Abstract
Background: Surgical site infection (SSI) has been estimated to occur in up to 5% of all procedures, accounting for up to 0.5% of all hospital costs. Bacterial biofilms residing on implanted foreign bodies have been implicated as contributing or causative factors in a wide variety of infectious scenarios, but little consideration has been given to the potential for implanted, submerged suture material to act as a host for biofilm and thus serve as a nidus of infection.
Methods: We report a series of 15 patients who underwent open Roux-en-Y gastric bypass (with musculofascial closure with permanent, multifilament sutures) who developed longstanding and refractory SSIs in the abdominal wall. Explanted suture material at subsequent exploration was examined for biofilm with confocal laser-scanning microscopy (CLSM) and fluorescence in situ hybridization (FISH).
Results: All 15 patients at re-exploration were found to have gross evidence of a “slimy” matrix or dense reactive granulation tissue localized to the implanted sutures. Confocal laser-scanning microscopy revealed abundant biofilm present on all sutures examined; FISH was able to identify the presence of specific pathogens in the biofilm. Complete removal of the foreign bodies (and attendant biofilms) resulted in all cases in cure of the SSI.
Conclusion: Bacterial biofilms on implanted suture material can manifest as persistent surgical site infections that require complete removal of the underlying foreign body substrata for resolution.
Surgical site infections (SSIs) are a frequent complication of operative procedures, occurring in up to 5% of all operations [1], and accounting for up to 0.5% of annual hospital budgetary expenditures [2]; an estimated 750,000 cases annually result in almost four million extra hospital days in some estimations [3]. Surgical site infections can be classified into three categories: Superficial incisional, deep incisional, and organ/space or intracavitary [4]. Superficial incisional SSIs can usually be managed successfully by simple opening and drainage (with local incision care), whereas deep incisional SSIs may necessitate more formal surgical debridement as well as adjuvant antibiotic therapy. Intracavitary SSI will also often require formal surgical intervention.
Although SSIs are a problem encountered commonly, usually acutely or sub-acutely after surgery, in some cases chronic SSI can persist despite multiple interventions, typically manifesting as a chronic non-healing incision, often with drainage. The pathophysiology underlying such persistence is not well understood, but presumably devolves from both host and microbial factors. Surgical site infections can frequently (up to 30%) be “culture-negative” on attempted microbiological evaluation, complicating further their treatment. Multiple reasons have been advanced for such culture-negativity, including use of prior antibiotics, presence of slow-growing or fastidious microorganisms, and the dismissal of extant pathogenic bacteria as “contaminants” [5]. Another underappreciated cause of both culture-negativity and chronic persistent infection is the presence of bacteria in a biofilm configuration.
Bacterial biofilms are communities of microorganisms that are attached to an underlying foreign body or tissue substratum. Biofilms have been demonstrated in association with a wide variety of implanted materials such as neurosurgical catheters [6], heart valves [7], and orthopedic joint prostheses [8,9]. The physiology of bacteria in biofilms differs radically from that of their counterparts in “planktonic” mode, which are found typically in most acute infection scenarios. Biofilm bacteria are more resistant to antibiotics by orders of magnitude, and are difficult to culture using standard techniques. They can persist as a nidus of chronic infection engendering a localized inflammatory response despite medical therapies, and can disperse planktonic showers of microorganisms of infection that can lead to acute exacerbations both locally and (less often) distantly [10]. Although the importance of biofilms in multiple disciplines is recognized, the full relevance of biofilms to surgical incisions and materials is still being explored.
We herein report on a cohort of patients status post-bariatric surgery who suffered from chronic SSIs that appeared to conform to all the characteristics typical of biofilm infections: Chronic localized inflammation typically presenting as a draining sinus, refractory to conventional antibiotic treatment, usually (although not always) culture-negative. We surmised that the root cause of the persistent SSI in these patients was infectious foci in biofilm configuration situated on permanent implanted materials, specifically multifilament polyester sutures, used at the time of musculofascial closure.
Patients and Methods
A series of 15 consecutive patients who presented with chronic non-healing incisions after Roux-en-Y gastric bypass (RYGB) were included in this study. Each patient had undergone closure of the abdominal wall at the musculofascial level with large, interrupted, multifilament polyester sutures at the time of RYGB (chosen for their ability to appose tissues and withstand the substantial distracting forces in these morbidly obese patients). In each case, the patient thereafter developed a chronic draining incision site (or several) that persisted for many months or even years despite multiple treatments. On surgical exploration, in all cases, at least one (but usually multiple) grossly infected polyester sutures were noted and removed, with explanted sutures examined by confocal laser-scanning microscopy (CLSM) and bacterial 16S fluorescence in situ hybridization (FISH) as described below. Ventral hernias were repaired, concomitant panniculectomy was performed, and patients were then followed closely for any complications. Two patients did require subsequent re-exploration; they are discussed at greater length below. Two patients, with major post-RYGB complications beyond a deep incisional SSI, have been described in greater detail [11,12].
