Abstract
Background: Postexercise protein or amino acid ingestion restores muscle protein synthesis in older adults and represents an important therapeutic strategy for aging muscle. However, the precise nutritional factors involved are unknown.
Objective: The purpose of this study was to determine the role of increased postexercise Leu ingestion on skeletal muscle myofibrillar protein synthesis (MyoPS), mammalian/mechanistic target of rapamycin complex 1 signaling, and amino acid transporter (AAT) mRNA expression in older men over a 24-h post–resistance exercise (RE) time course.
Methods: During a stable isotope infusion trial (l-[ring-13C6]Phe; l-[1-13C]Leu), older men performed RE and, at 1 h after exercise, ingested 10 g of essential amino acids (EAAs) containing either a Leu content similar to quality protein (control, 1.85 g of Leu, n = 7) or enriched Leu (LEU; 3.5 g of Leu, n = 8). Muscle biopsies (vastus lateralis) were obtained at rest and 2, 5, and 24 h after exercise.
Results: p70 S6 kinase 1 phosphorylation was increased in each group at 2 h (P < 0.05), whereas 4E binding protein 1 phosphorylation increased only in the LEU group (P < 0.05). MyoPS was similarly increased (∼90%) above basal in each group at 5 h (P < 0.05) and remained elevated (∼90%) at 24 h only in the LEU group (P < 0.05). The mRNA expression of select AATs was increased at 2 and 5 h in each group (P < 0.05), but AAT expression was increased at 24 h only in the LEU group (P < 0.05).
Conclusions: Leu-enriched EAA ingestion after RE may prolong the anabolic response and sensitivity of skeletal muscle to amino acids in older adults. These data emphasize the potential importance of adequate postexercise Leu ingestion to enhance the response of aging muscle to preventive or therapeutic exercise-based rehabilitation programs. This trial was registered at clinicaltrials.gov as NCT00891696.
Introduction
Advancing age is associated with a gradual and involuntary loss of skeletal muscle mass (1, 2) that can accelerate declines in muscle strength and function. This collective loss of skeletal muscle mass and function, commonly referred to as sarcopenia (3), is a major contributor to frailty and mobility limitations and places older adults at greater risk of functional impairments, dependent living, and mortality (3, 4). It is estimated that the population of individuals aged ≥65 y will rapidly expand to comprise ∼20% (∼71 million people) of the U.S. population by 2030 (U.S. Census Bureau; National Institutes on Aging statistics). These statistics, coupled with the rising health care costs associated with sarcopenia (5), underscore the need to identify practical evidence-based preventive and treatment strategies for age-related sarcopenia.
Resistance exercise (RE)11 is a common strategy recommended for older adults to enhance muscle size and function. However, we showed previously that, relative to younger individuals, older adults have an impaired muscle protein synthesis response to RE (6). Conversely, our laboratory and others showed that the impaired muscle protein synthesis response to RE in older adults can be overcome by ingesting a sufficient amount of essential amino acids (EAAs) or protein after a bout of RE (6–8), thus highlighting the therapeutic potential of strategic postexercise nutrition for older adults relative to the use of exercise without any nutritional support (9). However, to enhance the effects on aging skeletal muscle, there remains a need to better understand the specific nutritional factors and cellular mechanisms that facilitate the stimulation of muscle protein synthesis in older adults when amino acids or protein are ingested after exercise.
The amino acid Leu has received considerable attention as a key regulator of protein synthesis (10, 11). It is well understood that Leu (and EAAs) stimulate protein synthesis through activation of the mammalian/mechanistic target of rapamycin (mTOR) Complex 1 (mTORC1) signaling pathway (12, 13). Activation of mTORC1 stimulates translation initiation through phosphorylation of its direct downstream effectors, p70 S6 kinase 1 (S6K1) and 4E binding protein 1 (4E-BP1) (14–16). The ability of amino acids to trigger mTORC1 activation is regulated in part through the involvement of specific amino acid transporters (17–19). In particular, system L amino acid transporter 1 (LAT1), which forms a heterodimer with cluster of differentiation 98 (CD98), and system A amino acid transporter 2 (SNAT2) appear to work in concert at the cell membrane to facilitate the transport of amino acids (including Leu) into the cell (20), whereas the amino acid transporter (AAT) proton-assisted amino acid transporter 1 (PAT1) appears to have a role in the stimulation of mTORC1 activity in the presence of increased intracellular amino acids (18, 21).
Previous research has demonstrated that Leu has a unique ability to restore the capacity for EAA ingestion to stimulate muscle protein synthesis in older adults (22), but whether Leu provides the same metabolic trigger for older adults after exercise is unknown. This level of investigation is needed to enhance the overall therapeutic impact of exercise for older adults. Therefore, the purpose of this study was to determine the role of postexercise Leu ingestion by examining the effect of ingesting different Leu quantities (3.5 vs. 1.85 g) within an isonitrogenous mixture of EAAs on skeletal muscle myofibrillar protein synthesis (MyoPS) in older men over a 24-h post-RE time course. In addition, throughout this time course, we examined the response of mTORC1 signaling and AAT mRNA expression to obtain mechanistic insight into the role of postexercise Leu ingestion on MyoPS. We hypothesized that Leu-enriched EAA ingestion after RE would enhance MyoPS.
Materials and Methods
Participants.
Fifteen healthy older men (aged 72 ± 2 y) volunteered for this study. All participants were considered recreationally active but not engaged in regularly scheduled exercise training. Screening was completed by performing a clinical history, physical examination, and laboratory tests, including a complete blood count with differential, liver and kidney function, coagulation profile, fasting blood glucose, oral glucose tolerance, hepatitis B and C screening, HIV testing, thyroid-stimulating hormone, urinalysis, and drug screening. All participants gave informed written consent before participation, which was approved by the Institutional Review Board of the University of Texas Medical Branch (which is in compliance with the Declaration of Helsinki as revised in 1983).
Bilateral maximal knee extensor muscle strength was determined for each participant on 2 separate occasions (separated by ∼7 d) using a 1-repetition maximum (1RM) test performed on a leg extension device (Cybex-VR2; Medway). The 1RM measurement was obtained during the initial screening visit, and a second 1RM measurement was obtained ∼1 wk before study participation. The heaviest weight lifted between the 2 measurements was considered the participant’s 1RM.
Experimental design.
Participants were randomly assigned to 1 of 2 experimental groups: control or LEU. Participant characteristics for each group are presented in Table 1. Each group completed an experimental trial over 2 consecutive days, which was identical for each group with the exception of the composition of the EAA solution that was ingested after exercise (Table 2). Participants were housed in the Institute for Translational Sciences Clinical Research Center (ITS-CRC) of the University of Texas Medical Branch for the entirety of the trial.
TABLE 1.
