Abstract
Background: Previous human studies reported inconsistent effects of dietary protein and branched-chain amino acids (BCAAs) on insulin action and glucose metabolism. Similarly, it is unclear whether saturated fat (SF) intake influences these metabolic variables.
Objective: The objective of this study was to test the effects of high [30% of energy (%E)] vs. moderate (20%E) intakes of protein (primarily whey) on insulin action and lipid and lipoprotein concentrations in the context of both high (15%E) and low (7%E) SF diets.
Methods: The study was conducted as a randomized controlled trial in 158 overweight and obese men and women. After a 4-wk baseline diet [55%E carbohydrate, 15%E protein, 30%E fat (7%E SF)], participants were randomly assigned to 4 wk of either the baseline diet or 1 of 4 test diets containing 35%E carbohydrate and either 20%E or 30%E protein and either 7%E or 15%E SF. Frequently sampled i.v. glucose tolerance tests were administered after each dietary period.
Results: Other than significantly higher fasting glucose concentrations for high vs. moderate protein intakes with a low-fat diet (difference ± SE: 0.47 ± 0.14 mmol/L; P = 0.001), there were no significant effects of dietary protein or SF on glucose metabolism, plasma insulin, or concentrations of lipids and lipoproteins. Changes in plasma BCAAs across all diets were negatively correlated with changes in the metabolic clearance rate of insulin (ρ = −0.18, P = 0.03) and positively correlated with changes in the acute insulin response to glucose (ρ = 0.15, P = 0.05).
Conclusions: These findings suggest that short-term intake of BCAAs can influence insulin dynamics. However, in this group of overweight and obese individuals, neither high protein nor SF intake affected insulin sensitivity or plasma concentrations of lipids and lipoproteins. This trial was registered at clinicaltrials.gov as NCT00508937.
Introduction
Insulin resistance increases the risk of developing type 2 diabetes. High-protein diets have been hypothesized to improve insulin resistance, but such diets may also promote weight loss, and weight loss, even if modest, can improve insulin sensitivity. Whether high-protein diets can improve glucose tolerance and insulin sensitivity independent of weight loss remains unclear. Although some studies showed improved glucose homeostasis with high-protein vs. conventional hypocaloric diets with similar weight loss (1–3), others showed that high protein intake produced either no improvement in (4–7) or worsening of (8, 9) glucose homeostasis. The few trials that directly tested the effects of higher protein diets on insulin sensitivity in the absence of weight loss also produced mixed results (9–11).
These discrepancies may be due in part to different protein sources used in the studies, which can differ in their effects on glucose homeostasis (12). Higher intakes of dairy products have been associated with lower diabetes risk (13–15). Although it is not known which component of dairy confers reduced risk, there is evidence that dairy protein, especially whey, may improve glucose homeostasis by stimulating insulin secretion (16, 17). On the other hand, metabolomic studies found that high plasma concentrations of BCAAs, which are abundant in whey, are associated with insulin resistance and obesity (18–20); and in mice, BCAA feeding induces insulin resistance (19).
High-protein diets, particularly those enriched with animal protein, typically are high in saturated fat (SF)8. There is, however, no compelling evidence that dietary SF influences insulin sensitivity in humans. Specifically, several large intervention trials produced no difference in insulin sensitivity with isocaloric replacement of SF with monounsaturated fat when total fat remained high [>37% of energy (%E)] (21–23).
We therefore conducted a randomized controlled trial to determine whether a high-protein weight-maintenance diet altered insulin sensitivity and/or cardiovascular disease risk factors compared with a lower protein weight-maintenance diet at 2 amounts of SF intake (7% vs. 15%E). We also tested whether plasma biomarkers of protein and SF intake were associated with measures of insulin sensitivity and dynamics.
Participants and Methods
Study population.
Participants were recruited through Internet and direct mail solicitations. Eligibility criteria were as follows: age ≥18 y, no present use of tobacco products, BMI of 25–40 kg/m2, HOMA-IR (glucose × insulin/22.5) ≥2.5, fasting blood glucose <7.0 mmol/L, plasma TGs <5.65 mmol/L, and total and LDL cholesterol (LDL-C) ≤95th percentile for age and sex. Exclusion criteria were history of diabetes, cardiovascular disease, or other chronic disease or taking drugs known to affect glucose or lipid metabolism, blood thinning agents, dietary supplements, or hormones. Participants’ characteristics at screening are presented in Supplemental Table 1.
All participants provided written informed consent. The protocol was reviewed and approved by the institutional review boards of Children’s Hospital and Research Center Oakland and the University of California, San Francisco.
Study design.
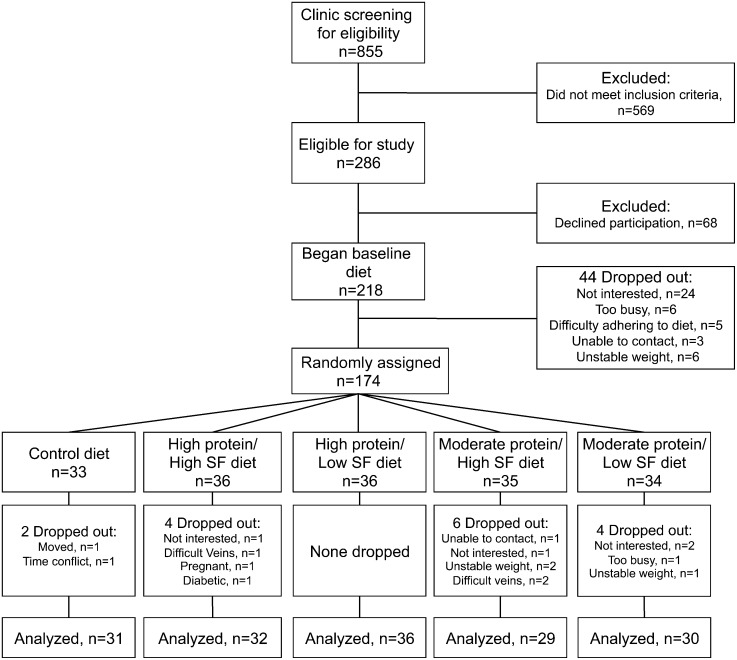
This outpatient study in free-living participants was carried out at 2 research clinics: the Cholesterol Research Center (Berkeley, CA) and San Francisco General Hospital (San Francisco, CA). All participants consumed a baseline run-in diet for 4 wk, after which they were randomly assigned to the baseline diet (control) or 1 of the following 4 diets for 4 wk: 1) high protein, high SF; 2) high protein, low SF; 3) moderate protein, high SF; or 4) moderate protein, low SF (Fig. 1). They were monitored weekly to maintain a relatively stable weight throughout the study [±3% of initial weight up to ±5 pounds (2.3 kg)] and their usual level of physical activity as monitored by a pedometer and activity log.