Patient histories and clinical reports were reviewed for the following: demographic information; risk factors for SSI present at either time of RYGB or time of panniculectomy; clinical timeline of infection and therapies; antibiotics used to treat the SSIs (unsuccessfully); bacteria cultured ultimately from the SSIs; and operative details and postoperative complications of the panniculectomy and incision exploration procedure. These studies were conducted in accordance with the ethical standards of the Helsinki Declaration of 1975 and with Institutional Review Board approval.
Confocal microscopy, FISH, and detection of biofilm
In addition to standard microbiological culture, specimens from surgery (both suture and tissue) were examined with confocal laser scanning microscopy and FISH as described previously [11] to determine if bacterial biofilms were present. Specimens were examined or fixed the same day as the surgery or no later than the following morning.
To identify live bacteria, we used the BacLight Live/Dead kit (Invitrogen, Carlsbad, CA). The BacLight kit consists of the nucleic acid stains Syto9 (green) and propidium iodide (red). Nuclei in human cells also take up both stains, but virtually all cells rapidly turn red (within 30 min). Human cells are distinguishable readily from bacterial cells by morphology and size. The polyester sutures themselves were imaged using the natural autofluorescence of the material or using reflected 488 nm light. Our technique on fresh specimens using a water immersion microscope objective ensures that a) the samples were never dehydrated, and b) we only observed bacteria attached firmly (unattached planktonic cells were removed by rinsing).
The FISH was performed as described previously [11] using the 16S ribosomal probe: Eub338 5′ – (GCTGCCTCCCGTAGGAGT) – 3′ which stains all bacteria [13] in combination with either the staphylococcus genus probe Sta [5′ – (TCCTCCATATCTCTGCGC) – 3′] [14] or the S. aureus specific 16S ribosomal probe Sau [5′- (GAAGCAAGCTTCTCGTCCG) – 3′] [15]. The probes were labeled with either the green fluorescent dye Cy3 ™ or the red fluorescent dye Cy5 ™ (Integrated DNA Technologies Inc., Coralville, Iowa). We also used the nucleic acid stain propidium iodide (Invitrogen) as a counter-stain to stain all bacteria and the nuclei of human cells.
Results
Characteristics of our patient population are given in Table 1. All patients were female, unsurprising because bariatric surgery (and especially post-bariatric body contouring) remains more popular among females. The majority (9/15) had primary RYGB procedures, but a substantial minority (6/15) had open conversion of vertical banded gastroplasty (VBG) to RYGB. Seven of the 15 had extant hernias at the time of RYGB that were repaired by direct closure with large multi-filament polyester sutures; the remaining eight patients had no hernias at the time of RYGB, but the same polyester sutures were used to close directly the musculofascial incision. Six of these 15 patients had major post-RYGB complications apart from the chronic SSIs that are the subject of this report.
Table 1.
Clinical Characteristics
| Patient Demographics | |
|---|---|
| Mean age, y | 41.1 |
| Female | 15/15 |
| Male | 0/15 |
| Mean BMI prior to RYGB, kg/m2 | 57 (low 34, high 81) |
| Mean BMI at time of panniculectomy, kg/m2 | 33 (low 23, high 46) |
| Open RYGB | 9/15 |
| Open conversion of VBG to RYGB | 6/15 |
| Post-RYGB complications* | 6/15 |
| Hernia repair at time of RYGB | 7/15 |
| Risk Factors for Development of SSI | |
| Diabetes mellitus at time of RYGB | 5/15 |
| Diabetes mellitus at time of panniculectomy | 1/15 |
| Tobacco use at time of RYGB | 11/15 |
| Tobacco use at time of panniculectomy | 6/15 |
| Steroids at time of RYGB | 1/15 |
| Steroids at time of panniculectomy | 1/15 |
| Malnutrition (albumin <3.5 mg/dL) | 9/15 |
| Pre-operative cultivation of Staphylococcus aureus | 2/15 |
| Microorganisms Obtained from Wound Culture | |
| Acinetobacter | 1 |
| S. aureus | 10 |
| MRSA | 19 |
| Klebsiella | 1 |
| Corynebacterium | 6 |
| Pseudomonas aeruginosa | 2 |
| Coagulase-negative Staphylococcus | 5 |
| Streptococcus viridans group | 2 |
| Streptococcus milleri group | 2 |
| Enterococcus | 2 |
| Proteus | 1 |
| Beta-hemolytic Streptococcus | 1 |
| Candida | 1 |
| Operative Treatment | |
| Mean weight of pannus removed, g | 3,168 |
| Estimated blood loss, mL | <100 |
| Fleur-de-lis pattern of repair | 15/15 |
| Herniorrhaphy at time of panniculectomy | 11/15 |
| Foreign body removed (suture) | 15/15 |
| Post-operative Care | |
| Mean length of post-operative follow up | 2.5 y |
| Post-operative drainage | 2/15 |
| Wound dehiscence | 2/15 |
| Fever | 1/15 |
| Oral antibiotic use | 2/15 |
| Surgical revision | 2/15 |
wound dehiscence, anastomotic stricture, gastrocutaneous fistula, intraabdominal abscess.
BMI=body mass index; RYGS=Roux-en-Y gastric bypass; VBG=vertical banded gastroplasty.
