Abstract
Background: Knowing family history is important for understanding cancer risk, yet communication within families is suboptimal. Providing strategies to enhance communication may be useful.
Methods: Four hundred ninety women were recruited from urban, safety-net, hospital-based primary care women's health clinics. Participants were randomized to receive the KinFact intervention or the control handout on lowering risks for breast/colon cancer and screening recommendations. Cancer family history was reviewed with all participants. The 20-minute KinFact intervention, based in communication and behavior theory, included reviewing individualized breast/colon cancer risks and an interactive presentation about cancer and communication. Study outcomes included whether participants reported collecting family history, shared cancer risk information with relatives, and the frequency of communication with relatives. Data were collected at baseline, 1, 6, and 14 months.
Results: Overall, intervention participants were significantly more likely to gather family cancer information at follow-up (odds ratio [OR]: 2.73; 95% confidence interval [CI]: 2.01, 3.71) and to share familial cancer information with relatives (OR: 1.85; 95% CI: 1.37, 2.48). Communication frequency (1=not at all; 4=a lot) was significantly increased at follow-up (1.67 vs. 1.54). Differences were not modified by age, race, education, or family history. However, effects were modified by pregnancy status and genetic literacy. Intervention effects for information gathering and frequency were observed for nonpregnant women but not for pregnant women. Additionally, intervention effects were observed for information gathering in women with high genetic literacy, but not in women with low genetic literacy.
Conclusions: The KinFact intervention successfully promoted family communication about cancer risk. Educating women to enhance their communication skills surrounding family history may allow them to partner more effectively with their families and ultimately their providers in discussing risks and prevention.
Introduction
Family health history is an important tool for health communication, genetic testing interpretation, and patient–provider interaction about risk, health behaviors, and screening.1–8 The burden of familial risk for breast, colorectal, and other cancers is estimated to be greater than 20%.9 Having a cancer family history increases risk and may inform screening decision making.10,11 Increased knowledge and communication about risk may reduce health disparities in disadvantaged, underscreened patients, resulting in earlier diagnosis and initiation of treatment.6,12–15 Despite the potential utility of family history, communication about familial cancer risk is variable. In one study, only 50% of individuals with cancer discussed their risks with relatives.16–20 Opportunities to use family history–based prevention and screening strategies are lost if individuals do not know or share their family history.14,21–24
A focus on family history may deserve particular emphasis in minority populations who may have inadequate health care access, medical distrust, lower genetic literacy,25,26 and lower perceived cancer risks.27 Although African Americans have an overall higher cancer incidence and mortality, their generally lower perceived risks28–31 may hinder cancer screening or other prevention behaviors. Public health efforts have focused on the development of tools to help families collect history,32 but strategies to enhance the skills to collect this information have not been well addressed. Individuals may also need support in understanding the benefits and risks (e.g., distress) of disclosure in family communication.33,34 Helping people to do a better job gathering and sharing family history could increase individual risk awareness and create opportunities for relatives to improve their health.14,16,31
The KinFact (Keeping Information about Family Cancer Tune-up Program) intervention was designed to address the communication challenges of gathering and sharing information about cancer risk and prevention in diverse families. These challenges can include a range of matters such as loss of contact, lack of closeness, worry about upsetting relatives, cancer-related distress, and lack of knowledge. These barriers may contribute to a general lack of direction or confidence in initiating conversations about familial cancer.
The intervention was not guided by one unifying theory; rather, it borrowed from the coordinated management of meaning theory,35 our prior work based in the expanded Health Belief Model,34,36,37,38 and the six-step process adapted by Daly39 from Buckman's “breaking bad news” technique.40 These frameworks emphasize that individuals in social communication want to understand what is going on, apply rules to figure things out, and act given their understanding of what is appropriate.35 More specifically, health belief models and theories strive to understand family risk communication by raising awareness, perceived risk/vulnerability, and self-efficacy. The intervention was then designed as a stepwise, skills-based strategy utilizing the aforementioned concepts for gathering and sharing familial cancer risk and health history information. It was hypothesized that the intervention would increase participants' gathering and sharing of family history and cancer prevention information with relatives. Because the intervention was designed to be simple, easy to follow, and not require high school level literacy, it was hypothesized that the effects would not differ by race, education, or genetic literacy.
Materials and Methods
KinFact is an internal review board–approved, parallel-group, randomized controlled trial comparing the impact of a theory-driven intervention on family communication about cancer family history and risk to an educational handout about breast and colon cancer prevention in a racially diverse sample of women.
Participants
Female patients within the Virginia Commonwealth University Women's Health Clinics in Richmond, Virginia were eligible to participate if they were English-speaking and 18 years or older. Of the study population, 45.3% of were recruited from a faculty practice and 54.7% from a resident practice. In 2011, nearly 30,000 women, 63% percent African American, were seen with widely varying primary care issues. The sample of study participants reflected the clinic's population.
Sample recruitment
The two study recruiters had teaching and counseling experience. Patients were recruited from July 2010 to January 2012 and were not recruited by visit reason, cancer family history, or any specific health condition. Following consent, genetic literacy assessments were performed, pedigrees obtained—specifically inquiring about cancer diagnoses in first- and second-degree relatives, and baseline measures completed. All pedigrees were entered into the risk-generating software, CAGene v. 5.1. Only intervention group participants were given their personalized risks. Participants in both arms were asked about baseline and follow-up genetic testing and counseling. There was no mechanism to identify or refer high-risk individuals in the control group. Participants could earn $40 in gift cards, with $10 given after finishing each of four time points (baseline and 1, 6, and 14 months).
Randomization
Following the participant's medical appointment and baseline measure completion, participants were randomized into control or intervention groups when they opened a sealed envelope with their group assignment. Prior to recruitment, the biostatistician prepared two sets of sequentially numbered envelopes (one per clinic) containing assignment to study arm. Assignment of study arm used 1:1 allocation and was generated via the nQuery program. Stratification (family or resident clinic) with blocks of size 2 and 4 were used, the order of which were randomly assigned by the nQuery program. Recruiters were blinded to the randomization. Follow-up interviewers and data entry personnel were blinded to study arm.