Participant characteristics1
| CTRL | LEU | |
| Participants, n | 7 | 8 |
| Age, y | 74 ± 2 | 71 ± 3 |
| Height, cm | 174 ± 2 | 172 ± 3 |
| Weight, kg | 80 ± 4 | 80 ± 3 |
| BMI, kg/m2 | 26 ± 1 | 27 ± 1 |
| 1RM, kg | 69 ± 2 | 76 ± 3 |
| 1RM during exercise trial,2 % | 64 ± 1 | 65 ± 1 |
Values are means ± SEMs unless otherwise indicated. CTRL, control group; LEU, Leu group; 1RM, 1 repetition maximum.
Mean 1RM of all 8 exercise sets.
TABLE 2.
EAA beverage composition for the CTRL and LEU groups1
| Amino Acid | CTRL | LEU |
| g | g | |
| Total EAAs | 10.0 | 10.0 |
| l-His | 1.10 | 0.80 |
| l-Lys | 1.55 | 1.20 |
| l-Met | 0.30 | 0.30 |
| l-Thr | 1.45 | 1.00 |
| l-Phe | 1.55 | 1.40 |
| l-Val | 1.20 | 1.00 |
| l-Ile | 1.00 | 0.80 |
| l-Leu | 1.85 | 3.50 |
| l-[13C6]Phe | 0.12 | 0.11 |
| l-1-[13C]Leu | 0.11 | 0.21 |
CTRL, control group; EAA, essential amino acid; LEU, Leu group.
The evening before the experimental trial was initiated, participants were admitted to the ITS-CRC, and a dual-energy x-ray absorptiometry scan (QDR 4500W; Hologic) was performed after the bladder was voided to measure body composition and lean mass. Participants were then fed a standard dinner (12 kcal/kg body weight; 60% carbohydrate, 20% fat, and 20% protein) and a snack (5 kcal/kg body weight; 60% carbohydrate, 20% fat, and 20% protein) at 2200, both prepared by the Bionutrition Division of the ITS-CRC. Both days of the trial took place after an overnight fast, under basal conditions. All participants were studied during the same time of day (0600–1630) to avoid potential circadian changes, and participants were asked to refrain from exercise for at least 48 h before beginning the trial.
On morning 1 of the experimental trial, while the subjects were resting in a supine position, an 18-gauge polyethylene catheter was inserted into an antecubital vein for tracer infusion. Another 18-gauge polyethylene catheter was inserted retrograde in a hand vein of the contralateral arm, which was kept in a heated pad for arterialized blood sampling. After drawing a background blood sample, a primed continuous infusion of isotopically labeled l-[ring-13C6]Phe and l-[1-13C]Leu tracers (Cambridge Isotope Laboratories) was initiated. Each tracer was dissolved in sterile 0.9% saline and passed through a 2-μm filter. The priming doses for l-[ring-13C6]Phe and l-[1-13C]Leu were 2 and 4.8 μmol/kg, respectively. The constant infusion rates for l-[ring-13C6]Phe and l-[1-13C]Leu were 0.05 and 0.08 μmol · kg−1 · min−1, respectively, and these rates were maintained throughout the trial day (13). Two hours after the initiation of the tracer infusion, muscle biopsy 1 was obtained from the lateral portion of the vastus lateralis of the leg with the biopsy site between 15 and 25 cm from the midpatella. The biopsy was performed using a 5-mm Bergström biopsy needle (23) with suction under sterile procedure and local anesthesia (1% lidocaine). At 2.5 h after biopsy 1, biopsy 2 was obtained from the same incision. The biopsy needle was inclined at a different angle so that biopsy 2 was obtained ∼5 cm proximal to biopsy 1. After muscle biopsy 2, participants were seated on the leg-extension device to begin the exercise portion of the trial. Participants completed 8 sets of 10 repetitions at a mean intensity of 65% 1RM with 3 min of rest between each set. Total time for the exercise period was ∼45 min. At 1 h after exercise, participants in the control group ingested 10 g of EAAs containing 1.85 g of Leu, whereas participants in the LEU group ingested 10 g of EAAs containing 3.5 g of Leu (Table 2) (24). Blood was obtained at 0, 0.5, 1, 1.25, 1.5, 1.75, 2, 2.5, 3, 4, and 5 h after exercise, and muscle biopsies were sampled at 2 h after exercise (1 h after the ingestion of the EAA solution) and 5 h after exercise. The biopsy obtained at 2 h after exercise was sampled from an incision on the opposite leg from the basal biopsies, and the 5-h postexercise biopsy was obtained from a new incision on the same leg made ∼5 cm proximal to incision 2. Throughout the trial, subjects were instructed to remain in their hospital beds in a supine position, with the exception of the exercise portion and restroom visits. After collection of the 5-h postexercise muscle biopsy, day 1 was concluded, tracers were stopped, and participants were given a standard lunch. Participants remained in the ITS-CRC and were encouraged to move around the unit to compile between 1000 and 1500 steps (to avoid prolonged periods of inactivity or bed rest), which was monitored (but not directly measured) by a study nurse. Participants were fed a dinner and snack identical to those of the previous night before an overnight fast in preparation for day 2 of the trial.
On morning 2 of the experimental trial, an infusion protocol identical to that described above was initiated, which was preceded by a blood draw. Muscle biopsies were obtained from a single incision at 2 and 4.5 h after initiation of the tracer infusion. The biopsy at 4.5 h corresponded to 24 h after exercise. After collection of the 24-h postexercise biopsy, participants were given a lunch and discharged from the unit.
Muscle tissue from all biopsies was immediately blotted, frozen in liquid nitrogen, and stored at −80°C until analysis.
EAA composition.
The composition of the EAA solution for each group is presented in Table 2. The Leu quantities were chosen based on previous research indicating a Leu threshold of ∼2 g in older adults (22, 25), and the use of an isonitrogenous mixture of EAAs was used to control for any influence of EAA total content (8). To minimize tracer dilution with the addition of the EAAs, we added l-[ring-13C6]Phe and l-[1-13C]Leu tracer to the oral EAA solution at 7.5% and 6.0% of the Phe and Leu total content, respectively. EAAs (Sigma-Aldrich) for the two groups were individually weighed and fully dissolved by continuous overnight stirring at room temperature in a noncaloric and caffeine-free flavored beverage (350 mL) to increase palatability (24).
Tissue processing for protein fractions.