We examined whether our patient cohort manifested known risk factors for SSI [16] (both at time of RYGB and at time of panniculectomy/incision exploration) to see if any condition was associated uniformly with the biofilm-based SSIs we observed. A minority of our patients had diabetes mellitus, with only 1/15 remaining diabetic at the time of panniculectomy. Tobacco use was common but not universal; steroid use was rare (1/15). Malnutrition (as inferred by hypoalbuminemia) was also common but not universal, and only 2/15 patients were known to have harbored S. aureus preoperatively. There were several patients who lacked any of the above-described risk factors but still developed the biofilms and chronic SSIs investigated here.
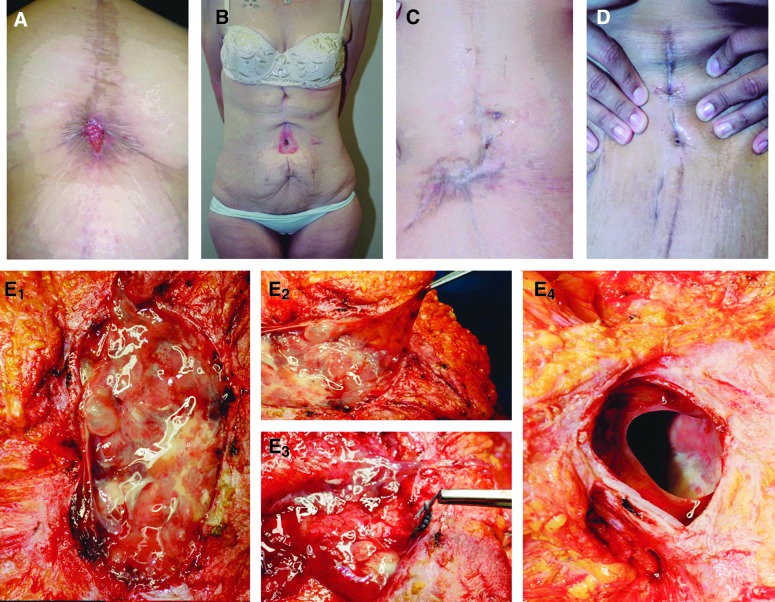
Figure 1 shows pre-operative and intra-operative views of some patients in this series. All patients had open wounds, most associated with drainage. Panels E1 – E4 depict a slimy accumulation surrounding a mass of polyester suture material; such slime is a typical characteristic of biofilms, which are encased in a matrix of viscous “extracellular polymeric substance” (EPS) [10]. On removal of the sutures and associated infected and reactive tissue, a clear abdominal wall defect is revealed, demonstrating the erosive effect of the localized inflammatory process, which appeared to have resulted in a frank hernia.
FIG. 1.
Pre- and intra-operative photographs of patients presenting with chronic surgical site infections after Roux-en-Y gastric bypass. A–D: Pre-operative views of multiple chronic SSIs with varying presentations. Note that the chronic wounds associated with the SSI could appear, among other things, granular (A), excoriated (B), involved with dense reactive scar (C), or unimpressive with just scant drainage (D). E1 – E4: “Slimy” accumulation consistent with biofilm infection situated on a mass of suture material in the abdominal wall. E2 depicts the viscosity of the material. E3 shows that this process is centered on embedded green polyester suture. E4 depicts the full-thickness muscle/fascia defect left after removal (by curettage) of sutures and associated infected tissues. Note the smooth, remodeled edges, indicating that chronic suture-associated SSI can be a cause of hernia formation. Color image is available online at www.liebertpub.com/sur
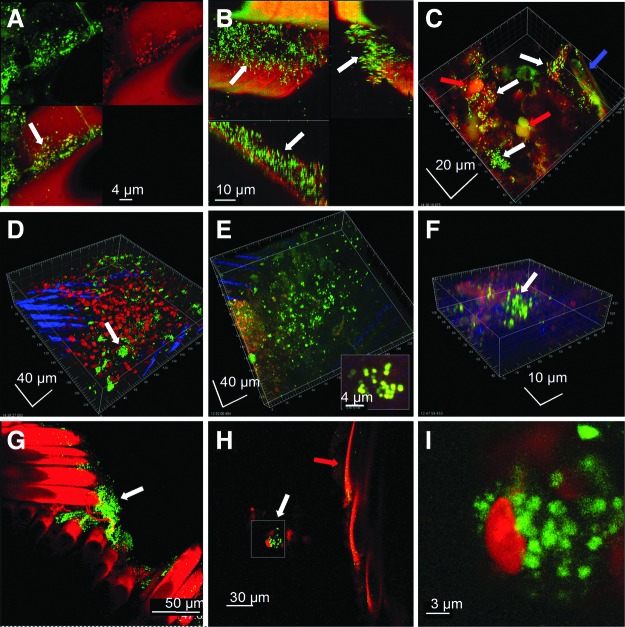
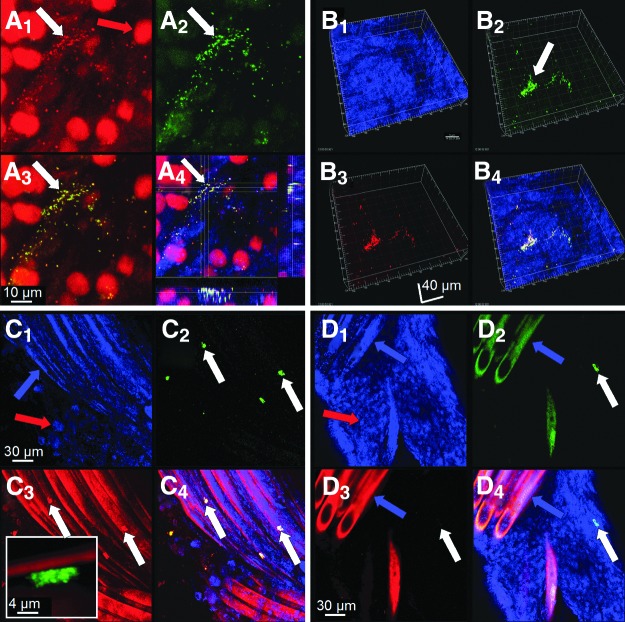
Confocal microscopic examination of the polyester suture material (and the associated reactive tissue) obtained at the time of revisional surgery revealed evidence of live biofilms in all patients examined (Fig. 2). The extent of biofilm formation was variable, in some cases covering millimeters of suture, whereas in others appearing as small clusters of bacteria. Biofilms were attached directly to suture braids (particularly in the crevices made by the interlocking braids), and were also demonstrated on the fibrous sheath and reactive tissues enveloping the sutures. Some