Control conditions
Control participants were given a handout promoting ways to lower breast and colon cancer risks, screening recommendations, and services contact information (see Supplementary Data, DataKinFact Controls Info Sheet; Supplementary Data are available online at www.liebertpub.com/jwh). The control arm handout was developed by us for a previous study with population-based screening recommendations. Reading level was 7.2 on the Rapid Estimate of Adult Literacy in Genetics (REAL-G).4,42 There was no information on the sheet about the importance of communicating with relatives about family history. There was a statement indicating that questions should be addressed to their doctor or the principal investigator.
KinFact intervention design
The intervention was designed to equip women with knowledge, strategies, and resources to heighten awareness of the importance of family communication about cancers' genetic aspects and ultimately adopt health-promoting behaviors. Participants in the intervention group were taken to a private room for the intervention. Using basic communication skills and steps for addressing cancer-risk results, the intervention included components that assessed knowledge and motivation for cancer communication, enabled women to identify relatives to talk to and ascertained the optimal approaches and settings in which to discuss family history with their relatives.43,44 Consciousness raising and perceived susceptibility were highlighted with several early pages entitled, “Did you know?” Later pages gave specific suggestions for phrases participants could use when talking with relatives.
KinFact intervention description
The intervention aimed to improve the participants' capacity to gather and share information about family history overall and share prevention information about colon and breast cancer. Recruiters worked individually with women randomized to the intervention group during a 20-minute session to (a) provide tailored risk information and prevention recommendations; (b) review their pedigree for breast and colon cancer within the CAGene v.5.1 program; (c) identify any missing information in the family history; (d) coach women in communication skills to obtain needed information; and (e) develop a plan for collection and follow-up of this information. Recruiters displayed a printed 27-page personalized booklet, “Talking about Cancer in your Family can Keep You and Your Family Healthy,” (see Supplementary Data, KinFact Intervention Booklet) and explained risks for breast and colon cancer based on the cancer risk assessment models available in CAGene (Claus, Gail, BRCApro, and MMRpro). Also available were hereditary risk probabilities (i.e., inherited mutation for strong risk for breast and/or colon cancer). If chances of a genetic mutation were ≥10%, if lifetime risk for developing breast cancer was ≥20%, or if lifetime risk for colon cancer was ≥10%, participants were referred to cancer genetic counseling clinic for a no-cost visit. The intervention's latter half, an interactive presentation on cancer and communication, focused on fostering discussion between women and their families about breast, colon, and other cancers, including how to obtain needed information to complete the family history and developing a plan for collection and follow-up of this information. The intervention contains many opportunities to discuss the risks and benefits of communication and provides skills and tools to assist women in making an informed decision about whether to communicate and in communicating if that is appropriate in their situation. There was no explicit connection or communication with providers.
Outcome measures
Family communication outcomes were measured at 1, 6, and 14 months post-baseline. The main outcomes were a modification of the U.S. Centers for Disease Control and Prevention (CDC)'s HealthStyles survey family communication measure, used extensively in health communication.1,45 Participants were asked, “Have you ever actively collected cancer information from your relatives for the purpose of creating a family health history?” and “Have you ever actively given your relatives information about hereditary cancer risk? (Hereditary cancer risk is cancer that tends to run in the family.)”
Frequency of communication about family cancer history was measured by asking, “How much have you spoken about family history of cancer with each of the following family members?”46 Frequency of communication with all listed first- and second-degree relatives was rated on a 4-point Likert scale, from 1 (not at all) to 4 (a lot). An option for “I do not have a family member of this type or they are not living” was provided. The average response over all living relatives was the outcome measure.
Demographic/other measures
Participants were asked about their birthdate, race, ethnicity, highest educational level, and personal cancer history. Given that the majority of participants reported their race as black, race was categorized as black and other (i.e., participants reporting white, other, or more than one race category). Pedigree information was used to determine if participants had a first- or second-degree relative with any cancer. A positive family history was defined as at least one first- or second-degree relative with cancer. Pregnancy status at time of enrollment was obtained utilizing International Classification of Diseases, Ninth Revision data from recruitment date and medical records. To assess health literacy, participants completed the validated REAL-G, an 8-item measure yielding a score of 0–8.42 Scores ≤3 indicate reading at or below a sixth grade level.
Analytic plan
The distributions of baseline variables between intervention and control groups were compared using chi-squared test for categorical variables or t-tests for continuous variables. Mixed model analysis of variance was used to compare intervention and control arms simultaneously at each follow-up period. All subjects with at least one follow-up observation were included in analysis. All observations available were used in analysis. The baseline value of the outcome variable was used as a covariate to control for possible differences at baseline and increase precision. Initial models included a term for the interaction of study arm and time to assess whether the pattern of intervention effect was the same at all three time points. This term was not statistically significant for the three outcome measures, either overall or for any of the subsets of the sample presented in the tables. Thus, analysis summarizes results over all follow-up time periods, and only the OR and 95% confidence interval [95% CI] for the analysis summarizing over all time periods is presented in the tables.
For the binary outcome measures, gathering and sharing information, the mixed model analysis specifically assumed a binary distribution, incorporated a logit link and was implemented using GLIMMIX in SAS 9.3. For the communication frequency variable, analysis assumed a normal distribution and was implemented with MIXED in SAS 9.3. Odds ratios and confidence intervals were used as a measure of treatment effect for the binary outcome; means differences and confidence intervals were used for the continuous outcome. Analyses were conducted by principles of intent-to-treat and performed between May and July 2013.
The next models considered the a priori hypothesized effect modification by race, education, and genetic literacy, as well as exploratory analysis of modification by family history of any cancer, having first-degree relatives with breast or colon cancer, age (in years), and pregnancy status. Analysis was done separately for each of these potential effect modifiers. For pregnancy, only participants of child-bearing age (age ≤40 years) were used. Due to possible interaction between age and pregnancy, in the analysis for age only nonpregnant women were considered. To test for effect measure modification (additive on the difference scale, and multiplicative on the odds ratio scale) of the intervention effect, an interaction term between study arm and the potential modification variable was added to the model. Significant interaction indicated that intervention effect differed at levels of the modifying variable. If a significant interaction was discovered then analysis was performed separately for subgroups defined by levels of the modification variable. Otherwise, unstratified analysis was sufficient. Summary measures comparing intervention and control groups for gathering and sharing information were presented as odds ratios and 95% CIs, adjusted for baseline values of the outcome measure, while adjusted mean differences and 95% CIs were calculated for communication frequency.
