Abstract
Secretions of the uterus support survival and growth of the conceptus (embryo/fetus and associated membranes) during pregnancy. Galectin-15, also known as OVGAL11 and a previously uncharacterized member of the galectin family of secreted β-galactoside lectins containing a conserved carbohydrate recognition domain and a separate putative integrin binding domain, was discovered in the uterus of sheep. In endometria of cyclic and pregnant sheep, galectin-15 mRNA was expressed specifically in the endometrial luminal epithelium but not in the conceptus. In pregnant sheep, galectin-15 mRNA expression appeared in the epithelia between days 10 and 12 and increased between days 12 and 16. Progesterone induced and IFN-τ stimulated galectin-15 mRNA in the endometrial epithelium. Galectin-15 protein was concentrated near and on the apical surface of the endometrial luminal epithelia and localized within discrete cytoplasmic crystalline structures of conceptus trophectoderm (Tr). In the uterine lumen, secreted galectin-15 protein increased between days 14 and 16 of pregnancy. Galectin-15 protein was functional in binding lactose and mannose sugars and immunologically identical to the unnamed Mr 14,000 (14K) protein from the ovine uterus that forms crystalline inclusion bodies in endometrial epithelia and conceptus Tr. Based on the functional studies of other galectins, galectin-15 is hypothesized to function extracellularly to regulate Tr migration and adhesion to the endometrial epithelium and intracellularly to regulate Tr cell survival, growth, and differentiation. Galectins may be useful as cellular and molecular markers for endometrial function and receptivity, to enhance conceptus survival and development, and to evaluate and enhance fertility.
Epithelia of the uterine endometrium synthesize and secrete or selectively transport a variety of substances, collectively termed histotroph, necessary for conceptus (embryo/fetus and associated membranes) survival, growth, and implantation (1–5). Histotroph is a rather undefined, complex mixture of transport proteins, ions, cytokines, growth factors, lymphokines, hormones, protease inhibitors, and other molecules (1, 3, 4). In rodents, colony-stimulating factor one, leukemia inhibitory factor, and calcitonin are examples of proteins in histotroph that mediate establishment of uterine receptivity and stimulate conceptus implantation (6–8). In humans, histotroph appears to be the primary source of nutrition for conceptus development during the first trimester, when mechanisms for hematotrophic nutrition are being established (9). Uterine secretions are of particular importance for conceptus survival and growth in domestic animals because of their protracted periimplantation period and noninvasive types of placentation (3, 4, 10). Available evidence from humans, laboratory animals, and domestic animals indicates that a reduction in uterine histotroph compromises periimplantation conceptus survival and growth, which can lead to infertility and intrauterine growth retardation (9, 11, 12). Recent studies correlate inadequate expression of cell-surface and secretory proteins with retarded endometrial differentiation and defective endometrial secretory phases (13–15). Therefore, identification of useful molecular and cellular markers of endometrial function and receptivity to the conceptus are of utmost importance, because they can be used for evaluation, regulation, and enhancement of fertility (15).
To understand the periimplantation pregnancy defect, the uterine gland knockout sheep model (11) was used for a gene expression profiling project using an endometrial cDNA library from the uterus of day-14 pregnant sheep. Interestingly, ≈1% of the 5,000 ESTs sequenced from the cDNA library were highly similar to ovgal11, a previously uncharacterized member of the galectin family of secreted animal lectins (16). OVGAL11 was originally shown to be induced in gastrointestinal tissue and secreted into the intestinal lumen in response to inflammation and eosinophil infiltration after infection of sheep with the helminth Haemonchus contortus (16). The sequence of OVGAL11 protein displayed highest similarity to human galectin-10 (also known as Charcot–Leyden crystal protein) (17, 18) and human galectin-13 (also known as placental protein-13 or PP13) (19). Galectins are proteins with a conserved carbohydrate recognition domain (CRD) that bind β-galactosides, thereby cross-linking glycoproteins as well as glycolipid receptors on the surface of cells and initiating biological responses (20, 21). Functional studies on the extracellular and intracellular roles of galectins have implicated them in cell growth, differentiation, and apoptosis, in addition to cell adhesion, chemoattraction, and migration. Because OVGAL11 from the intestine and endometrium of sheep does not have a known orthologue, it is proposed to be a new family member and renamed galectin-15. Of particular interest is the fact that galectin-15 appears to be the long-sought-after Mr 14,000 (14K) protein from sheep endometrium initially characterized as a progesterone-modulated protein associated with crystalline-inclusion bodies in uterine epithelia and conceptus trophectoderm (Tr) (22).