of the biofilms were identified morphologically as polymicrobial by the presence of both rods and cocci. Staphylococci were detected specifically in multiple patients that were examined using FISH (Fig. 3), which also demonstrated a polymicrobial infection in one patient by detecting a clump of attached bacteria that hybridized to the eubacterial probe, but failed to hybridize to the S. aureus probe, indicating that another species of bacteria was resident (Fig. 3, panel D). The complete list of microorganisms recovered by culture from these SSIs at any time along their clinical course (which may underappreciate the actual complement of bacteria present) is given in Table 1. We have also examined sutures obtained from several post-RYGB patients undergoing panniculectomy in whom no SSI had occurred; none of these demonstrated any evidence of attached biofilm bacteria (data not shown).
FIG. 2.
Confocal microscopic examination of suture and associated reactive tissue stained with the BacLight Live/Dead kit, showing various aspects of viable bacteria in biofilm configuration (indicated by white arrows). Images shown are representative from five patients. A) Biofilm consisting largely of cocci growing between the suture braids. The upper left panel shows live bacteria (green), and the upper right panel shows dead bacteria (red) and the autofluorescent sutures. The lower left panel is an overlay. B) Cross-section through the braids of a multifilament suture shows that the biofilm bacteria had penetrated the crevice formed by individual braids. The upper left panel is a maximum projection XY “plan” view and saggital sections in the XY and XZ planes are to the right and below respectively. The biofilm was up to 30 microns thick. C) The patient shown in panel B also had clusters of cocci in biofilms attached to the associated reactive tissue that had invested the suture material (blue arrow). The nuclei of host inflammatory cells were also seen (red arrow). D) Clusters of live coccal biofilm attached to the fibrous sheath (the nuclei of host cells are red) which was itself overlying the braids of the sutures (evident as blue striations). E) Extensive coccal biofilm attached to the fibrous sheath investing the suture. Inset is high magnification of a cluster of biofilm cocci. F) Biofilm clusters of live cocci attached to sclerotic fibrous tissue that was in intimate association with the suture. G) Clusters of viable bacteria attached to the suture braids (red). H) In some cases clusters of bacteria (white arrow) were in close vicinity to the sutures (red arrow) but not directly attached. This is consistent with the presence of EPS surrounding the bacterial cluster and attaching it to the suture surface. I) Higher magnification showed that the cluster in panel H was made up of live coccal cells (green) in close proximity to a host cell (red). Some of these bacteria appear to be in the process of division. Color image is available online at www.liebertpub.com/sur
FIG. 3.
