes served as control. The clamps were removed and the abdominal incision closed. For endotoxemia induction, mice were administered LPS (10 mg/kg) intraperitoneally. Blood was drawn at 6h following reperfusion or LPS administration for serum enzyme measurement. The livers were excised, washed in ice-cold PBS and portions were fixed in 10% buffered formalin or paraformaldehyde, or snap-frozen in liquid nitrogen.
Histological determinations
The sections of formalin-fixed tissue were stained with hematoxylin and eosin (H/E) for histopathological examination, with TUNEL-stain (ApopTag Peroxidase kit, Chemicon) to detect apoptotic cells, or immunostained using rat-anti-mouse F4/80 Ab (Serotec), biotinylated goat-anti-rat secondary Ab (Jackson Immunoresearch) and ABC Elite kit (Vector Laboratories) to detect KCs.
The sections of paraformaldehyde-fixed frozen tissue were immunostained with anti-desmin rabbit polyclonal Ab (Abcam), anti-GFAP rabbit polyclonal Ab (DakoCytomation), rat anti-mouse F4/80 Ab (BioLegend) or rat anti-mouse TNF-α Ab (R&D Systems) as described previously [8, 10].
Neutrophils were identified immunohistochemically using Naphthol As-D Chloroacetate Esterase Kit (Sigma-Aldrich). Neutrophil accumulation was quantified in at least 4 randomly selected high-power fields (400X) of each liver section.
mRNA analysis
RNA was prepared from the snap-frozen tissue using TRIzol Reagent (Invitrogen), and cDNA was prepared using high-capacity cDNA reverse transcription kit (Applied Biosystems). Quantitative real-time-PCR (qRT-PCR) was performed using the Sybr green master mix and 7500 Fast Real-Time PCR System (Applied Biosystems) with PCR primers listed in Table 1.
Table 1.
Primers used in qPCR reactions
| GFAP | 5’-ACCGCATCACCATTCCTGTAC-3’(F) 5’-TTGCCTTCTGACACGGATTT-3’(R) |
| IL-6 | 5’-CCGGAGAGGAGACTTCACAG-3’(F) 5’-TCCACGATTTCCCAGAGAAC-3’(R) |
| TNFα | 5’-CCCAGGTATATGGGCTCATACC-3’(F) 5’-GCCGATTTGCTATCTCATACCAGG-3’(R) |
| CXCL1 | 5’-CTGCACCCAAACCGAAGTC-3’(F) 5’-AGCTTCAGGGTCAAGGCAAG-3’(R) |
| ET-1 | 5’-GTGTCTACTTCTGCCACCTGGACAT-3’(F) 5’-GGGCTCGCACTATATAAGGGATGAC-3’(R) |
| ETaR | 5’-GATGGATAAGAACCGGTGTGAAC-3’(F) 5’-GAGCTATTGGGTTTATGCAAGAATTC-3’(R) |
| ETbR | 5’-ACCAGAGCAATCCACACAGG-3’(F) 5’-AGAGCGATTGGATTGATGCAG-3’(R) |
| GAPDH | 5’-TGTTGAAGTCACAGGAGACAACCT-3’(F) 5’-AACCTGCCAAGTATGATGACATCA-3’(R) |
Statistics
Statistical significance was determined by non-parametric Kruskal-Wallis One-Way ANOVA on Ranks. A ‘p’ value <0.05 was considered statistically significant.
Results
Characterization of the CCl4 effect on WT mice
To ensure that at the end of GCV treatment of mice, there is no residual hepatic injury due to earlier CCl4 administration before subjecting them to I/R or endotoxemia, WT-B6 mice were sacrificed a day after the third CCl4 injection or after 10 days of GCV treatment following termination of CCl4. There was significant hepatic injury the day after third CCl4 administration (Fig. 1A,B-middle panels), in both centrilobular and periportal areas (Supplementary Fig. 2), accompanied by inflammatory infiltration (Fig. 1B,C), and increased serum ALT (Fig. 1D). However, at the end of GCV treatment, liver histology and inflammation (Fig. 1A,B-lower panels; Fig. 1C) as well as serum ALT (Fig. 1D) had returned to normal and were identical to that in control mice.
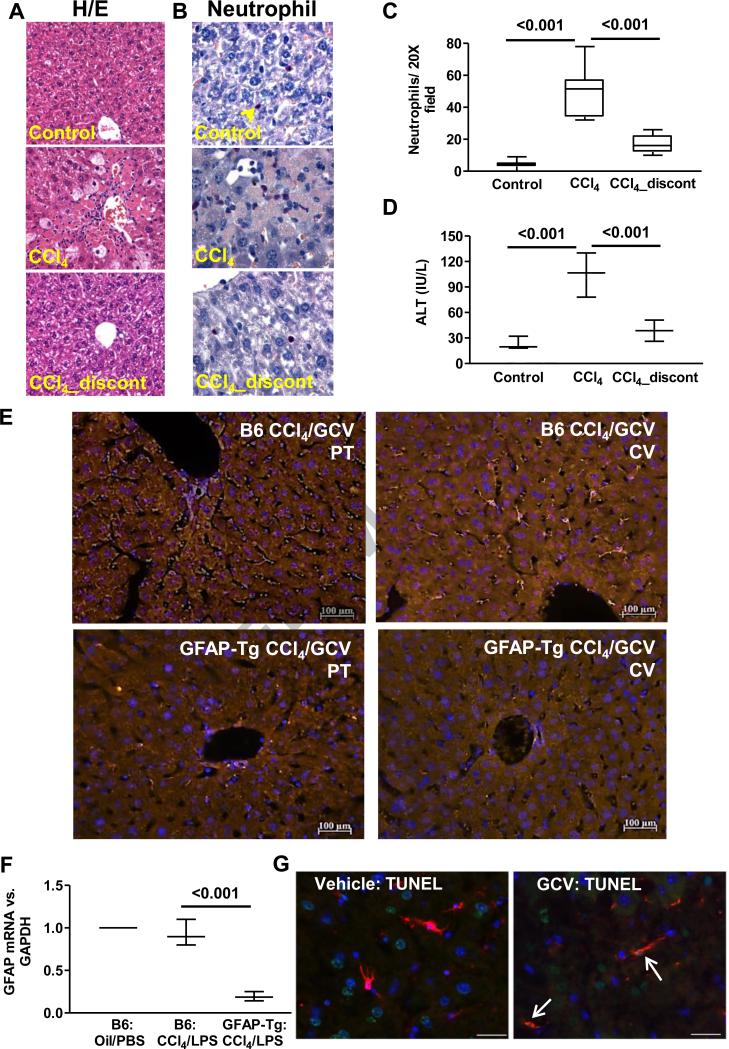
Fig. 1. Recovery of the liver from CCl4-induced injury.