FIGURE 1.
Participant enrollment. SF, saturated fat.
After each 4-wk dietary period, body weight was measured and percentage body fat was assessed by bioimpedance (TBF 551 body weight scale; Tanita) and a blood sample was collected after a 12- to 14-h overnight fast. A frequently sampled i.v. glucose tolerance test was performed as previously described (24). Briefly, an i.v. catheter was inserted into each arm, and after 45 min of rest 2 baseline blood samples were drawn (−15 and −5 min). At time 0, a 0.3 g/kg bolus of 50% dextrose was given; and blood samples were drawn at 2, 4, 6, 8, 14, and 19 min. At 20 min, an i.v. bolus of human insulin (0.03 U/kg) was administered. Blood sampling continued at 22, 24, 30, 40, 60, 80, 100, 120, 140, 160, 180, 210, and 240 min. The insulin sensitivity index (SI) was calculated by using minimal model analysis (25). The acute insulin response to glucose (AIRg) was calculated as the AUC in the first 8 min after infusing the dextrose. The disposition index (DI) was calculated as the product of SI and AIRg, and the metabolic clearance rate of insulin (MCRi) was calculated by dividing the dose of insulin normalized for body weight by the AUC of insulin above basal, the latter estimated by fitting an exponential decay curve to the insulin profile between t = 20 and 120 min (MLAB; Civilized Software) (26).
Dietary interventions.
Table 1 presents the nutritional composition of the prescribed study diets over their 7-d rotating menus. Participants were provided with frozen entrées (Lifespring Home Nutrition) for lunch and dinner and were required to purchase foods and prepare breakfast and snacks according to menu instructions and shopping lists. Whey protein isolate (Provon 290; Glanbia Nutritionals) was used to partially meet the increased protein content of the moderate- and high-protein diets. High-, low-, and nonfat dairy products (milk, cheese, yogurt, butter) were primarily used to achieve differences in SF between the high- and low-SF diets. Body weight was measured weekly and, if needed, energy intake was adjusted to achieve stable weight. All diets met the RDA for vitamins and minerals (27). A 5-point compliance score was assigned by the dietitian using weekly interviews, menu checklists, and grocery receipts.
TABLE 1.
Composition of baseline and experimental diets1
| High protein |
Moderate protein |
||||
| Baseline control | High SF | Low SF | High SF | Low SF | |
| Carbohydrate, %E | 55 | 35 | 35 | 35 | 35 |
| GI | 55 | 61 | 61 | 58 | 57 |
| GL | 213 | 146 | 144 | 138 | 136 |
| Protein, %E | 15 | 30 | 30 | 20 | 20 |
| Whey isolate, g | 0 | 51 | 51 | 15 | 9 |
| Total fat, %E | 30 | 35 | 35 | 45 | 45 |
| SFAs | 7 | 15 | 7 | 15 | 7 |
| MUFAs | 13 | 10 | 18 | 20 | 29 |
| PUFAs | 7 | 7 | 8 | 7 | 7 |
| Cholesterol, mg | 357 | 355 | 314 | 358 | 356 |
Values shown are for the 12,540-kJ menus. GI, glycemic index; GL, glycemic load; SF, saturated fat; %E, percentage of energy.
Laboratory measurements.
Insulin was measured by ELISA (Millipore). Total cholesterol, HDL cholesterol (HDL-C), TGs, and glucose were measured by enzymatic endpoint measurements by using enzyme reagent kits (Ciba Corning Diagnostics) on a Ciba Corning Express Plus 550 analyzer. LDL-C was calculated by using the Friedewald equation (28). apoB and apoAI were measured by immunoturbidimetric assays (Bacton Assay Systems and Express Plus 550 analyzer) (29, 30). Lipoprotein(a) was measured by immunoassay (Myriad RBM). Lipoprotein subclass concentrations were measured by ion mobility, which uses gas-phase differential electrophoretic macromolecular mobility to directly measure lipoprotein particle concentration (31). Plasma concentrations of SFAs (10:0, 12:0, 14:0, 15:0, 16:0, and 18:0) and BCAAs (isoleucine, leucine, and valine) were quantified by Lipomics, as previously described (32). FAs are expressed as mole percentages.
Statistical procedures.
ANCOVA was used to compare the baseline means when adjusted for sex, with Supplemental Table 2 presenting the baseline means ± SDs for each diet. ANCOVA was also used to test whether the sex-adjusted mean 4-wk changes from baseline differed across diets. Those results are presented in Table 2 and Supplemental Table 3, which include the adjusted means ± SEs for each diet. To assess the effect of protein and SF, linear contrasts were used to estimate the mean (95% CI) difference in 4-wk changes from baseline for the high- vs. moderate-protein diets when averaged over the 2 amounts of SF to assess the effect of protein and for the high- and low-SF diets when averaged over the moderate- and high-protein diets to assess the effects of SF. Interactions between SF and protein intake were compared by using linear contrast. Differences between individual diets were tested by using the Tukey-Kramer honestly significant difference test. The distributions of the variables after the baseline diet and their changes after the experimental diets were examined for departures from normality and log-transformed as required. As expected, the differences during the dietary interventions were more normally distributed than the cross-sectional measurements and required fewer transformations. Spearman’s correlation coefficients (ρ) were used to evaluate the relations between changes in BCAAs and glucose metabolism. Baseline values in the text are presented as means ± SDs, and changes in text and figures are presented as means ± SEs. A P value <0.05 was considered significant. All statistical procedures were performed by using JMP 9.0 (SAS Institute).
