Abstract
Background: Isothiocyanates in cruciferous vegetables modulate signaling pathways critical to carcinogenesis, including nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), a central regulator of inflammation. Glutathione S-transferase (GST) M1 and GSTT1 metabolize isothiocyanates; genetic variants may result in differences in biologic response. Objective: The objective of this study was to test whether consumption of cruciferous or cruciferous plus apiaceous vegetables altered serum concentrations of interleukin (IL)-6, IL-8, C-reactive protein (CRP), tumor necrosis factor (TNF) α, and soluble TNF receptor (sTNFR) I and II, and whether this response was GSTM1/GSTT1 genotype dependent. Methods: In a randomized crossover trial, healthy men (n = 32) and women (n = 31) aged 20–40 y consumed 4 14-d controlled diets: basal (vegetable-free), single-dose cruciferous (1xC) [7 g vegetables/kg body weight (BW)], double-dose cruciferous (2xC) (14 g/kg BW), and cruciferous plus apiaceous (carrot family) (1xC+A) vegetables (7 and 4 g/kg BW, respectively), with a 21-d washout period between each intervention. Urinary isothiocyanate excretion was also evaluated as a marker of systemic isothiocyanate exposure. Fasting morning blood and urine samples were collected on days 0 and 14 and analyzed. Results: IL-6 concentrations were significantly lower on day 14 of the 2xC and 1xC+A diets than with the basal diet [−19% (95% CI: −30%, −0.1%) and −20% (95% CI: −31%, -0.7%), respectively]. IL-8 concentrations were higher after the 1xC+A diet (+16%; 95% CI: 4.2%, 35.2%) than after the basal diet. There were no effects of diet on CRP, TNF-α, or sTNFRI or II. There were significant differences between GSTM1-null/GSTT1+ individuals for several biomarkers in response to 1xC+A compared with basal diets (CRP: −37.8%; 95% CI: −58.0%, −7.4%; IL-6: −48.6%; 95% CI: −49.6%, −12.0%; IL-8: 16.3%; 95% CI: 6.7%, 57.7%) and with the 2xC diet compared with the basal diet (IL-8: −33.2%; 95% CI: −43.0%, −1.4%; sTNFRI: −7.5%; 95% CI: −12.7%, −2.3%). There were no significant reductions in biomarker concentrations in response to diet among GSTM1+/GSTT1+ or GSTM1-null/GSTT1-null individuals. Twenty-four-hour urinary isothiocyanate excretion was not associated with any of the inflammation markers overall; however, IL-6 was inversely associated with total isothiocyanate excretion in GSTM1-null/GSTT1-null individuals (β = −0.12; 95% CI: −0.19, −0.05). Conclusions: In this young, healthy population, consumption of cruciferous and apiaceous vegetables reduced circulating IL-6; however, results for other biomarkers of inflammation were not consistent.
Introduction
Higher consumption of cruciferous (i.e., broccoli family) vegetables is associated with lower risk of several cancers (1). Isothiocyanates, the bioactive components derived from glucosinolates in cruciferous vegetables, are thought to exert their effects through several distinct mechanisms. One mechanism is through modulation of enzymes that metabolize exogenous compounds (e.g., various carcinogens) and endogenous compounds (e.g., sex steroid hormones) implicated in cancer risk. Isothiocyanates are thought to interact with the cytoplasmic-anchoring protein Kelch-like ECH-associated protein 1 (Keap1)6 and the nuclear transcription factor erythroid-derived related-factor 2-like 2 (Nrf2) (2). However, there is growing evidence for a role for isothiocyanates in the modulation of other signaling pathways critical to carcinogenesis, including stimulation of cell cycle arrest, apoptosis, epigenetic regulation, and inhibition of NF-κB (2, 3).
NF-κB is a central mediator in the inflammatory process. It regulates a battery of genes coding for cytokines, chemokines, adhesion molecules, and other soluble factors involved in the immune response (4). Upon release of this cytoplasmic-bound transcription factor from its inhibitor, inhibitor of kappa B (I-κB), NF-κB is free to translocate to the nucleus where the active subunit can bind the κB site in the promoter region of target genes to initiate transcription (4). Isothiocyanates inhibit NF-κB–mediated processes in vitro (5–7), and in vivo in animal models (8–10), and therefore may reduce inflammation in humans (11). Substantial research over the past few decades has linked chronic, low-grade inflammation with a number of human diseases including many cancers. For example, chronic infection with hepatitis or long-standing inflammation seen with inflammatory bowel diseases are associated with increased risk of hepatic and colon cancers, respectively (12). Furthermore, higher circulating concentrations of many inflammatory mediators, including C-reactive protein (CRP) (13, 14), IL-6 (15, 16), IL-8 (17), and TNF-α (18), have been associated prospectively with the risk of several cancers.
Isothiocyanates are conjugated by glutathione S-transferases (GSTs) and subsequently metabolized mainly via the mercapturic acid pathway to water-soluble end products (19). GSTs are a multigene family of enzymes involved in the conjugation of a wide range of compounds, including environmental carcinogens, chemotherapeutic agents, and reactive oxygen species, as well as potentially beneficial dietary compounds, such as isothiocyanates (20, 21). Null genotypes for GSTM1 and GSTT1 result in the absence of their respective enzymes. Several studies reported genotypic differences in biologic response to cruciferous vegetable consumption and isothiocyanate pharmacokinetics (22, 23). However, the contribution of these variants to differences in response is not clear because pharmacokinetic studies indicate that individuals with a GST-null genotype metabolize and excrete isothiocyanates at a faster, rather than slower, rate than their intact counterparts (23). This apparent paradox may reflect isothiocyanate effects on other targets or differences in the types or amounts of crucifers consumed. Alternatively, other GSTs may become more efficient in isothiocyanate metabolism among those individuals with GSTM1-null or GSTT1-null genotypes.
This study evaluated the effect of cruciferous vegetables on biomarkers of systemic inflammation and addressed whether variation in genes that encode for enzymes that metabolize isothiocyanates (i.e., GST) altered effects of these plant foods. Glucosinolates are also metabolized by gut microbiota; therefore, we also evaluated the associations between the panel of inflammation biomarkers and urinary total isothiocyanate excretion, a measure of systemic exposure. Because the parent study also included evaluation of another botanical family, Apiaceae (e.g., carrot family), which are also a rich source of several flavonoids, the effects of consuming these vegetables on biomarkers of systemic inflammation were also examined.
Participants and Methods
Research design.