H/E staining (A), neutrophil staining (B) and quantification (C), and serum ALT levels (D) demonstrating liver-injury in WT-B6 mice (n=4) a day after 3 CCl4 injections, and its resolution on day 11 after termination of CCl4. Effect of CCl4/GCV treatment on HSC-depletion in GFAP-Tg mice. Mice received CCl4 (3 injections) followed by its discontinuation and GCV treatment of 10 days. (E) GFAP-immunostaining in livers showing depletion of HSCs from portal (PT) and centrilobular (CV) regions of the GFAP-Tg mice at the end of CCl4/GCV treatment. (F) Strong reduction in GFAP mRNA expression in HSC-depleted mice. (G) TUNEL (green)- and GFAP (red)-stained cells are seen in CCl4/GCV-treated GFAP-Tg mice and not in CCl4/vehicle-treated mice at 2 days during vehicle or GCV treatment. Several apoptotic hepatocytes can also be seen at this time in both sections, which are replaced by day 10 after CCl4 discontinuation.
Depletion of HSCs from the GFAP-Tg mice
Similar to WT-B6 mice (Fig. 1), no evidence of liver injury was observed in the GFAP-Tg mice after completion of CCl4/GCV treatment as determined by histopathology and serum ALT (Supplementary Fig. 3A,B). GFAP immunostaining (Fig. 1E) demonstrated effective elimination of GFAP-positive cells from CCl4/GCV-treated GFAP-Tg mice in periportal and centrilobular areas (64-72%; Supplementary Fig. 3C); qRT-PCR confirmed strong reduction in GFAP expression (~80%) (Fig. 1F). Several apoptotic cells were observed in the hepatic sinusoids of CCl4/GCV-treated GFAP-Tg mice, but not in the CCl4/PBS-treated mice (Fig. 1G). We performed desmin staining since nearly 80-90% of HSCs express both GFAP and desmin, while the remainder cells express either GFAP or desmin [1]. Thus, desmin+ but GFAP− HSCs will not be eliminated due to GCV treatment. Indeed, there were significant desmin+ HSCs in CCl4/GCV-treated GFAP-Tg liver, and no effect of these treatments was observed on KCs (Supplementary Fig. 4).
Morphological (not shown) and histopathological examination showed no apparent injury to other organs including intestine (Supplementary Fig. 5) or other gastrointestinal compartments indicating that GCV (40 μg/g/d:ip) treatment affected the liver selectively.
Amelioration I/R- and endotoxema-induced injury in HSC-depleted mice
The mechanisms of hepatic I/R injury involve damage due to ischemia as well as reactive oxygen species (ROS) and inflammatory mediators produced by infiltrating monocytes and neutrophils, and KCs upon reperfusion [27,28]. HSCs, located in close association with hepatocytes, also produce ROS, proinflammatory mediators and chemokines [7-10], and thus may contribute to I/R injury. The production of the inflammatory mediators and chemokines by HSCs increases upon exposure to LPS [8,12-14]. Therefore, we investigated whether HSC-depletion affects warm I/R- or endotoxin-induced liver injury.
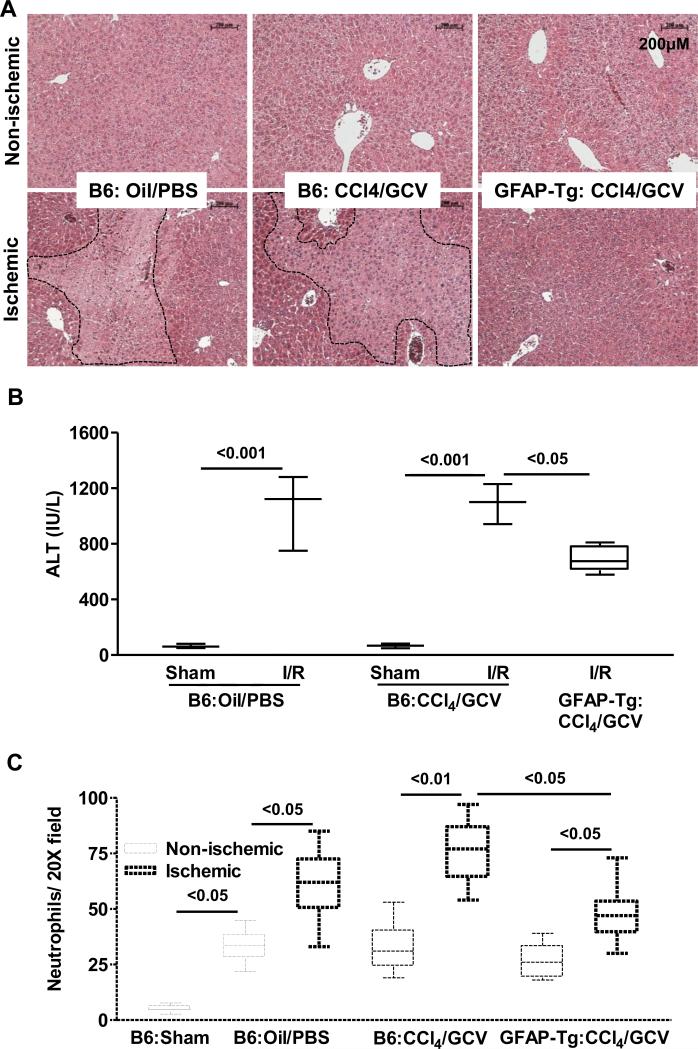
As expected, I/R caused profound parenchymal injury in the WT-B6 mice (Fig. 2A) with corresponding increase in serum ALT (Fig. 2B). The WT-B6 mice pretreated with CCl4/GCV had normal liver architecture and serum ALT levels, and their liver injury due to I/R was similar to that in vehicle-treated WT-B6 mice. However, I/R injury to HSC-depleted mice was markedly reduced (Fig. 2A,B). I/R injury in B6-WT mice treated with CCl4/PBS or oil/GCV, and GFAP-Tg mice treated with oil/PBS, CCl4/PBS or oil/GCV demonstrated injury similar to that in B6-WT mice treated with oil/PBS (Supplementary Fig. 6A). Furthermore, the number of GFAP+ cells in all of these treatment groups was similar (Supplementary Figure 6B, 6C), and statistically not different from B6-WT mice treated with CCl4/GCV. Neutrophil infiltration in the CCl4/GCV-treated WT-B6 mouse I/R lobes was similar to that in the control I/R lobes, but was modestly reduced in the I/R lobes of HSC-depleted mice (Fig. 2C). The number of neutrophils in the non-ischemic lobes increased similarly in all treatment groups as compared to sham control liver (Fig. 2C).