TABLE 2.
Changes in body weight, waist circumference, glucose metabolism, and plasma lipid and lipoprotein concentrations in overweight and obese adults after 4 wk of consuming diets containing different amounts of protein and SF1
| High protein |
Moderate protein |
Mean (95% CI) difference for protein and SF effects2 |
|||||
| Control (n = 31) | High SF (n = 32) | Low SF (n = 36) | High SF (n = 29) | Low SF (n = 30) | High–moderate protein | High–low SF | |
| Weight, kg | −0.6 ± 0.5 | −0.7 ± 0.4 | −0.3 ± 0.4 | −0.1 ± 0.5 | 0.3 ± 0.5 | −0.6 (−1.4, 0.3) | −0.4 (−1.3, 0.5) |
| BMI, kg/m2 | −0.1 ± 0.1 | −0.1 ± 0.1 | −0.0 ± 0.1 | −0.0 ± 0.1 | 0.1 ± 0.1 | −0.1 (−0.2, 0.1) | −0.1 (−0.2, 0.1) |
| Body fat, % | −0.6 ± 0.3 | −0.2 ± 0.3 | 0.4 ± 0.3 | −0.4 ± 0.3 | −0.3 ± 0.3 | 0.3 (−0.2, 0.9) | −0.4 (−0.9, 0.2) |
| Waist circumference, cm | 0 ± 1 | −2 ± 1 | −2 ± 1 | −2 ± 1 | 0 ± 1 | −1 (−3, 1) | −1 (−2, 1) |
| Glucose, mmol/L | 0.07 ± 0.10a,b | −0.17 ± 0.10b,c | 0.14 ± 0.09a | 0.01 ± 0.10a,b | −0.32 ± 0.10c | 0.14 (−0.05, 0.34) | 0.01 (−0.18, 0.21) |
| Insulin, pmol/L | −1.6 ± 1.1 | −0.2 ± 1.1 | 1.3 ± 1.0 | −0.0 ± 1.1 | −2.1 ± 1.1 | 1.6 (−0.5, 3.7) | 0.3 (−1.8, 2.5) |
| SI,3 ×10−5 min−1 per pmol/L | 0.2 ± 0.3 | −0.2 ± 0.3 | −0.1 ± 0.5 | −0.5 ± 0.3 | −0.7 ± 0.4 | 0.5 (−0.3, 1.1) | 0.1 (−0.5, 0.6) |
| AIRg,3 pmol/L × 10 min | −62 ± 60 | 49 ± 32 | 56 ± 46 | −15 ± 21 | 35 ± 43 | 47 (−55, 149) | −36 (−140, 67) |
| DI3 | 4 ± 193 | −72 ± 190 | 227 ± 181 | −95 ± 201 | −76 ± 196 | 163 (−213, 539) | −159 (−539, 222) |
| MCRi, L/min | 0.5 ± 0.6 | −0.2 ± 0.6 | 0.3 ± 0.6 | 0.3 ± 0.6 | 0.6 ± 0.6 | −0.4 (−1.6, 0.8) | −0.4 (−1.6, 0.8) |
| TGs,3 mmol/L | −0.11 ± 0.10 | −0.12 ± 0.10 | −0.31 ± 0.10 | −0.26 ± 0.11 | −0.21 ± 0.10 | 0.02 (−0.18, 0.22) | 0.07 (−0.13, 0.27) |
| TC, mmol/L | −0.04 ± 0.08 | −0.18 ± 0.08 | −0.20 ± 0.08 | −0.09 ± 0.09 | −0.08 ± 0.09 | −0.11 (−0.27, 0.06) | 0.00 (−0.17, 0.17) |
| LDL-C, mmol/L | 0.01 ± 0.08 | −0.11 ± 0.08 | −0.09 ± 0.07 | 0.01 ± 0.08 | −0.01 ± 0.08 | −0.10 (−0.25, 0.05) | 0.00 (−0.15, 0.15) |
| HDL-C, mmol/L | 0.00 ± 0.02 | −0.01 ± 0.02 | 0.00 ± 0.02 | 0.02 ± 0.02 | 0.01 ± 0.02 | −0.02 (−0.06, 0.05) | −0.01 (−0.04, 0.03) |
Values for diets are means ± SEs, adjusted for sex. Means within a row without a common letter are significantly different, P < 0.05. AIRg, acute insulin response to glucose; DI, disposition index; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; MCRi, metabolic clearance rate of insulin; SF, saturated fat; SI, insulin sensitivity index; TC, total cholesterol.
For comparison of high- vs. moderate-protein and comparison of high- vs. low-SF diets.
ANCOVAs were repeated with the use of log-transformed data, and levels of significance only negligibly improved.
Results
Study participants.
One hundred fifty-nine participants completed the study. One participant who became diabetic during the study was excluded from analysis. Figure 1 displays details of participant recruitment and withdrawal. Age and BMI of participants at baseline were 38 ± 12 y and 33.9 ± 3.8 kg/m2, respectively, and did not differ between diet groups. Ninety-five percent of participants had a dietary compliance score of at least 4 of 5 and the inclusion of compliance score as a covariate did not significantly alter results.
Effects of the diets on glucose metabolism and plasma lipid and lipoprotein concentrations.