Data and biologic samples for this ancillary study are from the 2EAT (Enzyme Activation Trial 2). The 2EAT study was a randomized, controlled, crossover feeding trial carried out at the Fred Hutchinson Cancer Research Center (FHCRC), Seattle, WA, as described previously (24). Participants were recruited on the basis of GSTM1 and GSTT1 genotypes so as to obtain a good distribution of the combined GST genotypes within an individual as follows: GSTM1+ and GSTT1+; GSTM1-null and GSTT1+; and GSTM1-null and GSTT1-null, where “+” designates at least 1 positive (e.g., intact) allele. Participants were randomly assigned to GSTM1 and GSTT1 genotype-by-sex by using a minimal balanced design, which has a Latin square consisting of the 4 treatment sequences (24). Exclusion criteria and recruitment were described in detail elsewhere (24). Briefly, exclusion criteria included factors known to influence biotransformation enzyme induction and inflammation, e.g., chronic disease, current use of medications [both prescription (including oral contraceptives) and over-the-counter (including use of aspirin or nonsteroidal anti-inflammatory drugs)], alcohol, smoking, habitual heavy exercise, and obesity. The institutional review board at FHCRC approved the study, and all participants gave informed consent.
Participants.
Participants were healthy, nonsmoking men and women, aged 20–40 y, recruited from the greater Seattle area. Of the 73 participants randomly assigned, 2 did not consent to use of their samples for future studies and archived serum samples were no longer available for 6 participants. An additional 2 women with a GSTM1+/GSTT1-null (vs. GSTM1-null/GSTT1+) genotype, who were recruited for their more rare cytochrome P450 1A2 (CYP1A2) genotype related to other aims of the parent study, were also excluded. Thus, 63 (32 men and 31 women) participants were included in the final IL-6/CRP analyses. Forty-nine participants completed all 4 diet periods; 9 completed 3 and 5 completed 2 diets. Data for all completed diet periods were included in the analysis. Archived samples were no longer available for an additional 9 participants for IL-8, TNF-α, and soluble TNF receptor (sTNFR) I and II assays. The 2 women with the GSTM1+/GSTT1-null genotype were included in overall diet analyses for IL-8, TNF-α, and sTNFRI and II to increase sample size, providing a total of 56 participants (26 men and 30 women), but they were not included in genotype contrasts.
Study diets.
Participants consumed the following 4 different diets with the amount of vegetables provided determined according to body weight (BW) to minimize confounding by BW between sexes: 1) a basal fruit- and vegetable-free diet; 2) a basal diet supplemented with 7 g/(kg BW · d) cruciferous vegetables [a mixture of minimally cooked broccoli and cauliflower (<3 min), cabbage, and radish sprouts; 1xC], 3) a basal diet supplemented with 14 g/(kg BW · d) cruciferous vegetables (2xC), and 4) a basal diet supplemented with 7 g/(kg BW · d) cruciferous vegetables plus 4 g/(kg BW · d) apiaceous vegetables (a mixture of raw carrots, celery, dill weed, parsley, and parsnips; 1xC+A). Each of the 4 diet periods lasted 14 d with a 21-d washout between diet periods. Further study diet details were published elsewhere (24).
Specimen collection and analyses.
Biologic samples were collected at baseline and during each 2-wk feeding period at days 0 and 14 in the morning after a 12-h minimum overnight fast (24). Tubes without additive were allowed to clot at room temperature for 30 min before they were centrifuged to separate the serum. Serum was separated into aliquots and stored at −80°C. Buccal cells, collected before random assignment, were isolated and DNA was extracted for determination of GSTM1/GSTT1 genotype and participant eligibility (21).
On day 13 of each feeding period, participants collected urine for 24 h. All urine samples were refrigerated at 4°C until delivery in the morning (day 14) to the FHCRC. The total volume and pH value were recorded and the sample was placed in aliquots and stored at −80°C. These urine samples were used to measure isothiocyanates for compliance (25).
All inflammation assays were performed on never-thawed samples with the exception of CRP and sTNFRs. These proteins are stable through freeze-thaw cycles and were measured in samples that had been thawed once (26). There were no observations in the present analysis that were below the limit of detection for any markers. Assays for all inflammation markers were described elsewhere (27). All samples from the same individual for all diet periods were analyzed on the same plate in duplicate, with kits having the same lot numbers. Intra- and interassay CVs for IL-6, CRP, IL-8, TNF-α, and sTNFRI and II were 5.1% and 2.9%, 5.9% and 3.1%, 6.3% and 15.3%, 9.1% and 16.9%, 2.3% and 11%, and 2.1% and 6%, respectively.
Statistical analysis.
All inflammatory biomarkers were log-transformed to improve the normality of the distributions before further analysis. Accordingly, geometric means with 95% CIs are presented for these markers. Comparisons across genotypes were performed for descriptive purposes by using chi-square (categorical variables) and ANOVA (continuous variables) to determine significant differences. Generalized estimating equations (GEEs), which account for correlated data due to repeated measures, were used to assess the effects of vegetable diets on surrogate markers of inflammation. Analyses were adjusted a priori for day 0 serum inflammatory biomarker concentrations for each diet period and for genotype. Sex, age, and BW category (BW divided into 4 categories in 15-kg increments) were other covariates adjusted in the model. We also evaluated the effect of diet order (i.e., the order in which participants received the 4 controlled diets), but because it did not affect the point estimates, we did not include diet order in the models. Responses to vegetable-containing diet treatments were compared with the basal diet. The same model was used to evaluate the association between total isothiocyanate excretion as a continuous variable and biomarkers of inflammation. Because isothiocyanate excretion is a continuous variable, β-coefficients and corresponding 95% CIs are presented. Because the serum inflammation biomarkers are influenced by minor infections (e.g., colds) and other illnesses (28, 29), we used daily logs completed by the participants to track possible illnesses; corresponding serum samples taken during any reported illnesses were not included in the present analyses to account for acute inflammatory conditions that affect serum inflammatory biomarkers (a total of 2 participants, both at day 0 time points). These time points were classified as missing data. Similarly, any other time points that were 1.5 IQRs below the first quartile or above the third quartile were also entered as missing data (a total of 6 samples). GEE analyses using the available data were applied to handle the missing data. In the secondary analysis, the GEE analysis referenced above was performed within each subgroup, stratified by GST genotype. The 2-sided P value for significance was set at <0.05. Analyses were performed by using Stata statistical software (version 10.0; StataCorp).
Results
Table 1 presents demographic and baseline characteristics of the participants by GSTM1 and GSTT1 genotypes. Baseline serum IL-6 concentrations were significantly lower among individuals with GSTM1-null and GSTT1+ genotypes than in those with GSTM1+ and GSTT1+ genotypes.
TABLE 1.