Methods
Animals. Experimental and surgical procedures on crossbred Suffolk sheep (Ovis aries) complied with the Guide for Care and Use of Agriculture Animals and were approved by the Institutional Animal Care and Use Committees of Texas A&M University.
In study 1, cyclic ewes were mated at estrus (day 0) to either a vasectomized or an intact ram and were hysterectomized (n = 5 sheep per day) on days 10, 12, 14, or 16 of the estrous cycle or on days 10, 12, 14, 16, 18, or 20 of pregnancy (gestation period is 147 days). On days 10–16, the uterine lumen was flushed with saline and examined for the presence of a morphologically normal conceptus to confirm pregnancy. Flushes were not possible on days 18 or 20, because the conceptus firmly adheres to the endometrial luminal epithelium (LE) and basal lamina. Cross sections of the uterine horn ipsilateral to the ovary bearing the corpus luteum were fixed in 4% paraformaldehyde in PBS for 24 h, dehydrated in 70% ethanol, and then embedded in Paraplast-Plus (Oxford Labware, St. Louis). The remaining endometrial tissues were dissected from myometrium and frozen at –80°C. Uterine flushes were clarified by centrifugation (3,000 × g for 30 min at 4°C) and frozen at –80°C.
In study 2, cyclic ewes (n = 20) were checked daily for estrus and then ovariectomized and fitted with indwelling uterine catheters on day 5 as described (23). Sheep were then assigned randomly (n = 5 per treatment) to receive daily i.m. injections of progesterone and/or a progesterone receptor (PR) antagonist (ZK 136,317; Schering) and intrauterine infusions of control serum proteins and/or recombinant ovine IFN (oIFN)-τ protein as follows: (i) 50 mg of progesterone (P, days 5–16) and 200 μg of control (CX) serum proteins (days 11–16) (P+CX); (ii) P and 75 mg of ZK 136,317 (days 11–16) and CX proteins (P+ZK+CX); (iii) P and IFN-τ (2 × 107 antiviral units, days 11–16) (P+IFN); or (iv) P and ZK and IFN-τ (P+ZK+IFN). Steroids were administered daily in corn oil vehicle. Both uterine horns of each ewe received twice-daily injections of either CX proteins (50 μg per horn per injection) or IFN-τ (5 × 106 antiviral units per horn per injection). Recombinant oIFN-τ was produced in Pichia pastoris and purified as described (24). Proteins were prepared for intrauterine injection as described (23). This regimen of progesterone and recombinant oIFN-τ mimics the effects of progesterone and the conceptus on endometrial expression of hormone receptors and IFN-τ -stimulated genes during early pregnancy in ewes (25–27). All ewes were hysterectomized on day 17, and the uterus and endometrium were processed as described in study 1.
In study 3, uterine secretions, termed uterine milk, were collected from the nongravid uterine horn of unilateral pregnant sheep (n = 4) on day 80 of pregnancy by flushing the uterine horn with 100 ml of saline by using methods described initially by Bazer et al. (28). The uterine flushing was clarified by centrifugation and stored at –80°C.
RNA Analysis. Total cellular RNA was isolated from frozen endometrial tissue by using TRIzol reagent (GIBCO/BRL). Steady-state levels of galectin-15 mRNA were assessed in the endometrium from Studies One and Two by slot-blot hybridization as described (29). A radiolabeled antisense cRNA probe was generated from a linearized ovine endometrial galectin-15 cDNA by in vitro transcription with [α-32P]UTP and hybridized with denatured endometrial total RNA (20 μg) from each ewe affixed to a slot-blot membrane. To correct for variation in total RNA loading, a duplicate total endometrial RNA slot-blot membrane was hybridized with a radiolabeled antisense 18S rRNA cRNA (pT718S; Ambion, Austin, TX). After washing, the blots were digested with ribonuclease A. The radioactivity associated with each slot was quantified by using a Typhoon 8600 MultiImager (Molecular Dynamics) and is expressed as relative units.
In Situ Hybridization Analysis. Galectin-15 mRNA was localized in uterine tissue sections (5 μm) by in situ hybridization analysis as described (29). Deparaffinized, rehydrated, and deproteinated uterine tissue sections were hybridized with radiolabeled anti-sense or sense galectin-15 cRNA probes generated by in vitro transcription with 5′-[α-[35S]thio]UTP. After hybridization, washing, and ribonuclease A digestion, slides were dipped in NTB-2 liquid photographic emulsion (Kodak), stored at 4°C for 1 week, and developed in D-19 developer. Sections were then counterstained with Harris-modified hematoxylin (Fisher Scientific), and protected with a coverslip.