A FISH examination of suture and associated reactive tissue from two patients. Specimens were stained with either 1) the general 16S rRNA probe Eub388, or 2) the nucleic acid stain propidium iodide and either the Staphylococcus genus probe (Sta) or the S. aureus specific probe (Sau). A) A1 shows clusters of cocci (white arrow) and the nuclei of host cells (red arrow). A2 shows that the bacteria were staphylococci (green). A3 shows an overlay of the previous panels, and A4 is a plan view and saggital sections through the staphylococcal biofilm cluster attached to fibrous tissue in this patient. In this last image, cytoskeletal elements of host cells were also visualized by staining f-actin blue with phalloidin. B–D) Specimens from a second patient stained for S. aureus (Sau). B1 shows the surface of reactive tissue visualized by reflected light. B2 shows the Eub388 eubacterial probe specifically staining bacteria in a biofilm cell cluster green (white arrow). B3 shows that the biofilm cluster was composed of S. aureus (in this case red). B4 is an overlay; the mixed red and green signals from the doubly-stained organisms appear yellow. C) S. aureus biofilm clusters adherent directly to the braids of a suture. C1 is a reflected image showing the suture braids (blue arrow) and some of the associated host cells (red arrow). C2 shows four clusters of biofilm stained with Eub388 (green). C3 shows that they were S. aureus (red). Inset shows a high magnification image of one cluster of cocci attached directly to the suture. C4 is an overlay. D) Evidence that the infection in this patient was polymicrobial. D1 is imaged using reflected light, showing suture braids and host cells. D2 shows a cluster of biofilm stained with the Eub388 probe (white arrow). However, the same cluster fails to stain with the Sau probe (D3), demonstrating that in addition to S. aureus the patient was infected with another species of bacteria. D4 is an overlay. Color images available online at www.liebertpub.com/sur
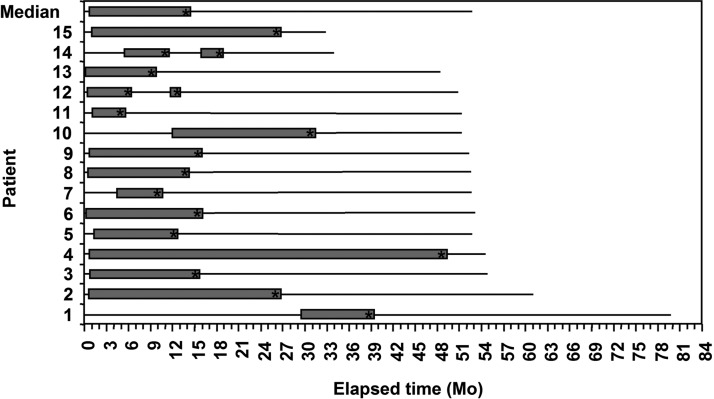
The clinical courses of the SSIs are displayed in Figure 4. Biofilm infections can establish themselves at any point after introduction of a foreign body substratum, but will often arise and persist from an initial acute infectious episode. This pattern fits our patient population, with the vast majority (12/15) first noting their SSI within six months of RYGB. Three patients, however, only presented with draining sinuses 6 mo or more after RYGB, and in one case more than two years after RYGB. Figure 4 demonstrates that 14/15 patients had open SSIs that lasted for more than six months, with 6/15 lasting more than one year. One patient had an open draining wound for almost four years.
FiG. 4.
Patient timelines, from the time of open RYGB surgery to time of writing. Time “0” represents the RYGB surgery at which polyester sutures were placed. The initial solid line represents an initial disease-free condition. The appearance of an open gray bar signifies the presentation of the SSI. The length of the gray bar is the length of persisting SSI. The asterisk (*) indicates the panniculectomy and abdominal wall exploration surgery at which suture material was removed. The solid lines thereafter represent a disease-free condition again. In two patients, (as discussed in Results) two surgical interventions were undertaken, hence the appearance of two gray bars. All patients, after complete removal of suture material, have remained free of SSI.
All of the patients did have some attempt to treat the SSIs undertaken prior to their presentation for plastic surgery. Seven of the 15 were given oral antibiotics, and 10/15 were treated with intravenous antibiotics. Often these interventions were not limited to one instance but were repeated. None succeeded in eradicating the chronic SSI, consistent with the known tendency of biofilm bacteria to be highly resistant to conventional antibiotic therapy. Eight of our 15 patients had also undergone some type of interval attempted debridement of their SSI, usually a limited and localized excision or curetting of affected soft tissue in the office setting, although several did visit the operating room. In all cases, quantities of remaining suture material left behind likely continued to propagate the SSI, ultimately necessitating much more extensive surgical exploration.
The post-operative courses of these patients are also evident in Figure 4. Although most patients healed uneventfully or with minimal transient wound care issues, two of the 15 patients came to a repeat surgical intervention, and their cases are instructive. Both patients presented some months from their initial suture explantation surgery with recurrent abdominal pain. One developed a subcutaneous fluid collection in her scar line that was drained, with no apparent foreign body. She was started on oral antibiotics but had persistent pain. The other complained of abdominal pain with no external findings, and an internal hernia was suspected. Both were ultimately returned to surgery, and in both cases small fragments of remaining suture material were found situated on the rectus fascia. It appeared that small pieces of suture material (<2 cm) that had been part of the intra-abdominal loop of the original tied suture must have emerged from the suture track in the abdominal wall. Since this material was most likely also host to bacterial biofilm, its continuing presence stimulated a recurrent localized infection. After its removal neither patient had further complaint or finding. Both of these revisional cases appear to have been necessitated by persistent retained biofilm-bearing foreign body, in one case with obvious superimposed acute infectious exacerbation, in the other with persistent pain as the only apparent manifestation.
Discussion
Surgical site infections remain an enormous clinical problem; most SSIs are noted in the weeks and months following surgery, that is, in the acute or sub-acute post-operative period, and are acted upon either medically or surgically. Comparatively, less attention has been paid thus far to the subset of infections described in this report that persist chronically despite such interventions.