Characteristics of study participants by GSTM1 and GSTT1 genotype1
| GSTM1+ and GSTT1+ (n = 24) | GSTM1-null and GSTT1+ (n = 28) | GSTM1-null and GSTT1-null (n = 11) | P2 | |
| Age, y | 30.8 ± 6.7 | 30.3 ± 6.1 | 28.9 ± 4.5 | 0.69 |
| Height, cm | 169 ± 10.0 | 172 ± 9.3 | 167 ± 9.3 | 0.19 |
| Weight, kg | 71 ± 16.2 | 70 ± 13.5 | 70 ± 12.9 | 0.89 |
| BMI, kg/m2 | 24.7 ± 3.6 | 23.4 ± 2.8 | 24.7 ± 3.9 | 0.26 |
| Females, % | 50 | 43 | 64 | 0.03 |
| Race, % | 0.002 | |||
| Caucasian | 63 | 61 | 46 | |
| Asian | 25 | 32 | 36 | |
| Other3 | 13 | 7 | 18 | |
| Baseline serum inflammatory biomarker concentrations4 | ||||
| CRP, mg/L | 0.6 (0.4, 1.0) | 0.4 (0.3, 0.6) | 0.5 (0.2, 0.8) | 0.23 |
| IL-6, ng/L | 1.2 (1.0, 1.5) | 0.9 (0.8, 1.0) | 1.0 (0.6, 1.3) | 0.04 |
| IL-8,5 ng/L | 3.9 (2.7, 4.0) | 3.4 (2.9, 5.2) | 2.8 (2.2, 3.7) | 0.57 |
| TNF-α,5 ng/L | 3.0 (2.3, 4.2) | 4.8 (3.6, 6.2) | 1.6 (1.5, 4.7) | 0.06 |
| sTNFRI,5 μg/L | 0.95 (0.84, 1.08) | 0.97 (0.82, 1.14) | 0.92 (0.79, 1.06) | 0.94 |
| sTNFRII,5 μg/L | 3.89 (3.49, 4.33) | 4.30 (3.95, 4.69) | 4.23 (3.78, 4.74) | 0.43 |
Values are means ± SDs unless otherwise indicated; n = 63. CRP, C-reactive protein; GST, glutathione S-transferase; sTNFR, soluble TNF receptor.
Tests for significant differences across genotypes: chi-square for categorical variables, ANOVA for continuous variables.
Other category includes unknown/refused (n = 1), mixed race (n = 2), African American (n = 3), American Indian (n = 1), and Pacific Islander (n = 2).
Values are geometric means (95% CIs) based on a composite of 4 day 0 values.
n = 22, 24, and 8 for GSTM1+ and GSTT1+, GSTM1-null and GSTT1+, and GSTM1-null and GSTT1-null, respectively.
Diet effects.
Overall, geometric mean serum IL-6 concentrations were significantly lower on day 14 of both 2xC and 1xC+A diet treatments than with the basal diet (Table 2). Geometric mean IL-8 concentrations were significantly higher on day 14 of the 1xC+A diet treatment than with the basal diet.
TABLE 2.
Effects of cruciferous and apiaceous vegetable diets on serum inflammatory biomarker concentrations in 63 healthy adults at the final day (day 14) of each diet period1
| Di et |
|||||
| Inflammatory marker | n | Basal | 1xC | 2xC | 1xC+A |
| CRP, mg/L | 63 | 0.43 (0.33, 0.54) | 0.53 (0.42, 0.68) | 0.41 (0.33, 0.53) | 0.35 (0.28, 0.45) |
| Change, % | — | 18.9 (−3.0, 63.0) | −4.9 (−25.0, 26.4) | −22.9 (−36.1, 7.5) | |
| IL-6, ng/L | 63 | 1.12 (0.98, 1.28) | 1.00 (0.87, 1.15) | 0.94 (0.82, 1.07)* | 0.93 (0.81, 1.07)* |
| Change, % | — | −12.0 (−25.3, 7.0) | −19.1 (−30.1, -0.1) | −20.4 (−31.0, −0.7) | |
| IL-8, ng/L | 56 | 2.94 (2.62, 3.29) | 3.21 (2.87, 3.59) | 3.28 (2.93, 3.66) | 3.49 (3.12, 3.90)* |
| Change, % | 8.4 (−4.2, 24.6) | 11.6 (−2.2, 27.0) | 15.8 (4.2, 35.2) | ||
| TNF-α, ng/L | 56 | 3.24 (2.89, 3.64) | 3.62 (3.20, 4.10) | 3.67 (3.28, 4.11) | 3.47 (3.08, 3.91) |
| Change, % | — | 10.4 (−5.1, 31.5) | 11.7 (−3.1, 32.0) | 6.6 (−8.8, 25.4) | |
| sTNFRI, μg/L | 56 | 0.93 (0.90, 0.96) | 0.96 (0.93, 0.99) | 0.92 (0.89, 0.95) | 0.94 (0.91, 0.97) |
| Change, % | — | 2.7 (−1.7, 7.4) | −1.8 (−6.1, 2.5) | 0.8 (−3.6, 5.3) | |
| sTNFRII, μg/L | 56 | 4.17 (4.02, 4.34) | 4.28 (4.12, 4.45) | 4.07 (3.92, 4.23) | 4.17 (4.00, 4.33) |
| Change, % | — | 2.6 (−3.0, 8.0) | −2.3 (−7.6, 2.9) | 0 (−5.6, 5.3) | |
Values are geometric means (95% CIs) adjusted for day 0 values, sex, age, body weight and GST genotype. The basal diet was devoid of fruits and vegetables. *Significantly different from basal, P < 0.05. CRP, C-reactive protein; GST, glutathione S-transferase; sTNFR, soluble TNF receptor; 1xC, single-dose cruciferous vegetable diet [7 g/(kg body weight · d)]; 1xC+A, single-dose cruciferous plus apiaceous vegetable diet [7 and 4 g/(kg body weight · d), respectively]; 2xC, double-dose cruciferous vegetable diet [14 g/(kg body weight · d)].
GSTM1/GSTT1 genotype effects.
There was a significant overall main effect of GST genotype (P = 0.03), with individuals with the combined GSTM1-null and GSTT1+ genotypes having lower IL-6 concentrations than GSTM1+ and GSTT1+ individuals, the referent group.
Table 3 shows the effects of the vegetable diet treatments on serum inflammatory biomarker concentrations at the final day (day 14) of the study, stratified by GST genotype. When looking at response to diet treatment within genotype, with the 1xC diet geometric mean IL-6 concentrations were significantly higher among individuals who were both GSTM1-null and GSTT1-null but tended to be lower among GSTM1-null and GSTT1+ individuals than with those consuming the basal diet (P < 0.08). Geometric mean CRP and sTNFRII concentrations were also significantly higher among GSTM1-null and GSTT1-null individuals. These higher inflammatory concentrations among individuals with a double-null genotype were driven largely by lower circulating concentrations during the basal diet, to which comparisons were made.
TABLE 3.
