Abstract
The zebrafish is an important model to understand the cell and molecular biology of organ and appendage regeneration. However, molecular strategies to employ reverse genetics have not yet been adequately developed to assess gene function in regeneration or tissue homeostasis during larval stages after zebrafish embryogenesis, and several tissues within the zebrafish larva are difficult to target. Intraventricular injections of gene-specific morpholinos offer an alternative method for the current inability to genomically target zebrafish genes in a temporally controlled manner at these stages. This method allows for complete dispersion and subsequent incorporation of the morpholino into various tissues throughout the body, including structures that were formerly impossible to reach such as those in the larval caudal fin, a structure often used to noninvasively research tissue regeneration. Several genes activated during larval finfold regeneration are also present in regenerating adult vertebrate tissues, so the larva is a useful model to understand regeneration in adults. This morpholino dispersion method allows for the quick and easy identification of genes required for the regeneration of larval tissues as well as other physiological phenomena regulating tissue homeostasis after embryogenesis. Therefore, this delivery method provides a currently needed strategy for temporal control to the evaluation of gene function after embryogenesis.
Keywords: Developmental Biology, Issue 88, zebrafish, larva, regeneration, intraventricular injection, heart, morpholino, knockdown, caudal fin
Introduction
Regeneration of organs and appendages is fundamentally important for survival and fitness; however, several vertebrates including man have limited regenerative abilities. While several animal models exist that have extensive regenerative capacity, reverse genetic techniques to assess gene function during organ and appendage regeneration remain very limited or non-existent. Therefore, new approaches are required to dissect the molecular biology of regeneration in these model organisms.
The zebrafish is a well-established model for understanding the cell and molecular biology of organ and appendage regeneration1, not only because of its significant ability to regenerate multiple organs, tissues and appendages, but also because several transgenic fish lines exist to track cells and to overexpress gene constructs2,3. However, gene inhibition in larval zebrafish is mainly limited to the overexpression of dominant-negative constructs, which are not available to all genes of interest or whose transgene product can acquire gain-of-function effects that do not reflect the endogenous activity of the gene. Thus, an alternative method to specifically remove gene expression by knockout or knockdown is needed to overcome these issues.
Gene-specific targeting using TALENS exists as a reverse genetic means to knockout gene function; however, this knockout strategy is very frequently limited to functional assessments during early embryogenesis, because initial requirements of the gene prevent further progression of embryonic development. Thus, studying later phenomena such as regeneration or organ homeostasis after development using TALENS is precluded4,5. Therefore, an alternate gene removal strategy is needed that targets gene function after early development to assess gene requirements in fully formed organs and structures.
Morpholino injection has been shown to be effective in targeting genes in a few adult organs and the adult regenerating fin6-8, but these methods require electroporation and many internal organs are difficult to electroporate either due to their location or due to their sensitivity to electrical disruption. Furthermore, some tissues in the larva are difficult to inject directly, because direct injection may disrupt their structural integrity or because their size is limiting. The caudal fin of the larva is one such structure, because direct injection into the finfold is not possible. Thus, an alternative to electroporation and direct injection was needed to target genes in tissues that are either too small to inject or can't be electroporated.
In order to target and inhibit the function of specific genes during the regeneration of the larval caudal fin, we have modified existing morpholino technologies allowing the assessment of gene function during caudal fin regeneration in late-staged larva. This method employs intraventricular delivery9 of fluorescein-tagged morpholinos together with Endo-Porter transfection reagent10. Once in the ventricle, the morpholino-Endo-Porter mixture quickly spreads throughout the larva via the vasculature and enters tissues that have been previously impossible to target. This injection method may be modified to target genes in specific tissues and quite possibly can be applied in other animal models that currently lack reverse genetic methods to inhibit gene function. Thus, it offers a quick and easy method with the potential for broad range use to immediately study gene function during general organ homeostasis and regeneration at larval stages.
Protocol
1. Preparation of the Glass Needles (Figure 1)
Use glass capillaries with a 0.75 mm diameter to prepare injection needles (Figure 1A).
Place the glass capillary into a needle puller and pull the needle with the following parameter: heating cycle value: 463; pulling cycle value: 230; velocity: 150 msec; time: 150 msec (Figure 1B).
