Abstract
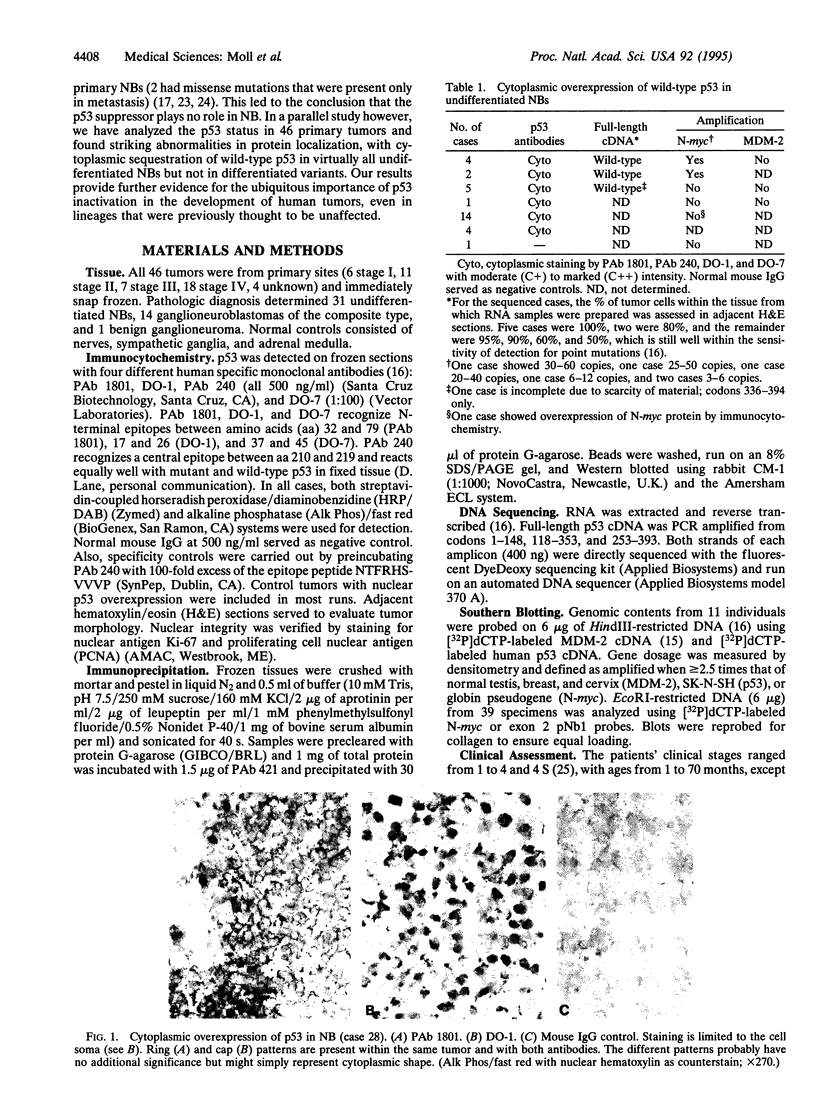
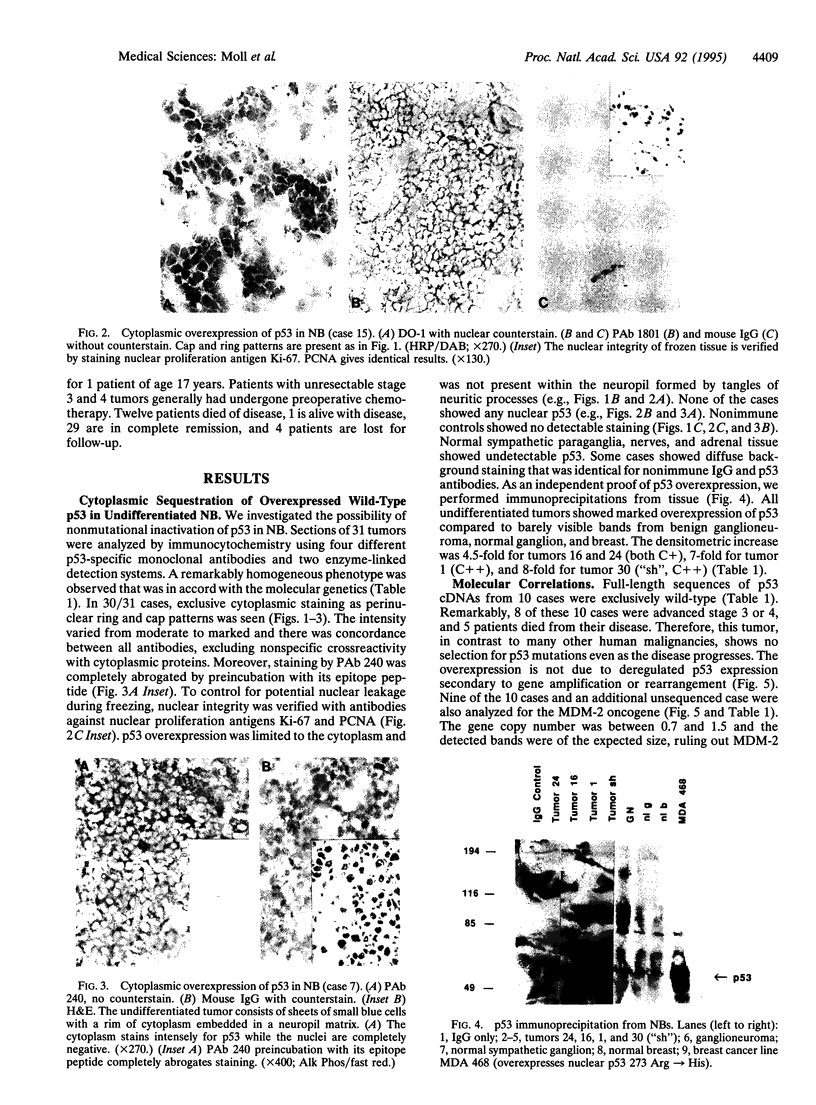
Neuroblastoma (NB), a tumor arising from the sympathetic nervous system, is one of the most common malignancies in childhood. Several recent reports on the p53 genotype found virtually exclusive wild-type status in primary tumors, and it was postulated that p53 plays no role in the development of NB. Here, however, we report that the vast majority of undifferentiated NBs exhibit abnormal cytoplasmic sequestration of wild-type p53. This inability of p53 to translocate to the nucleus presumably prevents the protein from functioning as a suppressor. Thirty of 31 cases (96%) of undifferentiated NB showed elevated levels of wild-type p53 in the cytoplasm of all tumor cells concomittant with a lack of nuclear staining. p53 immunoprecipitation from tumor tissues showed a 4.5- to 8-fold increase over normal protein levels. All of 10 tumors analyzed harbored wild-type p53 by direct sequencing of full-length cDNA and Southern blot. In addition, no MDM-2 gene amplification was seen in all 11 tumors analyzed. In contrast, no p53 abnormality was detected in 14 differentiated ganglioneuroblastomas and 1 benign ganglioneuroma. We conclude that loss of p53 function seems to play a major role in the tumorigenesis of undifferentiated NB. This tumor might abrogate the transactivating function of p53 by inhibiting its access to the nucleus, rather than by gene mutation. Importantly, our results suggest that (i) this could be a general mechanism for p53 inactivation not limited to breast cancer (where we first described it) and that (ii) it is found in a tumor previously not thought to be affected by p53 alteration.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brodeur G. M., Azar C., Brother M., Hiemstra J., Kaufman B., Marshall H., Moley J., Nakagawara A., Saylors R., Scavarda N. Neuroblastoma. Effect of genetic factors on prognosis and treatment. Cancer. 1992 Sep 15;70(6 Suppl):1685–1694. doi: 10.1002/1097-0142(19920915)70:4+<1685::aid-cncr2820701607>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Brodeur G. M., Seeger R. C., Barrett A., Berthold F., Castleberry R. P., D'Angio G., De Bernardi B., Evans A. E., Favrot M., Freeman A. I. International criteria for diagnosis, staging, and response to treatment in patients with neuroblastoma. J Clin Oncol. 1988 Dec;6(12):1874–1881. doi: 10.1200/JCO.1988.6.12.1874. [DOI] [PubMed] [Google Scholar]
- Brodeur G. M., Seeger R. C., Schwab M., Varmus H. E., Bishop J. M. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984 Jun 8;224(4653):1121–1124. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- Davidoff A. M., Pence J. C., Shorter N. A., Iglehart J. D., Marks J. R. Expression of p53 in human neuroblastoma- and neuroepithelioma-derived cell lines. Oncogene. 1992 Jan;7(1):127–133. [PubMed] [Google Scholar]
