Abstract
Background: Secondary peritonitis is an advanced form of complicated intra-abdominal infection (cIAI) requiring hospitalization, surgical source control, and empiric antibiotic therapy against causative aerobic and anaerobic bacteria.
Methods: This pooled analysis of four prospective, active-controlled randomized clinical trials compared the efficacy and safety of moxifloxacin with that of comparator antibiotics in patients with confirmed secondary peritonitis. The primary efficacy endpoint was clinical success rate at test-of-cure (TOC) between day 10 and 45 post-therapy in the per-protocol (PP) population. Safety and clinical efficacy were assessed also in the intent-to-treat population (ITT). Bacteriological success was assessed at TOC in the microbiologically-valid population as a secondary efficacy endpoint.
Results: Overall clinical success rates at TOC were 85.3% (431 of 505 patients) in the moxifloxacin and 88.4% (459 of 519 patients) in the comparator treatment groups (PP population, point estimate for the difference in success rates: −3.0%; 95% CI −7.06%, 1.05%), respectively. Similar clinical success rates between moxifloxacin and comparators were observed by anatomical site of infection, and ranged from 80.6% to 100% for moxifloxacin and from 71.4% to 96.6% for comparators, respectively. Bacteriologic success rates were similar with moxifloxacin (82.4%) and comparators (86.8%), respectively. The proportion of patients experiencing any treatment-emergent adverse events was slightly higher with moxifloxacin (67.3%) versus comparators (59.8%). Rates of drug-related adverse events (20.9% versus 20.0%) and deaths (4.3% versus 3.4%) were similar in moxifloxacin and comparator groups; none of the deaths were drug-related.
Conclusions: The data suggests that once-daily IV (or IV/PO) moxifloxacin has a comparable efficacy and safety profile to antibiotic regimens approved previously in the subgroup of patients with secondary peritonitis of mild-to-moderate severity.
Secondary peritonitis can arise from perforation or penetrating injury of the gastrointestinal tract as well as from ischemic necrosis [1]. Complicated intra-abdominal infections (cIAIs) require hospitalization, adequate surgery, antibiotic therapy, and continuous supportive care and monitoring [2,3]. If these interventions are delayed or inadequate, secondary peritonitis can spread rapidly throughout the abdominal cavity; up to 40% of patients with secondary peritonitis may progress to sepsis or septic shock with high mortality rates approaching 30–40% [1].
The bacteriologic etiology of secondary peritonitis is frequently polymicrobial due to concurrent infection with gram-positive and gram-negative aerobic and anaerobic pathogens [1,3–7]. Escherichia coli is usually the major aerobic isolate, although Bacteroides fragilis and other Bacteroides spp. are also anaerobes isolated frequently [5,6].
Current guidelines recommend empirical broad-spectrum antibiotic therapy as exact information on the responsible bacterial species and susceptibility results are rarely known when the treatment is initiated. Adequate source control and prompt, appropriate empiric treatment is essential to improve outcome [3,4,8] and to reduce mortality [9]. Fluoroquinolones, which are broad-spectrum antibiotics with favorable efficacy and safety profiles for the management of mild-to-moderate cIAIs either as monotherapy (moxifloxacin) or in combination therapy (levofloxacin, ciprofloxacin), are recommended in the latest Surgical Infection Society (SIS)/Infection Infectious Diseases Society of America (IDSA) guidelines for the treatment of mild-to-moderate infections [3,4]. Moxifloxacin has a favorable pharmacokinetic/pharmacodynamic (PK/PD) profile with an excellent penetration into gastrointestinal tissues, including abdominal abscesses [10] and peritoneal exudates in patients with peritonitis [11]. It is available in intravenous (IV) and oral formulations for once-daily administration [12].
Complicated IAIs encompass a wide spectrum of diseases in terms of peritoneal expansion, systemic response, surgical approach, and mortality rate. Secondary peritonitis represents the most severe forms of cIAIs (as opposed to localized or uncomplicated disease) due to its extent, inoculum size, and systemic response, either acquired in the community or associated with healthcare institutions.
On the basis of pooled data from the four Phase III randomized, active-controlled, prospective clinical trials [13–16] comparing the efficacy and safety of moxifloxacin monotherapy with that of comparator regimens in mild-to-moderate cIAIs, we undertook a retrospective analysis of the efficacy and safety of moxifloxacin in this subgroup of patients with secondary peritonitis, reflecting an advanced form of peritonitis.
Patients and Methods
Design of studies used in the analysis
Of the four randomized controlled trials (conducted between 2000 and 2009), three were double-blind [13,15–16] and one study was open-label [14]. All studies were designed to demonstrate the non-inferiority of moxifloxacin to comparator antibiotic regimens and used a non-inferiority margin of either 10% [13–14,16] or 15% [15]. All four studies were performed in accordance with the Declaration of Helsinki, the rules of International Conference on Harmonization (ICH) Good Clinical Practice and relevant national guidelines. Independent Ethics Committees approved the respective study protocols and written consent prior to enrollment from all patients in each study was obtained. The main regions enrolling patients in these studies were the United States [13], Asia [15], and Europe [14,16]. The clinical diagnoses were described in detail in the clinical study protocol and approved by regulatory authorities prior to the initiation of the studies in each individual study. They were collected on the case report form (CRF) in the four studies, and stored in the respective individual trial database. Finally, they were transferred to the pooled database of the studies. The CRF was not standardized across the four studies that would have captured specific cIAI diagnosis. If multiple diagnoses were documented in the CRF for the same patient, the primary diagnosis and the site of infection were determined and evaluated for validity of secondary peritonitis. For the purpose of documentation, a statistical analysis plan was prepared prior to performing the pooled subgroup analysis.
Inclusion criteria
Eligible patients were adults (≥18 y) with a primary diagnosis of cIAI that was supported by radiologic evidence of gastrointestinal tract perforation and required surgical intervention by laparotomy or laparoscopy [13–16] or percutaneous aspiration [13–15] for source control in addition to IV antibiotic therapy. Patients also had evidence of gross peritoneal inflammation with purulent exudates into the peritoneal cavity. Diagnosis could also be suspected in patients with radiological evidence of gastrointestinal perforation, and the presence of at least one abdominal cavity symptom lasting ≥24 h, together with evidence of at least one of abdominal tenderness, absent or diminished bowel sounds, or abdominal wall rigidity and at least two systemic signs of infection that included elevated body temperature (>38.3OC rectal or tympanic membrane, >37.8OC oral, or >37.3OC axillary), heart rate of >90 beats/min, respiratory rate of >20 breaths/min, and a white blood cell count of >12,000 cells/mm3 or <4,000 cells/mm3. It had to be confirmed by surgical findings within 24 h of enrollment [13–16]. Severity of the disease was described by the Acute Physiology and Chronic Health Evaluation (APACHE) II score in the current analysis.