Break the pulled glass capillary with watchmaker tweezers to produce a 20 µm diameter needle under a stereoscope with a micrometer eyepiece.
Use a lathe with a wetted rubber spinning wheel to sharpen the needle and produce a 20 µm bevel (Figures 1C and 1D). Note: Sharp, beveled needles improve the ease of insertion into the ventricle and thus minimize the damage to the tissue and allow the perforated muscle to reseal after removal of the needle (Figures 1E-G).
2. Preparation of the Morpholino Solution
Prepare the morpholino stock by dissolving the lyophilized morpholino in 1x Phosphate-Buffered Saline (PBS) to a final concentration of 7.5 mM. [See manufacturer's instructions for details (Materials table)].
Prepare the morpholino injection solution by mixing 2.5 µl morpholino stock solution (7.5 mM) with 2.8 µl Endo-Porter stock solution (1 mM) (See Materials table) for a final concentration of 3.5 mM morpholino and 0.5 mM Endo-Porter.
3. Preparation of the Morpholino Injection (Figure 2)
Load the beveled glass needle with 5 µl of this solution using a micropipette with a 10 µl microloader pipette tip.
Insert the glass needle into the needle holder of the micromanipulator connected to the pneumatic pico pump (Figures 2A-C).
Place the needle holder next to the microscope so that the needle only needs to be moved in one planar direction to insert it into the cardiac ventricle of the larva (Figure 2A).
Adjust the angle for the injections at around 45°.
Set the microinjector values as follows: hold pressure: 20 pounds per square inch (psi); ejection pressure: 15 psi; 100 msec range of gating, period value of 1.9 (corresponds to 10.9 msec).
Melt agarose in 1x PBS using a standard microwave to produce 20 ml of a 1.5% (w/v) gel. Pour melted gel into a 10 cm Petri dish. Place a grooved injection form into the warm agarose so that once hardened, the agarose will have furrows in which the sedated larva will be placed11 (Figures 2D and 2E).
4. Injections (Figure 3)
Anesthetize larva in 100 ml of aquarium water with 20 mg/L tricaine (L-Ethyl-m-amino-benzoate-methane sulfonate) until they stop responding to touch.
Use a plastic Pasteur pipette to carefully transfer the sedated larva into a groove of the wet agarose mold so that the ventral side is facing against the vertical agarose wall of the groove (Figure 3A).
Place the agarose plate under the stereomicroscope so that the ventricle is facing away from the injection needle (Figure 3A).
Lower the needle to insert it into the heart ventricle. Insert the needle only 1-2 µm into the ventricle taking care not to insert the needle too deeply (Figures 3B and 3C).
Once the needle is inserted, inject the morpholino solution into the ventricle with 4-6 pulses, each delivering 3 nl of the solution, with waiting intervals to allow clearing of the heart (Figures 3D and 3E).
After injection, remove the needle (parallel to the plane of insertion) and then carefully transfer the larva using a plastic Pasteur pipette filled with E3 medium back into a Petri dish containing fresh E3 medium.
Place the needle into a Petri dish containing 1x PBS to prevent the drying of the needle when transferring the injected larva.
Repeat steps 4.1-4.6 every 12-24 hr for the duration of the experiment. Note: Repeating the injections ensures the uptake and maintenance of the morpholino in cells.
5. Analyses of Injections (Figure 4)
Anesthetize the fish in 100 ml of aquarium water with 20 mg/L tricaine until they stop responding to touch.
Place the larva laterally on a flat wet 1.5% (w/v) agarose plate covered with E3 medium.
Image larvae using a stereomicroscope with brightfield and fluorescence imaging. Note: As the larvae are transparent, the fluorescein-tagged morpholino should be visible as fluorescent green in the blood vessels throughout the animal directly after injection. This fluorescence will further disperse into the vascularized tissues by 15 min (Figures 4A-4C).
6. Assessment of Regenerative Outgrowth
Assess captured images using Fiji Image J free software (http://fiji.sc/Fiji). For investigating regeneration, use the line-tracing tool to determine the amount of regenerative growth that has occurred on control and morpholino-injected larva.