To assess potential bias due to incomplete follow-up, participants without follow-up surveys at specific time points were compared on baseline variables (i.e., race, genetic literacy, education, cancer family history, age, and pregnancy) with those who completed the surveys using logistic regressions. This analysis was done separately for each of the three follow-up time points. Possible differences by study arm were assessed by adding to the logistic regression model an interaction term between study arm and missing follow-up variable.
Sample size was chosen so that if effect modification was found, subsets of the sample could be analyzed with reasonable power. When analyzing data separately by race, the sample size of 490 participants (245 per study arm) had 80% power to detect a difference between study arms of at least 14%, assuming an intraclass correlation of 0.3 and a dropout rate of 20%. We also had power to analyze study sample subsets defined by cancer family history, education, genetic literacy, age, and pregnancy (difference of 13%, 25%, 25%, 25%, and 17% respectively).
For all but assessment of interactions, a significance level for analysis was set at α=0.05. Because interaction tests tend to have low power, the alpha level for tests of interaction was increased to 0.10 to improve power. Analyses were run using SAS v. 9.3
Results
Study participants
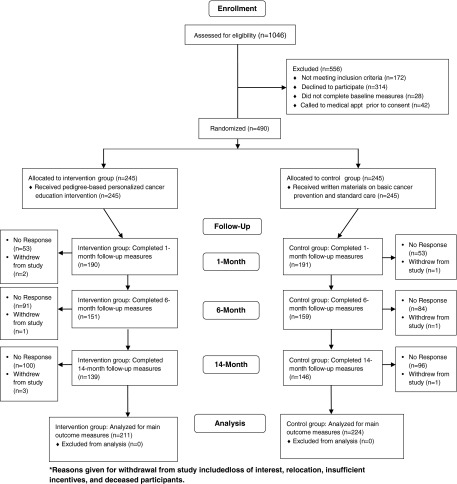
Figure 1 shows participant flow. In total, 1,046 women were assessed for eligibility. Of these, 490 completed baseline measures and were randomized to intervention and control groups, 245 per arm. Four hundred thirty-five (211 intervention, 224 control) participants completed at least one follow-up measure. Participants missing surveys were younger (28.6 vs. 33.5 years, p<0.001) than women providing follow-up data. There were no differences in race, genetic literacy, education, pregnancy, or family cancer history. The pattern of missing data was the same in both study arms.
FIG. 1.
A CONSORT flow chart showing the flow of patients through the KinFact study.
There were no significant baseline differences between intervention and control groups (Table 1). Mean age was 33.4 years, 59% were black, and about one-third were uninsured. One-third were pregnant at enrollment. Fifteen percent had less than a high school education, and 17% had a reading level of approximately the sixth grade or less based on the REAL-G. Most (74%) women reported at least one first- or second-degree relative with a diagnosis of any cancer, while 10% had at least one first-degree relative with breast or colon cancer.
Table 1.
Cohort Description
| Intervention (n = 245), n (%) | Control (n = 245), n (%) | Total (N = 490), N (%) | |
|---|---|---|---|
| Age [mean (SD)], years | 32.8 (11.6) | 34.0 (12.3) | 33.4 (11.9) |
| <20 | 8 (3) | 10 (4) | 18 (4) |
| 20–29 | 129 (53) | 107 (44) | 236 (48) |
| 30–39 | 48 (19) | 59 (24) | 107 (22) |
| 40–49 | 32 (13) | 35 (14) | 67 (13) |
| 50–59 | 24 (10) | 25 (10) | 49 (10) |
| 60+ | 4 (2) | 9 (4) | 13 (3) |
| Race | |||
| Black | 134 (55) | 154 (63) | 288 (59) |
| White | 87 (36) | 75 (31) | 162 (33) |
| Other | 12 (5) | 9 (4) | 21 (4) |
| Multiple race | 11 (5) | 7 (3) | 18 (4) |
| Marital status | |||
| Never married | 121 (49) | 112 (46) | 233 (47) |
| Married | 99 (41) | 101 (41) | 200 (41) |
| Formerly married | 25 (10) | 32 (13) | 57 (12) |
| Living arrangements | |||
| Partner/husband | 91 (37) | 95 (39) | 186 (38) |
| Partner/husband and parents | 7 (3) | 9 (4) | 16 (3) |
| Parents | 33 (14) | 35 (14) | 68 (14) |
| Alone | 75 (31) | 65 (26) | 140 (29) |
| Other | 39 (15) | 41 (17) | 80 (16) |
| Education levela | |||
| <High school | 38 (16) | 33 (14) | 71 (15) |
| High school graduate/GED | 59 (24) | 67 (27) | 126 (26) |
| Some college | 76 (31) | 61 (25) | 137 (28) |
| College graduate or more | 35 (14) | 41 (16) | 76 (15) |
| Missing | 37 (15) | 43 (18) | 80 (16) |
| Genetic literacy score [mean (SD)] | 5.8 (2.2) | 5.8 (2.4) | 5.8 (2.3) |
| ≤3 | 42 (17) | 42 (17) | 86 (17) |
| >3 | 202 (82) | 202 (82) | 402 (82) |
| Missing | 1 (1) | 1 (1) | 2 (1) |
| Insurance | |||
| Commercial | 73 (30) | 61 (25) | 134 (27) |
| Indigent care | 18 (7) | 18 (7) | 36 (7) |
| Medicaid/managed care | 133 (54) | 134 (55) | 267 (55) |
| Medicare | 4 (2) | 13 (5) | 17 (3) |
| Self-pay/Other | 17 (7) | 19 (8) | 36 (8) |
| Pregnancy status | |||
| Not pregnant | 164 (67) | 165 (67) | 329 (67) |
| Pregnant | 81 (33) | 80 (33) | 161 (33) |
| First- or second-degree relative with any cancer | |||
| Yes | 182 (74) | 183 (75) | 365 (75) |
| No | 63 (26) | 62 (25) | 125 (25) |
| First-degree relative with breast or colon cancer | |||
| Yes | 28 (11) | 23 (9) | 51 (10) |
| No | 217 (89) | 222 (91) | 439 (90) |
Education level was added as a measure after recruitment had begun. To minimize missing data, this question was added to all 6-month surveys of those not previously queried. Missing data resulted from participants who did not complete 6-month surveys.