Exclusion criteria
Main exclusion criteria were: Spontaneous bacterial peritonitis, conditions not requiring antibiotic therapy for a minimum of three days (e.g., non-complicated acute appendicitis, acute cholecystitis with infection confined to the gallbladder, transmural necrosis of the intestine due to acute embolic, thrombotic, or obstructive occlusions); the presence of known prolongation of the electrocardiogram (ECG) QT interval, uncorrected hypokalemia, concomitant antiarrhythmic drugs; severe infection requiring high-dose vasopressor drugs; acute kidney injury; and contraindications to study drugs (e.g., pregnancy, breastfeeding, or hypersensitivity) [13–16].
Antibiotic regimens
In all four studies, patients were randomized to receive IV moxifloxacin 400 mg (Avelox®, GP) once daily (QD) for up to 14 d [13–16], with the option to switch to per os (PO) moxifloxacin [12] 400 mg after a minimum of three days in two studies [13–14]. The comparator treatments included the following antibiotics: i, Piperacillin-tazobactam [17], 3.0g/0.375g IV, four times daily (QID), followed by amoxicillin/clavulanic acid [18], 800mg/114mg PO, twice daily (BID) [13]; ii, ceftriaxone [19], 2.0g QID plus metronidazole [20], 500mg IV, three times daily (TID), followed by amoxicillin/clavulanic acid [18], 500mg/125mg PO, TID [14]; iii, ceftriaxone [19], 2.0g QD plus metronidazole [20], 500mg IV, BID [15]; and iv ertapenem [21], 1.0 g IV, QID [16], respectively. The minimum length of antibiotic therapy was either five days in studies conducted by Malangoni et al. [13] and De Waele et al. [16] or three days in studies conducted by Weiss et al. [14] and Solomkin et al. [15].
Outcome parameters
The primary efficacy endpoint in all four studies was clinical success at test-of-cure (TOC) that occurred between day 10 and 45 after antibiotic therapy in the per-protocol (PP) population (according to individual studies: 11–45 d [13]; 14–37 d [14]; 10–14 d [15]; and 21–28 d [16]. Clinical success was defined as continued resolution or improvement of clinical signs and symptoms related to the infection not requiring any antibiotic therapy, and without the occurrence of any surgical infection requiring a systemic antibiotic treatment at TOC. Secondary efficacy outcome parameters were clinical success rate at TOC by anatomic site of infection, bacteriologic success (eradication plus presumed eradication) rate at TOC by anatomical site of infection (for overall and by bacteria type) and bacteriologic success rate for main organisms at TOC by the anatomic site of the infection [13–16].
Statistical analyses
Clinical efficacy assessments were based primarily on the PP population, which included all patients who met study specific criteria that were common to all four studies. These criteria included: 1, cIAI requiring surgery and supportive management; 2, no other systemic antimicrobial agent administered concomitantly with the study drug unless the subject was a treatment failure; 3, documented compliance with ≥80% of the study medication administered; 4, no protocol violations influencing the treatment efficacy; and 5, successful completion of an assessment at the test-of-cure (TOC) visit. Safety parameters were assessed in the intent-to-treat (ITT) population that included all randomized patients who had received at least one dose of study medication and had at least one observation after study drug intake. This pooled analysis also compared the clinical efficacy of moxifloxacin treatment with that of pooled comparator treatment in the ITT population. Secondary efficacy assessments included bacteriologic outcome parameters in the microbiologically-valid (MBV) population that included all patients who met the inclusion criteria in the PP population and had a causative organism at baseline as well as a bacteriological evaluation at the TOC visit. For the primary efficacy analysis, differences in clinical success rates for moxifloxacin versus pooled active comparator treatment in the PP population were calculated using a Mantel-Haenszel type analysis stratified by study, with 95% confidence intervals (CIs) for the difference in success rates computed according to Rothman et al. [22]. To assess possible heterogeneity of clinical success rates across the four studies, the Q test was applied where significance at the 10% level indicated statistical heterogeneity [23]. Forest plots were created to visualize the variability of the difference in success rates across trials. All analyses were exploratory in nature; confirmatory statistics were not carried out.
Results
Patients' demographic characteristics
Across the four studies, 1,229 patients had a confirmed diagnosis of secondary peritonitis in the ITT population, of whom 1,024 met the inclusion criteria in the PP population. A large proportion of patients (52.2%, 642 of 1,229 patients) included in these pooled analyses originated from the PROMISE study [16]. Baseline demographic and disease characteristics were similar for moxifloxacin-treated patients and those in the comparator arms in both the ITT and PP populations (Table 1). The whole pooled study population was predominantly under 50 y of age, approximately 71% of the patients had a comorbid illness and 37% of the patients were treated previously with a different antibiotic agent prior to study drugs. Most patients had community-acquired (>95%) cIAIs of polymicrobial etiology (>60%) that were of mild-to-moderate severity on the basis of APACHE II scores (mean±SD, 7.0±5.0). Approximately 3% of the patients had bacteremia. Consistent with a diagnosis of secondary peritonitis, patients showed signs of infection such as fever (median 38.5°C), raised white blood cell count, and high concentrations of C-reactive protein (Table 1).
Table 1.