To calibrate images, open one of the original image files in Fiji by dragging and dropping the file into the main Fiji window.
To set the scale for all following images, go to "Analyze" in the main menu.
In the drop down menu click on "Set scale".
In this window, turn on the "Global" option by clicking the check box.
Now open the image to measure the amount of regenerative growth again by drag and drop (see step 6.1).
Choose the "freehand selections" tool in the tool bar.
Encircle the area to be measured (Figures 4J-4L).
Press Ctrl+M. This will open a new "Results" window showing the value of the encircled area in square pixels.
Representative Results
The sharp, beveled injection needle is easily placed in the cardiac ventricle of the zebrafish larva when approached dorsally (Figure 3A). The heart continues pumping and blood flow is maintained despite the presence of the needle (Figures 3B and 3C). Careful injections do not disrupt the morphology of the ventricle or cardiac contractions (Figure 3D) despite injection of the morpholino into the heart (Figure 3E).
Within minutes, the morpholino-Endo-Porter solution distributes throughout the body via the vasculature (Figures 4A and 4B). The morpholino is then distributed from the vasculature into different tissues such as the finfold and brain for at least 12 hr or more as observed from the fluorescence (Figure 4C).
In this approach, we remove the distal tip of the finfold, the distal tip of the spinal cord, distal trunk muscle, the distal notochord and pigment cells (Figures 4D and 4H); all of which are difficult to specifically target by direct injection due to compactness (muscle), small size (spinal cord and notochord) and thinness (finfold), but appear to incorporate the morpholino after serial ventricular injections, which is seen by fluorescence within these tissues (Figure 4E) compared to uninjected animals (Figures 4F and 4G). Thus, this method relatively easily promotes the delivery of morpholino to tissues that are difficult to individually target, and it permits the assessment of gene function in several tissues in the regenerating fin at once. To assess the importance of specific genes involved in regeneration, one surgically partially amputates the larval caudal fin (Figure 4H), resecting all the tissues that have incorporated the morpholino (Figure 4I). The larval finfold regenerates its structure within a few days post amputation (dpa) (Figure 4J). Morpholino targeting a gene required for regeneration (unpublished data) results in a perturbed regeneration response (Figure 4K) compared to uninjected (Figure 4J) and mismatch morpholino controls (Figure 4L). The total effect of these morpholino experiments on regenerative outgrowth can be measured using standard morphometric tools in the publically available Fiji programs12 by tracing the regenerated tissues and comparing the areas (Figures 4J-4L). Thus, this gene-targeting method provides a quick and relatively easy assay to test the importance of genes in the regeneration process.
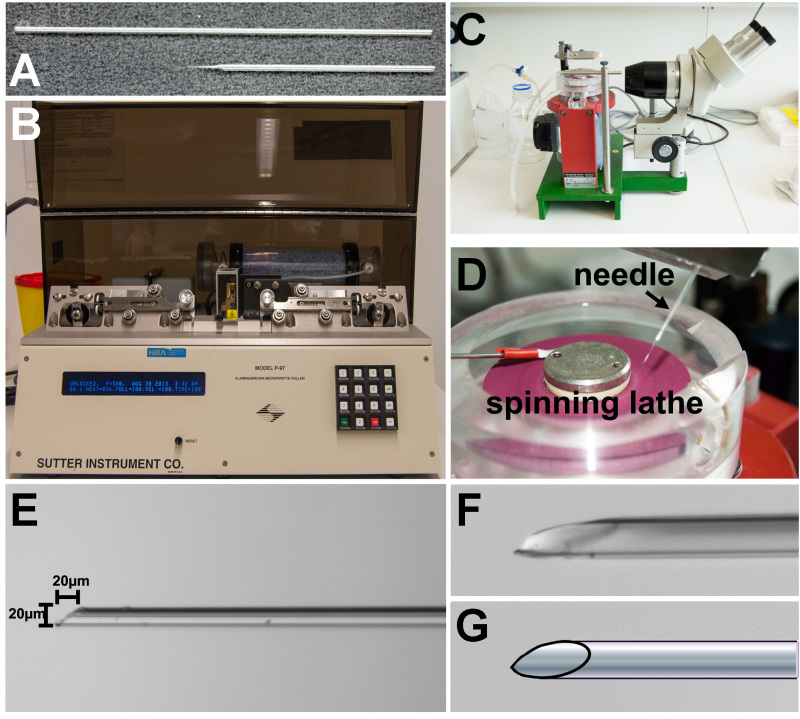 Figure 1. Preparation of glass capillary needles for injection. A) Glass capillary tube before pulling (above) and pulled needle (below). B) Needle pulling apparatus. C) Lathe for beveling and sharpening the glass needle. The needle is viewed through the binoculars. D) The lathe is a wetted rubber spinning disc. The needle is slowly lowered onto the disc. E) Sharpened needle must have a short bevel that is no longer (20 µm) than is wide (20 µm) to minimize the space needed to insert the entire end of the needle into the larval cardiac ventricle. F) Higher magnification image showing the tip of the needle. G) Schematic view of the needle tip showing the shape of the bevel after sharpening.