SD, standard deviation.
Effect of intervention on family communication
At baseline, 19.6% of intervention participants and 17.1% of control participants reported having ever gathered family cancer information; 11.8% of intervention and 11.0% of control participants reported having ever shared information about familial cancer with their relatives (see Table 2). These differences were not statistically significant (p=0.484 and 0.776 respectively). At follow-up, intervention participants were significantly more likely to report having gathered family cancer information (OR: 2.73; 95% CI: 2.00 to 3.71) and having shared familial cancer information with relatives (OR: 1.85; 95% CI: 1.37 to 2.48). There were no significant differences in communication frequency at baseline p=0.276 (see Table 3 for means). At follow-up, intervention participants reported a significantly higher average frequency of communication with relatives (mean difference: 0.14; 95% CI: 0.05 to 0.23).
Table 2.
Frequency (Percent) of Participants Who Reported Gathering or Sharing Cancer Risk Information with Relatives
| Baseline (n=490) | 1 month (n=381) | 6 months (n=310) | 14 months (n=285) | OR (95% CI) | p-Valuea | |
|---|---|---|---|---|---|---|
| Gather information | ||||||
| All subjects | 2.73 (2.01,3.71) | <0.001 | ||||
| Control | 48 (19.6) | 33 (17.3) | 37 (23.3) | 35 (24.0) | ||
| Intervention | 42 (17.1) | 64 (33.7) | 61 (40.7) | 59 (42.4) | ||
| Not pregnantb | 3.40 (2.02,5.72) | <0.001 | ||||
| Control | 15 (15.0) | 11 (14.7) | 12 (18.5) | 10 (16.4) | ||
| Intervention | 14 (13.0) | 25 (30.1) | 23 (36.5) | 22 (42.3) | ||
| Pregnantb | 1.08 (0.61,1.89) | 0.793 | ||||
| Control | 14 (17.5) | 15 (24.6) | 12 (26.7) | 15 (39.5) | ||
| Intervention | 13 (16.2) | 15 (27.3) | 11 (25.6) | 15 (36.6) | ||
| REAL-G>3b | 3.02 (2.16,4.21) | <0.001 | ||||
| Control | 42 (20.8) | 26 (15.9) | 33 (23.9) | 31 (24.8) | ||
| Intervention | 37 (18.3) | 56 (35.2) | 56 (44.8) | 49 (43.0) | ||
| REAL-G≤3b | 0.403 | |||||
| Control | 6 (14.3) | 7 (26.9) | 4 (20.0) | 4 (20.0) | 1.40 (0.64, 3.07) | |
| Intervention | 5 (11.9) | 8 (25.8) | 5 (20.0) | 9 (37.5) | ||
| Share information | ||||||
| All subjects | 1.85 (1.37,2.48) | <0.001 | ||||
| Control | 29 (11.8) | 34 (17.9) | 34 (21.7) | 36 (24.7) | ||
| Intervention | 27 (11.0) | 57 (30.3) | 38 (25.5) | 58 (41.7) | ||
p-Value comparing intervention and control groups, using mixed models analysis with logit link, controlling for baseline value of outcome measure. Only the odds ratio (OR) and 95% confidence interval (95% CI) for the analysis summarizing over all time periods is presented.
Significant effect modification for pregnancy (age ≤40 years) and genetic literacy: p-Value, coefficient, and 95% CI of interaction terms: p=0.003, −1.15 (−1.91, −0.39); p=0.078, −0.77 (−1.62, 0.08), respectively.
REAL-G, Rapid Estimate of Adult Literacy in Genetics.
Table 3.
Average Reported Frequencies of Communication with Relatives About a Family History of Cancer
| Baselinea(n=489) | 1 month(n=374) | 6 months(n=299) | 14 months(n=282) | Difference (95% CI)b | p-Valueb | |
|---|---|---|---|---|---|---|
| All subjects | 0.14 (0.05, 0.23) | <0.001 | ||||
| Control | 1.45 (0.47) | 1.41 (0.50) | 1.54 (0.62) | 1.57 (0.56) | ||
| Intervention | 1.50 (0.58) | 1.55 (0.60) | 1.75 (0.70) | 1.78 (0.71) | ||
| Not pregnantc | 0.15 (0.03, 0.27) | 0.018 | ||||
| Control | 1.38 (0.42) | 1.37 (0.40) | 1.50 (0.59) | 1.47 (0.47) | ||
| Intervention | 1.46 (0.60) | 1.52 (0.52) | 1.71 (0.63) | 1.63 (0.64) | ||
| Pregnantc | −0.03 (−0.17, 0.11) | 0.699 | ||||
| Control | 1.34 (0.37) | 1.35 (0.45) | 1.45 (0.42) | 1.56 (0.57) | ||
| Intervention | 1.37 (0.43) | 1.35 (0.45) | 1.39 (0.37) | 1.52 (0.48) |
Frequency of communication [mean (SD)] with all listed first- and second-degree relatives on a four-point Likert scale ranging from 1 (not at all) to 4 (a lot).
p-Value and confidence interval comparing intervention and control groups using mixed model analysis controlling for baseline value of outcome measure. Only the OR and 95% CI for the analysis summarizing over all time periods is presented.
Significant effect moderation: pregnancy (age ≤40 years), p=0.064, coefficient and 95% CI of interaction term, −0.18 (−0.36, −0.01).