Demographics and Baseline Characteristics of Patients with Secondary Peritonitis in the Pooled Analysis of cIAIs
| PP population | ITT population | |||||
|---|---|---|---|---|---|---|
| Characteristic | All patients (N=1,024) | Moxifloxacin (N=505) | Comparators (N=519) | All patients (N=1,229) | Moxifloxacin (N=609) | Comparators (N=620) |
| Gender, male, n (%) | 673 (65.7) | 330 (65.3) | 343 (66.1) | 807 (65.7) | 396 (65.0) | 411 (66.3) |
| Mean age±SD, y | 46.7±18.4 | 46.3±18.3 | 47.0±18.6 | 47.6±18.7 | 47.6±18.7 | 47.7±18.6 |
| Age ≥65 y, n (%) | 208 (20.3) | 94 (18.6) | 114 (22.0) | 269 (21.9) | 128 (21.0) | 141 (22.7) |
| Mean BMI, kg/m2 (SD) | 25.7±4.9 | 25.9±4.8 | 25.5±5.0 | 25.9±5.1 | 26.2±5.1 | 25.7±5.1 |
| Coexisting illnesses, n (%) | 700 (68.4) | 353 (69.9) | 347 (66.9) | 872 (71.0) | 443 (72.7) | 429 (69.2) |
| Origin of infection, community-acquired, n (%) | 983 (96.0) | 486 (96.2) | 497 (95.8) | 1,174 (95.5) | 584 (95.9) | 590 (95.2) |
| Mean duration of symptoms, d (SD) | 4.2±2.5 | 4.2±2.7 | 4.1±2.3 | 4.2±2.6 | 4.3±2.6 | 4.2±2.5 |
| >2 d, n (%) | 881 (86.0) | 433 (85.7) | 448 (86.3) | 1,057 (86.0) | 521 (85.6) | 536 (86.5) |
| Previous antibiotic therapy*, n (%) | 348 (34.0) | 167 (33.1) | 181 (34.9) | 463 (37.7) | 223 (36.6) | 240 (38.7) |
| Mean core temperature, °C (SD) | 38.5±0.92 | 38.5±0.90 | 38.5±0.94 | 38.5±0.93 | 38.5±0.92 | 38.5±0.94 |
| Mean WBC, (×109/L (SD) | 13.4±5.2 | 13.4±5.1 | 13.4±5.4 | 13.4±5.5 | 13.5±5.7 | 13.4±5.4 |
| Mean C-reactive protein, mg/dL (SD) | 19.8±18.3 | 20.2±17.7 | 19.5±18.8 | 20.2±18.7 | 20.9±19.2 | 19.4±18.2 |
| Mean APACHE II score (SD) | 6.9±5.0 | 6.9±4.7 | 6.9±5.2 | 7.0±5.0 | 7.1±4.8 | 6.9±5.2 |
| Anatomic site of infection, n (%) | ||||||
| Gallbladder and biliary tract | 55 (5.4) | 26 (5.1) | 29 (5.6) | 63 (5.1) | 29 (4.8) | 34 (5.5) |
| Stomach or duodenum | 189 (18.5) | 91 (18.0) | 98 (18.9) | 228 (18.6) | 116 (19.0) | 112 (18.1) |
| Appendix | 534 (52.1) | 269 (53.3) | 265 (51.1) | 621 (50.5) | 308 (50.6) | 313 (50.5) |
| Large bowel | 166 (16.2) | 86 (17.0) | 80 (15.4) | 213 (17.3) | 107 (17.6) | 106 (17.1) |
| Small bowel | 71 (6.9) | 31 (6.1) | 40 (7.7) | 90 (7.3) | 43 (7.1) | 47 (7.6) |
| Others | 5 (0.5) | 1 (0.2) | 4 (0.8) | 8 (0.7) | 4 (0.7) | 4 (0.6) |
| Unknown | 4 (0.4) | 1 (0.2) | 3 (0.6) | 6 (0.5) | 2 (0.3) | 4 (0.6) |
| Polymicrobial infection, n (%) | 629 (61.4) | 319 (63.2) | 310 (59.7) | 741 (60.3) | 381 (62.6) | 360 (58.1) |
| Bacteremia, n (%) | 29 (2.8) | 15 (3.0) | 14 (2.7) | 37 (3.0) | 22 (3.6) | 15 (2.4) |
APACHE=Acute Physiology and Chronic Health Evaluation; BMI=body mass index; cIAI=complicated intra-abdominal infections; ITT=intent-to-treat; PP=per-protocol; SD=standard deviation; WBC=white blood cell count. *Antibiotic administered within the week preceding the start of study therapy.
Efficacy analyses
Clinical efficacy
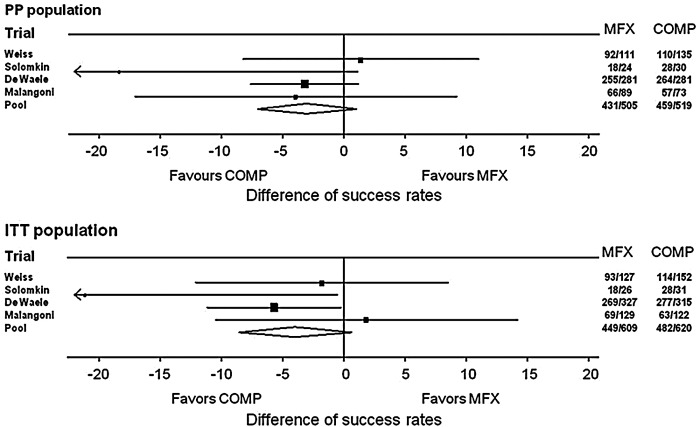
The primary endpoint, namely that moxifloxacin was non-inferior with regards to clinical efficacy versus comparator regimens in the cIAI patient populations as defined in the individual study protocols, was achieved in all four clinical studies. In the subgroup of patients with secondary peritonitis, the clinical success rates at TOC were 85.3% and 88.4% in the moxifloxacin and comparator treatment arms of the PP population, respectively, (point estimate for the difference in success rates: −3.01%; 95% CI: −7.06%, 1.05%) (Table 2, Fig. 1). Corresponding clinical success rates for the ITT population were 73.7% and 77.7%, respectively (point estimate −3.96%; 95% CI: −8.54%, 0.61%) (Table 2, Fig. 1). The point estimates for the difference in clinical success rates in the PP population ranged from −18.33% [15] to 1.4% [13], but the test of heterogeneity showed no significance at the 10% level indicating that the point estimates were consistent across the four studies included in the analysis (Table 2).
Table 2.