Figure 1. Preparation of glass capillary needles for injection. A) Glass capillary tube before pulling (above) and pulled needle (below). B) Needle pulling apparatus. C) Lathe for beveling and sharpening the glass needle. The needle is viewed through the binoculars. D) The lathe is a wetted rubber spinning disc. The needle is slowly lowered onto the disc. E) Sharpened needle must have a short bevel that is no longer (20 µm) than is wide (20 µm) to minimize the space needed to insert the entire end of the needle into the larval cardiac ventricle. F) Higher magnification image showing the tip of the needle. G) Schematic view of the needle tip showing the shape of the bevel after sharpening.
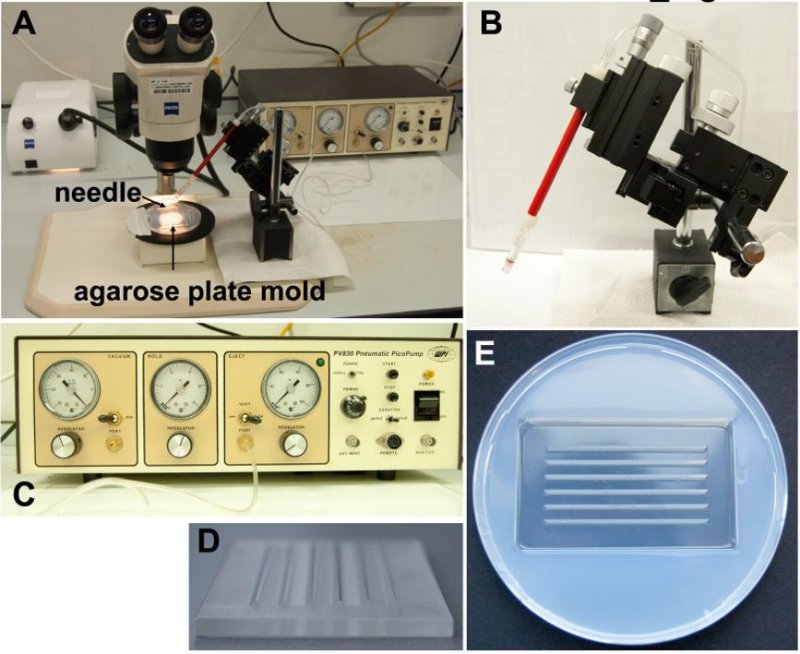 Figure 2. Injection apparatus set up. A) Complete apparatus for injection of morpholino into zebrafish larva. B) Stand to control the placement of the needle during injections. C) The pico pump that regulates pneumatic pressure for injections. D) Form press used to create agarose mold. E) Agarose mold used to stabilize larva for injection.
Figure 2. Injection apparatus set up. A) Complete apparatus for injection of morpholino into zebrafish larva. B) Stand to control the placement of the needle during injections. C) The pico pump that regulates pneumatic pressure for injections. D) Form press used to create agarose mold. E) Agarose mold used to stabilize larva for injection.