Effect modification
For all outcome variables, the intervention was similarly effective for different races, education level, age, family history of any cancer, or whether participants had a first-degree relative with breast or colon cancer (see Tables 2 and 3). Pregnancy status modified the intervention effect for gathering information and frequency of family communication (coefficients and 95% CI of interaction terms are −1.15 [−1.91 to −0.39] and −0.18 [−0.36 to −0.01] respectively). Women 40 years old and under in the intervention group who were not pregnant gathered significantly more information (OR: 3.40; 95% CI: 2.02 to 5.72) and communicated more frequently with family members (mean: 0.15; 95% CI: 0.03 to 0.27) than control participants. Pregnant women did not gather more information (OR: 1.08; 95% CI: 0.61 to 1.89) nor communicate more frequently (mean: −0.03, 95% CI: −0.17 to 0.11) between study arms. Finally, genetic literacy modified the intervention effect on gathering information (coefficient and 95% CI of interaction term: −0.77 [−1.62 to 0.08]). More intervention participants with higher genetic literacy (REAL-G score >3) gathered family information (OR: 3.02; 95% CI: 2.16 to 4.21) than control participants. Yet, intervention and control participants with low genetic literacy (REAL-G score ≤3) displayed similar information gathering (OR: 1.40; 95% CI: 0.64 to 3.07).
Discussion
The KinFact intervention improved family history collection, sharing of hereditary cancer risk information, and frequency of related communication. Previous studies and expert groups have demonstrated a need for improvements in family history communication.1,3 To our knowledge, KinFact is one of the first interventions to demonstrate an effective method for increasing this communication. While several interventions have been designed to increase cancer family history awareness in community settings47–49 and use genetic counseling support to enhance family communication about specific genetic risks in higher risk settings,39,50–55 we are not aware of studies assessing the gathering and sharing of cancer information in the families of primary care patients after communication skill-building intervention. As such, it may serve as the basis for future dissemination research and as a benchmark for studies aiming to improve the effectiveness of family health history interventions.
While acknowledging the positive impact of KinFact as reflected in intervention versus control group differences, relatively few intervention participants gathered or shared family history information, and family communication frequency was low. The general patient population, who were not selected for cancer risk or motivation to seek care, could partially explain the overall low communication after the intervention. Overall, women of lower socioeconomic status have been found to be less knowledgeable about their cancer family histories and are less likely to initiate related questions with their providers.56–58 Family health history communication about cancer is relatively low despite its known benefits for cancer screening and early detection. The KinFact intervention provides a blueprint to increase awareness of such history and promote familial cancer risk communication. Whether the 25%–30% increase in women gathering health information from and sharing health information with family members is clinically significant is unclear; however, this increase represents a move in the right direction. Knowledge of family history is critical to ensuring patients are appropriately screened.59 The fact that less than 20% of women at baseline engaged in collecting or sharing health information suggests that interventions like KinFact are needed to better understand patients' family health risks. The National Institutes of Health consensus development conference on family history recognized its potentially important role in medical practice to motivate positive lifestyle changes, enhance individual empowerment, and influence clinical interventions.3 Consequently, improvement in family communication of genetic risk information may lead to increased attention to high-risk women's cancer screening, particularly women from disadvantaged backgrounds.
Communication within families about cancer diagnoses and risk remains difficult and complex, even after sharing individual risk information, general prevention guidance, and communication techniques. The intervention provided a rule-based approach to guide conversations with relatives. Consistent with theories addressing health communication behaviors, intervention participants may have gained insight on their attitudes about family history discussion, relevance of the information for relatives, responsibilities to discuss and minimize distress, and the usefulness of communicating. The intervention may have influenced the participants' perceived control over sharing and gathering of information. Despite these important findings, questions about what is driving these results remain. Additional empirical analyses are needed to better understand family communication processes.60,61
The fact that KinFact was implemented in a primary care setting is important because few effective methods for family history collection in this setting have been identified.3 Moreover, in a clinical setting that was majority African American, KinFact improved family history outcomes for women regardless of race. Enhancing women's communication skills regarding their family cancer risk and cancer prevention could address disparities relating to self-efficacy and knowledge and lead to more personalized care and better health outcomes. Clinic-based family history approaches are important because they may immediately lead to medical interventions.59 Our parallel approach of waiting room recruitment and no direct incorporation of the KinFact intervention in clinical care was intentional, to ensure that the research did not impede clinic flow. Integrating the intervention with decision support for providers would be optimal. Adapting the intervention to electronic patient portals, navigator-led education in preclinical settings, or interactive phone or online incorporation of the skill-building modules with a guiding person or avatar as part of more patient-centered health care might be considered.62,63 KinFact-type skill-building could theoretically help women less knowledgeable about cancer initiate family history concerns with their providers more readily.
Along with understanding how and where KinFact can be more efficiently and effectively utilized, future studies might also investigate the extent of the intervention's impact. The endpoint of this study was family communication; however, family communication is only a surrogate for other important social and health outcomes. For example, new cancer diagnoses within a family likely impact patterns of family communication.64 Communication interventions like KinFact might enable women to initiate difficult conversations.65 Communication within families has been associated with greater problem-solving abilities, social skills, and competence.66,67
Interestingly, in exploratory subgroup analyses, the intervention was less effective for pregnant women. Pregnant women were included because pregnancy was viewed as a time when family history could have relevance. Family communication about cancer risk may be less of a priority during pregnancy. The intervention also appeared less effective for gathering family history among women with lower genetic literacy, while there was no interaction effect with overall educational background. It is possible that helping these women gather risk information may require more skill building or that educational background and genetic literacy are separate constructs with respect to family communication skills. Given the importance of reaching low literacy populations to reduce health disparities, future research will need to track this variable and consider focusing on how best to reach these individuals.
Despite the significant findings, there are some limitations. The results are limited by the sample makeup, relatively low response rate for follow-up, and the KinFact intervention's scope. It is possible that patients who agreed to participate in the study differed from those who declined participation, introducing a selection bias in the intervention group. This study's diversity in participant age, race, and education level are strengths that seek to address the concern of increasing disparities in genetics. Still, our study was not all inclusive. We were intentional in our focus on women have not evaluated it in men or other ethnicities. Study findings may also be compromised by missing data. For example, the lowest response rate was at 14 months, when only 58% (282) of participants completed a follow-up survey. No significant patterns were identified to suggest bias among responders, other than age; communication behavior specific to younger women could possibly bias results. For example, if younger women consider family history less relevant, they might be less likely to communicate, thus resulting in an overestimation of communication for the participants in our sample. The study is also limited in that it focused on breast and colon cancer, so application to other health concerns is uncertain.