Other than a small difference in total cholesterol, there were no significant differences in mean plasma glucose, lipid, or lipoprotein measurements between the experimental groups after the 4-wk baseline run-in diet (Supplemental Table 2). We used linear contrasts to assess the effects of dietary protein and SF on outcome measures (see Methods). Table 2 shows that fasting plasma glucose concentrations increased significantly after consuming the high-protein diets relative to the moderate-protein diets, with a significant interaction between protein and SF intake (P = 0.001) such that there was a significant increase in fasting glucose with the high- vs. moderate-protein diet when SF intake was low. There were no other significant effects of either protein or SF on changes in glucose metabolism or plasma lipids (Table 2), apolipoproteins, or lipoprotein subclass concentrations from the baseline diet (Supplemental Table 3).
Verification of dietary compliance on the basis of plasma amino and FA concentrations.
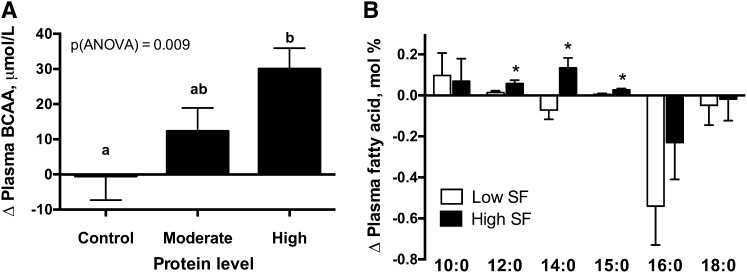
Plasma BCAAs (sum of leucine, isoleucine, and valine) were measured as biomarkers of protein intake. Higher protein intake was associated with a greater increase in plasma BCAAs from the baseline diet (Fig. 2A).
FIGURE 2.
Changes in plasma concentrations of BCAAs (A) and SFAs (B) in overweight and obese adults after 4 wk of consuming diets containing different amounts of protein and SF. Data from high-SF and low-SF diets were combined for each protein group (control: n = 31; moderate: n = 59; high: n = 68), and data from moderate- and high-protein diets were combined for each SF group (low SF: n = 66; high SF: n = 61). Values are means ± SEs. Groups without a common letter are significantly different, P < 0.05 (A). *Different from low-SF diet, P < 0.05 (B). SF, saturated fat.
Plasma concentrations of a panel of FAs were used as biomarkers of dietary fat intake. Compared with participants consuming the low-SF diets, participants consuming the high-SF diets had significantly greater increases from the baseline run-in diet in mean plasma concentrations of lauric (12:0), myristic (14:0), and pentadecanoic (15:0) acids, which are enriched in dairy fat (Fig. 2B). Among these, 15:0 is a specific marker for dairy fat intake (33). There was no significant effect of SF on changes in other measured plasma SFAs (10:0, 16:0, and 18:0) from the baseline diet. SFAs 16:0 and 18:0 were reported to have weaker correlations with dietary intake, in part due to endogenous production (34, 35). Mean ± SE plasma total MUFAs decreased after consumption of the high-SF diet in comparison with the low-SF diet (high-SF diet: −0.91 ± 0.28 mol%; low-SF diet: 0.23 ± 0.32 mol%; P = 0.009). There were no significant differences in changes in plasma total PUFA concentrations from the baseline diet between the high-SF and low-SF groups.
Relations of plasma BCAAs to measures of glucose metabolism.
Despite overall increases in mean plasma BCAA concentrations from the baseline diet with increasing protein amount in the experimental diets, there was considerable individual variation in the response. We used this variation to explore the relation of changes in plasma BCAAs and changes in measures of glucose metabolism independent of the experimental diets. In cross-sectional analysis, BCAAs measured after the baseline diet were positively correlated with fasting plasma concentrations of insulin (ρ = 0.26, P = 0.001) and glucose (ρ = 0.26, P = 0.001) and with HOMA-IR (ρ = 0.30, P = 0.0001) and were negatively correlated with AIRg (ρ = −0.17, P = 0.03) and DI (ρ = −0.25, P = 0.002) but not SI (ρ = −0.06, P = 0.42) or MCRi (ρ = −0.1, P = 0.19). Changes in BCAAs were inversely correlated with changes in MCRi (ρ = −0.18, P = 0.03) and positively correlated with changes in AIRg (ρ = 0.15, P = 0.05) (Supplemental Fig. 1). Notably, the inverse correlation between changes in MCRi and plasma BCAAs was driven by the individuals who consumed the high-SF diet (P–interaction = 0.05). BCAA response was not significantly correlated with changes in fasting glucose (ρ = 0.08, P = 0.32), insulin (ρ = 0.05, P = 0.52), SI (ρ = −0.10, P = 0.20), or DI (ρ = −0.06, P = 0.45).
Discussion
Epidemiologic studies suggest that high protein intake may be associated with increased risk of developing type 2 diabetes, a condition usually characterized by insulin resistance (36, 37). Further studies indicated that the protein source may be an important determinant, with red and processed meat conferring greater risk (38–40). Although a high intake of dairy products has been associated with reduced risk of diabetes (13–15), and dairy products are a major source of protein, less is known about the relation of dairy protein to diabetes risk.
There have been few intervention studies that tested the effects of isocaloric substitution of protein for carbohydrate, or protein for fat, on measurements of glucose homeostasis in nondiabetic individuals. Long-term consumers of high-protein diets were reported to have greater glucose-stimulated insulin secretion and slightly lower insulin sensitivity (8) than that observed in individuals with lower protein intake. In a study comparing an isoenergetic high-protein diet (25–30%E) with a conventional-protein (15%E) control diet and 2 high-cereal-fiber diets with conventional (15%E) or moderate protein (20–25%E), there was a decrease in insulin sensitivity as measured by euglycemic hyperinsulinemic clamp when compared with either baseline or the high-cereal-fiber diets. However, changes in insulin sensitivity from baseline in the high-protein diet vs. the conventional-protein control diet were not different, suggesting that protein content per se was not the causative factor (9).