Clinical Success Rates at TOC (10 to 45 Days Post-Therapy) in Patients with Secondary Peritonitis in the Pooled Analysis of cIAIs
| Study | Moxifloxacin n/N (%) | Comparators n/N (%) | 95% CI (%, %) | Point estimate (%) | Relative weight | Heterogeneity |
|---|---|---|---|---|---|---|
| PP populations | ||||||
| Malangoni (2000–2003, USA)13 | 66/89 (74.2) | 57/73 (78.1) | −17.07–9.22 | −3.92 | 15.74% | 0.02 (0.58%) |
| Weiss (2001–2002, Europe)14 | 92/111 (82.9) | 110/135 (81.5) | −8.19–10.99 | 1.40 | 23.90% | 0.81 (25.25%) |
| Solomkin (2005–2007, Asia)15 | 18/24 (75.0) | 28/30 (93.3) | −37.82–1.15 | −18.33 | 5.23% | 2.38 (73.93%) |
| De Waele (2006–2009, Europe)16 | 255/281 (90.7) | 264/281 (94.0) | −7.59–1.18 | −3.20 | 55.13% | < 0.01 (0.24%) |
| Total | 431/505 (85.3) | 459/519 (88.4) | −7.06–1.05 | −3.01 | Chi2=3.21 p value=0.360* |
|
| ITT populations | ||||||
|---|---|---|---|---|---|---|
| Malangoni (2000–2003, USA)13 | 69/129 (53.5) | 63/122 (51.6) | −10.51–14.21 | 1.85 | 20.46% | 0.85 (20.94%) |
| Weiss (2001–2002, Europe)14 | 93/127 (73.2) | 114/152 (75.0) | −12.10–8.56 | −1.77 | 22.58% | 0.17 (4.27%) |
| Solomkin (2005–2007, Asia)15 | 18/26 (69.2) | 28/31 (90.3) | −41.66–−0.52 | −21.09 | 4.61% | 2.66 (65.61%) |
| De Waele (2006–2009, Europe)16 | 269/327 (82.3) | 277/315 (87.9) | −11.16–−0.19 | −5.67 | 52.35% | 0.37 (9.18%) |
| Total | 449/609 (73.7) | 482/620 (77.7) | −8.54–0.61 | −3.96 | Chi2=4.06 p value=0.255* |
|
CI=confidence interval; cIAI=complicated intra-abdominal infections; ITT=intent-to-treat; PP, per-protocol; TOC=test-of-cure. *P value refers to heterogeneity test and not to comparison of clinical efficacy between treatment groups.
Fig. 1.
Forest plots for clinical efficacy in individual studies and overall (PP and ITT populations).
When analyzed by anatomical site of infection across the studies, moxifloxacin monotherapy achieved similar clinical success rates to comparators in infections localized in the gallbladder and biliary tract, stomach or duodenum, appendix, large bowel, small bowel, or in other locations (Table 3).
Table 3.
Clinical Success at TOC (10–45 Days Post-Therapy) by Anatomical Site of Infection in Patients with Secondary Peritonitis in the Pooled Analysis of cIAIs (PP Population)
| Site of infection | Moxifloxacin n/N (%) | Comparator n/N (%) |
|---|---|---|
| Gallbladder and biliary tract | 26/26 (100) | 28/29 (96.6) |
| Stomach or duodenum | 79/91 (86.8) | 91/98 (92.9) |
| Appendix | 229/269 (85.1) | 244/265 (92.1) |
| Large bowel | 70/86 (81.4) | 59/80 (73.8) |
| Small bowel | 25/31 (80.6) | 32/40 (80.0) |
| Others/Unknown | 2/2 (100) | 5/7 (71.4) |
cIAI=complicated intra-abdominal infections; PP=per-protocol; TOC=test-of-cure.
Bacteriologic efficacy
Escherichia coli and Bacteroides fragilis were the pathogens isolated most commonly from patients with secondary peritonitis which is in accordance with previous studies [5,24]. This pooled analysis revealed that in the MBV population, E. coli was isolated in 67.6% (269/398) of moxifloxacin- and 68.5% (270/394) of comparator-treated patients across the four clinical trials. The second species cultured most frequently was B. fragilis in 26.9% (107/398) of moxifloxacin- and 28.9% (114/394) of comparator-treated patients. Other Bacteroides species such as B. thetaiotaomicron (14.1% vs. 14.2%), B. distasonis (4.5% vs. 4.1%), B. ovatus (6.0% vs. 3.8%), B. uniformis (4.3% vs. 5.8%), and B. vulgatus (5.0% vs. 2.3%) were isolated less frequently. P. aeruginosa was isolated from 13.1% (52/398) and 9.1% (36/394) of moxifloxacin- and comparator-treated patients, respectively. Enterococcus avium (6.3% vs. 5.8%), E. faecalis (9.0% vs. 10.7%), E. faecium (5.8% vs. 5.1%) were also isolated but in lower frequency from patients with secondary peritonitis.
The majority of infections in patients with secondary peritonitis were due to either aerobic bacteria (40.2% and 46.0% of moxifloxacin- and comparator-treated patients, respectively) or to a mix of aerobic and anaerobic bacteria (56.8% and 51.8% of moxifloxacin- and comparator-treated patients, respectively). In the MBV population, 11 moxifloxacin- and eight comparator-treated patients had only anaerobic bacteria isolated.
Consistent with the clinical success rates, pooled bacteriologic success rates suggested that moxifloxacin was as effective as comparators (82.4% vs. 86.8%, Table 4). Treatment of patients with only anaerobic bacteria resulted in lower but comparable eradication rates (Table 4). Moxifloxacin was as effective as comparator antibiotics against E. coli (84.4% vs. 87.4%, respectively) and B. fragilis (84.1% vs. 88.6%, respectively) (Table 5).
Table 4.
Bacteriological Success (Eradication/Presumed Eradication) Rates at TOC (10–45 D Post-Therapy) in Patients with Secondary Peritonitis in the Pooled Analysis of cIAIs (MBV Population)
| Bacteria type | Moxifloxacin n/N (%) | Comparator n/N (%) |
|---|---|---|
| Aerobic only | 133/160 (83.1) | 162/181 (89.5) |
| Anaerobic only | 8/11 (72.7) | 6/8 (75.0) |
| Mixed (aerobic and anaerobic) | 186/226 (82.3) | 173/204 (84.8) |
| Overall* | 328/398 (82.4) | 342/394 (86.8) |
MBV=microbiologically valid; TOC=test-of-cure. *One isolate in each treatment group of other type of organism was successfully eradicated.