Immunohistochemistry. Immunoreactive galectin-15 protein was detected in uterine tissue cross sections from study 1 with rabbit anti-OVGAL11 polyclonal antibody (16) at a 1:2,000 dilution and a Super ABC Rabbit IgG Kit (Biomedia) by using methods described (30). Antigen retrieval was performed by using Pronase E digestion as described (31). Negative controls included substitution of the primary antibody with purified rabbit IgG at the same final concentration.
Western Blot Analyses. Uterine flushes from study 1 were concentrated by using Centricon-3 columns (Amicon). Protein content of concentrated flushes (study 1) and uterine milk (study 3) was determined by using a Bradford protein assay (Bio-Rad) with BSA as the standard. Proteins were denatured and separated by SDS/15% PAGE, and Western blots were conducted as described (30) by using enhanced chemiluminescence detection. Immunoreactive galectin-15 was detected by using the rabbit anti-ovine OVGAL11 antibody (16) at a 1:10,000 dilution. Negative control blots were performed in which primary antibody was replaced by rabbit IgG at the same concentration.
Carbohydrate Binding Analysis. This analysis was a modified version of methods for the in vitro analysis of saccharide binding by galectin-10 (32). All binding experiments were performed in Dulbecco's PBS (Sigma). Briefly, uterine flush proteins from day-16 pregnant ewes (200 μg) were incubated overnight at 4°C with a 50-μl bed volume of lactose-conjugated agarose (Sigma). For competition, lactose, mannose, or fucose (Sigma) was added at a final concentration of 500 mM. After incubation, the resin was washed three times in 1 ml of PBS, and the bound protein was eluted with SDS/PAGE-reducing sample buffer. The relative amount of galectin-15 protein bound to lactose was determined by SDS/15% PAGE and Western blot analysis using the rabbit anti-ovine OVGAL11 antibody.
Immunoprecipitation. Proteins (40 μg) from uterine flushings from day-16 pregnant ewes (study 1) or uterine milk (study 3) were incubated overnight at 4°C with 1 μl of normal rabbit serum (Sigma), rabbit anti-OVGAL11 antibody (16), or rabbit antiovine trophoblast protein 1 (oTP-1/oIFN-τ) (33) (kindly provided by James D. Godkin, University of Tennessee, Knoxville) and 10 μl of protein A/G plus-agarose (Santa Cruz Biotechnology) in immunoprecipitation lysis buffer (30). Proteins bound to the washed beads were separated on an SDS/8–16% PAGE gradient gel (Bio-Rad) and detected by Western blot analysis. Antibodies used in the analyses were rabbit anti-OVGAL11 (1:10,000) or rabbit anti-oTP-1/oIFN-τ (1:1,000). As a positive control, each gel contained 5 μg of total protein from a day-16 pregnant uterine flush and from uterine milk.
Statistical Analyses. Data from slot-blot hybridization and protein slot-blot analyses were subjected to least-squares analysis of variance by using the General Linear Models procedures of the Statistical Analysis System (SAS Institute, Cary, NC). Slot-blot hybridization data were corrected for differences in sample loading by using the 18S rRNA data as a covariate. Data from study 1 were analyzed for effects of day, pregnancy status (cyclic or pregnant), and their interaction. Data from study 2 were analyzed by using preplanned orthogonal contrasts (P+CX versus P+IFN, P+CX versus P+ZK+CX, and P+IFN versus P+ZK+IFN). Data are presented as the least-squares means with overall SE.
Results
During the course of sequencing ESTs from a day-14 pregnant ovine endometrial cDNA library, ≈1% of the 5,000 sequences were determined to be highly similar (95% identity) to OV-GAL11 (GenBank accession no. AF252548) (16). Based on the nucleotide sequence of the coding region (414 nt), ovine endometrial galectin is composed of 137 aa predicted to yield a 15.4-kDa protein with a pI of 5.24. It displayed the greatest similarity/identity to human galectin-13 (≈44% identity) followed by human galectin-10 (≈40% identity) (Fig. 1). Subsequent fasta searches against human, mouse, and rat genome sequences detected no other similar genes or proteins. Given that it has no known orthologue, OVGAL11 from sheep intestine and endometrium is considered a previously uncharacterized galectin family member and named galectin-15.