Effects of cruciferous and apiaceous vegetable diets on serum inflammatory biomarker concentrations in 63 healthy adults at the final day (day 14) stratified by GSTM1 and GSTT1 genotype1
| Diet |
|||||
| GSTM1 and GSTT1 genotype | n | Basal | 1xC | 2xC | 1xC+A |
| CRP, mg/L | |||||
| GSTM1+, GSTT1+ | 24 | 0.48 (0.33, 0.71) | 0.62 (0.42, 0.90) | 0.48 (0.32, 0.71) | 0.50 (0.34, 0.73) |
| Change, % | 22.6 (−9.9, 113.6) | 0 (−35.6, 54.3) | 4.0 (−28.2, 70.9) | ||
| GSTM1-null, GSTT1+ | 28 | 0.45 (0.31, 0.63) | 0.46 (0.32, 0.65) | 0.39 (0.27, 0.55) | 0.28 (0.20, 0.40)* |
| Change, % | 1.3 (−30.6, 54.3) | −15.4 (−41.9, 27.7) | −37.8 (−58.0, −7.4) | ||
| GSTM1-null, GSTT1-null | 11 | 0.27 (0.15, 0.49) | 0.58 (0.33, 1.03)* | 0.35 (0.19, 0.64) | 0.31 (0.17, 0.55) |
| Change, % | 53.4 (5.6, 178.8) | 22.8 (−34.4, 74.9) | 12.9 (−38.2, 57.6) | ||
| IL-6, ng/L | |||||
| GSTM1+, GSTT1+ | 24 | 1.25 (1.00, 1.55) | 1.15 (0.92, 1.43) | 1.06 (0.85, 1.32) | 1.11 (0.89, 1.39) |
| Change, % | −8.7 (−32.6, 23.1) | −17.9 (−37.7, 13.6) | −12.6 (−35.2, 20.3) | ||
| GSTM1-null, GSTT1+ | 28 | 1.16 (0.95, 1.41) | 0.89 (0.73, 1.09) | 0.87 (0.71, 0.95)* | 0.78 (0.64, 0.95)* |
| Change, % | −30.3 (−41.7, 2.0) | −33.2 (−43.0, −1.4) | −48.6 (−49.6, −12.0) | ||
| GSTM1-null, GSTT1-null | 11 | 0.79 (0.56, 1.11) | 1.01 (0.74, 1.40)* | 0.89 (0.64, 1.25) | 1.01 (0.72, 1.42) |
| Change, % | 21.8 (1.0, 73.0) | 11.2 (−12.0, 51.2) | 21.8 (−0.1, 72.6) | ||
| IL-8, ng/L | |||||
| GSTM1+, GSTT1+ | 22 | 2.82 (2.38, 3.35) | 3.24 (2.72, 3.87) | 3.72 (3.12, 4.45)* | 3.32 (2.79, 3.96) |
| Change, % | 13.0 (−8.9, 30.9) | 24.2 (7.8, 54.9) | 15.0 (−7.1, 33.2) | ||
| GSTM1-null, GSTT1+ | 24 | 2.98 (2.51, 3.54) | 3.29 (2.77, 3.90) | 3.02 (2.56, 3.58) | 3.56 (3.01, 4.25)* |
| Change, % | 9.4 (−0.1, 47.8) | 1.3 (−9.9, 33.7) | 16.3 (6.7, 57.7) | ||
| GSTM1-null, GSTT1-null | 8 | 3.15 (2.27, 4.35) | 2.94 (2.18, 4.01) | 3.15 (2.36, 4.20) | 3.32 (2.45, 4.52) |
| Change, % | −7.1 (−31.5, 24.1) | 0 (−25.7, 33.9) | 5.2 (−29.2, 25.7) | ||
| TNF-α, ng/L | |||||
| GSTM1+, GSTT1+ | 22 | 3.32 (2.78, 3.98) | 3.56 (2.95, 4.33) | 3.63 (3.05, 4.34) | 3.24 (2.69, 3.90) |
| Change, % | 6.8 (−13.3, 39.7) | 8.5 (−10.1, 42.1) | −2.4 (−23.2, 22.6) | ||
| GSTM1-null, GSTT1+ | 24 | 3.18 (2.70, 3.76) | 3.74 (3.12, 4.47) | 3.42 (2.87, 4.05) | 3.82 (3.07, 4.55) |
| Change, % | 15.0 (−2.1, 50.1) | 7.0 (−13.0, 31.6) | 16.7 (−1.3, 51.6) | ||
| GSTM1-null, GSTT1-null | 8 | 2.86 (1.97, 4.15) | 3.35 (2.31, 4.87) | 4.57 (3.43, 6.11) | 3.13 (2.30, 4.28) |
| Change, % | 14.7 (−45.4, 109.2) | 37.5 (−26.8, 158.7) | 8.7 (−50.7, 83.5) | ||
| sTNFRI, μg/L | |||||
| GSTM1+, GSTT1+ | 22 | 0.95 (0.90, 1.00) | 0.93 (893,987) | 0.97 (0.93, 1.02) | 0.93 (0.88, 0.98) |
| Change, % | −1.7 (−8.5, 6.4) | 2.2 (−5.6, 9.8) | −2.0 (−9.7, 5.6) | ||
| GSTM1-null, GSTT1+ | 24 | 0.93 (0.88, 0.96) | 0.97 (0.93, 1.02) | 0.85 (0.81, 0.87)* | 0.95 (0.91, 1.00) |
| Change, % | 5.6 (−0.6, 10.9) | −7.5 (−12.7, −2.3) | 3.9 (−2.1, 9.6) | ||
| GSTM1-null, GSTT1-null | 8 | 0.93 (0.85, 1.02) | 0.97 (0.89, 1.06) | 0.93 (0.86, 1.01) | 0.92 (0.84, 0.99) |
| Change, % | 4.0 (−4.1, 14.7) | −0.9 (−9.2, 8.2) | −1.9 (−10.6, 6.0) | ||
| sTNFRII, μg/L | |||||
| GSTM1+, GSTT1+ | 22 | 4.15 (3.88, 4.39) | 4.19 (3.93, 4.43) | 4.19 (3.97, 4.46) | 4.15 (3.87, 4.40) |
| Change, % | 1.0 (−10.8, 12.2) | 1.0 (−9.3, 14.0) | 0 (−12.1, 11.4) | ||
| GSTM1-null, GSTT1+ | 24 | 4.23 (4.02, 4.48) | 4.32 (4.08, 4.58) | 3.94 (3.72, 4.19)* | 4.23 (3.99, 4.49) |
| Change, % | 2.0 (−4.1, 7.0) | −7.3 (−12.4, −2.9) | 0 (−6.3, 4.8) | ||
| GSTM1-null, GSTT1-null | 8 | 4.02 (3.64, 4.49) | 4.45 (4.01, 4.94)* | 4.02 (3.64, 4.41) | 4.06 (3.68, 4.47) |
| Change, % | 9.5 (2.5, 19.7) | 0 (−8.7, 6.4) | 1.0 (−7.8, 7.0) | ||
Values are geometric means (95% CIs) adjusted for day 0 values, sex, age, and body weight. The basal diet was devoid of fruits and vegetables. *Significantly different from basal within genotype, P < 0.05. CRP, C-reactive protein; GST, glutathione S-transferase; sTNFR, soluble TNF receptor; 1xC, single-dose cruciferous vegetable diet [7 g/(kg body weight · d)]; 1xC+A, single-dose cruciferous plus apiaceous vegetable diet [7 and 4 g/(kg body weight · d), respectively]; 2xC, double-dose cruciferous vegetable diet [14 g/(kg body weight · d)].