Fig. 2. Hepatic I/R injury in HSC-depleted mice.
WT-B6 and GFAP-Tg mice (n=4-6) were treated with oil or CCl4 (3 injections) followed by its discontinuation and PBS or GCV treatment of 10 days. They were then subjected to hepatic I/R. (A) Histopathology, (B) ALT and (C) neutrophil infiltration are shown.
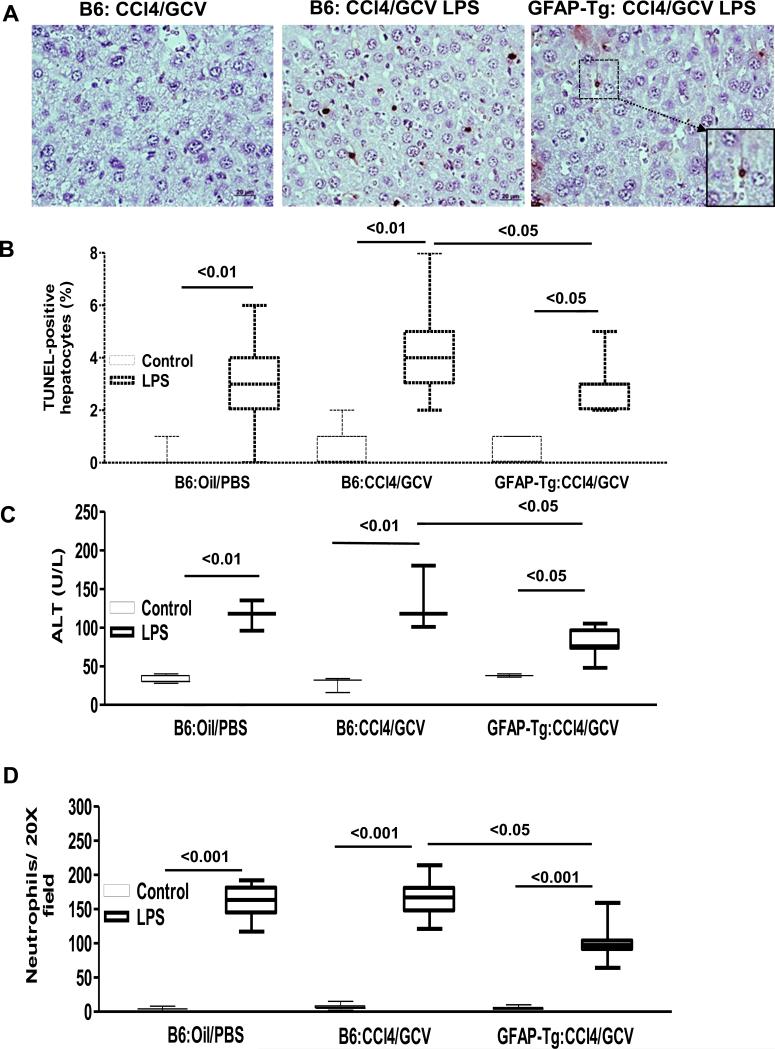
TUNEL-staining demonstrated modest apoptosis of hepatocytes in vehicle and CCl4/GCV-treated WT-B6 mice upon LPS administration (Fig. 3A,B). Hepatocyte apoptosis was significantly, but not completely, abrogated in HSC-depleted mice (Fig. 3A,B). These observations were consistent with similarly modest increase in serum ALT in vehicle- or CCl4/GCV-pretreated WT-B6 mice upon LPS administration; endotoxin-induced injury was significantly lower in HSC-depleted GFAP-Tg mice (Fig. 3C). Furthermore, hepatic neutrophil infiltration was lower in LPS-administered HSC-depleted mice than in vehicle- or CCl4/GCV-pretreated control mice (Fig. 3D). Interestingly, apoptotic cells were observed in the sinusoids of CCl4/GCV-treated GFAP-Tg mouse liver (Fig. 3A-inset) indicating continued elimination of HSCs.
Fig. 3. Hepatic endotoxemic injury in HSC-depleted mice.
WT-B6 and GFAP-Tg mice (n=4-6) received CCl4 (3 injections) followed by its discontinuation and GCV treatment of 10 days, then subjected to LPS treatment. (A) Histopathology and (B) quantification of TUNEL- positive hepatocytes; (C) serum ALT; and (D) neutrophil infiltration. Compared to CCl4/GCV then LPS-treated B6 mice very few apoptotic hepatocytes were observed in the livers of CCl4/GCV then LPS-treated GFAP-Tg mice; inset shows high magnification of the marked area to show apoptotic HSC.