In the present study we found no significant effects on insulin sensitivity of short-term increases in intake of protein, in conjunction with reduced dietary carbohydrate, in nondiabetic overweight and obese individuals. We used whey protein isolate to partially meet the increased protein content of the moderate- and high-protein diets. Acutely, milk proteins, specifically whey, consumed with glucose or standardized meals increase postprandial insulin response, resulting in improved glucose excursion (12, 16, 41, 42), with reported effects on both markers of insulin secretion (12) and hepatic extraction of insulin (42). Whey protein is rich in BCAAs, which appear in the plasma postprandially (12). Recently, metabolomic profiling showed that elevated basal concentrations of BCAAs are associated with obesity and surrogate measures of insulin resistance such as HOMA-IR (19, 20). In cross-sectional analysis, a cluster of amino acids including BCAAs was inversely related to more specific measures of insulin action, including SI and DI (18). In prospective studies, baseline BCAA concentrations predicted ∼6-y HOMA-IR (43) and 2-h glucose (44) values and diabetes incidence (45). Moreover, skeletal insulin resistance can be induced by acute amino acid infusion in humans (46) and by BCAA feeding in mice (19). Although we observed cross-sectional relations of BCAAs with fasting glucose, insulin, HOMA-IR, AIRg, and DI, changes in BCAA concentrations were not associated with changes in fasting glucose, insulin sensitivity (SI), or DI. Rather, we found that increases in BCAAs over the 4-wk diet intervention were correlated with decreased insulin clearance (MCRi) and increased secretion (AIRg). Notably, reduced insulin clearance was reported to predict the incidence of type 2 diabetes (47) and was associated with glucose intolerance, abdominal obesity, and nonalcoholic fatty liver disease. Thus, it is possible that BCAA effects on insulin dynamics may precede the development of insulin resistance and type 2 diabetes. We also observed that the relation between plasma BCAAs and insulin clearance was attenuated with the low-SF diet, suggesting that either low SF or high monounsaturated fat intake blunts the association of BCAAs and insulin action. In this regard, Newgard et al. (19) found that in mice dietary BCAAs reduced insulin sensitivity only on the background of a diet high in total and SF and not when fed a standard chow diet. Greater BCAA flux and catabolism in muscle and liver are postulated to contribute to incomplete FA oxidation (48), which is associated with reduced insulin action (49). This may be exacerbated by the increase in FA oxidation with a high-fat diet (50); however, it unknown whether SF may preferentially promote this process.
Studies in animal models indicated that insulin sensitivity is impaired by diets high in SF [reviewed in (51–53)], and some human observational studies reported positive associations between SF intake and hyperinsulinemia, independent of body fat (54–58). However, in the majority of human intervention studies, changes in dietary fat quality had no effects on insulin sensitivity (52, 53, 59), including several large trials comparing replacement of monounsaturated fat for SF in the context of a higher fat diet. Our data support the evidence that high SF intake does not have a major impact on insulin sensitivity.
Measures of biomarkers of SF and protein intake suggest that our results are not due to poor dietary compliance. Plasma concentrations of pentadecanoic acid (15:0), a specific marker of intake of dairy fat (33, 60), the primary source of added SF in the high-SF diet, were significantly higher in the high-SF group; and plasma concentrations of BCAAs were correlated with protein content of the prescribed diets.
Replacement of monounsaturated fat by SF is known to increase plasma LDL-C and often HDL-C (61, 62). However, we observed no difference in these measurements between the high-SF and low-SF diets. It is possible that this is related to the selection of overweight and obese individuals for this study. There are reports that individuals with higher BMI values exhibit smaller than expected reductions in LDL-C in response to reductions in dietary SF compared with those with lower BMI values (63–66), as well as those with evidence for insulin resistance (67). Attenuated LDL-C lowering with reduction in dietary SF is particularly evident in women (65, 66), who made up the majority of our population. Moreover, in the recent LIPGENE study, an ∼7%E substitution of monounsaturated fat for SF in individuals with the metabolic syndrome (mean BMI: ∼32 kg/m2) resulted in no significant changes in total cholesterol, LDL-C, or HDL-C, despite evidence for dietary compliance as assessed by plasma FAs (22). Although there is no known basis for reduced responsiveness of obese individuals to changes in dietary SF, it may be that very high tissue concentrations of SFAs or cholesterol dampen the effect of exogenous FAs on hepatic cholesterol content (63, 65, 67).
Strengths of our study include a comprehensive design for testing effects of changes in both protein and SF intake, lack of potential confounding by weight loss, detailed measurements of insulin action, and demonstration of dietary compliance by plasma biomarkers of both protein and SF intake. Limitations include the short-term dietary intervention and the restriction of the study population to overweight and obese individuals. In addition, because higher protein intake was achieved with the addition of whey protein to a mixed-protein diet, and higher SF intake was achieved primarily by using whole-fat dairy products, it is possible that the present findings would not apply to comparable dietary amounts of protein and SF from other food sources. Finally, physiologically meaningful effects of the diets may have been smaller than those that the study was statistically powered to detect.
In conclusion, our results show that, in the absence of weight loss, increased consumption of protein or SF primarily from dairy sources does not significantly alter insulin sensitivity or insulin action in nondiabetic overweight and obese individuals. However, we found that diet-induced increases in plasma BCAAs correlate with increased insulin secretion and reduced insulin clearance, with the latter relation being influenced by the type of fat. Additional studies are warranted to better understand how BCAA metabolism influences insulin dynamics in humans.
Supplementary Material
Acknowledgments
The authors thank Katie Wojnoonski for technical support, Robin Rawlings and Cam-Tu Tran for administering clinical protocols, Sue Fernstrom for help with development and administration of the dietary intervention, and Linda Abe for data management. R.M.K. designed the research; S.C. and R.M.K. conducted the research; S.C., P.T.W., R.N.B., D.S., S.M.W., and R.M.K analyzed the data; S.C., P.T.W., T.D., and R.M.K. wrote the manuscript; and S.C. had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AIRg, acute insulin response to glucose; DI, disposition index; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; MCRi, metabolic clearance rate of insulin; SF, saturated fat; SI, insulin sensitivity index; %E, percentage of energy.