Muscle samples were processed to obtain general protein and myofibrillar protein fractions as described previously (26). Briefly, ∼30–50 mg of frozen muscle tissue was placed in buffer (27), homogenized (1:9, wt:v), and centrifuged at 3400 × g for 10 min at 4°C. The resulting supernatant was collected and used for general cytosolic protein immunoblotting as described below. The resulting pellet was then suspended in isolation buffer (1 mol/L sucrose, 1 mol/L Tris-HCl, 1 mol/L KCl, and 0.5 mol/L EDTA, pH 7.4) containing protease and phosphatase inhibitors and centrifuged for 10 min at 4°C and 700 × g. After a series of 3 PBS buffer suspensions and 5-min centrifugations of 15,000 × g at 4°C, the pellet was resuspended and agitated on ice 2 times for 20 min and in a 4°C sonication bath in high-salt buffer (1:4, wt:v). The slurry was centrifuged at 15,000 × g for 10 min at 4°C. The resulting pellet was fully suspended in double-distilled water and centrifuged at 15,000 × g for 5 min at 4°C. To precipitate the myofibrillar proteins, 1 mL of 0.3 mol/L NaOH was added to resuspend the pellet and heated at 50°C for 30 min with frequent mixing by vortex. After centrifugation at 10,000 × g for 5 min at 4°C, the supernatant was collected, and an additional 1 mL of 0.3 mol/L NaOH was added to resuspend the pellet and heated at 37°C for 10 min with frequent mixing by vortex. After centrifugation at 10,000 × g for 5 min at 4°C, the supernatant was collected as the myofibrillar protein fraction and the collagen pellet was discarded. Precipitate was created by addition of 1 mL of perchloric acid to the collected supernatant and pelleted at 805 × g for 10 min at 4°C. This pellet was washed 2 times with 70% ethanol and then hydrolyzed overnight in 1.5 mL of 6 mol/L HCl for determination of myofibrillar protein–bound enrichment.
Amino acid enrichments and concentrations.
A separate piece of muscle (∼20–30 mg) was homogenized and used to obtain intracellular free amino acids as described previously (27). Myofibrillar protein–bound l-[ring-13C6]Phe enrichment (26) and muscle intracellular fluid l-[ring-13C6]Phe and l-[1-13C]Leu enrichment were determined via GC-MS (6890 Plus GC, 5973N MSD, 7683 autosampler; Agilent Technologies). Myofibrillar protein–bound l-[ring-13C6]Phe enrichment was determined by GC-MS in triplicate after protein hydrolysis and amino acid extraction (27) using the m+6:m+4 ratio and an external standard curve of known m+6:m+0 ratios (28, 29). Muscle intracellular free l-[ring-13C6]Phe and l-[1-13C]Leu enrichments were determined by GC-MS in triplicate using the m+6:m+0 and m+1:m+0 ratios, respectively. Blood l-[ring-13C6]Phe and l-[1-13C]Leu enrichments were determined from deproteinized blood samples in duplicate using the m+6:m+0 and m+1:m+0 ratios, respectively. All tracer enrichments were determined using tert-butyldimethylsilyl amino acid derivatives.
Concentrations of Phe and Leu were determined in blood and muscle intracellular fluid using tracer enrichments and l-[15N]Phe and l-[5,5,5-2H3]Leu as internal standards for Phe and Leu, respectively, as described previously (30). Basal intracellular amino acid concentrations were taken as the mean from the 2 pre-exercise muscle biopsies.
Calculation of muscle protein fractional synthesis rate.
The fractional synthesis rate of myofibrillar protein was determined by examining the rate of l-[ring-13C6]Phe incorporated into myofibrillar protein using following the precursor product model:
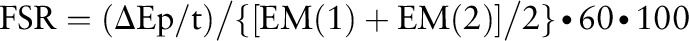 |
where FSR is the fractional synthesis rate, ∆Ep is the increment in myofibrillar protein–bound l-[ring-13C6]Phe enrichment between 2 muscle biopsies, t is the time between the 2 muscle biopsies, and EM(1) + EM(2) are the l-[ring-13C6]Phe enrichments in the free intracellular pool in the 2 muscle biopsies. Data are expressed as percentage per hour.
Immunoblot analysis.
Immunoblot analysis on the general cytosolic protein fractions was performed as detailed previously (27). To maintain consistency with our previous publications most relevant to this study (6, 24), immunoblot data are presented as phosphorylation status relative to an internal control sample (rat soleus muscle) that was loaded on every gel for comparison across blots and then expressed as a fold change from basal.
Antibodies.
The phosphorylated and total antibodies used for immunoblotting were purchased from Cell Signaling Technologies: phosphorylated mTOR (Ser2448; 1:250), phosphorylated S6K1 (Thr389; 1:500), phosphorylated 4E-BP1 (Thr37/46; 1:1000), phosphorylated eukaryotic elongation factor 2 (eEF2; Thr56; 1:5000), and total eEF2 (1:2000). mTOR, S6K1, and 4E-BP1 total protein were detected using an antibody dilution of 1:1000. Anti-rabbit IgG HRP-conjugated secondary antibody was purchased from GE Healthcare (1:2000).
RNA extraction and semiquantitative real-time PCR.
RNA isolation, cDNA synthesis, and real-time qPCR were performed as we described previously (31, 32). Real-time qPCR was performed with an iQ5 Multicolor Real-Time PCR cycler (Bio-Rad). cDNA was analyzed with SYBR green fluorescence (iQ SYBR green supermix; Bio-Rad). Primer sequences for the current investigation were published previously (31, 33). β2-Microglobulin was used as a normalization/housekeeping gene. Relative fold changes were determined from the cycle threshold values using the 2−ΔΔCt method (34).
Statistical analysis.
Independent t tests were used to compare participant characteristics between groups. A 2-factor ANOVA with repeated measures on the time factor was used to test time × group differences. Post hoc testing was performed using Bonferroni correction when appropriate. All data were analyzed using SigmaPlot version 12.5 (Systat Software). Significance for all analyses was set to P ≤ 0.05. Data are presented as means ± SEMs.
Results
Participant characteristics and RE.
Groups were similar in age, height, weight, BMI, and 1RM (P > 0.05) (Table 1). The 2 groups performed leg-extension exercises at a similar percentage 1RM (taken as the mean among all 8 sets) during the experimental trial (P > 0.05) (Table 1). Blood lactate values increased in the 2 groups after the exercise bout (P < 0.05) (data not shown), but no differences in blood lactate were observed between groups at any time during the experimental trial (P > 0.05) (data not shown).
Blood and intracellular amino acid concentrations.