Expression of TNF-α, IL-6 and CXCL1
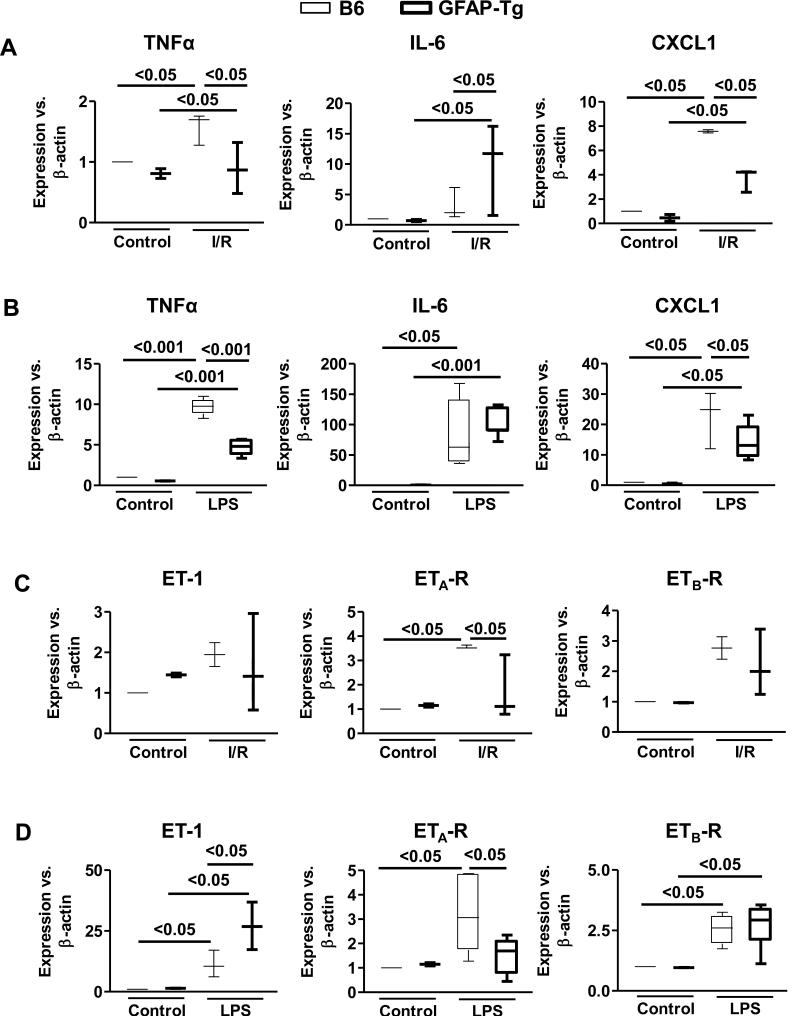
TNF-α and IL-6 are implicated in I/R- and LPS-induced hepatic injury. Hepatic mRNA expression of TNF-α, but not IL-6, increased significantly upon I/R in control mice (Fig. 4A). While the expression of TNF-α was lower in HSC-depleted I/R liver that of IL-6 increased significantly (Fig. 4A). LPS treatment increased TNF-α and IL-6 mRNA expression in control and HSC-depleted mice, and although this effect was reduced in the HSC-depleted mice, it was statistically significant for TNF-α and not IL-6 (Fig. 4B). Given the prominent role of TNF-α in I/R- and LPS-induced liver injury, we determined its protein expression immunohistochemically. The immunostaining for TNF-α increased strongly in the WT-B6 but not in HSC-depleted mice following I/R or endotoxemia (Supplementary Fig. 7). Hepatic mRNA expression of CXCL1, which plays an important role in neutrophil chemotaxis in the injured tissue, increased in mice subjected to I/R as well as endotoxin treatment, but the increase was significantly lower in HSC-depleted livers (Fig. 4A,B).
Fig. 4. Hepatic mRNA expression of molecules of I/R- or endotoxin-induced injury in HSC-depleted mice.
WT-B6 and GFAP-Tg mice (n=4-6) received CCl4 (3 injections) followed by its discontinuation and GCV treatment of 10 days, then subjected to I/R or LPS treatment. The mRNA expression of (A, B) TNF-α, IL-6 and CXCL1 or (C, D) ET-1, ETa-R and ETb-R were quantified in the nonischemic and ischemic lobes (A, C) and livers of control and LPS-treated mice (B, D).
Expression of ET-1 and its receptors
ET-1, through its vasoconstrictor activity, also contributes to I/R- [29,30] and LPS-induced [31] liver injury. The mRNA expression of ET-1 was not altered significantly upon I/R in control and HSC-depleted mice (Fig. 4C). ET-1 mRNA expression was up-regulated upon LPS treatment in control mice and, interestingly, increased further in LPS-treated HSC-depleted mice (Fig. 4D). ET-1 acts through two major receptors on various cell types: ETa-R and ETb-R. The direct effect of ET-1 on ETa-R induces vasoconstriction while ETb-R mediates vasodilation by stimulating release of nitric oxide and prostacyclin [32]. We found significant increase in ETa-R expression in WT-B6 but not in HSC-depleted mice subjected to I/R or endotoxin (Fig. 4C,D). In contrast, ETb-R expression increased similarly in both WT-B6 and HSC-depleted mice upon I/R or endotoxin treatment.
Discussion
The role of activated HSCs in hepatic fibrosis and cirrhosis has been established [5,33]. Recent research with cell culture studies and some indirect in vivo evidence indicate that quiescent or transiently activated HSCs (found in the liver during early phase of acute injury) can significantly influence metabolic and hemodynamic properties of the liver [7]. However, the lack of animal models in which HSCs are selectively depleted has been a major drawback to demonstrate unequivocally their precise contribution to acute liver injury. Herein, we demonstrate, for the first time, that HSCs play important role in I/R or endotoxin-induced hepatic injury using HSC-depleted mice.
Nearly 80% of the murine HSCs express GFAP [1], and thus the transgenic mouse in which HSV-TK is expressed under the GFAP promoter [25] provides a tool to generate an effective HSC-depleted model. However, in order for the toxic phosphorylated GCV to integrate into cellular DNA and induce cell death [34], the cells must leave their quiescent G0 state and enter the cell cycle. To achieve this, we treated GFAP-Tg mice with CCl4 to induce proliferation of HSCs. The CCl4-induced liver injury was repaired during the 10 days of GCV treatment while causing profound loss of HSCs. However, the liver was not completely devoid of HSCs as determined by desmin immunostaining, which may be due to the fact that about 10-15% of the cells do not express GFAP [1] and some GFAP+ cells may have escaped GCV-induced death. All of the other organs, including the gastrointestinal compartments were found to have no evidence of any injury. We note that GFAP-Tg mice that demonstrated severe inflammation and necrosis of the jejunum and ileum were treated continuously with 100 μg/g/d GCV [25], while we administered 40 μg/g/day GCV as bolus ip injections. We also note that bolus administrations of 100 μg/g/d GCV to GFAP-Tg mice did not elicit injury to other organs [24].