With the 2xC diet treatment, geometric mean IL-6 and sTNFRI and II concentrations were significantly lower among individuals with a GSTM1-null and GSTT1+ genotype, and IL-8 concentrations were significantly higher among individuals with GSTM1+ and GSTT1+ genotypes. With the 1xC+A diet treatments, geometric mean IL-6 and CRP concentrations were both significantly lower among individuals with GSTM1-null and GSTT1+ genotypes.
The majority of these diet-genotype relations persisted when GSTM1 genotype (i.e., GSTM1+ vs. GSTM1-null, irrespective of GSTT1) was analyzed separately. Geometric mean sTNFRI and II were significantly lower with the 2xC diet treatment [−5.1% (95% CI: −10.1%, −0.9%) and −6.0% (95% CI: −10.5%, −1.5%), respectively] among GSTM1-null individuals. Geometric mean IL-6 and CRP concentrations were significantly lower and IL-8 concentrations higher with the 1xC+A diet treatment [−22.8% (95% CI: −45.5%, −0.2%) for IL-6, −34.3% (95% CI: −66.8%, −1.7%) for CRP, and 22.4% (95% CI: 5.2%, 39.6%) for IL-8; data not shown] among GSTM1-null individuals.
Effects by isothiocyanate excretion.
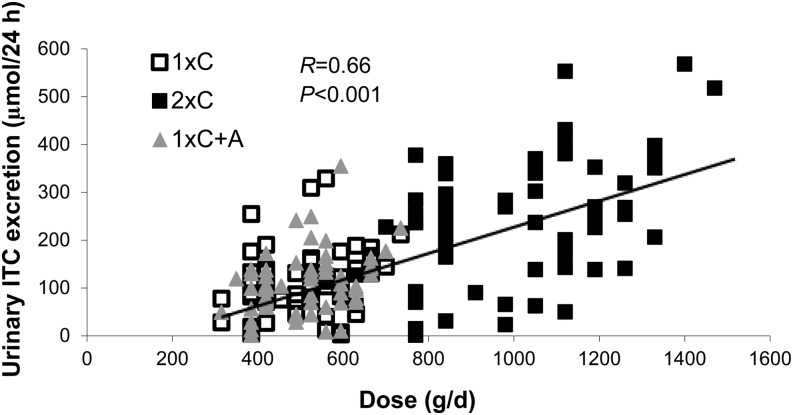
Because individuals metabolize glucosinolates and isothiocyanates differently, actual exposure of these constituents may vary. Thus, we also evaluated the association between urinary excretion of total isothiocyanates on the panel of inflammation biomarkers. Urinary total isothiocyanate excretion on day 14 for all 63 participants on the basis of amount of cruciferous vegetables provided is shown in Figure 1. There were significantly lower concentrations of IL-6 with increasing excretion of total isothiocyanates among individuals with a GSTM1-null/GSTT1-null genotype (β = −0.12; 95% CI: −0.19, −0.5, on a log scale), even though the amount of vegetables consumed among this group was not greater. Mean ± SD cruciferous vegetable amount (g/d) and urinary total isothiocyanate excretion (μmol/24 h) by genotype were as follows: GSTM1+/GSTT1+, 682 ± 291 and 167 ± 111; GSTM1-null/GSTT1+, 661 ± 268 and 140 ± 102; and GSTM1-null/GSTT1-null, 672 ± 262 and 169 ± 122, respectively.
FIGURE 1.
Urinary total ITC excretion on day 14 on the basis of cruciferous vegetable dose in healthy adults (n = 63). Because individuals metabolize glucosinolates and ITCs differently, actual exposure of these constituents may vary. Thus, we evaluated the association between urinary excretion of total ITCs on the panel of surrogate inflammation biomarkers. There were no associations between ITC excretion and any inflammation biomarkers (data not shown). ITC, isothiocyanate; 1xC, single-dose cruciferous vegetable diet [7 g/(kg body weight · d)]; 1xC+A, single-dose cruciferous plus apiaceous vegetable diet [7 and 4 g/(kg body weight · d), respectively] 2xC, double-dose cruciferous vegetable diet [14 g/(kg body weight · d)].
Discussion
In this healthy population, the consumption of diets high in cruciferous and a mixture of cruciferous and apiaceous vegetables reduced serum IL-6 by ∼19% and ∼20%, respectively, compared with a basal diet devoid of fruits and vegetables. Similar reductions were reported with statin therapy among individuals with higher baseline CRP concentrations (e.g., >3.0 mg/L) (30). Other inflammatory markers were not altered significantly or, in the case of IL-8, tended to increase, particularly with the mixture of cruciferous plus apiaceous vegetables, although concentrations remained relatively low compared with other healthy cohorts (31, 32).
Several studies in animal models reported decreased activation of NF-κB–regulated pathways and lower concentrations of many cytokines with administration of isolated isothiocyanates or dried broccoli preparations (7–9, 33, 34). Because the inflammatory markers we chose were all shown to be NF-κB–regulated (4), we hypothesized that a mixed cruciferous vegetable diet would reduce biomarkers of systemic inflammation in humans. Although we observed a reduction in circulating IL-6 concentrations with high cruciferous vegetable intake, we did not see a consistent reduction in the other NF-κB–mediated targets measured.
Overlap with other pro- or anti-inflammatory pathways that may be differentially regulated may have obscured an effect of our dietary protocol on the NF-κB–mediated biomarkers measured and may have contributed to the disparate effects of diet on IL-6 and IL-8. Although both are proinflammatory under the influence of the transcription factor NF-κB, they differ in other aspects of biologic function, tissue expression, and regulation by other mediators and signaling pathways.
Binding of IL-6 to its receptor and signal-transducer gp130 leads to the activation of the Janus kinase/signal transducer and activator of transcription (JAK/STAT) complex, and subsequent expression of various target genes, including IL-6 in a positive forward-feed loop (35). A number of negative regulators, including suppressor of cytokine signaling (SOCS), inhibit STAT signaling to dampen this response. There is evidence that dietary compounds may modulate SOCS family members, apart from the regulation that occurs in the inflammation cascade (36, 37). Although limited, these studies indicate that diet may affect different pathways converging on IL-6 regulation, and that modulation may vary on the basis of dietary constituent and is situation- and cell-type specific. Furthermore, because IL-6 was the only inflammation marker in which a reduction was consistently observed, it is possible that this marker is more responsive than other markers to environmental factors, or that it responds more quickly. These considerations are consistent with the observation that physical activity acutely alters serum IL-6 concentrations more than any other inflammation biomarker (38).