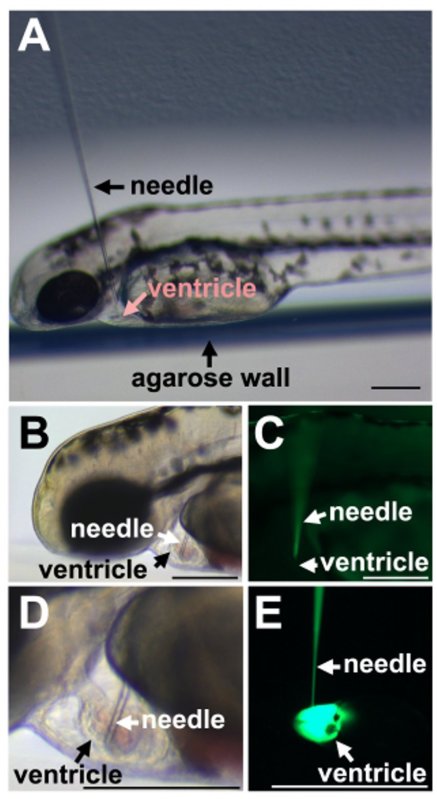 Figure 3. Injection of fish larva. A) Placement of the needle in relation to the zebrafish larva. B) Insertion into cardiac ventricle. C) Fluorescence of the morpholino in the needle but absent in the ventricle before injection. D) Brightfield image shows the size relationship between the glass needle and the larval ventricle. E) Injection of the fluorescent morpholino into the heart ventricle. Scale bars equal 100 µm.
Figure 3. Injection of fish larva. A) Placement of the needle in relation to the zebrafish larva. B) Insertion into cardiac ventricle. C) Fluorescence of the morpholino in the needle but absent in the ventricle before injection. D) Brightfield image shows the size relationship between the glass needle and the larval ventricle. E) Injection of the fluorescent morpholino into the heart ventricle. Scale bars equal 100 µm.
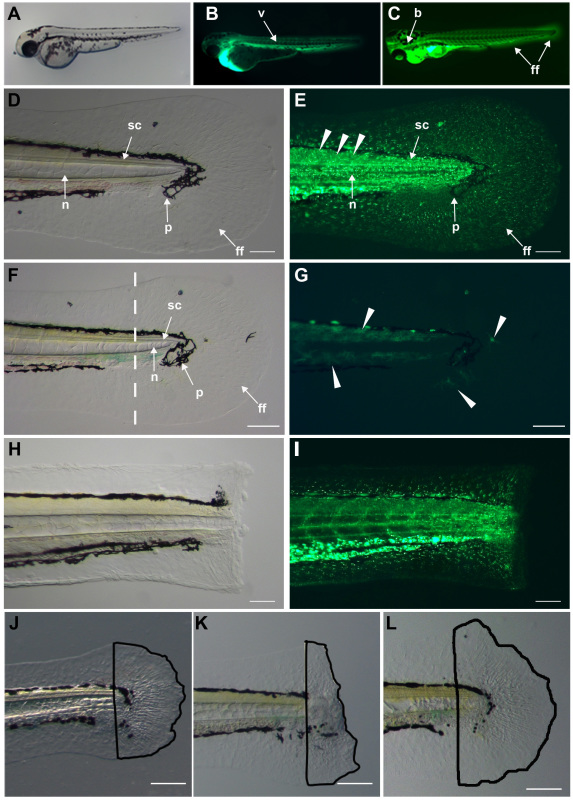 Figure 4. Dispersion of morpholino throughout fish. A) Zebrafish 3 day old larva. B) Fluorescence of fluorescein-tagged morpholino throughout the body vasculature (v) 5 min post ventricular injection. C) Dispersion of the morpholino throughout the animal including finfold (ff), brain (b) in older larva after injections of morpholino every 12 hr. D) Tissues in the larval caudal fin and trunk: notochord (n), spinal cord (sc), pigment (p), and finfold (ff). E) Dispersed morpholino several minutes after injection into larval trunk tissues including the finfold. F) Brightfield image of uninjected larval trunk and caudal fin. Dashed line indicates the prospective amputation plane. G) Uninjected fin imaged with the GFP filter showing autofluorescence in green (arrowheads). The dendritic shape of the fluorescing structures suggests that these are pigment cells. H) Brightfield image of a surgically amputated larval caudal fin through the notochord, muscle and spinal cord. I) Fluorescent image showing morpholino distribution in the stump tissues. Morpholino incorporation into muscle, spinal cord, notochord and finfold. J) Regenerated caudal finfold structures of wild-type larva. Black line traces the regenerated tissues for quantification analysis. K) Morpholino-mediated inhibition of regeneration. Black line tracing clearly highlights the reduced regeneration response after morpholino knockdown. L) Regenerated caudal finfold of larva injected with a mismatch control morpholino showing a similar regeneration response as in uninjected larva (J). Scale bars equal 100 µm.