Fig. 1.
clustalw alignment of the amino acid sequences of ovine galectin-15 with human galectin-10 and -13. The asterisks denote the conserved residues forming the CRD found in prototypical galectin family members. The underlined amino acids indicate a predicted cell attachment sequence that is a conserved integrin binding site.
The amino acid sequence of endometrial galectin-15 was searched for conserved domains, which revealed the presence of a CRD characteristic of galectins (34). The CRD is a consensus motif that consists of 13 aa (35), of which 8 (H-N-R-------V-N------W--E-R) play a critical role in sugar binding (32, 36). Comparison of the galectin-15 CRD with the conserved CRD of other galectins indicates that four residues are identical (V62, N64, W71, and E74) and two are conservatively substituted (R53 and K76). The C57 is different from prototypical galectins but appears to allow for binding of mannose in galectin-10 (37). Consistent with other galectins, galectin-15 had no apparent or predicted signal peptide, transmembrane domain, or glycosylation sites. A prosite search revealed two putative cell attachment sequences at positions 123 (LDV) and 126 (RGD) that are conserved integrin-binding domains (38).
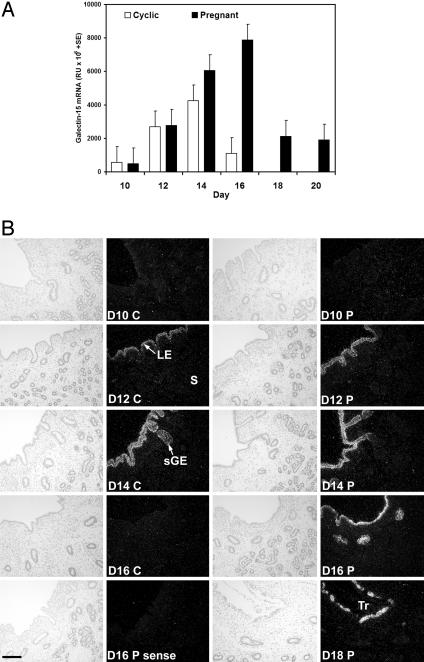
Northern blot analysis of total RNA from endometria of cyclic and pregnant ewes detected a single transcript of ≈0.8 kb (data not shown). Steady-state levels of galectin-15 mRNA were very low on day 10 and then increased ≈13-fold between days 10 and 14 in both cyclic and pregnant ewes (Fig. 2A). However, galectin-15 mRNA levels decreased between days 14 and 16 in cyclic ewes but increased in pregnant ewes (day × status, P < 0.001). Galectin-15 mRNA levels in endometrium of day-16 pregnant ewes were ≈7-fold greater than for day-16 cyclic ewes. In pregnant ewes, galectin-15 mRNA levels declined between days 16 and 18 of pregnancy but remained detectable to at least day 20.
Fig. 2.
Galectin-15 mRNA expression in the uterus (study 1). (A) Steady-state levels of galectin-15 mRNA in the endometrium as determined by slot-blot hybridization analysis. (B) In situ hybridization analysis of galectin-15 mRNA in the uterus. C, cyclic; D, day postestrus/mating; P, pregnant; S, stroma. (Bar = 10 μm.)
Galectin-15 mRNA was specifically expressed in endometrial LE and superficial ductal glandular epithelium (sGE) in both cyclic and pregnant ewes (Fig. 2B). In cyclic ewes, expression of galectin-15 mRNA was low in endometrial LE and sGE of cyclic ewes on day 10, increased to day 14, and then markedly decreased on day 16. Galectin-15 mRNA was not detected in the endometrium on days 1, 3, 5, 7, or 9 of the estrous cycle (data not shown). In pregnant ewes, galectin-15 mRNA was very low or undetectable in endometrial LE and sGE on day 10 and increased between days 10 and 16 (Fig. 2B). Galectin-15 mRNA expression was abundant in endometrial LE and sGE on days 18 and 20 of pregnancy but was not detected in the conceptus. Indeed, the decline in galectin-15 mRNA between days 16 and 20 of pregnancy is most likely due to ablation of LE by the conceptus as binucleate cells form a syncytium with the LE, which occurs as the conceptus elongates and attaches along the length of the uterine horns (39). After syncytium formation with the LE, the binucleate cells migrate and adhere to the epithelial basement membrane.