- Debbas M., White E. Wild-type p53 mediates apoptosis by E1A, which is inhibited by E1B. Genes Dev. 1993 Apr;7(4):546–554. doi: 10.1101/gad.7.4.546. [DOI] [PubMed] [Google Scholar]
- Fong C. T., White P. S., Peterson K., Sapienza C., Cavenee W. K., Kern S. E., Vogelstein B., Cantor A. B., Look A. T., Brodeur G. M. Loss of heterozygosity for chromosomes 1 or 14 defines subsets of advanced neuroblastomas. Cancer Res. 1992 Apr 1;52(7):1780–1785. [PubMed] [Google Scholar]
- Funk W. D., Pak D. T., Karas R. H., Wright W. E., Shay J. W. A transcriptionally active DNA-binding site for human p53 protein complexes. Mol Cell Biol. 1992 Jun;12(6):2866–2871. doi: 10.1128/mcb.12.6.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert F., Feder M., Balaban G., Brangman D., Lurie D. K., Podolsky R., Rinaldt V., Vinikoor N., Weisband J. Human neuroblastomas and abnormalities of chromosomes 1 and 17. Cancer Res. 1984 Nov;44(11):5444–5449. [PubMed] [Google Scholar]
- Harris C. C., Hollstein M. Clinical implications of the p53 tumor-suppressor gene. N Engl J Med. 1993 Oct 28;329(18):1318–1327. doi: 10.1056/NEJM199310283291807. [DOI] [PubMed] [Google Scholar]
- Harvey D. M., Levine A. J. p53 alteration is a common event in the spontaneous immortalization of primary BALB/c murine embryo fibroblasts. Genes Dev. 1991 Dec;5(12B):2375–2385. doi: 10.1101/gad.5.12b.2375. [DOI] [PubMed] [Google Scholar]
- Imamura J., Bartram C. R., Berthold F., Harms D., Nakamura H., Koeffler H. P. Mutation of the p53 gene in neuroblastoma and its relationship with N-myc amplification. Cancer Res. 1993 Sep 1;53(17):4053–4058. [PubMed] [Google Scholar]
- Kastan M. B., Zhan Q., el-Deiry W. S., Carrier F., Jacks T., Walsh W. V., Plunkett B. S., Vogelstein B., Fornace A. J., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992 Nov 13;71(4):587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Komuro H., Hayashi Y., Kawamura M., Hayashi K., Kaneko Y., Kamoshita S., Hanada R., Yamamoto K., Hongo T., Yamada M. Mutations of the p53 gene are involved in Ewing's sarcomas but not in neuroblastomas. Cancer Res. 1993 Nov 1;53(21):5284–5288. [PubMed] [Google Scholar]
- Landers J. E., Haines D. S., Strauss J. F., 3rd, George D. L. Enhanced translation: a novel mechanism of mdm2 oncogene overexpression identified in human tumor cells. Oncogene. 1994 Sep;9(9):2745–2750. [PubMed] [Google Scholar]
- Livingstone L. R., White A., Sprouse J., Livanos E., Jacks T., Tlsty T. D. Altered cell cycle arrest and gene amplification potential accompany loss of wild-type p53. Cell. 1992 Sep 18;70(6):923–935. doi: 10.1016/0092-8674(92)90243-6. [DOI] [PubMed] [Google Scholar]