Figure 4. Dispersion of morpholino throughout fish. A) Zebrafish 3 day old larva. B) Fluorescence of fluorescein-tagged morpholino throughout the body vasculature (v) 5 min post ventricular injection. C) Dispersion of the morpholino throughout the animal including finfold (ff), brain (b) in older larva after injections of morpholino every 12 hr. D) Tissues in the larval caudal fin and trunk: notochord (n), spinal cord (sc), pigment (p), and finfold (ff). E) Dispersed morpholino several minutes after injection into larval trunk tissues including the finfold. F) Brightfield image of uninjected larval trunk and caudal fin. Dashed line indicates the prospective amputation plane. G) Uninjected fin imaged with the GFP filter showing autofluorescence in green (arrowheads). The dendritic shape of the fluorescing structures suggests that these are pigment cells. H) Brightfield image of a surgically amputated larval caudal fin through the notochord, muscle and spinal cord. I) Fluorescent image showing morpholino distribution in the stump tissues. Morpholino incorporation into muscle, spinal cord, notochord and finfold. J) Regenerated caudal finfold structures of wild-type larva. Black line traces the regenerated tissues for quantification analysis. K) Morpholino-mediated inhibition of regeneration. Black line tracing clearly highlights the reduced regeneration response after morpholino knockdown. L) Regenerated caudal finfold of larva injected with a mismatch control morpholino showing a similar regeneration response as in uninjected larva (J). Scale bars equal 100 µm.
Discussion
The intraventricular injection provides a quick and reliable assessment method for testing gene function at later stages of development or of body homeostasis without affecting the gene function during embryogenesis. To ensure the success of this technique, one should be aware of four critical points: 1) needle size, 2) drying out of the needle, 3) minimizing volume, and 4) minimal exposure time in the sedation solution. Needles that are too small will clog frequently, while needles that are too large will damage the ventricle and cause excessive bleeding. While 20 µm is recommended, the needle should never be more than one-fifth the size of the ventricular chamber. A second critical point is to keep the needle from drying out and clogging. The small size of the needle and the inevitable frequent pauses to sedate, transfer and position the larva can provide enough time to allow the morpholino at the needle tip to dry out and clog the needle. To prevent this, one can immerse the needle in a Petri dish of 1x PBS. A third critical point is to minimize bloating of the ventricle by too high of a volume. If the dosage is limited by the size of the needle, then increasing the number of injections is always preferable to increasing the volume: larger volumes can cause stretch injury to the heart. A fourth critical point is the extent of sedation. The sedation time is based on the length of time needed for the procedure. Larvae can remain in the sedation solution and remain sedated for several minutes, because they are rather robust and resuscitate rather easily. However, maintaining the fish too long in the anesthetic will impair its recovery or too short will cause them to revive during the injection. Therefore, one should sedate only the number of larva that one can inject within a 4 min period.
One limitation of this current method is that the morpholino does not target gene function in a tissue-specific manner. Two different modifications can provide tissue specificity: One modification is to use caged morpholinos. After activation by laser light from a confocal microscope, these morpholinos inhibit their gene target, so timed confocal exposure can provide temporal and spatial control to morpholino activity. Caged morpholinos have been used to target genes during early development after injection into the one-cell embryo. While delivery at single-cell stage can work to target genes during embryogenesis, the concentration of the morpholino will likely be too low at later larval stages due to the number of successive cell divisions as development progresses to the larval stage13. The amount of morpholino one can inject at the one-cell-stage is also limited by toxicity13-15. A second possible modification is to directly inject a morpholino into the organ or tissue to be studied when size and imaging allow. The zebrafish larva is translucent, so viewing most internal organs after they have developed should not be a problem. The fluorescein-tagged morpholinos work with this protocol; therefore, fluorescence can be used to track the distribution and tissue-specific localization of the morpholino. The incorporation of the fluorescein-tagged morpholino into tissue cells requires the presence of the Endo-Porter reagent, so another modification would be to limit the spatial distribution of the Endo-Porter reagent by injecting it at lower amounts directly into the target tissue. Thus, spatial resolution should be possible with this technique.