I/R injury is a complex phenomenon with inflammatory mediators and ROS produced by resident macrophages (KCs) and infiltrating blood cells such as neutrophils playing an important role. Several laboratories have observed that HSCs also produce the same mediators that can induce hepatocyte injury [7]. We have shown that LPS-stimulated HSCs produce inflammatory mediators and chemokines [8,10,12-14], and induce apoptosis of hepatocytes [13]. With their close proximity to hepatocytes and number similar to KCs, we hypothesized that HSCs may play a significant role both in I/R and LPS-induced liver injury.
In contrast to the highly defined and extensive injury to untreated as well as CCl4/GCV-pretreated WT mice, the I/R injury to HSC-depleted mice was diffuse and significantly reduced. Likewise, LPS-induced injury was also significantly reduced in HSC-depleted mice. Furthermore, neutrophil infiltration was lower in the I/R lobes and endotoxemic liver of HSC-depleted mice than in control mice suggesting that HSC-derived chemokines may contribute to their chemotaxis. Indeed, hepatic mRNA expression of a potent neutrophil chemoattractant CXCL1, which is produced by HSCs [8,10], was significantly lower in HSC-depleted mice. Both I/R- and endotoxemia-induced neutrophil infiltration also involve chemokines released by KCs [28,35], which might explain incomplete abrogation of neutrophil infiltration in HSC-depleted mice. Curiously, we observed significant, but similar in magnitude, neutrophil infiltration in the non-ischemic lobes of WT and HSC-depleted mice following reperfusion suggesting cross-trafficking of chemoattractants from the ischemic lobes.
It has been demonstrated that TNF-α causes injury to hepatocytes [36,37], while IL-6 protects them from I/R and acetaminophen-induced liver damage [38]. The smaller increase in TNF-α expression in HSC-depleted livers following I/R and endotoxin treatment suggests that HSCs might be major producers of TNF-α. Contrarily, IL-6 expression increased in HSC-depleted liver after I/R, and was similarly increased in LPS-treated control and HSC-depleted livers. Since LPS stimulates IL-6 synthesis in HSCs [8,12-14], our observations suggest that other cell types (e.g., KCs) may compensate for IL-6 production. Alternatively, it is plausible that HSCs might down-regulate IL-6 expression in other cells types. Nevertheless, increased IL-6 and reduced TNF-α in HSC-depleted liver appear to be a protective mechanism of I/R or endotoxemia-induced hepatic injury.
Amelioration of increased portal pressure in CCl4-induced acute liver injury [39] and I/R [29,30] by antagonism by ET-1 action suggested that up-regulation of ET-1 and its receptors is a mechanism of this effect. LPS also up-regulates ET-1 expression in HSCs [11], and in the liver [31,40]. The role of HSCs in hepatic vasoconstriction is supported by the finding that ET-1 antagonism attenuates contraction of HSCs as well as increased portal pressure in experimental animals [31,41]. ET-1-induced contraction of quiescent (nontransformed) HSCs in vivo [42] and in cell culture [43] suggests that sinusoidal narrowing due to the action of ET-1 on HSCs may contribute to I/R- as well as endotoxin-induced liver injury. An important mechanism of increased hepatic ET-1 expression in I/R and endotoxemia could be the stimulatory effect of KC-derived TNF-α and/or TGF-β, which increase ET-1 release by endothelial cells and HSCs [44-46]. Blocking KCs activity by gadolinium chloride (GdCl3) reduced ET-1 secretion and improved liver function after warm I/R in rats [47]. Furthermore, treatment with neutralizing TNF-α antibody ameliorated I/R injury [48,49], and carbon monoxide inhalation by recipient animals caused inhibition of hepatic graft TNF-α and IL-6 expression and reduced I/R injury [50]. LPS also stimulates production of TNF-α and IL-6 by KCs [51-54]. Together, these observations suggest that ET-1 contributes to I/R as well as endotoxin-mediated liver damage. However, the results of this study show that the increase in ET-1 mRNA expression after I/R in WT mice was statistically not significant and did not change in the HSC-depleted mice. On the other hand, hepatic ET-1 mRNA increased further in HSC-depleted mice after endotoxin treatment. These data are paradoxical as LPS increases ET-1 expression in HSCs [11] and the whole liver [31]. A plausible interpretation of this finding is that LPS also induces counter-regulatory mechanisms that abrogate ET-1 expression. For example, synthesis of NO, which down-regulates ET-1 expression (55,56), is increased strongly in HSCs upon exposure to LPS and LPS-stimulated HSCs elicit a similar response in hepatocytes [11-14]. In the HSC-depleted liver, the loss of such mechanism(s) may be responsible for increased ET-1 expression in other cells (e.g., endothelial cells). Nevertheless, the results suggest that enhanced ET-1 release from the endogenous stores and/or up-regulation of ET-1 receptors during I/R and endotoxemia might be the major factors in hepatic injury. Indeed, whereas I/R and endotoxin increased ETa-R and ETb-R expression significantly in WT-B6 mice, only ETa-R expression was significantly lower in HSC-depleted mice. These results suggest that ET-1-related (ETa-R-mediated) contraction plays an important role in I/R and endotoxin-induced liver injury, and that this probably overrides the effect of vasodilatory mediators released in response to ETb-R stimulation in the presence of HSCs.