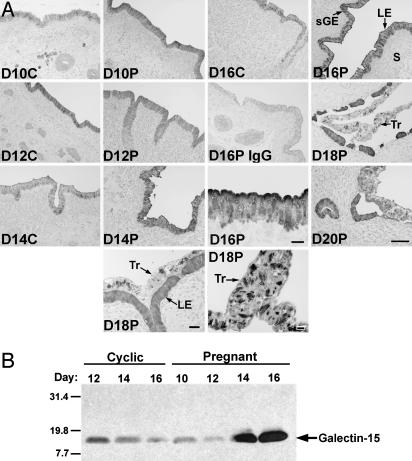
Overall changes in immunoreactive galectin-15 protein abundance in endometrial LE/sGE paralleled changes in galectin-15 mRNA in cyclic and pregnant ewes (Fig. 3A). On days 10 and 12 of the cycle and pregnancy, galectin-15 protein was localized primarily in the cytoplasm of epithelial cells. However, on days 14–20 of pregnancy, galectin-15 protein was readily apparent near and on the apical surface of endometrial LE. Further, galectin-15 protein was localized within discrete cytoplasmic structures in the conceptus Tr on days 16, 18, and 20 of pregnancy.
Fig. 3.
Galectin-15 protein in the endometrium and lumen of the uterus (study 1). (A) Immunohistochemical localization of galectin-15 protein in the endometrium. Sections were not counterstained. (B) Western blot analysis of proteins in the lumen of the uterus. C, cyclic; D, day postestrus/mating; P, pregnant; S, stroma. (Bar = 10 μm.)
Western blot analyses of uterine flushings revealed a single immunoreactive protein of ≈15 kDa (Fig. 3B), which is the predicted size based on the inferred amino acid sequence and similar to OVGAL11 in sheep intestine (16). Galectin-15 protein levels were low in the uterine lumen of cyclic ewes on days 10 and 12 of pregnancy but abundant in the uterine lumen on days 14 and 16, which corresponds to the onset of conceptus implantation and firm adherence of the Tr to LE (39).
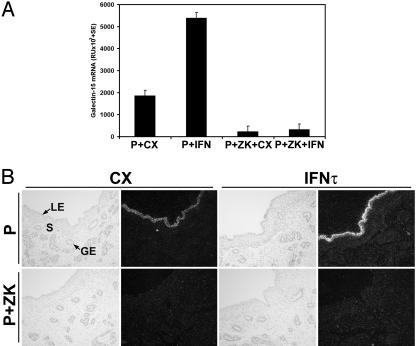
The regulation of galectin-15 gene expression in the endometrium by progesterone and oIFN-τ was determined in study 2 (Fig. 4). In ewes receiving intrauterine injections of CX proteins, progesterone increased galectin-15 mRNA in the endometrium (P+CX vs. P+ZK+CX, P < 0.01). In progesterone-treated ewes, intrauterine injections of recombinant oIFN-τ increased galectin-15 mRNA levels ≈4-fold (P+CX vs. P+IFN, P < 0.03). However, this effect of IFN-τ depended on progesterone (P+ZK+CX vs. P+ZK+IFN, P > 0.10). Galectin-15 mRNA was detected in LE and sGE of endometria from P+CX and P+IFN ewes (Fig. 4B); however, it was not detected in ewes receiving the ZK 136,317 PR antagonist, regardless of intrauterine protein treatment.
Fig. 4.
Effects of progesterone and IFN-τ on galectin-15 mRNA in the uterus (study 2). (A) Steady-state levels of galectin-15 mRNA in the endometrium as determined by slot-blot hybridization analysis. (B) In situ hybridization localization of galectin-15 mRNA in the endometrium. S, stroma. (Bar = 10 μm.)
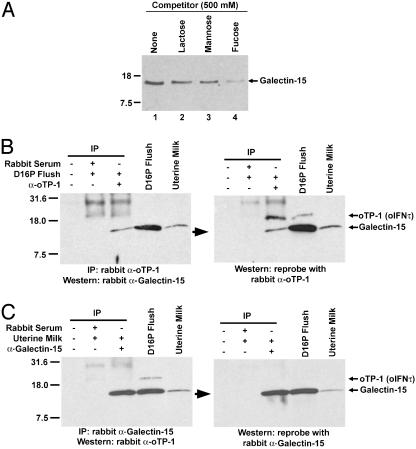
The carbohydrate-binding ability of ovine endometrial galectin-15 was assessed by using lactose-agarose (Fig. 5A). Galectin-15 from day-16 pregnant uterine flushes bound lactose (lane 1), and this binding could be partially blocked by competition with free lactose (lane 2), mannose (lane 3), or fucose (lane 4). Specific binding of galectin-15 from uterine flushes and uterine milk was also detected by using mannose-agarose and fetuinagarose (data not shown).