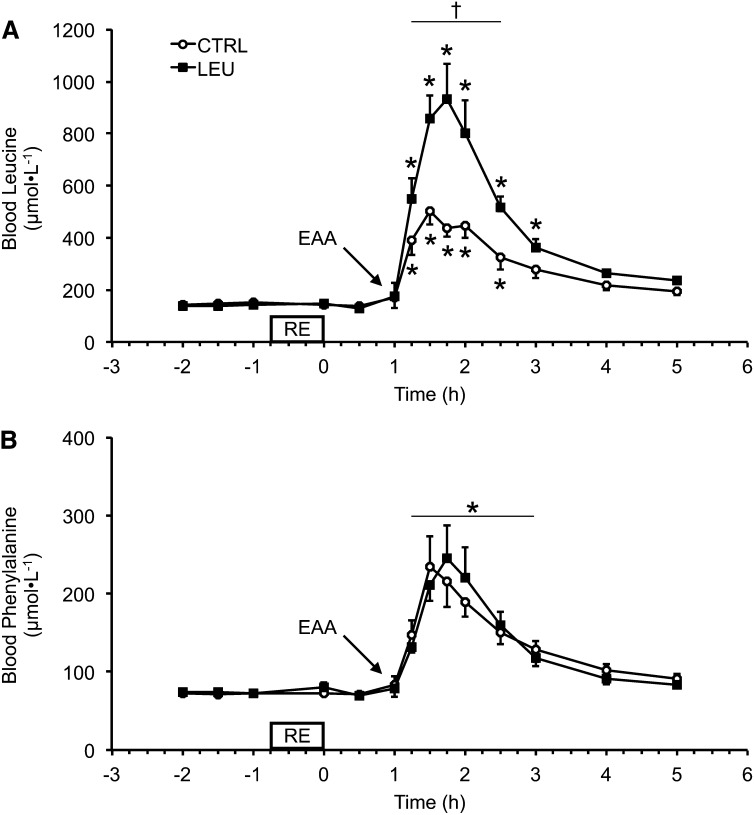
There were no differences between groups in blood Leu or Phe concentrations during the basal, exercise, or immediate 1 h postexercise periods. Blood Leu concentrations increased above basal values (mean of pre-RE time points) for the 2 groups after EAA ingestion at 1 h after exercise (Fig. 1A). Blood Leu concentrations remained elevated in the control group for 1.5 h after EAA ingestion (2.5 h after exercise), whereas blood Leu concentrations remained elevated in the LEU group for 2 h after EAA ingestion [3 h after exercise (P < 0.05)] (Fig. 1A). Furthermore, blood Leu concentrations were higher in the LEU group than in the control group for 1.5 h after EAA ingestion (2.5 h after exercise) (P < 0.05) (Fig. 1A). Blood Phe concentrations were elevated in each group for 2 h after EAA ingestion (3 h after exercise) (P < 0.05), and no group differences were observed for blood Phe concentrations at any time point (Fig. 1B).
FIGURE 1.
Time course of arterialized blood Leu (A) and Phe (B) concentrations in older men after the combination of RE and postexercise (1 h) ingestion of 10 g of EAAs containing 1.85 g of Leu (CTRL; n = 7) or 3.5 g of Leu (LEU; n = 8). Data are means ± SEMs. *Different from basal (−2 h), P < 0.05; †group difference, P < 0.05. CTRL, control group; EAA, essential amino acid; LEU, Leu group; RE, resistance exercise.
Basal intracellular Leu and Phe concentrations were similar between groups (Table 3). Intracellular Leu concentrations increased only in the LEU group at 2 h after exercise (1 h after EAA ingestion) (P < 0.05), and intracellular Leu concentration was higher in the LEU group than in the control group at this time point (P < 0.05) (Table 3). Intracellular Leu concentrations were similar to basal values in the 2 groups at 5 and 24 h after exercise (P > 0.05). Intracellular Phe concentrations increased in the 2 groups at 2 h after exercise (1 h after EAA ingestion) (P < 0.05) and were similar to basal values in each group at 5 and 24 h after exercise (P > 0.05) (Table 3). There were no group differences in intracellular Phe concentrations at any time point during the experimental trial.
TABLE 3.
Intracellular Leu and Phe concentrations under basal conditions and after resistance exercise and the ingestion of 10 g of EAAs in older men1
| Basal |
2 h |
5 h |
24 h |
|||||
| EAA | LEU | CTRL | LEU | CTRL | LEU | CTRL | LEU | CTRL |
| Leu, μmol/L | 115 ± 8.0 | 126 ± 19.4 | 395 ± 52.9*,† | 167 ± 17.5 | 161 ± 15.2 | 135 ± 11.8 | 105 ± 10.5 | 110 ± 9.9 |
| Phe, μmol/L | 57.0 ± 3.2 | 63.2 ± 6.0 | 109 ± 10.2* | 95.4 ± 14.8* | 57.2 ± 5.6 | 68.8 ± 6.9 | 55.5 ± 4.6 | 57.8 ± 4.5 |
Values are means ± SEMs. CTRL, n = 7; LEU, n = 8. *Different from basal, P < 0.05; †group difference, P < 0.05. CTRL, control group; EAA, essential amino acid; LEU, Leu group.
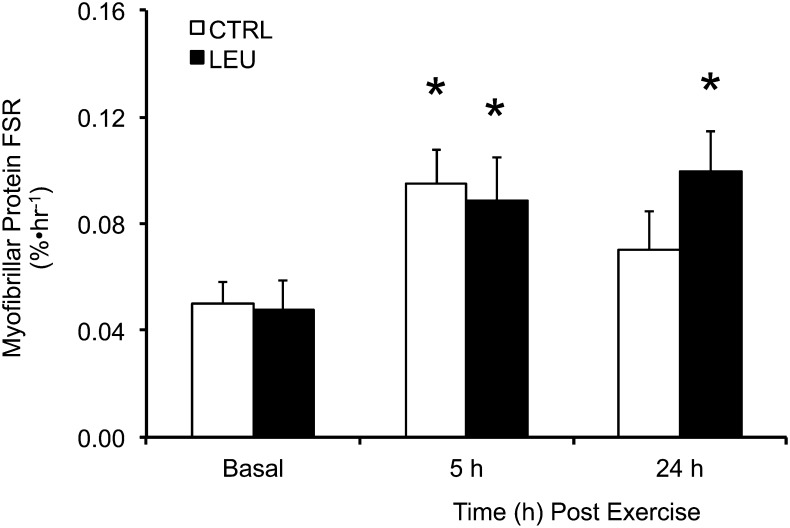
MyoPS rate.
The basal MyoPS rate was similar between groups (Fig. 2). After the combination of RE and EAA ingestion (1 h after exercise), the MyoPS rate was increased by ∼90% in the 2 groups from 2 to 5 h after exercise (P < 0.05), with no difference between groups (P > 0.05) (Fig. 2). In contrast, the MyoPS rate was elevated above basal values only in the LEU group at 24 h after exercise (P < 0.05) (Fig. 2).
FIGURE 2.
Time course of myofibrillar protein FSR in older men after the combination of resistance exercise and postexercise (1 h) ingestion of 10 g of essential amino acids containing 1.85 g of Leu (CTRL; n = 7) or 3.5 g of Leu (LEU; n = 8). Data are means ± SEMs. *Different from basal, P < 0.05. CTRL, control group; FSR, fractional synthesis rate; LEU, Leu group.
Cell signaling.