While this work was in progress, depletion of HSCs during concomitant treatment with CCl4 and GCV was reported to ameliorate fibrosis and the on-going CCl4-induced hepatic injury in GFAP-Tg mice [24]. This is an interesting observation as hepatocytes metabolize CCl4 (via cytochrome P450) to CCl3, which then elicits cellular injury. Thus the findings of Puch et al [24] suggest that HSCs may contribute to CCl4-induced hepatocyte death. In this regard, it was reported that GdCl3-induced blockade of KCs reduced neutrophil infiltration and almost completely prevented CCl4-induced hepatocyte death, without altering cytochrome P450-mediated CCl4 metabolism [57]. Since HSCs also produce chemokines that induce neutrophil infiltration (Fig. 4) [8,10], a similar mechanism of reduced CCl4-induced liver injury in HSC-depleted mice [24] is a likely possibility. Although the precise mechanisms of how HSCs mediate CCl4-induced hepatic parenchymal damage [24] remain to be determined, our results indicate that HSCs are major participants of clinically relevant liver damage via inflammation, cytokines, chemokines and vasoactive mediators. Thus the blockade of the mechanisms by which HSCs induce acute liver injury has potential therapeutic relevance.
Supplementary Material
Acknowledgement
We thank Ms. Rebecca B. Schuster for excellent technical assistance.
Financial Support: NIH PO1A1081678 and VA Merit Review Award 1IO1BX001174, and Department of Surgery, University of Cincinnati and Children's Hospital Medical Center, Cincinnati.
Abbreviations
- HSC
hepatic stellate cell
- GFAP
glial fibrillary acidic protein
- ECM
extracellular matrix
- ET-1
endothelin-1
- LPS
lipopolysaccharide
- NO
nitric oxide
- NOS
nitric oxide synthase
- TNF
tumor necrosis factor
- IL
interleukin
- I/R
ischemia/reperfusion
- KCs
Kupffer cells
- WT
wild type
- Tg
transgenic
- HSV
herpes simplex virus
- TK
thymidine kinase
- GCV
ganciclovir
- IP
intraperitoneal
- CCl4
carbon tetrachloride
- PBS
phosphate-buffered saline
- H/E
hematoxylin and eosin
- Ab
antibody
- qRT-PCR
quantitative real-time polymerase chain reaction
- ALT
alanine aminotransferase
- ROS
reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None of the authors participated in this study have anything to disclose regarding conflict of interest.
References
- 1.Geerts A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin Liver Dis. 2001;21:311–335. doi: 10.1055/s-2001-17550. [DOI] [PubMed] [Google Scholar]
- 2.Iredale JP. Hepatic stellate cell behavior during resolution of liver injury. Semin Liver Dis. 2001;21:427–436. doi: 10.1055/s-2001-17557. [DOI] [PubMed] [Google Scholar]
- 3.Gaca MD, Zhou X, Issa R, Kiriella K, Iredale JP, Benyon RC. Basement membrane-like matrix inhibits proliferation and collagen synthesis by activated rat hepatic stellate cells: evidence for matrix-dependent deactivation of stellate cells. Matrix Biol. 2003;22:229–239. doi: 10.1016/s0945-053x(03)00017-9. [DOI] [PubMed] [Google Scholar]
- 4.Henderson NC, Iredale JP. Liver fibrosis: cellular mechanisms of progression and resolution. Clin Sci. 2007;112:265–280. doi: 10.1042/CS20060242. [DOI] [PubMed] [Google Scholar]
- 5.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 6.Rockey DC. Hepatic blood flow regulation by stellate cells in normal and injured liver. Semin Liver Dis. 2001;21:337–349. doi: 10.1055/s-2001-17551. [DOI] [PubMed] [Google Scholar]
- 7.Gandhi CR. Hepatic Stellate Cells. In: Monga SP, editor. Molecular Pathology of Liver Diseases. Springer; 2010. pp. 53–80. [Google Scholar]
- 8.Dangi A, Sumpter TL, Kimura S, Stolz DB, Murase N, Raimondi G, et al. Selective expansion of allogeneic regulatory T cells by hepatic stellate cells: role of endotoxin and implications for allograft tolerance. J Immunol. 2012;188:3667–3677. doi: 10.4049/jimmunol.1102460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinzani M, Marra F. Cytokine receptors and signaling in hepatic stellate cells. Semin Liver Dis. 2001;21:397–416. doi: 10.1055/s-2001-17554. [DOI] [PubMed] [Google Scholar]
- 10.Sumpter TL, Dangi A, Matta B, Huang C, Stolz D, Vodovotz Y, et al. Hepatic stellate cells undermine the allostimulatory function of liver myeloid dendritic cells via STAT3-dependent induction of IDO. J Immunol. 2012;189:3848–3858. doi: 10.4049/jimmunol.1200819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gandhi CR, Uemura T, Kuddus RH. Endotoxin causes up-regulation of endothelin receptors in cultured hepatic stellate cells via nitric oxide-dependent and –independent mechanisms. Br J Pharmacol. 2000;131:319–327. doi: 10.1038/sj.bjp.0703577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uemura T, Gandhi CR. Inhibition of DNA synthesis in cultured hepatocytes by endotoxin-conditioned medium of activated stellate cells is transforming growth factor-β- and nitric oxide-independent. Br J Pharmacol. 2001;133:1125–1133. doi: 10.1038/sj.bjp.0704151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thirunavukkarasu C, Uemura T, Wang LF, Watkins S, Gandhi CR. Normal rat hepatic stellate cells respond to endotoxin in LBP-independent manner to produce inhibitor(s) of DNA synthesis in hepatocytes. J Cell Physiol. 2005;204:654–665. doi: 10.1002/jcp.20366. [DOI] [PubMed] [Google Scholar]