- Mietz J. A., Unger T., Huibregtse J. M., Howley P. M. The transcriptional transactivation function of wild-type p53 is inhibited by SV40 large T-antigen and by HPV-16 E6 oncoprotein. EMBO J. 1992 Dec;11(13):5013–5020. doi: 10.1002/j.1460-2075.1992.tb05608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll U. M., Riou G., Levine A. J. Two distinct mechanisms alter p53 in breast cancer: mutation and nuclear exclusion. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7262–7266. doi: 10.1073/pnas.89.15.7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momand J., Zambetti G. P., Olson D. C., George D., Levine A. J. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992 Jun 26;69(7):1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- Oliner J. D., Kinzler K. W., Meltzer P. S., George D. L., Vogelstein B. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature. 1992 Jul 2;358(6381):80–83. doi: 10.1038/358080a0. [DOI] [PubMed] [Google Scholar]
- Scheffner M., Werness B. A., Huibregtse J. M., Levine A. J., Howley P. M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990 Dec 21;63(6):1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- Seto E., Usheva A., Zambetti G. P., Momand J., Horikoshi N., Weinmann R., Levine A. J., Shenk T. Wild-type p53 binds to the TATA-binding protein and represses transcription. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12028–12032. doi: 10.1073/pnas.89.24.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaulsky G., Goldfinger N., Peled A., Rotter V. Involvement of wild-type p53 in pre-B-cell differentiation in vitro. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8982–8986. doi: 10.1073/pnas.88.20.8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaulsky G., Goldfinger N., Tosky M. S., Levine A. J., Rotter V. Nuclear localization is essential for the activity of p53 protein. Oncogene. 1991 Nov;6(11):2055–2065. [PubMed] [Google Scholar]
- Sidell N., Koeffler H. P. Modulation of Mr 53,000 protein with induction of differentiation of human neuroblastoma cells. Cancer Res. 1988 Apr 15;48(8):2226–2230. [PubMed] [Google Scholar]
- Vogan K., Bernstein M., Leclerc J. M., Brisson L., Brossard J., Brodeur G. M., Pelletier J., Gros P. Absence of p53 gene mutations in primary neuroblastomas. Cancer Res. 1993 Nov 1;53(21):5269–5273. [PubMed] [Google Scholar]
- Xiong Y., Hannon G. J., Zhang H., Casso D., Kobayashi R., Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993 Dec 16;366(6456):701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- Yin Y., Tainsky M. A., Bischoff F. Z., Strong L. C., Wahl G. M. Wild-type p53 restores cell cycle control and inhibits gene amplification in cells with mutant p53 alleles. Cell. 1992 Sep 18;70(6):937–948. doi: 10.1016/0092-8674(92)90244-7. [DOI] [PubMed] [Google Scholar]
- Zambetti G. P., Levine A. J. A comparison of the biological activities of wild-type and mutant p53. FASEB J. 1993 Jul;7(10):855–865. doi: 10.1096/fasebj.7.10.8344485. [DOI] [PubMed] [Google Scholar]
- el-Deiry W. S., Kern S. E., Pietenpol J. A., Kinzler K. W., Vogelstein B. Definition of a consensus binding site for p53. Nat Genet. 1992 Apr;1(1):45–49. doi: 10.1038/ng0492-45. [DOI] [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]