Table 5.
Bacteriological Success (Eradication/Presumed Eradication) Rates by Baseline Pathogen at TOC (10–45 D Post-Therapy) (MBV Population)
| Organism | Moxifloxacin n/N (%) | Comparator n/N (%) |
|---|---|---|
| Gram-positive aerobic | 155/197 (78.7) | 161/195 (82.6) |
| Enterococcus avium | 21/25 (84.0) | 18/23 (78.3) |
| Enterococcus faecalis | 28/36 (77.8) | 30/42 (71.4) |
| Enterococcus faecium | 17/23 (73.9) | 15/20 (75.0) |
| Streptococcus anginosus | 47/59 (79.7) | 56/65 (86.2) |
| Streptococcus constellatus | 42/54 (77.8) | 42/45 (93.3) |
| Gram-negative aerobic | 351/420 (83.6) | 335/387 (86.6) |
| Citrobacter freundii | 9/11 (81.8) | 12/13 (92.3) |
| Enterobacter cloacae | 10/13 (76.9) | 8/9 (88.9) |
| Escherichia coli | 227/269 (84.4) | 236/270 (87.4) |
| Klebsiella oxytoca | 21/24 (87.5) | 15/18 (83.3) |
| Klebsiella pneumoniae | 27/32 (84.4) | 25/31 (80.6) |
| Pseudomonas aeruginosa | 43/52 (82.7) | 30/36 (83.3) |
| Proteus mirabilis | 14/19 (73.7) | 9/10 (90.0) |
| Gram-positive anaerobic | 22/31 (71.0) | 33/39 (84.6) |
| Clostridium species | 18/23 (78.3) | 24/28 (85.7) |
| Peptostrepcococcus species | 4/8 (50.0) | 9/11 (81.8) |
| Gram-negative anaerobic | 237/279 (85.0) | 222/254 (87.4) |
| Bacteroides distasonis | 16/18 (88.9) | 14/16 (87.5) |
| Bacteroides fragilis | 90/107 (84.1) | 101/114 (88.6) |
| Bacteroides ovatus | 19/24 (79.2) | 14/15 (93.3) |
| Bacteroides thetaiotaomicron | 48/56 (85.7) | 49/56 (87.5) |
| Bacteroides uniformis | 16/17 (94.1) | 19/23 (82.6) |
| Bacteroides vulgatus | 17/20 (85.0) | 7/9 (77.8) |
| Other Bacteroides species | 31/37 (83.8) | 18/21 (85.7) |
MBV=microbiologically valid; TOC=test-of-cure.
Safety
Adverse events (AEs) occurred in 67.3% and 59.8% of the moxifloxacin and comparator treatment regimens in the ITT population, respectively (Table 6). The most common adverse events in moxifloxacin-treated patients (MXF) versus comparator-treated patients were nausea and diarrhea. Furthermore, as a consequence of open abdominal surgery, surgical infections (or these could be considered as surgical site infections) occurred in 10.7% (65/609) and 8.2% (51/620) of moxifloxacin- and comparator-treated patients, respectively. Approximately 20% of patients had drug-related AEs in each group (MXF: 20.9% vs. COMP: 20.0%), of which 19 (3.1%) and six (1.0%) experienced serious drug-related AEs in the moxifloxacin and comparator treatment arms, respectively. The difference in the frequency of serious drug-related AEs between moxifloxacin and comparators was driven mainly by gastrointestinal disorders, surgical infections, asymptomatic prolongation of the QT interval on the ECG, and increased liver enzymes. One patient (0.16%) in the MXF group and three patients (0.5%) in the COMP group had Clostridium difficile colitis as adverse event; and there was one patient with clostridial infection in the COMP group as reported adverse event in this subset of patients. The C. difficile colitis case was related to the study drug in the MXF-treated patient; however, this was not a serious AE. In the COMP group two cases of C. difficile colitis were serious AEs and these were both related to the study drug. Treatment was discontinued prematurely in 5.1% of MXF and in 4.0% of those in the comparator treatment arm (Table 6). Deaths, none of which were attributed to study medication, occurred with a similar frequency in both treatment arms (4.3% vs. 3.4% of the moxifloxacin- and comparator-treated patients, respectively) (Table 6) and were not attributed to study medication. All of these fatalities were treatment failures or indeterminate cases (patients in whom clinical evaluation was not possible to determine) prior to their deaths, and their infections developed into sepsis or septic shock and resulted in further complications such as acute respiratory distress syndrome, hemodynamic shock, multiple organ dysfunction syndrome, embolism, hemorrhage, respiratory or cardiac failure, or pneumonia.
Table 6.
Incidence of Treatment-Emergent AEs in Patients with Secondary Peritonitis (ITT Population)
| MedDRA PT Event | Moxifloxacin (N=609) n (%) | Comparator (N=620) n (%) |
|---|---|---|
| Any AEs | 410 (67.3) | 371 (59.8) |
| Nausea | 49 (8.0) | 28 (4.5) |
| Diarrhea | 39 (6.4) | 49 (7.9) |
| Abdominal pain | 26 (4.3) | 19 (3.1) |
| Constipation | 21 (3.4) | 19 (3.1) |
| Surgical site infection | 65 (10.7) | 51 (8.2) |
| Post-operative Surgical site infection | 10 (1.6) | 6 (1.0) |
| Drug-related AEs occurring in >5 patients in either treatment group Any | 127 (20.9) | 124 (20.0) |
| Serious AEs | 110 (18.1) | 88 (14.2) |
| Drug-related serious AEs | 19 (3.1) | 6 (1.0) |
| Premature discontinuations due to AEs | 31 (5.1) | 25 (4.0) |
| Deaths | 26 (4.3) | 21 (3.4) |
AE=adverse event; ITT=intent-to-treat; MedDRA PT=Medical Dictionary for Regulatory Activities Preferred Term.
Discussion
The results of these pooled analyses suggest that, for the treatment of secondary peritonitis, moxifloxacin has similar clinical and bacteriologic efficacy and a similar safety profile compared with other antibiotic agents currently approved for this indication in patients with infections of mild-to-moderate severity. This comparable efficacy was found across different sites of infection as well as causative bacteria. From all enrolled (N=2,444) patients with cIAIs in these four randomized clinical trials, secondary peritonitis occurred in 1,229 (50%) patients, reflecting the high prevalence of this more severe disease among patients with mild-to-moderate, community-acquired cIAI origin.