The role of suture material as a contributing factor to SSI has been the subject of speculation and investigation for more than 30 years; in 1979 Osterberg and Blomstedt noted that “capillary” (multifilament) suture material recovered from S. aureus-infected tissues in a rat model yielded significantly greater bacteria than “non-capillary” suture [17]. They concluded that “in the case of the capillary suture material the bacteria would tend to be protected through their enclosure in the interstices of the material.” Osterberg subsequently examined braided polyester (and twisted polyamide) suture material in a similar model, and noted granulation tissue and an inflammatory infiltrate associated with the braided polyester [18]. Osterberg commented further “bacteria which (sic) are enclosed in the interstices of multifilament suture material, and protected from the phagocytic activity of leukocytes, can sustain and prolong an infection.” These remarkable early observations dovetail exquisitely with the known features of bacterial biofilm formation and persistence, but bacterial biofilms on sutures as a pathogenic entity in patients have thus far been little studied, and then chiefly in the ophthalmologic literature. This report is, to our knowledge, the first to document comprehensively the clinical and microscopic characteristics of a series of patients with suture-associated biofilm infections in the abdominal wall.
A close examination of available clinical evidence regarding the effect of suture material on surgical site infection rates shows that our observations herein are congruent with data reported previously. Van't Riet et al. [19] conducted a meta-analysis of 15 randomized, prospective, controlled trials with at least 100 patients with a follow up of at least one year, comparing different suture materials or suture techniques used in closure of a midline abdominal incision. Although incisional hernia was their primary outcome, surgical site infection, wound dehiscence, and notably suture sinus formation were also scored. Overall, although they found no difference in surgical site infection rates between absorbable and non-absorbable sutures, they did note significantly more suture sinuses when non-absorbable suture was used. Similar results were obtained by Hodgson et al. [20]; they meta-analyzed 13 randomized clinical trials also comparing suture materials or techniques in the closure of various abdominal incisions. They, too, found no difference in the overall incidence of SSI, but also noted significantly more suture sinuses with non-absorbable sutures. Our findings explain these results by demonstrating directly the ability of bacterial biofilms to situate on implanted suture material and cause persistent infection that manifests as a suture sinus. The fact that overall SSI rates were not dissimilar between suture types may be because of the multifactorial nature of SSI, and may also result from the fact that even absorbable sutures may serve as a nidus for infection acutely, with only non-absorbable sutures generating a persistent chronic SSI and suture sinus.
We base our conclusion that these chronic SSIs derived from an underlying biofilm etiology by application of the criteria proposed by Parsek and Singh [21] for use as a clinical diagnostic guide. These are enumerated and explicated as follows: (1) “The infecting bacteria were adherent to some substratum or are surface-associated.” On both gross physical examination at surgery and on microscopic examination the infectious foci were adherent to suture material and/or its investing tissue surface. (2) “Direct examination of infected tissue shows bacteria living in cell clusters, or microcolonies, encased in an extracellular matrix. The matrix may often be composed of bacterial and host components.” Our confocal microscopy and FISH results demonstrate exactly this. Figure 2H is a particularly good example: A cluster of microbes remains attached to the suture substratum via a matrix of extracellular polymeric substance that is not visualized directly by these dyes, but must exist else the hydrated, unfixed bacteria would simply float away. (3) “The infection is generally confined to a particular location. Although dissemination may occur, it is a secondary phenomenon.” In all cases, the infection remained localized to the adjacent tissues of the abdominal wall. In one early patient, the localized infection actually did progress to a frank gastrocutaneous fistula [12]. (4) “The infection is difficult or impossible to eradicate with antibiotics despite the fact that the responsible organisms are susceptible to killing in the planktonic state.” In our patient series, numerous courses of antibiotics were tried, both oral and intravenous, with little lasting effect, although temporary improvement was sometimes seen. Most often the bacteria recovered (in planktonic form) from these SSIs did show susceptibility on standard microbial culture to antibiotics that had been employed against them (data not shown), but could not be eradicated with antibiotics alone whereas a nidus of persistent biofilm remained.
The clinical course of our patients is paradigmatic for a biofilm infection. In most instances, the biofilms established early, possibly even at the time of RYGB surgery itself, and presented shortly thereafter. However, in several cases, the SSI only declared itself many months after surgery; in these cases, either the biofilms in question had a lengthy initial asymptomatic phase, or they established in a delayed fashion after surgery (e.g., by hematogenous spread from another location). Each of these patterns is consistent with known patterns of biofilm behavior, that is, biofilms can situate at any time in a clinical course, and can have lengthy periods of latency. The two patients who required revisional abdominal wall exploration also demonstrate this last point: Each remained quiescent for months after our initial abdominal wall exploration before again becoming symptomatic as the biofilm infection reasserted itself. It is interesting to note that in one of these patients the only presentation of continued SSI was pain, although perhaps with more time another sinus tract may have appeared. This raises the possibility that patients with unexplained chronic abdominal pain following abdominal surgery may be suffering from unrecognized biofilm-based SSIs. It is also noteworthy that 11 of our 15 patients required herniorrhaphy to correct musculofascial defects at the sites of chronically infected sutures. It appears that a combination of the direct action of biofilm bacteria and the associated inflammatory response can lead to disordered wound healing and frank host tissue destruction (as seen in Fig. 1), suggesting, for the first time, an infectious etiology to the common problem of delayed hernia formation.