Neither the participants nor statistician were blinded to the arms of the study, and this could have led to potential bias. Because it was not possible to blind study participants as to whether they were in the intervention or control arms, it is possible that women receiving the intervention may have been more likely to report gathering or sharing family history, thus artificially inflating results. The individuals collecting follow-up data were blinded to whether participants were in the intervention or control arms and the staff who conducted the intervention did not conduct follow-up interviews, minimizing introduction of bias and participants' bias to please the interventionists. In theory (knowingly or not) the limitation of non-blinding could have influenced the statistician's analysis of the results to show a positive effect if she believed the intervention would work. In addition, the level of risk provided, enhanced self-efficacy, and other factors could have influenced the KinFact outcomes. Significant findings could also occur by chance. Future research could provide more insights into its mechanism of action.
Challenges for integrating KinFact into clinics could include time, the risk assessment tool, and the use of skilled facilitators. Potential enhancements such as more automation and briefer, streamlined, KinFact-type interventions could be assessed. However, it is unclear which intervention aspects would convey with fidelity or uptake to more automated or briefer approaches.68,69 Family history collection may be improved—or complemented—through alternative approaches besides clinical environments. Programs to enhance family history communication have included national initiatives such as the U.S. Surgeon General's Family History tool,70 community-based workshops,26 and other culturally adept family history campaigns.47
Finally, as with most survey-based research, this study is limited by the measures utilized. In particular, the processes of family communication and how these relate to participants' responses to survey items might vary. For example, women may perceive the question of “actively” gathering information to mean that they were primarily initiating asking questions. Yet, while sharing information, women may be primed to ask follow-up questions during a conversation and may not perceive this as actively gathering information. In fact, studies have found discrepancies in family history cancer discussions based on the way the question is asked.71
These limitations notwithstanding, the KinFact findings presented here break new ground in family health history research, and they open the way toward a promising research agenda.
Conclusion
The KinFact intervention, undertaken in a population at risk for disparities in cancer care and genetic services, may be useful in enhancing family discussions of cancer risk information. It may also serve as an important step in promoting patient-and family-centered care and could be adaptable in other settings and lead to improved public health. Educating women to enhance their “kinkeeping”72 skills may allow them to partner more effectively with relatives in discussing cancer risk and prevention.
Supplementary Material
Acknowledgments
We wish to thank the participants, staff of the Women's Health Clinic, the Virginia Breast Cancer Foundation, and research assistants for their support and collaboration in this study. This work was supported by National Cancer Institute, grant number R01-CA140959.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Yoon PW, Scheuner MT, Gwinn M, et al. Awareness of family health history as a risk factor for disease – United States. MMWR Morb Mortal Weekly Rep 2004;53:1044–1147 [PubMed] [Google Scholar]
- 2.Murff HJ, Peterson NB, Greevy RA, et al. Early initiation of colorectal cancer screening in individuals with affected first-degree relatives. J Gen Intern Med 2007;22:121–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berg AO, Baird MA, Botkin JR, et al. National Institutes of Health state-of-the-science conference statement: family history and improving health. Ann Intern Med 2009;151:872–877 [DOI] [PubMed] [Google Scholar]
- 4.Koehly LM, Peters JA, Kenen R, et al. Characteristics of health information gatherers, disseminators, and blockers within families at risk of hereditary cancer: Implications for family health communication interventions. Am J Public Health 2009;99:2203–2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herrington DM. Cardiovascular genomics: Outcomes and implications. Can J Cardiol 2010; 26:60A–63A [DOI] [PubMed] [Google Scholar]
- 6.Khoury MJ, Feero WG, Valdez R. Family history and personal genomics as tools for improving health in an era of evidence-based medicine. Am J Prev Med 2010;39:184–188 [DOI] [PubMed] [Google Scholar]
- 7.Valdez R, Yoon PW, Qureshi N, et al. Family history in public health practice: A genomic tool for disease prevention and health promotion. Annu Rev Public Health 2010;31:69–87 [DOI] [PubMed] [Google Scholar]
- 8.Edwards AG, Naik G, Ahmed H, et al. Personalised risk communication for informed decision making about taking screening tests. Cochrane Database Syst Rev 2013;28:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheuner MT, McNeel TS, Freedman AN. Population prevalence of familial cancer and common hereditary cancer syndromes. The 2005 California Health Interview Survey. Genet Med 2010;12:726–735 [DOI] [PubMed] [Google Scholar]
- 10.Acheson LS. Recording, interpreting, and updating the family history of cancer: implications for cancer prevention. JAMA 2011;306:208–210 [DOI] [PubMed] [Google Scholar]
- 11.Chowdhury S, Dent T, Pashayan N, et al. Incorporating genomics into breast and prostate cancer screening: assessing the implications. Genet Med 2013;15:423–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dove-Edwin L, Sasieni P, Adams J, Thomas HJ. Prevention of colorectal cancer by colonoscopic surveillance in individuals with a family history of colorectal cancer: 16-year, prospective, follow-up study. BMJ 2005;331:1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.FH01 Collaborative Teams. Mammographic surveillance in women younger than 50 years who have a family history of breast cancer: Tumour characteristics and projected effect on mortality in the prospective, single-arm, FH01 study. Lancet Oncol 2010;11:1127–1134 [DOI] [PubMed] [Google Scholar]