Fig. 5.
Biochemical characterization of ovine endometrial galectin-15. (A) Carbohydrate-binding analysis of galectin-15 from the uterine lumen of day-16 pregnant ewes by using lactose-agarose and competition with lactose, mannose, or fucose. (B) Immunological identification of galectin-15 from the uterine lumen of day-16 pregnant ewes (study 1) and as the previously uncharacterized 14K protein. (C) Immunological identification of galectin-15 from uterine milk (study 3) as the 14K protein. The anti-oTP-1/oIFN-τ antibody crossreacts with the 14K protein (22), which is ovine endometrial galectin-15.
The homology of galectin-15 to galectin-10, also known as Charcot–Leyden crystal protein in eosinophils and basophils (40), and its apparent molecular weight suggested that galectin-15 may be the 14K endometrial protein initially characterized as a progesterone-modulated, low-molecular-weight protein from the sheep uterus associated with crystalline inclusion bodies in uterine epithelia and conceptus Tr (22). The 14K protein was originally identified as a contaminant of native oTP-1/oIFN-τ protein that was purified from conceptus cultures and used to generate antiserum in rabbits (22). Galectin-15 from uterine flushes of day-16 pregnant ewes and from uterine milk (study 3) was immunoprecipitated with rabbit antiserum to oTP-1/oIFN-τ (Fig. 5 B and C). In the uterine flushing from a day-16 pregnant ewe, the rabbit anti-oTP-1/oIFN-τ antibody recognized two immunoreactive proteins that are oTP-1/oIFN-τ (19 kDa) and galectin-15 (15 kDa). Only galectin-15 is present in uterine milk, because oTP-1/oIFN-τ is produced only by the mononuclear trophectodermal cells of the ovine conceptus between days 10 and 21–25 of gestation.
Discussion
Temporal changes in expression of endometrial galectin-15 mRNA support the hypothesis that ovarian progesterone and conceptus IFN-τ regulate transcription of the galectin-15 gene (Fig. 2 A). The increase in galectin-15 mRNA in LE and sGE after day 10 postestrus/mating is coincident with the disappearance of PR mRNA and protein in the same epithelia (31). Similarly, the decrease in galectin-15 mRNA between days 14 and 16 of the cycle is coincident with the reappearance of PR protein in endometrial LE and a decline in circulating progesterone. Galectin-15 mRNA was induced in endometrial LE and sGE of ovariectomized ewes treated with progesterone for 12 days (Fig. 4), but this induction was prevented by the PR antagonist ZK 136,317. Continuous exposure of the sheep uterus to progesterone for 8–10 days down-regulates PR expression in endometrial LE and sGE but not stroma or myometrium (25). PR is present in the endometrial epithelia of P+ZK-treated sheep (41), because PR antagonists prevent the inhibitory effects of progesterone on the PR gene expression. These results support the hypothesis that progesterone binds to PR, which represses or silences transcription of the galectin-15 gene in the endometrial LE/sGE before day 10. Consequently, the progesterone modulation of galectin-15 mRNA may be attributed, at least in part, to down-regulation of the PR by progesterone that occurs in the LE and sGE between days 10 and 12 of the cycle and pregnancy. Thus, PR loss in the endometrial epithelia may reprogram these cells, allowing them to express genes associated with terminal differentiated function (42).
Galectin-15 is also an IFN-τ-stimulated gene. IFN-τ is the pregnancy recognition hormone in sheep that acts on the endometrium to prevent development of the luteolytic mechanism, thereby maintaining the corpus luteum and production of progesterone (42). The increase in galectin-15 expression in endometrial LE and sGE between days 12 and 16 of early pregnancy parallels the increase in production of IFN-τ by the conceptus, which is maximal between days 14 and 16 (43). Intrauterine administration of IFN-τ increased galectin-15 mRNA, but only in progesterone-treated ewes (Fig. 3). One hypothesis is that IFN-τ can only stimulate transcription of the galectin-15 gene in the absence of repression by liganded PR. Alternatively, the PR-positive stroma may produce a “progestamedin” that is required for the LE to respond to IFN-τ (42). The signaling pathway whereby IFN-τ regulates transcription of the galectin-15 gene is not known, but it certainly does not involve the classical Janus kinase/signal transducer and activator of transcription signaling pathway (26, 44). To date, Wnt7a is the only other gene identified in endometrial LE that is increased by IFN-τ (26).