References
- 1.Layman DK, Boileau RA, Erickson DJ, Painter JE, Shiue H, Sather C, Christou DD. A reduced ratio of dietary carbohydrate to protein improves body composition and blood lipid profiles during weight loss in adult women. J Nutr 2003;133:411–7. [DOI] [PubMed] [Google Scholar]
- 2.Piatti PM, Monti F, Fermo I, Baruffaldi L, Nasser R, Santambrogio G, Librenti MC, Galli-Kienle M, Pontiroli AE, Pozza G. Hypocaloric high-protein diet improves glucose oxidation and spares lean body mass: comparison to hypocaloric high-carbohydrate diet. Metabolism 1994;43:1481–7. [DOI] [PubMed] [Google Scholar]
- 3.Farnsworth E, Luscombe ND, Noakes M, Wittert G, Argyiou E, Clifton PM. Effect of a high-protein, energy-restricted diet on body composition, glycemic control, and lipid concentrations in overweight and obese hyperinsulinemic men and women. Am J Clin Nutr 2003;78:31–9. [DOI] [PubMed] [Google Scholar]
- 4.Claessens M, van Baak MA, Monsheimer S, Saris WH. The effect of a low-fat, high-protein or high-carbohydrate ad libitum diet on weight loss maintenance and metabolic risk factors. Int J Obes (Lond) 2009;33:296–304. [DOI] [PubMed] [Google Scholar]
- 5.Parker B, Noakes M, Luscombe N, Clifton P. Effect of a high-protein, high-monounsaturated fat weight loss diet on glycemic control and lipid levels in type 2 diabetes. Diabetes Care 2002;25:425–30. [DOI] [PubMed] [Google Scholar]
- 6.Brinkworth GD, Noakes M, Keogh JB, Luscombe ND, Wittert GA, Clifton PM. Long-term effects of a high-protein, low-carbohydrate diet on weight control and cardiovascular risk markers in obese hyperinsulinemic subjects. Int J Obes Relat Metab Disord 2004;28:661–70. [DOI] [PubMed] [Google Scholar]
- 7.Luscombe-Marsh ND, Noakes M, Wittert GA, Keogh JB, Foster P, Clifton PM. Carbohydrate-restricted diets high in either monounsaturated fat or protein are equally effective at promoting fat loss and improving blood lipids. Am J Clin Nutr 2005;81:762–72. [DOI] [PubMed] [Google Scholar]
- 8.Linn T, Santosa B, Gronemeyer D, Aygen S, Scholz N, Busch M, Bretzel RG. Effect of long-term dietary protein intake on glucose metabolism in humans. Diabetologia 2000;43:1257–65. [DOI] [PubMed] [Google Scholar]
- 9.Weickert MO, Roden M, Isken F, Hoffmann D, Nowotny P, Osterhoff M, Blaut M, Alpert C, Gogebakan O, Bumke-Vogt C, et al. Effects of supplemented isoenergetic diets differing in cereal fiber and protein content on insulin sensitivity in overweight humans. Am J Clin Nutr 2011;94:459–71. [DOI] [PubMed] [Google Scholar]
- 10.Gannon MC, Nuttall FQ, Saeed A, Jordan K, Hoover H. An increase in dietary protein improves the blood glucose response in persons with type 2 diabetes. Am J Clin Nutr 2003;78:734–41. [DOI] [PubMed] [Google Scholar]
- 11.Gadgil MD, Appel LJ, Yeung E, Anderson CA, Sacks FM, Miller ER., III The effects of carbohydrate, unsaturated fat, and protein intake on measures of insulin sensitivity: results from the OmniHeart trial. Diabetes Care 2013;36:1132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nilsson M, Stenberg M, Frid AH, Holst JJ, Bjorck IM. Glycemia and insulinemia in healthy subjects after lactose-equivalent meals of milk and other food proteins: the role of plasma amino acids and incretins. Am J Clin Nutr 2004;80:1246–53. [DOI] [PubMed] [Google Scholar]
- 13.Aune D, Norat T, Romundstad P, Vatten LJ. Dairy products and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Am J Clin Nutr 2013;98:1066–83. [DOI] [PubMed] [Google Scholar]
- 14.Elwood PC, Pickering JE, Givens DI, Gallacher JE. The consumption of milk and dairy foods and the incidence of vascular disease and diabetes: an overview of the evidence. Lipids 2010;45:925–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tong X, Dong JY, Wu ZW, Li W, Qin LQ. Dairy consumption and risk of type 2 diabetes mellitus: a meta-analysis of cohort studies. Eur J Clin Nutr 2011;65:1027–31. [DOI] [PubMed] [Google Scholar]
- 16.Frid AH, Nilsson M, Holst JJ, Bjorck IM. Effect of whey on blood glucose and insulin responses to composite breakfast and lunch meals in type 2 diabetic subjects. Am J Clin Nutr 2005;82:69–75. [DOI] [PubMed] [Google Scholar]
- 17.Pal S, Ellis V, Dhaliwal S. Effects of whey protein isolate on body composition, lipids, insulin and glucose in overweight and obese individuals. Br J Nutr 2010;104:716–23. [DOI] [PubMed] [Google Scholar]
- 18.Huffman KM, Shah SH, Stevens RD, Bain JR, Muehlbauer M, Slentz CA, Tanner CJ, Kuchibhatla M, Houmard JA, Newgard CB, et al. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care 2009;32:1678–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009;9:311–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah SH, Crosslin DR, Haynes CS, Nelson S, Turer CB, Stevens RD, Muehlbauer MJ, Wenner BR, Bain JR, Laferrere B, et al. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia 2012;55:321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jebb SA, Lovegrove JA, Griffin BA, Frost GS, Moore CS, Chatfield MD, Bluck LJ, Williams CM, Sanders TA, RISCK Study Group. Effect of changing the amount and type of fat and carbohydrate on insulin sensitivity and cardiovascular risk: the RISCK (Reading, Imperial, Surrey, Cambridge, and Kings) trial. Am J Clin Nutr 2010;92:748–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tierney AC, McMonagle J, Shaw DI, Gulseth HL, Helal O, Saris WH, Paniagua JA, Golabek-Leszczynska I, Defoort C, Williams CM, et al. Effects of dietary fat modification on insulin sensitivity and on other risk factors of the metabolic syndrome—LIPGENE: a European randomized dietary intervention study. Int J Obes (Lond) 2011;35:800–9. [DOI] [PubMed] [Google Scholar]