Broad spectrum antibiotic therapy is an important part of the management of secondary peritonitis. In accordance with previous reports [5,24–26] and consistent with a patient population with community-acquired mild-to-moderately severe cIAIs, E. coli, B. fragilis and other Bacteroides spp. were the most frequently isolated pathogens. Moxifloxacin achieved similar bacteriological eradication rates to comparator regimens against these (and other causative) bacteria. The anti-anaerobic activity of moxifloxacin observed in this subset of patients was similar to that reported in a recent review by Goldstein et al. [27] on a broader population. Against susceptible anaerobic pathogens (MIC ≤2 mg/L), moxifloxacin achieved high bacteriologic eradication and clinical success rates of 84.5% and 83.1%, respectively [27]. Importantly, the clinical success rate was maintained at more than 80% beyond the susceptibility breakpoint of 4 mg/L, and declining to 67.7% only at an MIC of 32 mg/L [27]. Antibiotic resistance among bacteria implicated in the pathogenesis of polymicrobial peritonitis is considered as a critical issue in empiric therapy, as patients treated with antibiotics to which the pathogens are resistant are more likely to experience treatment failure and worse outcome [28]. However, as observed in a previous analysis of cIAI patients [27], the rates of clinical and bacteriological success achieved with moxifloxacin appear to be maintained well beyond the susceptibility breakpoint for key pathogenic species; this was observed during the long interval between 2000 and 2009 including each Phase III study period. Thus, despite recent warnings about the occurrence of resistant bacteria to major classes of antibiotics used to treat cIAIs that include not only fluoroquinolones but also carbapenems and piperacillin-tazobactam [29–38], our results may support the empiric use of antibiotics with a spectrum of activity covering the main causative organisms in cIAI patients with mild-to-moderate disease.
Pseudomona aeruginosa was isolated as a potential pathogen in a small number of patients. Although moxifloxacin has weak activity against P. aeruginosa, patients from whom this species was isolated responded well to therapy. This suggests that P. aeruginosa might have been present either as a colonizer or as a co-pathogen, which is consistent with the view that the prevalence of strains tends to be low in community-acquired IAIs [7].
Enterococci were also isolated in a small number of patients. Although the use of antibiotic therapy in patients with cIAIs harboring enterococci has proved controversial, it is now accepted that for patients with community-acquired infections, coverage against enterococci is not necessary as these bacteria are found mainly in nosocomial cIAIs and after previous exposure to antibiotics [3,39].
Fluoroquinolones possess potent in vitro activity against aerobic gram-negative bacteria; however, ciprofloxacin and levofloxacin lack anti-anaerobic activity, therefore, they need to be used in combination with metronidazole to provide coverage against Bacteroides spp. and other anaerobes [3,4]. Moxifloxacin has the advantage that it is active against both aerobic and anaerobic bacteria [5,40–41] and can be administered once daily as monotherapy [3,4]. As secondary peritonitis is frequently polymicrobial [9], in accordance with data of the current analysis showing the presence of two or more pathogens in approximately 60% of patients, a broad-spectrum, single-agent therapy, such as moxifloxacin, offers practical advantages over multi-dose combination regimens.
The appendix is the most common source of infection in community-acquired cIAIs, followed by the colon and stomach [42]; similar findings were observed in our analysis showing that these sites accounted for more than 80% of all peritonitis cases reported across the four studies. Treatment with moxifloxacin resulted in similar clinical success rates to comparator regimens in secondary peritonitis originating from these and other less common anatomic sites. Most patients in this study had community-acquired cIAIs of mild-to-moderate severity on the basis of the APACHE II scoring system. Across the four trials there were relatively few patients at higher risk of clinical failure such as those with higher APACHE II scores (≥15; 7.7%), hospital-acquired infection (4.0%), renal dysfunction or disorders (4.8%), and the presence of other risk factors such as diabetes mellitus (10.4%), older age (20.3%), and previous antibiotic therapy (34%) were observed in a small proportion of patients. Thus, the population in this pooled analysis best reflects the moderately ill cIAI patients as described in recent SIS/IDSA guidelines and our clinical data support the use of moxifloxacin in patients with mild-to-moderate cIAIs as recommended in these guidelines [3,4].
Pooled safety and tolerability data indicated that moxifloxacin was generally well tolerated in cIAI patients with secondary peritonitis. Although the overall incidence of AEs was higher in the moxifloxacin treatment arm, the incidence of study drug-related AEs, serious AEs, and deaths were similar between treatment groups in the ITT/safety population. The most frequent treatment-emergent AEs were gastrointestinal disorders including diarrhea, nausea, and vomiting. Clostridium difficile infections may be associated with moxifloxacin or other FQ treatment [43]. However, in this pooled dataset C. difficile infection was rare and it was not reported with higher frequency in MXF-treated patients than in COMP-treated patients. Surgical infections are not infrequent complications following open surgery but there was no clinically meaningful difference in rates of surgical infections between the two treatment groups in this pooled analysis. Of note, cardiac adverse events that are included in the warning labeling of moxifloxacin (such as QT interval prolongation, [12]) occurred in low frequency (<1%) among patients. Most AEs were not attributable directly to study medications, which suggests that many were related to the fact that patients were hospitalized for surgery in addition to their need for IV antibiotic therapy.