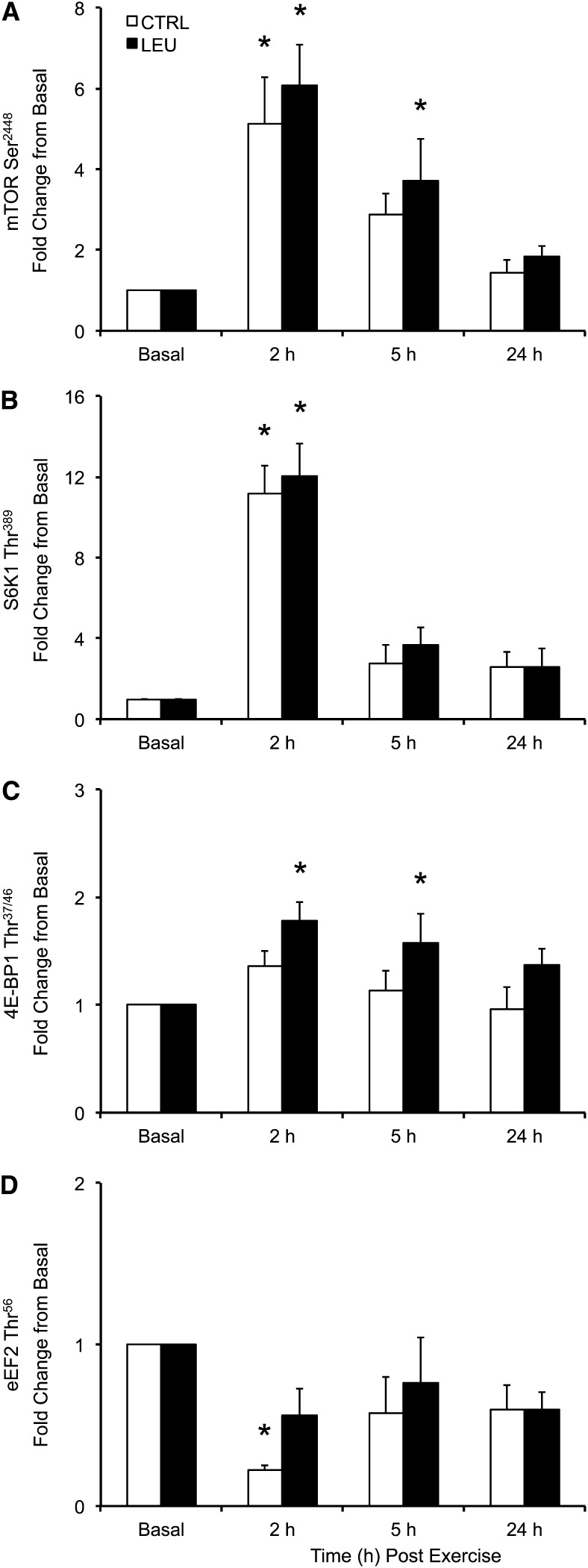
Total protein content did not change during the time course for any signaling protein (P > 0.05). Phosphorylation of mTOR (Ser2448) was increased above basal in the control group only at 2 h after exercise (1 h after EAA ingestion) (P < 0.05), whereas phosphorylation of mTOR was increased above basal in the LEU group at 2 and 5 h after exercise (P < 0.05) (Fig. 3A). Phosphorylation of mTOR was similar to basal in the 2 groups at 24 h after exercise (P > 0.05), and no group differences were observed in mTOR phosphorylation at any time point. Phosphorylation of S6K1 (Thr389) was increased above basal in the 2 groups at 2 h (P < 0.05) but was similar to basal at 5 and 24 h after exercise (P > 0.05) (Fig. 3B). No group differences were observed in S6K1 phosphorylation at any time point. Phosphorylation of 4E-BP1 (Thr37/46) was similar to basal at all postexercise time points in the control group (P > 0.05) (Fig. 3C). In contrast, phosphorylation of 4E-BP1 was increased above basal in the LEU group at 2 and 5 h after exercise (P < 0.05) but not at 24 h after exercise (P > 0.05) (Fig. 3C). No group differences were observed in 4E-BP1 phosphorylation at any time point. Phosphorylation of eEF2 (Thr56) was decreased below basal in the control group at 2 h after exercise (P < 0.05) but not at 5 or 24 h after exercise (P > 0.05) (Fig. 3D). Phosphorylation of eEF2 was similar to basal at all postexercise time points in the LEU group (P > 0.05) (Fig. 3D).
FIGURE 3.
Time course of phosphorylation of mTOR at Ser2448 (A), S6K1 at Thr389 (B), 4E-BP1 at Thr37/46 (C), and eEF2 at Thr56 (D) in older men after the combination of resistance exercise and postexercise (1 h) ingestion of 10 g of essential amino acids containing 1.85 g of Leu (CTRL; n = 7) or 3.5 g of Leu (LEU; n = 8). Immunoblot data were normalized to an internal control sample, and data are adjusted to represent fold change from basal in phosphorylated protein. Data are expressed as means ± SEMs. *Different from basal, P < 0.05. Representative blots are provided in Supplemental Figure 1. CTRL, control group; eEF2, eukaryotic elongation factor 2; LEU, Leu group; mTOR, mammalian/mechanistic target of rapamycin; S6K1, p70 S6 kinase 1; 4E-BP1, 4E binding protein 1.
AAT expression.