The adherence of bacteria to suture surfaces is obviously the first component of biofilm formation, and represents an opportunity for intervention to prevent the establishment of peri-sutural infection, both acute and chronic. One approach that has shown promise is the use of augmented suture materials, typically coated with the antimicrobial agent triclosan. In vitro and in vivo experiments in animal models have demonstrated that coated polyglactin 910 suture (braided) can inhibit the quantity of viable bacteria recovered from inoculated suture material compared to a non-coated control. Edmiston et al. [22] found that triclosan-coated polyglactin 910 suture significantly decreased the amount of S. aureus, S. epidermidis, and E. coli bacteria recovered after brief (up to two minutes) exposure to the microbes, likely signifying a significant decrease in bacterial adherence. Storch et al. [23] tested coated versus non-coated polyglactin 910 suture material in guinea pigs, inoculating each with S. aureus and allowing a 48-h incubation before recovering the suture materials and enumerating colony forming units (CFs) resident thereon. They found that coated suture material yielded an almost 97% reduction in the amount of recovered CFUs, although they did not specifically address possible biofilm formation, which in itself may result in decreased CFUs because of the poor culturability of bacteria in biofilm configuration. Some encouraging clinical results have also been obtained: Justinger et al. [24] compared triclosan-coated polyglactin 910 suture versus uncoated polidioxanone suture use in 2,088 patients undergoing midline laparotomy. With similar patient characteristics in each group, they found a SSI rate of 10.8% when polidioxanone was used, but only 4.9% with the coated polyglactin 910. Similarly, a reduced rate of SSI was found in patients undergoing hepatobiliary surgery utilizing a transverse abdominal incision (9.2% with polidioxanone alone versus 4.3% with triclosan-coated polyglactin 910) [25]. However, other studies exploring antibacterial sutures in other surgical settings have not shown any significant difference [26,27], suggesting these effects may be to some extent procedure-specific.
Our study conclusively demonstrates that suture material can host bacterial biofilms to malign clinical effect, but not all suture material is necessarily equivalent in this regard. Although this report describes a series of bariatric patients with multifilament sutures, we have also observed biofilm formation in the setting of chronic SSI on monofilament polyamide suture (and in non-bariatric patients), indicating that biofilms are capable of forming on multiple chemical suture substrates and do not require (although they may favor) an interstitial niche [28]. We find that chronic surgical site infection can derive from non-absorbable sutures which are as much a permanent implant as any of the larger medical devices typically recognized as being susceptible to biofilm infections, and which should be kept in mind as a source of potential infectious complications. Treatment of foreign body-associated biofilm infections remains problematic, usually requiring the complete removal of the offending foreign body before resolution of infection can be achieved, demonstrated also in these patients. In addition, the reactive soft tissue that can invest the biofilm-bearing suture may itself provide a substrate for biofilm propagation, and should be meticulously removed. It may be that bacterial adherence to and biofilm formation on even absorbable sutures is an important component of acute SSIs that are successfully managed (unlike the patients presented here), with resolution possible because of the dissoluble nature of the absorbable suture material, which functionally removes the substratum on which biofilms may persist with its resorption. More mechanistic studies and a better understanding of the host and microbial factors leading to sutural inoculation, biofilm formation, and surgical site infection will hopefully clarify this issue.
Acknowledgments
We thank Ms. Mary OToole for her assistance in the preparation of this manuscript. We would also like to acknowledge the assistance of Dr. Donald Moorman, and the support of the Allegheny-Singer Research Institute, the Allegheny General Hospital Department of Surgery, and grant support from NIH (DE014780- SK).
Author Disclosure Statement
The authors declare that they have no competing financial interests. Funding in support of this work was received in part from the National Institutes of Health.