- 14.Thirunavukkarasu C, Watkins S, Gandhi CR. Mechanisms of endotoxin-induced nitric oxide, interleukin-6 and tumor necrosis factor-α production in activated rat hepatic stellate cells: role of p38MAPK. Hepatology. 2006;44:389–398. doi: 10.1002/hep.21254. [DOI] [PubMed] [Google Scholar]
- 15.Yu MC, Chen CH, Liang X, Wang L, Gandhi CR, Fung JJ, et al. Inhibition of T cell responses by hepatic stellate cells via B7-H1 mediated T cell apoptosis. Hepatology. 2004;40:1312–1321. doi: 10.1002/hep.20488. [DOI] [PubMed] [Google Scholar]
- 16.Winau F, Hegasy G, Weiskirchen R, Weber S, Cassan C, Sieling PA, et al. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity. 2007;26:117–129. doi: 10.1016/j.immuni.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Jameel NM, Thirunavukkarasu C, Murase N, Cascio M, Prelich J, Yang S, et al. Constitutive release of powerful antioxidant scavenging activity by hepatic stellate cells: Protection of hepatocytes from ischemia/reperfusion injury. Liver Transplantation. 2010;16:1400–1409. doi: 10.1002/lt.22172. [DOI] [PubMed] [Google Scholar]
- 18.Wright MC, Issa R, Smart DE, Trim N, Murray GI, Primrose JN, et al. Gliotoxin stimulates the apoptosis of human and rat hepatic stellate cells and enhances the resolution of liver fibrosis in rats. Gastroenterology. 2001;121:685–698. doi: 10.1053/gast.2001.27188. [DOI] [PubMed] [Google Scholar]
- 19.Orr JG, Leel V, Cameron GA, Marek CJ, Haughton EL, Elrick LJ, et al. Mechanism of action of the antifibrogenic compound gliotoxin in rat liver cells. Hepatology. 2004;40:232–242. doi: 10.1002/hep.20254. [DOI] [PubMed] [Google Scholar]
- 20.Anselmi K, Nalesnik M, Watkins SC, Beer-Stolz D, Gandhi CR. Gliotoxin causes apoptosis and necrosis of rat Kupffer cells in vitro and in vivo in the absence of oxidative stress: Exacerbation by caspase and serine protease inhibition. J Hepatology. 2007;47:103–113. doi: 10.1016/j.jhep.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagens WI, Olinga P, Meijer DK, Groothuis GM, Beljaars L, Poelstra K. Gliotoxin non-selectively induces apoptosis in fibrotic and normal livers. Liver Int. 2006;26:232–239. doi: 10.1111/j.1478-3231.2005.01212.x. [DOI] [PubMed] [Google Scholar]
- 22.Ebrahimkhani MR, Oakley F, Murphy LB, Mann J, Moles A, Perugorria MJ, et al. Stimulating healthy tissue regeneration by targeting the 5-HT B receptor in chronic liver disease. Nat Med. 2011;17:1668–1673. doi: 10.1038/nm.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cassiman D, Libbrecht L, Desmet V, Denef C, Roskams T. Hepatic stellate cell/myofibroblast subpopulations in fibrotic human and rat livers. J Hepatol. 2002;36:200–209. doi: 10.1016/s0168-8278(01)00260-4. [DOI] [PubMed] [Google Scholar]
- 24.Puche JE, Lee YA, Jiao J, Aloman C, Fiel MI, Muñoz U, et al. A novel murine model to deplete hepatic stellate cells uncovers their role in amplifying liver damage in mice. Hepatology. 2013;57:339–350. doi: 10.1002/hep.26053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, et al. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189–201. doi: 10.1016/s0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- 26.Miller WH, Miller RL. Phosphorylation of acyclovir (acycloguanosine) monophosphate by GMP kinase. J Biol Chem. 1980;255:7204–7207. [PubMed] [Google Scholar]
- 27.Montalvo-Jave EE, Escalante-Tattersfield T, Ortega-Salgado JA, Piña E, Geller DA. Factors in the pathophysiology of the liver ischemia-reperfusion injury. J Surg Res. 2008;147:153–159. doi: 10.1016/j.jss.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J Gastroenterol Hepatol. 2011;26(Suppl 1):173–179. doi: 10.1111/j.1440-1746.2010.06592.x. [DOI] [PubMed] [Google Scholar]
- 29.Goto M, Takei Y, Kawano S, Nagano K, Tsuji S, Masuda E, et al. Endothelin-1 is involved in the pathogenesis of ischemia/reperfusion liver injury by hepatic microcirculatory disturbances. Hepatology. 1994;19:675–681. doi: 10.1002/hep.1840190319. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura S, Nishiyama R, Serizawa A, Yokoi Y, Suzuki S, Konno H, et al. Hepatic release of endothelin-1 after warm ischemia. Transplantation. 1995;59:679–684. doi: 10.1097/00007890-199503150-00006. [DOI] [PubMed] [Google Scholar]
- 31.Gandhi CR, Kuddus RH, Nemoto EM, Murase N. Endotoxin treatment causes up-regulation of endothelin system in the liver: Amelioration of increased portal resistance by endothelin receptor antagonism. J Gastroenterol Hepatol. 2001;6:61–69. doi: 10.1046/j.1440-1746.2001.02419.x. [DOI] [PubMed] [Google Scholar]
- 32.Gandhi CR, Berkowitz DE, Watkins WD. Endothelins: Biochemistry and Pathophysiologic Actions. Anesthesiology. 1994;80:892–905. [PubMed] [Google Scholar]
- 33.Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–456. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- 34.Heyman RA, Borrelli E, Lesley J, Anderson D, Richman DD, Baird SM, et al. Thymidine kinase obliteration: creation of transgenic mice with controlled immune deficiency. Proc Natl Acad Sci USA. 1989;86:2698–2702. doi: 10.1073/pnas.86.8.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sadik CD, Kim ND, Luster AD. Neutrophils cascading their way to inflammation. Trends Immunol. 2011;32:452–460. doi: 10.1016/j.it.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teoh NC, Farrell GC. Hepatic ischemia reperfusion injury: Pathogenic mechanisms and basis for hepatoprotection. J Gastroenterol Hepatol. 2003;18:891–902. doi: 10.1046/j.1440-1746.2003.03056.x. [DOI] [PubMed] [Google Scholar]