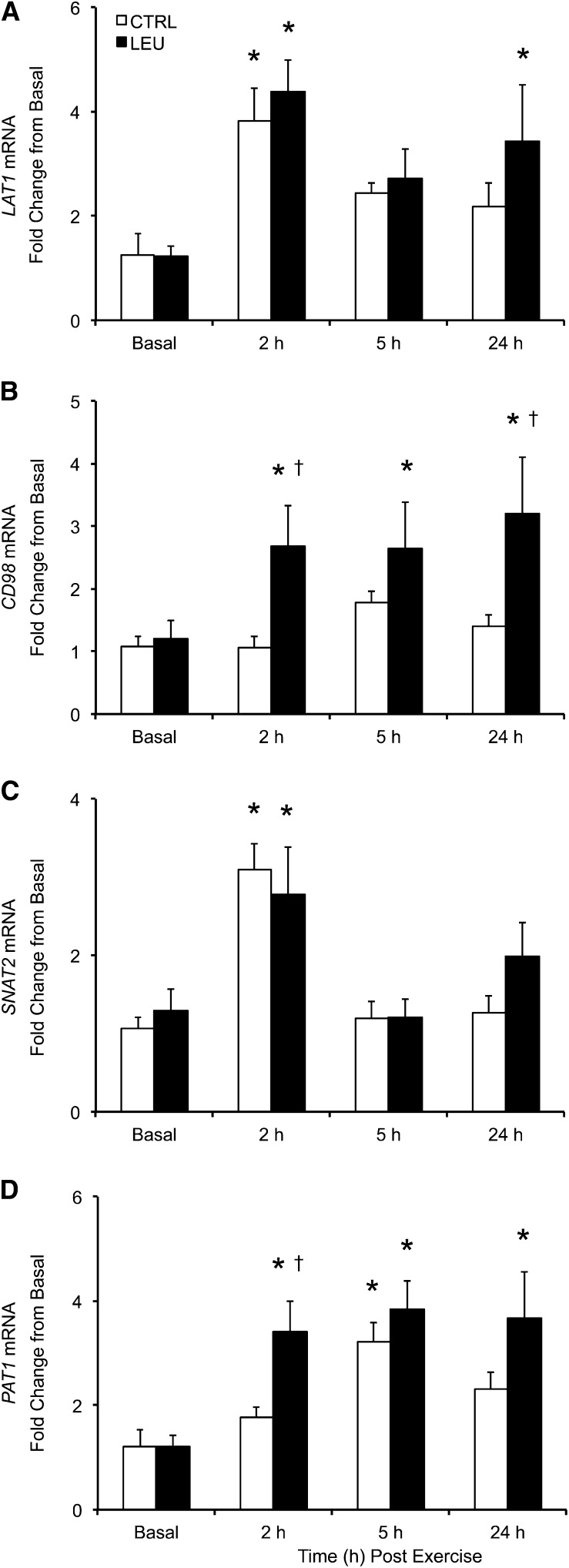
LAT1 mRNA expression was increased above basal in the control group at 2 h after exercise (1 h after EAA ingestion) (P < 0.05) but not at 5 h (4 h after EAA ingestion) or 24 h after exercise (P > 0.05) (Fig. 4A). LAT1 mRNA expression was elevated above basal in the LEU group at 2 and 24 h after exercise (P < 0.05) but not at 5 h after exercise (P > 0.05) (Fig. 4A). No group differences in LAT1 mRNA expression were observed at any time point. CD98 mRNA expression was unchanged from basal at any time point in the control group (P > 0.05), whereas CD98 mRNA expression was elevated at all postexercise time points in the LEU group (P < 0.05) (Fig. 4B). Furthermore, CD98 mRNA expression was higher in the LEU group than in the control group at 2 and 24 h after exercise (P < 0.05). SNAT2 mRNA expression was elevated in the 2 groups at 2 h after exercise (P < 0.05), whereas SNAT2 mRNA expression was similar to basal in the 2 groups at 5 and 24 h after exercise (P > 0.05) (Fig. 4C). No group differences in SNAT2 mRNA expression were observed at any time point. PAT1 mRNA expression was elevated above basal in the control group at 5 h after exercise (P < 0.05) but was similar to basal in the control group at 2 and 24 h after exercise (P > 0.05) (Fig. 4D). In contrast, PAT1 mRNA expression was elevated in the LEU group at all postexercise time points (P < 0.05). Furthermore, PAT1 mRNA expression was higher in the LEU group than in the control group at 2 h after exercise (P < 0.05).
FIGURE 4.
Time course of mRNA expression of LAT1 (A), CD98 (B), SNAT2 (C), and PAT1 (D) in older men after the combination of resistance exercise and postexercise (1 h) ingestion of 10 g of essential amino acids containing 1.85 g of Leu (CTRL; n = 7) or 3.5 g of Leu (LEU; n = 8). Relative fold changes were determined from the cycle threshold values using the 2−ΔΔCt method (34). Data are expressed as means ± SEMs. *Different from basal, P < 0.05; †group difference, P < 0.05. CD98, cluster of differentiation 98; CTRL, control group; LAT1, system L amino acid transporter1; LEU, Leu group; PAT1, proton-assisted amino acid transporter 1; SNAT2, system A amino acid transporter 2.
Discussion
In the current study, we sought to better understand the role of postexercise Leu ingestion for older adults, and, in particular, whether simply ingesting relatively higher quantities of Leu after exercise could enhance MyoPS. We observed that the increase in MyoPS from 2 to 5 h after RE was unaffected by the quantity of Leu ingested, but greater Leu ingestion after RE did prolong the increase in MyoPS rate measured 24 h after exercise. Furthermore, postexercise-enriched Leu ingestion was also associated with a more rapid and prolonged skeletal muscle mRNA expression of AATs. These findings indicate that ingesting sufficient quantities of Leu 1 h after exercise can prolong the anabolic effects of RE in older adults.
A novel aspect to this study design was to examine the effect of different postexercise nutritional strategies in older adults over a 24-h post-RE time course. We observed that greater postexercise Leu ingestion did not influence MyoPS rates in the immediate hours after exercise. Conversely, greater postexercise Leu ingestion was associated with a sustained increase in MyoPS rate that remained elevated 24 h after exercise. Although the ability for RE training to facilitate increases in muscle size and strength, even in older adults, is very well understood (35–38), a recent meta-analysis indicated that increases in muscle size and strength produced through RE training are enhanced when coupled with protein or amino acid supplementation (39). Because RE is not commonly performed on a daily basis (40), it is interesting to speculate, based on our data, that the ability for protein/amino acid supplementation to augment the response of the muscle to RE (at least in older adults) may be facilitated through a sustained increase in MyoPS related to the ingestion of proper amounts of Leu (≥3.5 g) after exercise. Collectively, these findings emphasize the importance of sufficient postexercise Leu/protein ingestion as a strategy to enhance the preservation and/or recovery of muscle function in older adults undergoing exercise training or exercise-based therapies for rehabilitation. Indeed, ingestion of an EAA mixture containing 3.6 g of Leu in combination with rehabilitation was shown recently to improve recovery from total knee replacement in older adults (41). However, whether an equivalent dose (Leu content) of intact protein is necessary to sustain MyoPS requires additional investigation (42).
In the current study, we observed that, despite the differences in Leu total content between the experimental groups, the rate of MyoPS during the immediate hours after RE (2–5 h) was similarly increased in older men when 1.85 or 3.5 g of Leu were ingested after RE, indicating that older men may become more sensitive to lower doses of Leu after RE (22). This sensitizing effect of MyoPS to lower quantities of Leu in the immediate hours after RE is in contrast to previous reports in older adults using intact protein (8, 43). Discrepancies between our findings and these previous studies could be due to differences in the source of amino acids (intact whey or casein protein vs. crystalline amino acids) (43), the intrinsic properties of intact protein, the total quantity of amino acids ingested (8), or the timing of the postexercise MyoPS measure. In addition, to maintain an isonitrogenous mixture in the LEU group, the total content of the remaining EAAs was decreased, which could have reduced our potential to observe a greater stimulation of MyoPS in the LEU group during the immediate hours after RE. Conversely, the ability for aging muscle to increase MyoPS in response to low concentrations of Leu ingestion in the current study could be related to the timing of protein/amino acid ingestion relative to the end of RE [1 h after in the current study vs. immediately after in previous studies (8, 43)]. Thus, it is interesting to speculate that delaying EAA ingestion may better coincide with an improved anabolic sensitivity of aging muscle to the transient increase in circulating amino acids through improved muscle perfusion and delivery of amino acids to muscle (44), and/or increased amino acid transporter expression (32) and transport into muscle (45) after exercise. In addition, relative to young individuals, older adults appear to experience a greater cellular stress response to RE (32, 46). Thus, delaying EAA ingestion could also better coincide with cellular recovery to stress in older adults. Collectively, delaying postexercise protein ingestion may be an important factor to consider for older adults and may be necessary for older adults to experience the nutrient-sensitizing effects of RE when lower quantities of EAAs are ingested (22), at least in the immediate hours after RE.