These studies identified galectin-15 as the previously uncharacterized 14K progesterone-modulated protein from the sheep uterus associated with crystalline inclusion bodies in endometrial LE and conceptus Tr (22). The 14K protein was originally identified as a component of conceptus-conditioned culture medium and uterine flushes (45). Release of the 14K protein was attributed to the cellular breakdown of concepti in culture (22). The 14K protein was confined to the endometrial LE and sGE but absent from the deep GE in the uteri of day-16 pregnant sheep and from sheep treated with progesterone for 14 or 30 days (22). In the uterine epithelia, immunoreactive 14K protein was most strongly detected over crystal-like structures but was also uniformly present over the cytoplasm and nucleoplasm (22). Immunogold electron microscopy revealed that within Tr, the 14K protein was localized to large, membrane-bound rhomboidal or needle-shaped crystal structures but not in the endoplasmic reticulum and Golgi body. Thus, Kazemi and coworkers (22) suggested that the protein was secreted by the endometrial epithelia and taken up by the conceptus from uterine histotroph. Interestingly, development of in vitro-produced bovine blastocysts transferred into the sheep uterus resulted in the presence of crystals in Tr cells (46). The presence of crystals in sheep uterine milk (or histotroph) present between maternal and fetal intercotyledonary membranes is well documented (47, 48). Reports of crystals in sheep Tr and uterine epithelia are numerous (48). Similar progesterone-induced crystal proteins are present in endometrium and conceptus Tr of many mammals, including rabbit, mouse, pig, and human (49–53). Accordingly, galectin-15-related family members are likely to be expressed in the endometrium of many mammals to facilitate conceptus–endometrium interactions. Although the biological role(s) of galectin-15 crystals in the conceptus is not known, the intracellular role of other galectins includes modulation of cell growth, differentiation, and apoptosis through functioning as pre-mRNA splicing factors and interacting with specific intracellular ligands such as Ras and Bcl-2 (53, 54).
Galectin-15 is the newest member of the multifunctional galectin family. Members of this family must fulfill two key criteria: (i) conserved sequence elements forming a CRD, and (ii) binding affinity for β-galactosides without requiring metal ions for activity. These studies indicate that galectin-15 fulfills the two key criteria to be considered a bona fide galectin family member, because it contains the CRD and binds lactose and mannose (Figs. 1 and 5). Although the CRD of galectin-15 differs slightly from that in the prototypical galectins, it does possess the “jellyroll” structural fold similar to that of the prototypical galectin-1 and -2 and found in galectin-10 and -13 (55), which share similarity with galectin-15. Galectins bind β-galactosides by means of the CRD, but the carbohydrate-binding specificity for each galectin appears to be different (56). In addition to the CRD, galectin-15 also contains predicted cell attachment sequences (LDV and RGD) that could mediate binding to integrins in extracellular matrix proteins (57, 58). Integrins are thought to be the dominant glycoproteins that regulate Tr adhesion to endometrial LE during implantation (58, 59). During the periimplantation period of pregnancy in sheep, integrin subunits αv, α4, α5, β1, β3, and β5 are constitutively expressed on conceptus Tr and apical surface of endometrial LE (60). In sheep, receptivity to implantation does not appear to involve changes in temporal or spatial patterns of integrin expression but may depend on changes in expression of extracellular matrix proteins, which are ligands for heterodimers of these integrins. Other galectins bind integrins, fibronectin, and laminin, because these extracellular matrix proteins are modified with β-galactoside sugars (20, 21). Functional studies of other galectins have implicated these proteins in cell growth, differentiation, and apoptosis as well as in cell adhesion, chemoattraction, and migration (20, 61, 62). The temporal and spatial alterations in galectin-15 mRNA and protein in endometrial LE and lumen of the ovine uterus during pregnancy, combined with the functional aspects of galectin-15 and its family members, make it a strong candidate for a mediator of conceptus–endometrium interactions during implantation. Therefore, the proposed extracellular role of galectin-15 in the uterine lumen is to functionally bind and cross-link β-galactosides on glycoproteins, such as integrins, fibronectin, and laminin, and glycolipids, thereby allowing it to function as a heterophilic cell adhesion molecule bridging the endometrial LE and conceptus Tr. The biological responses of the Tr to galectin-15 may also include migration, proliferation, and differentiation, which are critical for successful conceptus implantation. Understanding the extracellular and intracellular functions of galectin-15 in endometrial epithelia and conceptus Tr will broaden our knowledge of the roles of galectins in basic biological processes and facilitate development of therapeutic applications for galectins in infertility and cancer.