Patients with secondary peritonitis constitute an important sub-group within the cIAI patient population and for whom there is an increased risk of mortality from severe sepsis and septic shock if adequate antibiotic therapy is not administered promptly. Initiation of surgical source control in addition to the broad-spectrum antibiotic therapy, as performed in controlled clinical trials and in dedicated hospitals, prevented multiple organ dysfunction syndrome or sepsis in our patients which may count for the relatively low mortality rate (<5%). The strength of the data presented here is that the four studies included a large number of patients with secondary peritonitis who had an advanced disease but were not critically ill and thus representative of the moderately ill cIAI patient category as described in recent SIS/IDSA guidelines [3,4]. The APACHE II scoring system was used to describe the severity of the disease in this analysis, as it was in most other studies in this field. Originally devised as a score to predict mortality upon ICU admission and generally considered a good marker of severity in these patients, its value in peritonitis has recently been questioned because it does not take into account the effect of interventions that may alter physiologic paramaters [8]. Patients with mild-to-moderate cIAIs generally have APACHE II scores of <10 that are not associated with high mortality risk [25,44]. However, this evaluation is often performed before patients are operated on and remains stable hemodynamically. The APACHE II score may not be the most reliable way to assess the severity of surgical patients and a more specific scoring system—such as the Mannheim peritonitis index (MPI), which includes the description of peri-operative findings—may have been a more appropriate choice [8]. Another weakness of this pooled analysis was the heterogeneity of follow up periods after the end of antibiotic therapy. In the study by Solomkin et al., the TOC visit was carried out within 10–14 d of end-of-therapy, possibly precluding the identification of late clinical failures, recurrences or super-infections, resulting in the highest response rate for the comparator group [15]. Late clinical failures were captured in the other three larger studies where patients were monitored for longer periods post-therapy [13–14,16].
In conclusion, in this pooled analysis it has been observed that moxifloxacin has similar clinical and bacteriologic efficacy and a good safety profile compared with those of other previously approved antibiotic regimens in the treatment of mild-to-moderate secondary peritonitis. Despite certain limitations, the data from this pooled analysis provides support for the use of moxifloxacin as a valuable therapeutic option in the group of patients with advanced cIAIs, which is consistent with current treatment guidelines [3,4].
Acknowledgments
Highfield Communication (Oxford, UK), funded by Bayer HealthCare AG, provided editorial assistance. The authors thank all participating investigators (Refs. 13-16) in all four clinical trials included in this pooled analysis.
Author Disclosure Statement
This study was funded by Bayer HealthCare AG, Germany. Jan J. De Waele has provided consultancy services to AstraZeneca, Bayer Healthcare, MSD, and Pfizer. He has been the principal investigator and received research grants. The Ghent University Hospital was supported for participating in multi-center clinical studies sponsored by: AstraZeneca, Astellas, Bayer Healthcare, Pfizer, and MSD. Jose M. Tellado has provided consultancy services at Advisory Board meetings for MSD, Wyeth, AstraZeneca, Bayer Pharma AG, and Roche. Günter Weiss has no financial interest to disclose. Joseph S. Solomkin has been a consultant for Affinium, Astellas, Astra Zeneca, Basilea, Cubist, MedPace, Pfizer, PPDI, Rempex, and Tetraphase. He has been an Advisory Board member for Astra Zeneca, Bayer HealthCare, MedImmune, Merck, and Pfizer. Jeffrey Alder, Frank Kruesmann, Pierre Arvis, and Tajamul S. Hussain are Bayer Healthcare AG employees.
References
- 1.Pieracci FM, Barie PS. Management of severe sepsis of abdominal origin. Scand J Surg 2007;96:184–196 [DOI] [PubMed] [Google Scholar]
- 2.Solomkin JS, Mazuski JE, Baron EJ, et al. Guidelines for the selection of anti-infective agents for complicated intra-abdominal infections. Clin Infect Dis 2003;37:997–1005 [DOI] [PubMed] [Google Scholar]
- 3.Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis 2010;50:133–164 [DOI] [PubMed] [Google Scholar]
- 4.Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg Infect 2010; 11:79–109 [DOI] [PubMed] [Google Scholar]
- 5.Goldstein EJC. Intra-abdominal anaerobic infections: bacteriology and therapeutic potential of newer antimicrobial carbapenem, fluoroquinolone, and desfluoroquinolone therapeutic agents. Clin Infect Dis 2002;35(Suppl 1):S106–S111 [DOI] [PubMed] [Google Scholar]
- 6.Weigelt JA. Empiric treatment options in the management of complicated intra-abdominal infections. Cleve Clin J Med 2007;27(Suppl 4):S29–S37 [DOI] [PubMed] [Google Scholar]
- 7.Eckmann C, Dryden M, Montravers P, et al. Antimicrobial treatment of “complicated” intra-abdominal infections and the new IDSA guidelines? A commentary and an alternative European approach according to clinical definitions. Eur J Med Res 2011;16:115–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sartelli M. A focus on intra-abdominal infections. World J Emerg Surg 2010;5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blot S, De Waele JJ, Vogelaers D. Essentials for selecting antimicrobial therapy for intra-abdominal infections. Drugs 2012;72:e17–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rink AD, Stass H, Delesen H, et al. Pharmacokinetics and tissue penetration of moxifloxacin in intervention therapy for intra-abdominal abscess. Clin Drug Invest 2008;28:71–79 [DOI] [PubMed] [Google Scholar]
- 11.Stass H, Rink AD, Delesen H, et al. Pharmacokinetics and peritoneal penetration of moxifloxacin in peritonitis. J Antimicrob Chemother 2006;58:693–696 [DOI] [PubMed] [Google Scholar]
- 12.Avelox® 400 mg/250 ml solution for infusion. UK SmPC. Bayer PLC, September2010 [Google Scholar]
- 13.Malangoni MA, Song J, Herrington J, et al. Randomized controlled trial of moxifloxacin compared with piperacillin-tazobactam and amoxicillin-clavulanate for the treatment of complicated intra-abdominal infections. Ann Surg 2006;244:204–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss G, Reimnitz P, Hampel B, et al. Moxifloxacin for the treatment of patients with complicated intra-abdominal infections (the AIDA Study). J Chemother 2009;21:170–180 [DOI] [PubMed] [Google Scholar]