Although some discrepancies existed between groups in the mTORC1 signaling response, most notably in the phosphorylation of 4E-BP1, there was a trend in each group to indicate a general activation of the mTORC1 pathway in the immediate hours after RE and EAA ingestion. Given the well-described role of mTORC1 in the regulation of protein synthesis (14–16), the similar rate of MyoPS between groups in the immediate hours after RE and EAA ingestion is likely related to a general activation of mTORC1 signaling in each group. Interestingly, however, the prolonged increase in MyoPS rate when additional postexercise Leu was ingested was not accompanied by a sustained activation of mTORC1 signaling. Thus, pathways or other cellular mechanisms aside from those examined in the current study may facilitate the sustained increase in MyoPS after greater postexercise Leu ingestion in older men, perhaps through subtle alterations in translational capacity or the cellular recovery process, or through processes triggered through an increased postexercise 4E-BP1 phosphorylation. Conversely, we showed previously in young adults that the sustained increase in mixed muscle protein synthesis 24 h after RE is indeed associated with a prolonged phosphorylation of mTORC1 signaling proteins (6). Thus, we cannot discount the fact that markers of mTORC1 signaling were perhaps more active during a time outside that of muscle sampling. Nonetheless, more work is necessary to uncover the cellular mechanisms governing the sustained increase in MyoPS observed after additional postexercise Leu ingestion.
In addition to a sustained increase in MyoPS, we also observed that greater postexercise Leu ingestion produced a rapid and sustained increase in the skeletal muscle mRNA expression of the AATs LAT1, CD98, and PAT1. Although these AATs are known to be involved in the process of protein synthesis (17–19), the role of the prolonged elevation in the expression of these AATs for sustaining the increased MyoPS requires additional investigation. Conversely, it is well understood that aging is associated with an inability for lower quantities of ingested protein to stimulate muscle protein synthesis (47), which may contribute to, or even accelerate, the onset of sarcopenia (48). Thus, given the roles of LAT1/CD98 and PAT1 as amino acid delivery (17) and intracellular signaling (18) mechanisms, respectively, the sustained upregulation of these particular AATs after RE indicates that ingesting adequate Leu after RE may facilitate an improved sensitivity of aging skeletal muscle to amino acids. Although direct measures of amino acid sensitivity and transport were not examined in the current study, previous research indicated a greater sensitivity of the myofibrillar protein fraction to protein feeding in young adults 24 h after RE performed to failure (49). Thus, adequate postexercise Leu ingestion might also provide a strategy to improve the anabolic response of aging skeletal muscle, in particular to low protein meals, but additional research is necessary.
In summary, we observed that postexercise (1 h) ingestion of 10 g of EAAs containing either 1.85 or 3.5 g of Leu had a similar effect on MyoPS in the immediate hours after RE (2–5 h after exercise). However, our main novel finding was that postexercise ingestion of a higher concentration of Leu prolonged the increase in MyoPS, which persisted up to 24 h after RE. In addition, the higher dose of postexercise Leu produced a sustained increase in the mRNA expression of select AATs. Collectivity, ingesting sufficient quantities of Leu after RE may provide a necessary stimulus to prolong the anabolic response of aging muscle to RE and facilitate improved sensitivity of aging muscle to amino acids. Therefore, proper postexercise Leu ingestion may represent an important therapeutic strategy for the preservation and/or recovery of muscle function in older adults and clinical populations.
Supplementary Material
Acknowledgments
The authors thank Shelley Medina, Ming-Qian Zheng, and Junfung Hao for technical assistance. J.M.D., E.V., and B.B.R. designed the research; J.M.D., D.M.G., D.K.W., P.T.R., M.S.B., M.J.D., M.A., and E.V. conducted the research; J.M.D. and B.B.R. analyzed the data and performed the statistical analysis; J.M.D. and B.B.R. wrote the paper and had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AAT, amino acid transporter; CD98, cluster of differentiation 98; EAA, essential amino acid; eEF2, eukaryotic elongation factor 2; ITS-CRC, Institute for Translational Sciences Clinical Research Center; LAT1, system L amino acid transporter 1; LEU, Leu group; mTOR, mammalian/mechanistic target of rapamycin; mTORC1, mammalian/mechanistic target of rapamycin Complex 1; MyoPS, myofibrillar protein synthesis; PAT1, proton-assisted amino acid transporter 1; RE, resistance exercise; S6K1, p70 S6 kinase 1; SNAT2, system A amino acid transporter 2; 1RM, 1-repetition maximum; 4E-BP1, 4E binding protein 1.