- 23.Vessby B, Uusitupa M, Hermansen K, Riccardi G, Rivellese AA, Tapsell LC, Nalsen C, Berglund L, Louheranta A, Rasmussen BM, et al. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: the KANWU Study. Diabetologia 2001;44:312–9. [DOI] [PubMed] [Google Scholar]
- 24.Saad MF, Anderson RL, Laws A, Watanabe RM, Kades WW, Chen YD, Sands RE, Pei D, Savage PJ, Bergman RN. A comparison between the minimal model and the glucose clamp in the assessment of insulin sensitivity across the spectrum of glucose tolerance. Insulin Resistance Atherosclerosis Study. Diabetes 1994;43:1114–21. [DOI] [PubMed] [Google Scholar]
- 25.Pacini G, Bergman RN. MINMOD: a computer program to calculate insulin sensitivity and pancreatic responsivity from the frequently sampled intravenous glucose tolerance test. Comput Methods Programs Biomed 1986;23:113–22. [DOI] [PubMed] [Google Scholar]
- 26.Mittelman SD, Van Citters GW, Kim SP, Davis DA, Dea MK, Hamilton-Wessler M, Bergman RN. Longitudinal compensation for fat-induced insulin resistance includes reduced insulin clearance and enhanced beta-cell response. Diabetes 2000;49:2116–25. [DOI] [PubMed] [Google Scholar]
- 27.National Research Council. Dietary reference intakes: the essential guide to nutrient requirements. Washington: The National Academies Press; 2006. [Google Scholar]
- 28.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- 29.Rifai N, King ME. Immunoturbidimetric assays of apolipoproteins A, AI, AII, and B in serum. Clin Chem 1986;32:957–61. [PubMed] [Google Scholar]
- 30.Smith SJ, Cooper GR, Henderson LO, Hannon WH. An international collaborative study on standardization of apolipoproteins A-I and B. Part I. Evaluation of a lyophilized candidate reference and calibration material. Clin Chem 1987;33:2240–9. [PubMed] [Google Scholar]
- 31.Caulfield MP, Li S, Lee G, Blanche PJ, Salameh WA, Benner WH, Reitz RE, Krauss RM. Direct determination of lipoprotein particle sizes and concentrations by ion mobility analysis. Clin Chem 2008;54:1307–16. [DOI] [PubMed] [Google Scholar]
- 32.Watkins SM, Reifsnyder PR, Pan HJ, German JB, Leiter EH. Lipid metabolome-wide effects of the PPARgamma agonist rosiglitazone. J Lipid Res 2002;43:1809–17. [DOI] [PubMed] [Google Scholar]
- 33.Smedman AE, Gustafsson IB, Berglund LG, Vessby BO. Pentadecanoic acid in serum as a marker for intake of milk fat: relations between intake of milk fat and metabolic risk factors. Am J Clin Nutr 1999;69:22–9. [DOI] [PubMed] [Google Scholar]
- 34.Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, Johnson L, Franco OH, Butterworth AS, Forouhi NG, Thompson SG, et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med 2014;160:398–406. [DOI] [PubMed] [Google Scholar]
- 35.Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 2008;47:348–80. [DOI] [PubMed] [Google Scholar]
- 36.de Koning L, Fung TT, Liao X, Chiuve SE, Rimm EB, Willett WC, Spiegelman D, Hu FB. Low-carbohydrate diet scores and risk of type 2 diabetes in men. Am J Clin Nutr 2011;93:844–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sluijs I, Beulens JW, van der A D, Spijkerman AM, Grobbee DE, van der Schouw YT. Dietary intake of total, animal, and vegetable protein and risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-NL study. Diabetes Care 2010;33:43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.InterAct Consortium. Association between dietary meat consumption and incident type 2 diabetes: the EPIC-InterAct study. Diabetologia 2013;56:47–59. [DOI] [PubMed] [Google Scholar]
- 39.Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation 2010;121:2271–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan A, Sun Q, Bernstein AM, Manson JE, Willett WC, Hu FB. Changes in red meat consumption and subsequent risk of type 2 diabetes mellitus: three cohorts of US men and women. JAMA Intern Med 2013;173:1328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nuttall FQ, Mooradian AD, Gannon MC, Billington C, Krezowski P. Effect of protein ingestion on the glucose and insulin response to a standardized oral glucose load. Diabetes Care 1984;7:465–70. [DOI] [PubMed] [Google Scholar]
- 42.Lan-Pidhainy X, Wolever TM. The hypoglycemic effect of fat and protein is not attenuated by insulin resistance. Am J Clin Nutr 2010;91:98–105. [DOI] [PubMed] [Google Scholar]