IL-8, a chemotactic factor, was higher after the combination of cruciferous and apiaceous vegetables. Little is known about the effects of diet on IL-8. Although we did not have a specific hypothesis about apiaceous vegetables as we did with cruciferous vegetables, apiaceous vegetables are a rich source of several types of flavonoids and therefore may also have anti-inflammatory effects. For example, psoralens (found in plant foods in the Apiaceae family), like isothiocyanates, were shown to reduce NF-κB activity in vitro (39–41). However, apiaceous family members also contain polyacetylenes, which, in addition to possessing many beneficial properties, are known to induce allergic reactions (42). It may be that some amount of consumption of apiaceous vegetables is beneficial, but the effect is diminished or even reversed above a certain threshold [as in “hormesis” (43)]. In the present study, individuals with the highest IL-8 concentrations were male with higher BWs. Because the amount of vegetables was provided on the basis of weight, we cannot rule out the possibility of a hormetic response. Chronic daily consumption of 4 g of apiaceous vegetables per kilogram of BW (e.g., ∼3 servings/d for a 70-kg person) is a higher intake than what is observed in the general population (<1 serving/d) (44). Another possibility is that the higher amounts of cruciferous vegetables produce transient low levels of oxidative stress through isothiocyanate conjugation and subsequent depletion of intracellular glutathione, and this induced genes responsive to oxidative stress. Because IL-8 expression is enhanced in the presence of reactive oxygen species (45, 46), upregulation through oxidative stress is a plausible explanation.
Given the differential responses of IL-6 and IL-8, and the lack of response in the other biomarkers, our data suggest that the effects of cruciferous and apiaceous vegetables on inflammation may include pathways other than NF-κB. Moreover, although we hypothesized that isothiocyanates might be involved in the purported anti-inflammatory effects of crucifers observed in in vitro and animal studies, these vegetables also contain other phytochemicals that have been associated with decreased inflammation. They are high in dietary fiber, which can be fermented by gut bacteria to yield SCFAs, such as butyrate. Butyrate was shown to inhibit NF-κB translocation (47, 48) and may also contribute to lower systemic inflammation through improved intestinal barrier integrity (49). In addition, fermentable fiber is associated with beneficial shifts in gut microbial composition (50); gut microbes play an important role in immune function through interaction with Toll-like receptors (TLRs) (51). We showed previously that a mean intake of ∼28 g/d of total dietary fiber resulted in significant microbial community differences compared with the basal diet (52). Although participants all received the same gram per kilogram of BW “dose” of cruciferous vegetables, there was substantial variation in the actual exposure to isothiocyanates. This was evident when comparing biomarker concentrations among individuals with different GST genotypes by cruciferous vegetable “dose” vs. by isothiocyanate urinary excretion. Concentrations of several of the inflammatory markers in our panel were reduced to a greater extent among individuals with a GSTM1-null genotype (IL-6 by 30–49%, CRP by 38%, and sTNFRs I and II by ∼7% each) compared with their intact counterparts. However, when looking at isothiocyanate excretion, an inverse association with serum IL-6 was only significant among individuals who were null for both GST genotypes. Consequently, the variation in actual exposure to isothiocyanates may have also obscured our ability to detect an effect of diet on our panel of inflammation markers.
To our knowledge, this is the first controlled intervention study to evaluate the effects of cruciferous vegetables on surrogate biomarkers of systemic inflammation in humans. Jiang et al. (53) recently looked at this relation cross-sectionally in a cohort of ∼1000 middle-aged Chinese women. By using FFQs to estimate intake, the authors reported lower circulating TNF-α, IL-1β, and IL-6 concentrations among those women with higher intakes of cruciferous vegetables. Although this study reported lower concentrations for other inflammatory biomarkers, including TNF-α, which was measured in our panel, differences between extreme quintiles were more modest (∼13%), and IL-8 was not measured. Our results are similar to those of other dietary interventions that yielded inconsistent results across various inflammatory markers (54–56), suggesting that many circulating inflammation markers are modifiable by diet, but the response of the biomarkers to different dietary exposures may be highly variable.
A major strength of this study is the controlled feeding design, with evaluation of a botanical family of vegetables tested in 2 different amounts (Cruciferae) and in combination with another botanical family (Apiaceae). Additional strengths include the a priori selection on the basis of GST genotype and the stringent inclusion and exclusion criteria, which minimized potential confounding due to other factors that may affect inflammatory status (e.g., age, BMI, tobacco or medication use, chronic health conditions). Limitations of the study include a ubiquitous problem in studies of intermediate biology in humans, namely that it is not always possible to monitor the biologic changes in the most appropriate tissues. Future studies should evaluate inflammation status at the tissue level (e.g., within adipose tissue or colon, etc.). In this design, circulating inflammation markers were measured as surrogates of response in tissues. Furthermore, because this study was conducted in a younger, healthy population, baseline concentrations of the inflammation biomarkers were already low, without much potential for further reduction. For example, >95% of the participants had very low concentrations of CRP (<1 mg/L). Finally, although we had a large sample size by controlled feeding standards, we were not sufficiently powered to formally evaluate diet by genotype interactions.
In summary, in this healthy population, there was evidence of an effect of cruciferous and apiaceous vegetables on some circulating inflammatory biomarkers, particularly IL-6, but not others. Reductions in IL-6, CRP, and sTNFRI and II concentrations were most marked among individuals with GSTM1-null genotypes; however, these findings were not consistent with the isothiocyanate excretion data. Our hypothesis was based on a single pathway, i.e., that consumption of cruciferous vegetables attenuates NF-κB activity. This hypothesis is not supported by our data, given the lack of response for most biomarkers. The significance of these relatively modest and varied responses of inflammatory markers in healthy individuals is unknown. Further study is needed to determine whether there is greater potential for reduced systemic inflammation through dietary intervention in populations with higher baseline chronic low-grade inflammation.
Acknowledgments
The authors thank Kara Breymeyer in the Human Nutrition Laboratory at the Fred Hutchinson Cancer Research Center for her dedication to the study. S.L.N., Y.S., X.S., C.-Y.W., S.P.T., and J.W.L. designed the research; S.L.N., Y.S., X.S., C.C., and J.W.L., conducted the research; S.L.N., and C.-Y.W. analyzed the data; S.L.N., A.R.K., M.K., D.L.E., and J.W.L. wrote the manuscript; and S.L.N. and J.W.L. had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: BW, body weight; CRP, C-reactive protein; CYP1A2, cytochrome P450 1A2; FHCRC, Fred Hutchinson Cancer Research Center; GEE, generalized estimating equation; GST, glutathione S-transferase; I-κB, inhibitor of kappa B; JAK/STAT, Janus kinase/signal transducer and activator of transcription; Keap1, Kelch-like ECH-associated protein 1; Nrf2, nuclear transcription factor erythroid-derived related-factor 2-like 2; SOCS, suppressor of cytokine signaling; sTNFR, soluble TNF receptor; TLR, Toll-like receptor; 1xC, single-dose cruciferous vegetable diet; 1xC+A, single-dose cruciferous plus apiaceous vegetable diet; 2xC, double-dose cruciferous vegetable diet.