- 37.Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury - a fresh look. Exp Mol Pathol. 2003;74:86–93. doi: 10.1016/s0014-4800(03)00008-x. [DOI] [PubMed] [Google Scholar]
- 38.Jaruga B, Hong FH, Sun R, Radaeva S, Gao B. Crucial role of IL-4/STAT6 in T cell-mediated hepatitis: up-regulating eotaxins and IL-5 and recruiting leukocytes. J Immunol. 2003;171:3233–3244. doi: 10.4049/jimmunol.171.6.3233. [DOI] [PubMed] [Google Scholar]
- 39.Gandhi CR, Nemoto, Watkins SC, Subbotin VM. An endothelin receptor antagonist TAK-044 ameliorates carbon tetrachloride-induced acute liver injury and portal hypertension in rats. Liver. 1998;18:39–48. doi: 10.1111/j.1600-0676.1998.tb00125.x. [DOI] [PubMed] [Google Scholar]
- 40.Sonin NV, Garcia-Pagan JC, Nakanishi K, Zhang JX, Clemens MG. Patterns of vasoregulatory gene expression in the liver response to ischemia/reperfusion and endotoxemia. Shock. 1999;11:175–179. doi: 10.1097/00024382-199903000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Kawada N, Seki S, Kuroki T, Kaneda K. ROCK inhibitor Y-27632 attenuates stellate cell contraction and portal pressure increase induced by endothelin-1. Biochem Biophys Res Commun. 1999;266:296–300. doi: 10.1006/bbrc.1999.1823. [DOI] [PubMed] [Google Scholar]
- 42.Zhang JX, Pegoli W, Jr, Clemens MG. Endothelin-1 induces direct constriction of hepatic sinusoids. Am J Physiol. 1994;266:G624–G632. doi: 10.1152/ajpgi.1994.266.4.G624. [DOI] [PubMed] [Google Scholar]
- 43.Sakamoto M, Ueno T, Kin M, Ohira H, Torimura T, Inuzuka S, et al. Ito cell contraction in response to endothelin-1 and substance P. Hepatology. 1993;18:978–983. doi: 10.1002/hep.1840180432. [DOI] [PubMed] [Google Scholar]
- 44.Zhao RZ, Chen X, Yao Q, Chen C. TNF-alpha induces interleukin-8 and endothelin-1 expression in human endothelial cells with different redox pathways. Biochem Biophys Res Commun. 2005;327:985–992. doi: 10.1016/j.bbrc.2004.12.109. [DOI] [PubMed] [Google Scholar]
- 45.Rieder H, Ramadori G, Meyer zum Büschenfelde KH. Sinusoidal endothelial liver cells in vitro release endothelin--augmentation by transforming growth factor beta and Kupffer cell-conditioned media. Klin Wochenschr. 1991;69:387–391. doi: 10.1007/BF01647411. [DOI] [PubMed] [Google Scholar]
- 46.Gandhi CR. Kupffer Cells. In: Monga SP, editor. Molecular Pathology of Liver Diseases. Springer; 2010. pp. 81–96. [Google Scholar]
- 47.Frankenberg MV, Weimann J, Fritz S, Fiedler J, Mehrabi A, Büchler MW, et al. Gadolinium chloride-induced improvement of postischemic hepatic perfusion after warm ischemia is associated with reduced hepatic endothelin secretion. Transpl Int. 2005;18:429–436. doi: 10.1111/j.1432-2277.2004.00058.x. [DOI] [PubMed] [Google Scholar]
- 48.Shirasugi N, Wakabayashi G, Shimazu M, Oshima A, Shito M, Kawachi S, et al. Up-regulation of oxygen-derived free radicals by interleukin-1 in hepatic ischemia/reperfusion injury. Transplantation. 1997;64:1398–1403. doi: 10.1097/00007890-199711270-00004. [DOI] [PubMed] [Google Scholar]
- 49.Shito M, Wakabayashi G, Ueda M, Shimazu M, Shirasugi N, Endo M, et al. Interleukin 1 receptor blockade reduces tumor necrosis factor production, tissue injury, and mortality after hepatic ischemia-reperfusion in the rat. Transplantation. 1997;63:143–148. doi: 10.1097/00007890-199701150-00026. [DOI] [PubMed] [Google Scholar]
- 50.Tomiyama K, Ikeda A, Ueki S, Nakao A, Stolz DB, Koike Y, et al. Inhibition of Kupffer cell-mediated early proinflammatory response with carbon monoxide in transplant-induced hepatic ischemia/reperfusion injury in rats. Hepatology. 2008;48:1608–1620. doi: 10.1002/hep.22482. [DOI] [PubMed] [Google Scholar]
- 51.Decker K. Biologically active products of stimulated liver macrophages (Kupffer cells). Eur J Biochem. 1990;192:245–261. doi: 10.1111/j.1432-1033.1990.tb19222.x. [DOI] [PubMed] [Google Scholar]
- 52.Su GL. Lipopylysaccharides in liver injury: molecular mechanisms of Kupffer cell activation. Am J Physiol Gastrointest Liver Physiol. 2002;283:G256–G265. doi: 10.1152/ajpgi.00550.2001. [DOI] [PubMed] [Google Scholar]
- 53.Cutrn JC, Perrelli MG, Cavalieri B, Peralta C, Rosell Catafau J, et al. Microvascular dysfunction induced by reperfusion injury and protective effect of ischemic preconditioning. Free Radic Biol Med. 2002;33:1200–1208. doi: 10.1016/s0891-5849(02)01017-1. [DOI] [PubMed] [Google Scholar]
- 54.Tsukamoto H. Redox regulation of cytokine expression in Kupffer cells. Antioxid Redox Signal. 2002;4:741–748. doi: 10.1089/152308602760598882. [DOI] [PubMed] [Google Scholar]
- 55.Boulanger C, Lüscher TF. Release of endothelin from the porcine aorta. Inhibition by endothelium-derived nitric oxide. J Clin Invest. 1990;85:587–590. doi: 10.1172/JCI114477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kourembanas S, McQuillan LP, Leung GK, Faller DV. Nitric oxide regulates the expression of vasoconstrictors and growth factors by vascular endothelium under both normoxia and hypoxia. J Clin Invest. 1993;92:99–104. doi: 10.1172/JCI116604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Edwards MJ, Keller BJ, Kauffman FC, Thurman RG. The involvement of Kupffer cells in carbon tetrachloride toxicity. Toxicol Appl Pharmacol. 1993;119:275–279. doi: 10.1006/taap.1993.1069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.