- 43.Würtz P, Soininen P, Kangas AJ, Ronnemaa T, Lehtimaki T, Kahonen M, Viikari JS, Raitakari OT, Ala-Korpela M. Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care 2013;36:648–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Würtz P, Tiainen M, Makinen VP, Kangas AJ, Soininen P, Saltevo J, Keinanen-Kiukaanniemi S, Mantyselka P, Lehtimaki T, Laakso M, et al. Circulating metabolite predictors of glycemia in middle-aged men and women. Diabetes Care 2012;35:1749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, et al. Metabolite profiles and the risk of developing diabetes. Nat Med 2011;17:448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krebs M, Krssak M, Bernroider E, Anderwald C, Brehm A, Meyerspeer M, Nowotny P, Roth E, Waldhausl W, Roden M. Mechanism of amino acid-induced skeletal muscle insulin resistance in humans. Diabetes 2002;51:599–605. [DOI] [PubMed] [Google Scholar]
- 47.Lee CC, Haffner SM, Wagenknecht LE, Lorenzo C, Norris JM, Bergman RN, Stefanovski D, Anderson AM, Rotter JI, Goodarzi MO, et al. Insulin clearance and the incidence of type 2 diabetes in Hispanics and African Americans: the IRAS Family Study. Diabetes Care 2013;36:901–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab 2012;15:606–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 2008;7:45–56. [DOI] [PubMed] [Google Scholar]
- 50.Schrauwen P, Wagenmakers AJ, van Marken Lichtenbelt WD, Saris WH, Westerterp KR. Increase in fat oxidation on a high-fat diet is accompanied by an increase in triglyceride-derived fatty acid oxidation. Diabetes 2000;49:640–6. [DOI] [PubMed] [Google Scholar]
- 51.Rivellese AA, De Natale C, Lilli S. Type of dietary fat and insulin resistance. Ann N Y Acad Sci 2002;967:329–35. [DOI] [PubMed] [Google Scholar]
- 52.Riccardi G, Giacco R, Rivellese AA. Dietary fat, insulin sensitivity and the metabolic syndrome. Clin Nutr 2004;23:447–56. [DOI] [PubMed] [Google Scholar]
- 53.McAuley K, Mann J. Thematic review series: patient-oriented research. Nutritional determinants of insulin resistance. J Lipid Res 2006;47:1668–76. [DOI] [PubMed] [Google Scholar]
- 54.Parker DR, Weiss ST, Troisi R, Cassano PA, Vokonas PS, Landsberg L. Relationship of dietary saturated fatty acids and body habitus to serum insulin concentrations: the Normative Aging Study. Am J Clin Nutr 1993;58:129–36. [DOI] [PubMed] [Google Scholar]
- 55.Mayer EJ, Newman B, Quesenberry CP, Jr, Selby JV. Usual dietary fat intake and insulin concentrations in healthy women twins. Diabetes Care 1993;16:1459–69. [DOI] [PubMed] [Google Scholar]
- 56.Marshall JA, Bessesen DH, Hamman RF. High saturated fat and low starch and fibre are associated with hyperinsulinaemia in a non-diabetic population: the San Luis Valley Diabetes Study. Diabetologia 1997;40:430–8. [DOI] [PubMed] [Google Scholar]
- 57.Maron DJ, Fair JM, Haskell WL. Saturated fat intake and insulin resistance in men with coronary artery disease. The Stanford Coronary Risk Intervention Project Investigators and Staff. Circulation 1991;84:2020–7. [DOI] [PubMed] [Google Scholar]
- 58.Feskens EJ, Loeber JG, Kromhout D. Diet and physical activity as determinants of hyperinsulinemia: the Zutphen Elderly Study. Am J Epidemiol 1994;140:350–60. [DOI] [PubMed] [Google Scholar]
- 59.Manco M, Calvani M, Mingrone G. Effects of dietary fatty acids on insulin sensitivity and secretion. Diabetes Obes Metab 2004;6:402–13. [DOI] [PubMed] [Google Scholar]
- 60.Brevik A, Veierod MB, Drevon CA, Andersen LF. Evaluation of the odd fatty acids 15:0 and 17:0 in serum and adipose tissue as markers of intake of milk and dairy fat. Eur J Clin Nutr 2005;59:1417–22. [DOI] [PubMed] [Google Scholar]
- 61.Krauss RM, Blanche PJ, Rawlings RS, Fernstrom HS, Williams PT. Separate effects of reduced carbohydrate intake and weight loss on atherogenic dyslipidemia. Am J Clin Nutr 2006;83:1031–3. [DOI] [PubMed] [Google Scholar]
- 62.Mensink RP, Katan MB. Effect of dietary fatty acids on serum lipids and lipoproteins: a meta-analysis of 27 trials. Arterioscler Thromb 1992;12:911–9. [DOI] [PubMed] [Google Scholar]
- 63.Denke MA, Adams-Huet B, Nguyen AT. Individual cholesterol variation in response to a margarine- or butter-based diet: a study in families. JAMA 2000;284:2740–7. [DOI] [PubMed] [Google Scholar]
- 64.Jansen S, Lopez-Miranda J, Salas J, Castro P, Paniagua JA, Lopez-Segura F, Ordovas JM, Jimenez-Pereperez JA, Blanco A, Perez-Jimenez F. Plasma lipid response to hypolipidemic diets in young healthy non-obese men varies with body mass index. J Nutr 1998;128:1144–9. [DOI] [PubMed] [Google Scholar]
- 65.Hannah JS, Jablonski KA, Howard BV. The relationship between weight and response to cholesterol-lowering diets in women. Int J Obes Relat Metab Disord 1997;21:445–50. [DOI] [PubMed] [Google Scholar]
- 66.Clifton PM, Abbey M, Noakes M, Beltrame S, Rumbelow N, Nestel PJ. Body fat distribution is a determinant of the high-density lipoprotein response to dietary fat and cholesterol in women. Arterioscler Thromb Vasc Biol 1995;15:1070–8. [DOI] [PubMed] [Google Scholar]
- 67.Lefevre M, Champagne CM, Tulley RT, Rood JC, Most MM. Individual variability in cardiovascular disease risk factor responses to low-fat and low-saturated-fat diets in men: body mass index, adiposity, and insulin resistance predict changes in LDL cholesterol. Am J Clin Nutr 2005;82:957–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.