- 14.Zlot AI, Silvey K, Newell N, et al. Family history of colorectal cancer: Clinicians' preventive recommendations and patient behavior. Prev Chronic Dis 2012;9:100254. [PMC free article] [PubMed] [Google Scholar]
- 15.Mesher D, Dove-Edwin I, Sasieni P, et al. A pooled analysis of the outcome of prospective colonoscopic surveillance for familial colorectal cancer. Int J Cancer 2014;134:939–947 [DOI] [PubMed] [Google Scholar]
- 16.Kelly K, Sturm A, Kemp K, et al. How can we reach them? Information seeking and preferences for a cancer family history campaign in underserved communities. J Health Commun 2009;14:573–589 [DOI] [PubMed] [Google Scholar]
- 17.Eisinger F, Bouhnik A, Malavolti L, et al. Cancer survivors: Familial risk perception and management advice given to their relatives. Familial Cancer 2011;10:147–155 [DOI] [PubMed] [Google Scholar]
- 18.Maddock C, Schrijvers D, Turco M, et al. To know or not to know? Not the only question in familial breast cancer risk communication. Ecancermedicalscience 2011;5:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murthy V, Garza M, Almario D, et al. Using a family history intervention to improve cancer risk perception in a Black community. J Gen Counsel 2011;20:639–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell JA, Hawkins J, Watkins DC. Factors associated with cancer family history communication between African American men and their relatives. J Mens Studies 2013;21:97–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kenen R, Ardern-Jones A, Eeles R. We are talking, but are they listening? Communication patterns in families with a history of breast/ovarian cancer (HBOC). Psychooncology 2004;13:335–345 [DOI] [PubMed] [Google Scholar]
- 22.Nisker J. A public health education initiative for women with a family history of breast/ovarian cancer: why did it take Angelina Jolie. J Obstet Gynaecol Can 2013;35:689–691 [DOI] [PubMed] [Google Scholar]
- 23.Carney PA, O'Malley JP, Gough A, et al. Association between documented family history of cancer and screening for breast and colorectal cancer. Prev Med 2013;57:679–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cameron E, Rose S, Carey M. Assessment of family history of colorectal cancer in primary care: Perceptions of first degree relatives of people with colorectal cancer. Patient Educ Couns 2013;94:427–431 [DOI] [PubMed] [Google Scholar]
- 25.Catz DS, Green NS, Tobin JN, et al. Attitudes about genetics in underserved, culturally diverse populations. Community Genet 2005;8:161–172 [DOI] [PubMed] [Google Scholar]
- 26.Butty JM, Richardson F, Mouton CP, et al. Evaluation findings from genetics and family health history community-based workshops for African-Americans. J Community Genet 2012;3:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orom H, Kiviniemi M, Underwood W 3rd, et al. Perceived cancer risk: Why is it lower among nonwhites than whites? Cancer Epidemiol Biomarkers Prev 2010;19:746–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hughes C, Lerman C, Lustbader E. Ethnic differences in risk perception among women at increased risk for breast cancer. Breast Cancer Res Treat 1996;40:25–35 [DOI] [PubMed] [Google Scholar]
- 29.Honda K, Neugut A. Associations between perceived cancer risk and established risk factors in a national community sample. Cancer Detect Prev 2004;28:1–7 [DOI] [PubMed] [Google Scholar]
- 30.Haas JS, Kaplan CP, Des Jarlais G, et al. Perceived risk of breast cancer among women at average and increased risk. J Womens Health 2005;14:845–851 [DOI] [PubMed] [Google Scholar]
- 31.Griffith KA, McGuire DB, Royak-Schaler R, et al. Influence of family history and preventive health behaviors on colorectal cancer screening in African Americans. Cancer 2008;;113:276–285 [DOI] [PubMed] [Google Scholar]
- 32.Wang C, Gallo RE, Fleisher L, Miller SM. Literacy assessment of family health history tools for public health prevention. Public Health Genomics 2011; 14:222–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Etchegary H, Potter B, Perrier C, Wilson B. Cultural differences in family communication about inherited cancer: Implications for cancer genetics research. J Cult Divers 2013;20:195–201 [PubMed] [Google Scholar]
- 34.Wiseman M, Dancyger C, Michie S. Communicating genetic risk information within families: A review. Fam Cancer 2010;9:691–703 [DOI] [PubMed] [Google Scholar]
- 35.Cronen VE, Parce WB, Harris LM. The logic of the coordinated management of meaning: A rules-based-approach to the first course in interpersonal communication. Commun Educ 1979;28:22–38 [Google Scholar]
- 36.Wiens ME, Wilson BJ, Honeywell C, Etchegary H. A family genetic risk communication framework: guiding tool development in genetics health services. J Community Genet 2013;4:233–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burns AC. The expanded Health Belief Model as a basis for enlightened preventive health care practice and research. J Health Care Mark 1992;12:32–45 [PubMed] [Google Scholar]
- 38.Wilson BJ, Forrest K, van Teijlingen ER, et al. Family communication about genetic risk: The little that is known. Community Genet 2004;7:15–24 [DOI] [PubMed] [Google Scholar]
- 39.Daly MB, Barsevick A, Miller SM, et al. Communicating genetic test results to the family: A six-step, skills-building strategy. Fam Community Health 2001;24:13–26 [DOI] [PubMed] [Google Scholar]
- 40.Buckman R, ed. How to break bad news, a guide for health care professionals. Baltimore, MD: The Johns Hopkins University Press, 1992 [Google Scholar]
- 41.Kincaid JP, Fishburne RP, Rogers RL, Chissom BS. (1975). Derivation of new readability formulas (Automated Readability Index, Fog Count, and Flesch Reading Ease formula) for Navy enlisted personnel. Research Branch Report 8–75. Millington, TN: Chief of Naval Technical Training, U.S. Naval Air Station, Memphis [Google Scholar]
- 42.Erby LH, Roter D, Larson S, Cho J. The rapid estimate of adult literacy in genetics (REAL-G): a means to assess literacy deficits in the context of genetics. Am J Med Genet A 2008;;146A:174–181 [DOI] [PubMed] [Google Scholar]
- 43.Rogers LE, Escudero V, eds. Communication: The central dynamic in personal relationships—theoretical foundations. In: Friedley SA, ed. Foundations of interpersonal communication theory: A reader. Reno, NV: Bent Tree Press; 2005:51–67 [Google Scholar]