References
- 1.Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 1988;84:275–94. [DOI] [PubMed] [Google Scholar]
- 2.Doherty TJ. Invited review: aging and sarcopenia. J Appl Physiol 2003;95:1717–27. [DOI] [PubMed] [Google Scholar]
- 3.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci 2006;61:72–7. [DOI] [PubMed] [Google Scholar]
- 5.Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc 2004;52:80–5. [DOI] [PubMed] [Google Scholar]
- 6.Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Dhanani S, Volpi E, Rasmussen BB. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle 2011;1:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, Sheffield-Moore M, Volpi E, Rasmussen BB. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol 2008;104:1452–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Y, Breen L, Burd NA, Hector AJ, Churchward-Venne TA, Josse AR, Tarnopolsky MA, Phillips SM. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br J Nutr 2012;108:1780–8. [DOI] [PubMed] [Google Scholar]
- 9.Dickinson JM, Volpi E, Rasmussen BB. Exercise and nutrition to target protein synthesis impairments in aging skeletal muscle. Exerc Sport Sci Rev 2013;41:216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr 2000;130:139–45. [DOI] [PubMed] [Google Scholar]
- 11.Garlick PJ. The role of leucine in the regulation of protein metabolism. J Nutr 2005;135:1553S–6S. [DOI] [PubMed] [Google Scholar]
- 12.Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr 2000;130:2413–9. [DOI] [PubMed] [Google Scholar]
- 13.Dickinson JM, Fry CS, Drummond MJ, Gundermann DM, Walker DK, Glynn EL, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J Nutr 2011;141:856–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimball SR. The role of nutrition in stimulating muscle protein accretion at the molecular level. Biochem Soc Trans 2007;35:1298–301. [DOI] [PubMed] [Google Scholar]
- 15.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 2009;10:307–18. [DOI] [PubMed] [Google Scholar]
- 16.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 2011;12:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poncet N, Mitchell FE, Ibrahim AF, McGuire VA, English G, Arthur JS, Shi YB, Taylor PM. The catalytic subunit of the system l1 amino Acid transporter (slc7a5) facilitates nutrient signalling in mouse skeletal muscle. PLoS One 2014;9:e89547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ögmundsdóttir MH, Heublein S, Kazi S, Reynolds B, Visvalingam SM, Shaw MK, Goberdhan DC. Proton-assisted amino acid transporter PAT1 complexes with Rag GTPases and activates TORC1 on late endosomal and lysosomal membranes. PLoS One 2012;7:e36616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hyde R, Hajduch E, Powell DJ, Taylor PM, Hundal HS. Ceramide down-regulates System A amino acid transport and protein synthesis in rat skeletal muscle cells. FASEB J 2005;19:461–3. [DOI] [PubMed] [Google Scholar]
- 20.Baird FE, Bett KJ, MacLean C, Tee AR, Hundal HS, Taylor PM. Tertiary active transport of amino acids reconstituted by coexpression of System A and L transporters in Xenopus oocytes. Am J Physiol Endocrinol Metab 2009;297:E822–9. [DOI] [PubMed] [Google Scholar]
- 21.Goberdhan DC, Meredith D, Boyd CA, Wilson C. PAT-related amino acid transporters regulate growth via a novel mechanism that does not require bulk transport of amino acids. Development 2005;132:2365–75. [DOI] [PubMed] [Google Scholar]
- 22.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab 2006;291:E381–7. [DOI] [PubMed] [Google Scholar]
- 23.Bergstrom J. Muscle electrolytes in man. Scand J Med Sci Sports 1962;68:1–110. [Google Scholar]
- 24.Glynn EL, Fry CS, Drummond MJ, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women. J Nutr 2010;140:1970–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Symons TB, Schutzler SE, Cocke TL, Chinkes DL, Wolfe RR, Paddon-Jones D. Aging does not impair the anabolic response to a protein-rich meal. Am J Clin Nutr 2007;86:451–6. [DOI] [PubMed] [Google Scholar]
- 26.Reidy PT, Walker DK, Dickinson JM, Gundermann DM, Drummond MJ, Timmerman KL, Cope MB, Mukherjea R, Jennings K, Volpi E, et al. Soy-dairy protein blend and whey protein ingestion after resistance exercise increases amino acid transport and transporter expression in human skeletal muscle. J Appl Physiol 2014;116:1353–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E–BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol 2006;576:613–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calder AG, Anderson SE, Grant I, McNurlan MA, Garlick PJ. The determination of low d5-phenylalanine enrichment (0.002–0.09 atom percent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gas chromatography/electron ionization mass spectrometry. Rapid Commun Mass Spectrom 1992;6:421–4. [DOI] [PubMed] [Google Scholar]
- 29.Patterson BW, Zhang XJ, Chen Y, Klein S, Wolfe RR. Measurement of very low stable isotope enrichments by gas chromatography/mass spectrometry: application to measurement of muscle protein synthesis. Metabolism 1997;46:943–8. [DOI] [PubMed] [Google Scholar]
- 30.Wolfe RR, Chinkes DL. Isotopic tracers in metabolic research: principles and practice of kinetic analysis. 2nd ed. Hoboken (NJ): John Wiley & Sons; 2005.
- 31.Drummond MJ, Miyazaki M, Dreyer HC, Pennings B, Dhanani S, Volpi E, Esser KA, Rasmussen BB. Expression of growth-related genes in young and older human skeletal muscle following an acute stimulation of protein synthesis. J Appl Physiol 2009;106:1403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drummond MJ, Fry CS, Glynn EL, Timmerman KL, Dickinson JM, Walker DK, Gundermann DM, Volpi E, Rasmussen BB. Skeletal muscle amino acid transporter expression is increased in young and older adults following resistance exercise. J Appl Physiol 2011;111:135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drummond MJ, Glynn EL, Fry CS, Timmerman KL, Volpi E, Rasmussen BB. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab 2010;298:E1011–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Methods 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 35.Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA 1990;263:3029–34. [PubMed] [Google Scholar]
- 36.Frontera WR, Meredith CN, O’Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol 1988;64:1038–44. [DOI] [PubMed] [Google Scholar]
- 37.Latham NK, Bennett DA, Stretton CM, Anderson CS. Systematic review of progressive resistance strength training in older adults. J Gerontol A Biol Sci Med Sci 2004;59:48–61. [DOI] [PubMed] [Google Scholar]
- 38.Trappe TA, Carroll CC, Dickinson JM, LeMoine JK, Haus JM, Sullivan BE, Lee JD, Jemiolo B, Weinheimer EM, Hollon CJ. Influence of acetaminophen and ibuprofen on skeletal muscle adaptations to resistance exercise in older adults. Am J Physiol Regul Integr Comp Physiol 2011;300:R655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr 2012;96:1454–64. [DOI] [PubMed] [Google Scholar]
- 40.Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, Macera CA, Castaneda-Sceppa C. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc 2007;39:1435–45. [DOI] [PubMed] [Google Scholar]
- 41.Dreyer HC, Strycker LA, Senesac HA, Hocker AD, Smolkowski K, Shah SN, Jewett BA. Essential amino acid supplementation in patients following total knee arthroplasty. J Clin Invest 2013;123:4654–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katsanos CS, Chinkes DL, Paddon-Jones D, Zhang XJ, Aarsland A, Wolfe RR. Whey protein ingestion in elderly persons results in greater muscle protein accrual than ingestion of its constituent essential amino acid content. Nutr Res 2008;28:651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burd NA, Yang Y, Moore DR, Tang JE, Tarnopolsky MA, Phillips SM. Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolate v. micellar casein at rest and after resistance exercise in elderly men. Br J Nutr 2012;108:958–62. [DOI] [PubMed] [Google Scholar]
- 44.Timmerman KL, Dhanani S, Glynn EL, Fry CS, Drummond MJ, Jennings K, Rasmussen BB, Volpi E. A moderate acute increase in physical activity enhances nutritive flow and the muscle protein anabolic response to mixed nutrient intake in older adults. Am J Clin Nutr 2012;95:1403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol 1995;268:E514–20. [DOI] [PubMed] [Google Scholar]
- 46.Thalacker-Mercer AE, Dell’Italia LJ, Cui X, Cross JM, Bamman MM. Differential genomic responses in old vs. young humans despite similar levels of modest muscle damage after resistance loading. Physiol Genomics 2010;40:141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr 2005;82:1065–73. [DOI] [PubMed] [Google Scholar]
- 48.Houston DK, Nicklas BJ, Ding J, Harris TB, Tylavsky FA, Newman AB, Lee JS, Sahyoun NR, Visser M, Kritchevsky SB, et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr 2008;87:150–5. [DOI] [PubMed] [Google Scholar]
- 49.Burd NA, West DW, Moore DR, Atherton PJ, Staples AW, Prior T, Tang JE, Rennie MJ, Baker SK, Phillips SM. Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. J Nutr 2011;141:568–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.