References
- 1.Cheadle WG. Risk factors for surgical site infection. Surg Infect (Larchmt) 2006;7Suppl 1:S7–S11 [DOI] [PubMed] [Google Scholar]
- 2.Fry DE. The economic costs of surgical site infection. Surg Infect (Larchmt) 2002;3Suppl 1:S37–S43 [DOI] [PubMed] [Google Scholar]
- 3.Edmiston CE, Seabrook GR, Goheen MP, et al. Bacterial adherence to surgical sutures: Can antibacterial-coated sutures reduce the risk of microbial contamination? J Am Coll Surg 2006;203:481–489 [DOI] [PubMed] [Google Scholar]
- 4.Turina M, Cheadle WG. Management of established surgical site infections. Surg Infect (Larchmt) 2006; 7Suppl 3:S33–S4116895501 [Google Scholar]
- 5.Rasnake MS, Dooley DP. Culture-negative surgical site infections. Surg Infect (Larchmt) 2006;7:555–565 [DOI] [PubMed] [Google Scholar]
- 6.Fux CA, Quigley M, Worel AM, et al. Biofilm-related infections of cerebrospinal fluid shunts. Clin Microbiol Infect 2006;12:331–337 [DOI] [PubMed] [Google Scholar]
- 7.Cook G, Costerton JW, Darouiche RO. Direct confocal microscopy studies of the bacterial colonization in vitro of a silver-coated heart valve sewing cuff. Int J Antimicrob Agents. 2000;13:169–173 [DOI] [PubMed] [Google Scholar]
- 8.Tunney MM, Patrick S, Curran MD, et al. Detection of prosthetic hip infection at revision arthroplasty by immunofluorescence microscopy and PCR amplification of the bacterial 16S rRNA gene. J Clin Microbiol 1999;37:3281–3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stoodley P, Nistico L, Johnson S, et al. Direct demonstration of viable Staphylococcus aureus biofilms in an infected total joint arthroplasty. A case report. J Bone Joint Surg Am 2008;90:1751–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol 2004; 2:95–108 [DOI] [PubMed] [Google Scholar]
- 11.Kathju S, Nistico L, Hall-Stoodley L, et al. Chronic surgical site infection because of suture-associated polymicrobial biofilm. Surg Infect (Larchmt) 2009;10:457–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kathju S, Lasko LA, Nistico L, et al. Cutaneous fistula from the gastric remnant resulting from a chronic suture-associated biofilm infection. Obes Surg 2010;20:251–256 [DOI] [PubMed] [Google Scholar]
- 13.Amann RI, Binder BJ, Olson RJ, et al. Combination of 16 S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol 1990;56:1919–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trebesius K, Leitritz L, Adler K, et al. Culture independent and rapid identification of bacterial pathogens in necrotising fasciitis and streptococcal toxic shock syndrome by fluorescence in situ hybridisation. Med Microbiol Immunol 2000;188:169–175 [DOI] [PubMed] [Google Scholar]
- 15.Kempf VAJ, Trebesius K, Autenrieth IB. Fluorescence in situ hybridization allows rapid identification of microorganisms in blood cultures. J. Clin Microbiol 2000;38:830–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mangram AJ, Horan TC, Pearson ML, et al. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Guideline for Prevention of Surgical Site Infection, 1999. See also Infect Control Hosp Epidemiol 1999;20:250–278; Am J Infect Control 1999;27:97–132. [DOI] [PubMed] [Google Scholar]
- 17.Osterberg B, Blomstedt B. Effect of suture materials on bacterial survival in infected incisions. An experimental study. Acta Chir Scand 1979;145:431–434 [PubMed] [Google Scholar]
- 18.Osterberg B. Influence of capillary multifilament sutures on the antibacterial action of inflammatory cells in infected incisions. Acta Chir Scand 1983;149:751–757 [PubMed] [Google Scholar]
- 19.van ‘t Riet M, Steyerberg EW, Nellensteyn J, et al. Meta-analysis of techniques for closure of midline abdominal incisions. Br J Surg 2002;89:1350–1356 [DOI] [PubMed] [Google Scholar]
- 20.Hodgson NC, Malthaner RA, Ostbye T. The search for an ideal method of abdominal fascial closure: a meta-analysis. Ann Surg 2000;231:436–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parsek MR, Singh PK. Bacterial biofilms: an emerging link to disease pathogenesis. Annu Rev Microbiol 2003;57:677–701 [DOI] [PubMed] [Google Scholar]
- 22.Edmiston CE, Seabrook GR, Goheen MP, et al. Bacterial adherence to surgical sutures: can antibacterial-coated sutures reduce the risk of microbial contamination? J Am Coll Surg 2006;203:481–489 [DOI] [PubMed] [Google Scholar]
- 23.Storch ML, Rothenburger SJ, Jacinto G. Experimental efficacy study of coated VICRYL plus antibacterial suture in guinea pigs challenged with Staphylococcus aureus. Surg Infect (Larchmt) 2004;5:281–288 [DOI] [PubMed] [Google Scholar]
- 24.Justinger C, Moussavian MR, Schlueter C, et al. Antibacterial [corrected] coating of abdominal closure sutures and incision infection. Surgery 2009;145:330–334 [DOI] [PubMed] [Google Scholar]
- 25.Justinger C, Schuld J, Sperling J, et al. Triclosan-coated sutures reduce incision infections after hepatobiliary surgery–a prospective non-randomized clinical pathway driven study. Langenbecks Arch Surg 2011;396:845–850 [DOI] [PubMed] [Google Scholar]
- 26.Williams N, Sweetland H, Goyal S, et al. Randomized trial of antimicrobial-coated sutures to prevent surgical site infection after breast cancer surgery. Surg Infect 2011;12:469–474 [DOI] [PubMed] [Google Scholar]
- 27.Chen SY, Chen TM, Dai NT, et al. Do antibacterial-coated sutures reduce incision infection in head and neck cancer reconstruction? Eur J Surg Oncol 2011;37:300–304 [DOI] [PubMed] [Google Scholar]
- 28.Kathju S, Nistico L, Lasko LA, et al. Bacterial biofilm on monofilament suture and porcine xenograft after inguinal herniorrhaphy. FEMS Immunol Med Microbiol 2010;59:405–409 [DOI] [PubMed] [Google Scholar]