- 44.Gaff CL, Clarke AJ, Atkinson P, et al. Process and outcome in communication of genetic information within families: a systematic review. Eur J Hum Genet 2007;15:999–1011 [DOI] [PubMed] [Google Scholar]
- 45.Centers for Disease Control and Prevention. HealthStyles88 Fact Sheet. Available at /www.orau.gov/cdcynergy/soc2web/Content/activeinformation/resources/Healthstyles.pdf Accessed June30, 2014
- 46.Bowen DJ, Bourcier E, Press N, et al. Effects of individual and family functioning on interest in genetic testing. Community Genet 2004;7:25–32 [DOI] [PubMed] [Google Scholar]
- 47.O'Leary J, Edelson V, Gardner N, et al. Community-centered family health history: a customized approach to increased health communication and awareness. Prog Community Health Partnersh 2011;5:113–122 [DOI] [PubMed] [Google Scholar]
- 48.Wallace JP, Baugh C, Cornett S, et al. A family history demonstration project among women in an urban Appalachian community. Prog Community Health Partnersh 2009;3:155–163 [DOI] [PubMed] [Google Scholar]
- 49.Mays D, Sharff ME, DeMarco TA, et al. Outcomes of a systems-level intervention offering breast cancer risk assessments to low-income underserved women. Fam Cancer 2012;11:493–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roshanai AH, Roseqnuist R, Lampic C, Nordin K. Does enhanced information at cancer genetic counseling improve counselees' knowledge, risk perception, satisfaction and negotiation of information to at-risk relatives? Acta Oncol 2009;48:999–1009 [DOI] [PubMed] [Google Scholar]
- 51.Seymour KC, Addington-Hall J, Lucassen AM, Foster CL. What facilitates or impedes family communication following genetic testing for cancer risk? A systematic review and meta-synthesis of primary qualitative research. J Genet Counsel 2010;19:330–342 [DOI] [PubMed] [Google Scholar]
- 52.Forrest L, Delatycki M, Curnow L, et al. An audit of clinical service examining the uptake of genetic testing by at-risk family members. Gen in Med 2012;14:122–128 [DOI] [PubMed] [Google Scholar]
- 53.Kardashian A, Fehniger J, Creasman J, et al. A pilot study of the sharing risk information tool (ShaRIT) for families with hereditary breast and ovarian cancer syndrome. Hered Cancer Clin Pract 2012;10:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Montgomery SV, Barsevick AM, Egleston BL, et al. Preparing individuals to communicate genetic test results to their relatives: report of a randomized control trial. Fam Cancer 2013;12:537–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gaff C. and Hodgson J. A genetic counseling intervention to facilitate family communication about inherited conditions. J Genet Counsel 2014;23:814–823 [Epub 2014 Mar 1] [DOI] [PubMed] [Google Scholar]
- 56.Kinney AY, Croyle RT, Dudley WN, et al. Knowledge, attitudes, and interest in breast-ovarian cancer gene testing: A survey of a large African-American kindred with a BRCA1 mutation. Prev Med 2001;33:543–551 [DOI] [PubMed] [Google Scholar]
- 57.Russell KM, Champion VL, Monahan PO, et al. Randomized trial of a lay health advisor and computer intervention to increase mammography screening in African American women. Cancer Epidemiol Biomarkers Prev 2010;19:201–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fair AM, Monahan PO, Russell K, et al. The interaction of perceived risk and benefits and the relationship to predicting mammography adherence in African American women. Oncology Nursing Forum 2012; 39:53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berg AO. Family history gets a boost. Ann Intern Med 2012;156:315–316 [DOI] [PubMed] [Google Scholar]
- 60.Kaphingst KA, Goodman M, Pandya C, et al. Factors affecting frequency of communication about family health history with family members and doctors in a medically underserved population. Patient Educ Couns 2012;88:291–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ashida S, Kaphingst KA, Goodman M, Schafer EJ. Family health history communication networks of older adults: importance of social relationships and disease perceptions. Health Educ Behav 2013;40:612–619 [DOI] [PubMed] [Google Scholar]
- 62.Sarfaty M, Wender R, Smith R. Promoting cancer screening within the patient centered medical home. CA Cancer J Clin 2011;61:397–408 [DOI] [PubMed] [Google Scholar]
- 63.Smith ML, Sosa ET, Hochhalter AK, et al. Correlates of family health history discussions between college students and physicians: Does family cancer history make a difference? J Prim Prev 2011;32:311–322 [DOI] [PubMed] [Google Scholar]
- 64.Forrest LE, Curnow L, Delatycki MB, et al. Health first, genetics second: Exploring families' experiences of communicating genetic information. Eur Jnl Hum Gen 2008;16:1329–1335 [DOI] [PubMed] [Google Scholar]
- 65.Afifi WA, Morgan SE, Stephenson MT, et al. Examining the decision to talk with family about organ donation: applying theory of motivated information management. Commun Monogr 2006;73:188–215 [Google Scholar]
- 66.Coughlin C, Vuchinich S. Family experience in preadolescence and the development of male delinquency. J Marriage Fam 1996;58:491–501 [Google Scholar]
- 67.Smith EP, Prinz RJ, Dumas JE, Laughlin JE. Latent models of family processes in African American families: Relationships to child competence, achievement, and problem behavior. J Marriage Fam 2001;63:967–980 [Google Scholar]
- 68.Simon C, Acheson L, Burant C, et al. Patient interest in recording family histories of cancer via the Internet. Genet Med 2008;10:895–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McBride CM, Alford SH, Reid RJ, et al. Characteristics of users of online personalized genomic risk assessments: Implications for physician-patient interaction. Genet Med 2009;11:582–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guttmacher AE, Collins FS, Carmona RH. The family history—more important than ever. N Engl J Med 2004;351:2333–2336 [DOI] [PubMed] [Google Scholar]
- 71.Corona R, Rodriguez V, Quillin J, et al. Talking (or not) about family health history in families of Latino young adults. Health Educ Behav 2013;40:571–580 [DOI] [PubMed] [Google Scholar]
- 72.Gallagher SK, Gerstel N. Kinkeeping and friend keeping among older women: The effect of marriage. Gerontologist 1993;33:675–681 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.