There are transgenic overexpression methods that provide for temporal and spatial control of exogenous gene expression by combining tissue-specific promoters with heat-shock or tamoxifen-inducible promoters which enables the analysis of gene function at later stages of development or in certain tissues by using tissue specific promoters16-19. This includes the transgenic expression of dominant-negative constructs designed to create a product that can outcompete the endogenous gene. This knockdown strategy is susceptible to off-target effects when a transgene produces disproportionate expression levels of a transgenic product that acquires gain-of-function effects20 or when it integrates into a gene and disrupts the gene's endogenous function21,22. While morpholinos can also generate off-target effects, one can more easily control and limit these effects by using mismatch control morpholinos with similar but slightly altered target sequences that do not bind the target mRNA, by using multiple antisense morpholinos that target different sequences within the same mRNA and by testing different morpholino concentrations13-15. Moreover, morpholinos can be produced and tested much faster and cheaper than designing, raising and testing different dominant-negative transgenic lines. Therefore, the intraventricular injection of morpholinos provides a useful tool to knockdown genes at later stages of development, when dominant-negative transgenesis is not possible. When a dominant-negative transgenic strategy is possible, intraventricular morpholino injection is an alternative approach to confirm the specificity of the overexpressed dominant-negative transgene.
It is likely that effective concentrations between different morpholinos will vary, as they do when using them for gene knockdown during embryogenesis13,15. For the gene targeted in this study, we did not observe an effect on regeneration at doses lower than 3.5 mM, and non-specific effects were not observed in mismatch controls with this morpholino concentration.
Intraventricular injection allows for the distribution of small molecules to assess activity and efficacy in a whole animal system in real time when efficacy is limited by penetration through the skin or digestive system or by the amounts of compound available. It also allows the targeting of tissues when they can´t be targeted by direct injections, as is the case for the finfold. This method does not only provide a tool to study gene function during fin regeneration at larval stages. Since the morpholino integrates into the larval finfold comprising mesenchymal cells and epidermis, it might also provide a suitable technique to investigate the role of genes that play part in the process of wound healing or the occurrence of skin cancer. The injection of morpholino together with Endo-Porter may also be applied to target organs or tissues in adult zebrafish, which are amenable to surgery and translucent adult zebrafish lines are available. Furthermore, this method is not limited to zebrafish; dispersion by intraventricular injection can be used on other vertebrate models as well, especially those models that still lack effective recessive gene methods such as Xenopus, Axolotl and newt, which are also important models for regeneration studies.
Thus, intraventricular injection in the zebrafish offers the ability to temporally control gene expression with a relatively quick protocol to target multiple tissues, and the versatility of this method allows for its use with zebrafish transgenic lines as well as for other organisms that lack functional transgenesis or gene knockout strategies.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (DFG), we wish to thank Ayele Tsedeke Taddese for technical support.