References
- 1.Talalay P, Fahey JW. Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. J Nutr 2001;131:3027S–33S. [DOI] [PubMed] [Google Scholar]
- 2.Navarro SL, Li F, Lampe JW. Mechanisms of action of isothiocyanates in cancer chemoprevention: an update. Food Funct 2011;2:579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayes JD, Kelleher MO, Eggleston IM. The cancer chemopreventive actions of phytochemicals derived from glucosinolates. Eur J Nutr 2008;47 Suppl 2:73–88. [DOI] [PubMed] [Google Scholar]
- 4.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 1999;18:6853–66. [DOI] [PubMed] [Google Scholar]
- 5.Nair S, Li W, Kong AN. Natural dietary anti-cancer chemopreventive compounds: redox-mediated differential signaling mechanisms in cytoprotection of normal cells versus cytotoxicity in tumor cells. Acta Pharmacol Sin 2007;28:459–72. [DOI] [PubMed] [Google Scholar]
- 6.Clarke JD, Dashwood RH, Ho E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett 2008;269:291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilmore TD, Herscovitch M. Inhibitors of NF-kappaB signaling: 785 and counting. Oncogene 2006;25:6887–99. [DOI] [PubMed] [Google Scholar]
- 8.Youn HS, Kim YS, Park ZY, Kim SY, Choi NY, Joung SM, Seo JA, Lim KM, Kwak MK, Hwang DH, et al. Sulforaphane suppresses oligomerization of TLR4 in a thiol-dependent manner. J Immunol 2010;184:411–9. [DOI] [PubMed] [Google Scholar]
- 9.Wu L, Noyan Ashraf MH, Facci M, Wang R, Paterson PG, Ferrie A, Juurlink BH. Dietary approach to attenuate oxidative stress, hypertension, and inflammation in the cardiovascular system. Proc Natl Acad Sci USA 2004;101:7094–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giacoppo S, Galuppo M, Iori R, De Nicola GR, Cassata G, Bramanti P, Mazzon E. Protective role of (RS)-glucoraphanin bioactivated with myrosinase in an experimental model of multiple sclerosis. CNS Neurosci Ther 2013;19:577–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohshima H, Bartsch H. Chronic infections and inflammatory processes as cancer risk factors: possible role of nitric oxide in carcinogenesis. Mutat Res 1994;305:253–64. [DOI] [PubMed] [Google Scholar]
- 12.Aggarwal BB. Inflammation, a silent killer in cancer is not so silent! Curr Opin Pharmacol 2009;9:347–50. [DOI] [PubMed] [Google Scholar]
- 13.Allin KH, Nordestgaard BG. Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Crit Rev Clin Lab Sci 2011;48:155–70. [DOI] [PubMed] [Google Scholar]
- 14.Aggarwal BB. Targeting inflammation-induced obesity and metabolic diseases by curcumin and other nutraceuticals. Annu Rev Nutr 2010;30:173–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heikkilä K, Ebrahim S, Lawlor DA. Systematic review of the association between circulating interleukin-6 (IL-6) and cancer. Eur J Cancer 2008;44:937–45. [DOI] [PubMed] [Google Scholar]
- 16.Krzystek-Korpacka M, Diakowska D, Kapturkiewicz B, Bebenek M, Gamian A. Profiles of circulating inflammatory cytokines in colorectal cancer (CRC), high cancer risk conditions, and health are distinct: possible implications for CRC screening and surveillance. Cancer Lett 2013;337:107–14. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann TK, Sonkoly E, Homey B, Scheckenbach K, Gwosdz C, Bas M, Chaker A, Schirlau K, Whiteside TL. Aberrant cytokine expression in serum of patients with adenoid cystic carcinoma and squamous cell carcinoma of the head and neck. Head Neck 2007;29:472–8. [DOI] [PubMed] [Google Scholar]
- 18.Il'yasova D, Colbert LH, Harris TB, Newman AB, Bauer DC, Satterfield S, Kritchevsky SB. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev 2005;14:2413–8. [DOI] [PubMed] [Google Scholar]
- 19.International Agency for Research on Cancer. Cruciferous vegetables, isothiocyanates and indoles. Lyon (France): International Agency for Research on Cancer; 2004.
- 20.Kolm RH, Danielson UH, Zhang Y, Talalay P, Mannervik B. Isothiocyanates as substrates for human glutathione transferases: structure-activity studies. Biochem J 1995;311:453–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Navarro SL, Chang J, Peterson S, Chen C, King IB, Schwarz Y, Li SS, Potter JD, Lampe JW. Modulation of human serum glutathione S-transferase-A1/2 concentration by cruciferous vegetables in a controlled feeding study is influenced by GSTM1 and GSTT1 genotypes. Cancer Epidemiol Biomarkers Prev 2009;18:2974–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ketterer B. Dietary isothiocyanates as confounding factors in the molecular epidemiology of colon cancer. Cancer Epidemiol Biomarkers Prev 1998;7:645–6. [PubMed] [Google Scholar]
- 23.Gasper AV, Al-Janobi A, Smith JA, Bacon JR, Fortun P, Atherton C, Taylor MA, Hawkey CJ, Barrett DA, Mithen RF. Glutathione S-transferase M1 polymorphism and metabolism of sulforaphane from standard and high-glucosinolate broccoli. Am J Clin Nutr 2005;82:1283–91. [DOI] [PubMed] [Google Scholar]
- 24.Navarro SL, Peterson S, Chen C, Makar KW, Schwarz Y, King IB, Li SS, Li L, Kestin M, Lampe JW. Cruciferous vegetable feeding alters UGT1A1 activity: diet- and genotype-dependent changes in serum bilirubin in a controlled feeding trial. Cancer Prev Res (Phila) 2009;2:345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang JL, Bigler J, Schwarz Y, Li SS, Li L, King IB, Potter JD, Lampe JW. UGT1A1 polymorphism is associated with serum bilirubin concentrations in a randomized, controlled, fruit and vegetable feeding trial. J Nutr 2007;137:890–7. [DOI] [PubMed] [Google Scholar]
- 26.Aziz N, Fahey JL, Detels R, Butch AW. Analytical performance of a highly sensitive C-reactive protein-based immunoassay and the effects of laboratory variables on levels of protein in blood. Clin Diagn Lab Immunol 2003;10:652–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navarro SL, Brasky TM, Schwarz Y, Song X, Wang CY, Kristal AR, Kratz M, White E, Lampe JW. Reliability of serum biomarkers of inflammation from repeated measures in healthy individuals. Cancer Epidemiol Biomarkers Prev 2012;21:1167–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uwe S. Anti-inflammatory interventions of NF-kappaB signaling: potential applications and risks. Biochem Pharmacol 2008;75:1567–79. [DOI] [PubMed] [Google Scholar]
- 29.Muriel P. NF-kappaB in liver diseases: a target for drug therapy. J Appl Toxicol 2009;29:91–100. [DOI] [PubMed] [Google Scholar]