- 15.Solomkin J, Zhao YP, Ma EL, et al. Moxifloxacin is non-inferior to combination therapy with ceftriaxone plus metronidazole in patients with community-origin complicated intra-abdominal infections. Int J Antimicrobial Agents 2009;34:439–445 [DOI] [PubMed] [Google Scholar]
- 16.De Waele JJ, Tellado JM, Alder J, et al. Efficacy and safety of moxifloxacin vs. ertapenem in complicated intra-abdominal infections: results of the PROMISE study. Int J Antimicrob Agents 2013;41:57–64 [DOI] [PubMed] [Google Scholar]
- 17.Zosyn® Piperacillin and Tazobactam for injection, USP, Pfizer [Google Scholar]
- 18.Augmentin® Intravenous Powder for solution for injection or infusion. GSK
- 19.Rocephin® 2g vials. Each 2g vial contains 2g ceftriaxone as 2.39g hydrated disodium ceftriaxone. Roche [Google Scholar]
- 20.Flagyl® Metronidazole Injection, USP RTU® 500mg/100mL intravenous solution. Baxter Healthcare Corporation [Google Scholar]
- 21.Invanz® 1 g powder for concentrate for solution for infusion. UK SmPC. Merck Sharp & Dohme Limited, August2010 [Google Scholar]
- 22.Rothman KJ, Greenland S. Modern Epidemiology. 2nd ed. Philadelphia: Lippincott Williams & Wilkins Publishers; 1998 [Google Scholar]
- 23.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188 [DOI] [PubMed] [Google Scholar]
- 24.Goldstein EJC, Snydman DR. Intra-abdominal infections: review of the bacteriology, antimicrobial susceptibility and the role of ertapenem in their therapy. J Antimicrob Chemother 2004;53(Suppl 2):ii29–ii36 [DOI] [PubMed] [Google Scholar]
- 25.Wittmann DH, Schein M, Condon RE. Management of secondary peritonitis. Ann Surg 1996;224:10–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solomkin J, Teppler H, Graham DR, et al. Treatment of polymicrobial infections: post hoc analysis of three trials comparing ertapenem and piperacillin-tazobactam. J Antimicrob Chemother 2004;53(Suppl 2):ii51–ii57 [DOI] [PubMed] [Google Scholar]
- 27.Goldstein EJ, Solomkin JS, Citron DM, et al. Clinical efficacy and correlation of clinical outcomes with in vitro susceptibility for anaerobic bacteria in patients with complicated intra-abdominal infections treated with moxifloxacin. Clin Infect Dis 2011;53:1074–1080 [DOI] [PubMed] [Google Scholar]
- 28.Falagas ME, Barefoot L, Griffith J, et al. Risk factors leading to clinical failure in the treatment of intra-abdominal or skin/soft tissue infections. Eur J Clin Microbiol Infect Dis 1996;15:913–921 [DOI] [PubMed] [Google Scholar]
- 29.Goldstein EJC, Citron DM, Hecht DW. Resistance in anaerobic bacteria. In: Fong IW, Drlica K, eds. Antimicrobial resistance and implications for the 21st century. New York, NY: Springer Publishing; 2008:207–229 [Google Scholar]
- 30.Liu CY, Huang YT, Liao CH, et al. Increasing trends in antimicrobial resistance among clinically important anaerobes and Bacteroides fragilis isolates causing nosocomial infections: emerging resistance to carbapenems. Antimicrob Agents Chemother 2008;52:3161–3168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seifert H, Dalhoff A. PRISMA Study Group. German multicentre survey of the antibiotic susceptibility of Bacteroides fragilis group and Prevotella species isolated from intra-abdominal infections: results from the PRISMA study. J Antimicrob Chemother 2010;65:2405–2410 [DOI] [PubMed] [Google Scholar]
- 32.Snydman DR, Jacobus NV, McDermott LA, et al. Lessons learned from the anaerobe survey: Historical perspective and review of the most recent data (2005–2007). Clin Infect Dis 2010;50(Suppl 1):S26–S33 [DOI] [PubMed] [Google Scholar]
- 33.Snydman DR, Jacobus NV, McDermott LA, et al. Update on resistance of Bacteroides fragilis group and related species with special attention to carbapenems 2006–2009. Anaerobe 2011;17:147–151 [DOI] [PubMed] [Google Scholar]
- 34.Nagy E, Urbán E, Nord CE, et al. Antimicrobial susceptibility of Bacteroides fragilis group isolates in Europe: 20 years of experience. Clin Microbiol Infect 2011;17:371–379 [DOI] [PubMed] [Google Scholar]
- 35.Brink AJ, Botha RF, Poswa X, et al. Antimicrobial susceptibility of gram-negative pathogens isolated from patients with complicated intra-abdominal infections in South African hospitals (SMART Study 2004–2009): Impact of the new carbapenem breakpoints. Surg Infect 2012;13:43–49 [DOI] [PubMed] [Google Scholar]
- 36.Lau YJ, Chen YH, Huang CT, et al. Role of moxifloxacin for the treatment of commmunity-acquired complicated intra-abdominal infections in Taiwan. J Microbiol Immunol Infect 2012;45:1–6 [DOI] [PubMed] [Google Scholar]
- 37.Karlowsky JA, Walkty AJ, Adam HJ, et al. Prevalence of antimicrobial resistance among clinical isolates of Bacteroides fragilis group in Canada in 2010–2011: CANWARD surveillance study. Antimicrob Agents Chemother 2012; 56:1247–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hombach M, Bloemberg GV, Böttger EC. Effects of clinical breakpoint changes in CLSI guidelines 2010/2011 and EUCAST guidelines 2011 on antibiotic susceptibility test reporting of Gram-negative bacilli. J Antimicrob Chemother 2012;67:622–632 [DOI] [PubMed] [Google Scholar]
- 39.Blot S, De Waele J. Critical issues in the clinical management of complicated intra-abdominal infections. Drugs 2005;66:1611–1620 [DOI] [PubMed] [Google Scholar]
- 40.Ackermann G, Schaumann R, Pless B, et al. Comparative activity of moxifloxacin in vitro against obligately anaerobic bacteria. Eur J Clin Microb Infect Dis 2000;19:228–232 [DOI] [PubMed] [Google Scholar]
- 41.Edmiston CE, Krepel CJ, Seabrook GR, et al. In vitro activities of moxifloxacin against 900 aerobic and anaerobic surgical isolates from patients with intra-abdominal and diabetic foot infections. Antimicrob Agents Chemother 2004;48:1012–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sartelli M, Catena F, Ansaloni L, et al. Complicated intra-abdominal infections observations European study (CIAO study). World J Emerg Surg 2011;6:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blondeau JM. What have we learned about antimicrobial use and the risks for Clostridium difficile-associated diarrhoea? J Antimicrob Chemother 2009;63(2):238–242 [DOI] [PubMed] [Google Scholar]
- 44.Mazuski JE. Antimicrobial treatment for intra-abdominal infections. Expert Opin Pharmacother 2007;8:2933–2945 [DOI] [PubMed] [Google Scholar]