References
- Gemberlin M, Bailey TJ, Hyde DR, Poss KD. The zebrafish as a model for complex tissue regeneration. Trends in Genetics. 2013;29:611–620. doi: 10.1016/j.tig.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suster ML, Abe G, Schouw A, Kawakami K. Transposon-mediated BAC transgenesis in zebrafish. Nature Protocols. 1998;6 doi: 10.1038/nprot.2011.416. [DOI] [PubMed] [Google Scholar]
- Skromne I, Prince VE. Current perspectives in zebrafish reverse genetics: moving forward. Developmental Dynamics. 2008;237:861–882. doi: 10.1002/dvdy.21484. [DOI] [PubMed] [Google Scholar]
- Zu Y, et al. TALEN-mediated precise genome modification by homologous recombination in zebrafish. Nature Protocols. 2013;10:329–332. doi: 10.1038/nmeth.2374. [DOI] [PubMed] [Google Scholar]
- Bedell VM, et al. In vivo genome editing using a high-efficiency TALEN system. Nature. 2012;491:114–118. doi: 10.1038/nature11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizil C, IItzsche A, Kaslin J, Brand M. Micromanipulation of gene expression in the adult zebrafish brain using cerebroventricular microinjection of morpholino oligonucleotides. J Vis Exp. 2013. [DOI] [PMC free article] [PubMed]
- Hyde D, Godwin A, Thummel R. In vivo electroporation of morpholinos into the regenerating adult zebrafish tail fin. J Vis Exp. 2012. [DOI] [PMC free article] [PubMed]
- Thummel R, Bailey T, Hyde D. In vivo electroporation of morpholinos into the adult zebrafish retina. J Vis Exp. 2011. [DOI] [PMC free article] [PubMed]
- Rieger S, Kulkarni RP, Darcy D, Fraser SE, Köster RW. Quantum dots are powerful multipurose vital labeling agents in zebrafish embryos. Developmental Dynamics. 2005;234:670–681. doi: 10.1002/dvdy.20524. [DOI] [PubMed] [Google Scholar]
- Madhavan M, et al. The role of Pax-6 in lens regeneration. Proceedings of the National Academy of Science U. 2006;103:14848–14853. doi: 10.1073/pnas.0601949103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour DT, Jessen JR, Lin S. Zebrafish: a practical approach. Vol. 261. Oxford: University Press; 2000. pp. 121–143. [Google Scholar]
- Schindelin J, et al. Fiji: an open-source platform for biological-image analysis. Nature Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen JS, Smith JC. Controlling morpholino experiments: don't stop making antisense. Development. 2008;135:1735–1743. doi: 10.1242/dev.001115. [DOI] [PubMed] [Google Scholar]
- Bill BR, Petzold AM, Clark KJ, Schimmenti LA, Ekker SC. A primer for morpholino use in zebrafish. Zebrafish. 2009;6:69–77. doi: 10.1089/zeb.2008.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt TM, Ekker SC. Vectors and techniques for ectopic gene expression in zebrafish. Methods Cell Biol. 1999;59:117–126. doi: 10.1016/s0091-679x(08)61823-3. [DOI] [PubMed] [Google Scholar]
- Hans S, Kaslin J, Freudenreich D, Brand M. Temporally controlled site-specific recombination in zebrafish. PLoS One. 2009;4 doi: 10.1371/journal.pone.0004640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Grill S, Sanchez A, Murphy-Ryan M, Poss KD. Fgf signaling instructs position-dependent growth rate during zebrafish fin regeneration. Development. 2005;132:5173–5183. doi: 10.1242/dev.02101. [DOI] [PubMed] [Google Scholar]
- Liu S, D LS. Zebrafish models for cancer. Annu. Re. Pathol. Mech. Dis. 2011;6:71–93. doi: 10.1146/annurev-pathol-011110-130330. [DOI] [PubMed] [Google Scholar]
- Stoick-Cooper CL, et al. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development. 2007;134:479–489. doi: 10.1242/dev.001123. [DOI] [PubMed] [Google Scholar]
- Shen SP, Aleksic J, Russell S. Identifying targets of the Sox domain proteins Dichaete in the Drosophila CNS via targeted expression of dominant negative proteins. BMC Dev. Biol. 2013;13 doi: 10.1186/1471-213X-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeish JD, Scott WJ, Potter Jr SS. Legless, a novel mutation found in PHT1-1 transgenic mice. Science. 1988;241:837–839. doi: 10.1126/science.3406741. [DOI] [PubMed] [Google Scholar]
- Covarrubias L, Nishida Y, Terao M, D'Eustachio P, Mintz B. Cellular DNA rearrangements and early developmental arrest caused by DNA insertion in transgenic mouse embryos. Mol. Cell. Biol. 1987;7:2243–2247. doi: 10.1128/mcb.7.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]