- 30.Nissen SE, Tuzcu EM, Schoenhagen P, Crowe T, Sasiela WJ, Tsai J, Orazem J, Magorien RD, O'Shaughnessy C, Ganz P, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med 2005;352:29–38. [DOI] [PubMed] [Google Scholar]
- 31.Esser D, Oosterink E, op't Roodt J, Henry RM, Stehouwer CD, Muller M, Afman LA. Vascular and inflammatory high fat meal responses in young healthy men; a discriminative role of IL-8 observed in a randomized trial. PLoS ONE 2013;8:e53474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sofi F, Buccioni A, Cesari F, Gori AM, Minieri S, Mannini L, Casini A, Gensini GF, Abbate R, Antongiovanni M. Effects of a dairy product (pecorino cheese) naturally rich in cis-9, trans-11 conjugated linoleic acid on lipid, inflammatory and haemorheological variables: a dietary intervention study. Nutr Metab Cardiovasc Dis 2010;20:117–24. [DOI] [PubMed] [Google Scholar]
- 33.Heiss E, Herhaus C, Klimo K, Bartsch H, Gerhauser C. Nuclear factor kappa B is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J Biol Chem 2001;276:32008–15. [DOI] [PubMed] [Google Scholar]
- 34.Guerrero-Beltrán CE, Mukhopadhyay P, Horvath B, Rajesh M, Tapia E, Garcia-Torres I, Pedraza-Chaverri J, Pacher P. Sulforaphane, a natural constituent of broccoli, prevents cell death and inflammation in nephropathy. J Nutr Biochem 2012;23:494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang J, Liao X, Agarwal MK, Barnes L, Auron PE, Stark GR. Unphosphorylated STAT3 accumulates in response to IL-6 and activates transcription by binding to NFkappaB. Genes Dev 2007;21:1396–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen CQ, Yu K, Yan QX, Xing CY, Chen Y, Yan Z, Shi YF, Zhao KW, Gao SM. Pure curcumin increases the expression of SOCS1 and SOCS3 in myeloproliferative neoplasms through suppressing class I histone deacetylases. Carcinogenesis 2013;34:1442–9. [DOI] [PubMed] [Google Scholar]
- 37.Park JH, Kim JW, Lee CM, Kim YD, Chung SW, Jung ID, Noh KT, Park JW, Heo DR, Shin YK, et al. Sulforaphane inhibits the Th2 immune response in ovalbumin-induced asthma. BMB Rep 2012;45:311–6. [DOI] [PubMed] [Google Scholar]
- 38.Pledge D, Grosset JF, Onambele-Pearson GL. Is there a morning-to-evening difference in the acute IL-6 and cortisol responses to resistance exercise? Cytokine 2011;55:318–23. [DOI] [PubMed] [Google Scholar]
- 39.Nam NH. Naturally occurring NF-kappaB inhibitors. Mini Rev Med Chem 2006;6:945–51. [DOI] [PubMed] [Google Scholar]
- 40.Kang OH, Lee GH, Choi HJ, Park PS, Chae HS, Jeong SI, Kim YC, Sohn DH, Park H, Lee JH, et al. Ethyl acetate extract from Angelica Dahuricae Radix inhibits lipopolysaccharide-induced production of nitric oxide, prostaglandin E2 and tumor necrosis factor-alphavia mitogen-activated protein kinases and nuclear factor-kappaB in macrophages. Pharmacol Res 2007;55:263–70. [DOI] [PubMed] [Google Scholar]
- 41.Sánchez Ruderisch H, Schwarz C, Shang J, Tebbe B. Trioxsalen in the presence of UVA is able to induce nuclear factor kappa B binding activity in HaCaT keratinocytes. Skin Pharmacol Appl Skin Physiol 2002;15:335–41. [DOI] [PubMed] [Google Scholar]
- 42.Hausen BM, Brohan J, Konig WA, Faasch H, Hahn H, Bruhn G. Allergic and irritant contact dermatitis from falcarinol and didehydrofalcarinol in common ivy (Hedera helix L.). Contact Dermat 1987;17:1–9. [DOI] [PubMed] [Google Scholar]
- 43.Mattson MP. Hormesis defined. Ageing Res Rev 2008;7:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wright ME, Park Y, Subar AF, Freedman ND, Albanes D, Hollenbeck A, Leitzmann MF, Schatzkin A. Intakes of fruit, vegetables, and specific botanical groups in relation to lung cancer risk in the NIH-AARP Diet and Health Study. Am J Epidemiol 2008;168:1024–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res 2008;14:6735–41. [DOI] [PubMed] [Google Scholar]
- 46.Paszti-Gere E, Csibrik-Nemeth E, Szeker K, Csizinszky R, Jakab C, Galfi P. Acute oxidative stress affects IL-8 and TNF-alpha expression in IPEC-J2 porcine epithelial cells. Inflammation 2012;35:994–1004. [DOI] [PubMed] [Google Scholar]
- 47.Usami M, Kishimoto K, Ohata A, Miyoshi M, Aoyama M, Fueda Y, Kotani J. Butyrate and trichostatin A attenuate nuclear factor kappaB activation and tumor necrosis factor alpha secretion and increase prostaglandin E2 secretion in human peripheral blood mononuclear cells. Nutr Res 2008;28:321–8. [DOI] [PubMed] [Google Scholar]
- 48.Place RF, Noonan EJ, Giardina C. HDAC inhibition prevents NF-kappa B activation by suppressing proteasome activity: down-regulation of proteasome subunit expression stabilizes I kappa B alpha. Biochem Pharmacol 2005;70:394–406. [DOI] [PubMed] [Google Scholar]
- 49.Brahe LK, Astrup A, Larsen LH. Is butyrate the link between diet, intestinal microbiota and obesity-related metabolic diseases? Obes Rev 2013;14:950–9. [DOI] [PubMed] [Google Scholar]
- 50.Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009;58:1091–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 2010;328:228–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li F, Hullar MA, Schwarz Y, Lampe JW. Human gut bacterial communities are altered by addition of cruciferous vegetables to a controlled fruit- and vegetable-free diet. J Nutr 2009;139:1685–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang Y, Wu SH, Shu XO, Xiang YB, Ji BT, Milne GL, Cai Q, Zhang X, Gao YT, Zheng W, et al. Cruciferous vegetable intake is inversely correlated with circulating levels of proinflammatory markers in women. J Acad Nutr Diet 2014;114:700–8, e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karlsen A, Paur I, Bohn SK, Sakhi AK, Borge GI, Serafini M, Erlund I, Laake P, Tonstad S, Blomhoff R. Bilberry juice modulates plasma concentration of NF-kappaB related inflammatory markers in subjects at increased risk of CVD. Eur J Nutr 2010;49:345–55. [DOI] [PubMed] [Google Scholar]
- 55.Monagas M, Khan N, Andres-Lacueva C, Casas R, Urpi-Sarda M, Llorach R, Lamuela-Raventos RM, Estruch R. Effect of cocoa powder on the modulation of inflammatory biomarkers in patients at high risk of cardiovascular disease. Am J Clin Nutr 2009;90:1144–50. [DOI] [PubMed] [Google Scholar]
- 56.Hallund J, Tetens I, Bugel S, Tholstrup T, Bruun JM. The effect of a lignan complex isolated from flaxseed on inflammation markers in healthy postmenopausal women. Nutr Metab Cardiovasc Dis 2008;18:497–502. [DOI] [PubMed] [Google Scholar]