Acknowledgments
Research was supported by U.S. Department of Agriculture National Research Initiative Grant 2001-02259, National Institutes of Health R01 Grant HD32534, and National Institute on Environmental Health Sciences Grant 5 P30 ES09106-03.
Abbreviations: 14K protein, Mr 14,000 protein; CRD, carbohydrate-recognition domain; LE, luminal epithelium; oIFN, ovine IFN; sGE, superficial ductal glandular epithelium; oTP-1, ovine trophoblast protein 1 (now IFN-τ); PR, progesterone receptor; Tr, trophectoderm.
References
- 1.Kane, M. T., Morgan, P. M. & Coonan, C. (1997) Hum. Reprod. Update 3, 137–157. [DOI] [PubMed] [Google Scholar]
- 2.Carson, D. D., Bagchi, I., Dey, S. K., Enders, A. C., Fazleabas, A. T., Lessey, B. A. & Yoshinaga, K. (2000) Dev. Biol. 223, 217–237. [DOI] [PubMed] [Google Scholar]
- 3.Bazer, F. W. (1975) J. Anim. Sci. 41, 1376–1382. [DOI] [PubMed] [Google Scholar]
- 4.Roberts, R. M. & Bazer, F. W. (1988) J. Reprod. Fertil. 82, 875–892. [DOI] [PubMed] [Google Scholar]
- 5.Fazleabas, A. T., Donnelly, K. M., Hild-Petito, S., Hausermann, H. M. & Verhage, H. G. (1997) Hum. Reprod. Update 3, 553–559. [DOI] [PubMed] [Google Scholar]
- 6.Stewart, C. L., Kaspar, P., Brunet, L. J., Bhatt, H., Gadi, I., Kontgen, F. & Abbondanzo, S. J. (1992) Nature 359, 76–79. [DOI] [PubMed] [Google Scholar]
- 7.Zhu, L. J., Bagchi, M. K. & Bagchi, I. C. (1998) Endocrinology 139, 330–339. [DOI] [PubMed] [Google Scholar]
- 8.Pollard, J. W., Hunt, J. S., Wiktor-Jedrzejczak, W. & Stanley, E. R. (1991) Dev. Biol. 148, 273–283. [DOI] [PubMed] [Google Scholar]
- 9.Burton, G. J., Watson, A. L., Hempstock, J., Skepper, J. N. & Jauniaux, E. (2002) J. Clin. Endocr. Metab. 87, 2954–2959. [DOI] [PubMed] [Google Scholar]
- 10.Roberts, R. M., Murray, M. K., Burke, M. G., Ketcham, C. M. & Bazer, F. W. (1987) Adv. Exp. Med. Biol. 230, 137–150. [DOI] [PubMed] [Google Scholar]
- 11.Gray, C. A., Burghardt, R. C., Johnson, G. A., Bazer, F. W. & Spencer, T. E. (2002) Reproduction 124, 289–300. [PubMed] [Google Scholar]
- 12.Herrler, A., von Rango, U. & Beier, H. M. (2003) Reprod. Biomed. Online 6, 244–256. [DOI] [PubMed] [Google Scholar]
- 13.Thornburgh, I. & Anderson, M. C. (1997) Histopathology 30, 11–15. [DOI] [PubMed] [Google Scholar]
- 14.Hambartsoumian, E. (1998) Am. J. Reprod. Immunol. 39, 137–143. [DOI] [PubMed] [Google Scholar]
- 15.Westergaard, L. G., Wiberg, N., Andersen, C. Y., Laursen, S. B., Kliem, A., Westergaard, J. G. & Teisner, B. (1998) Hum. Reprod. 13, 2612–2619. [DOI] [PubMed] [Google Scholar]
- 16.Dunphy, J. L., Balic, A., Barcham, G. J., Horvath, A. J., Nash, A. D. & Meeusen, E. N. (2000) J. Biol. Chem. 275, 32106–32113. [DOI] [PubMed] [Google Scholar]
- 17.Weller, P. F., Bach, D. S. & Austen, K. F. (1984) J. Biol. Chem. 259, 15100–15105. [PubMed] [Google Scholar]
- 18.Dvorak, A. M. (1996) Histol. Histopathol. 11, 711–728. [PubMed] [Google Scholar]
- 19.Bohn, H., Kraus, W. & Winckler, W. (1983) Oncodev. Biol. Med. 4, 343–350. [PubMed] [Google Scholar]
- 20.Yang, R. Y. & Liu, F. T. (2003) Cell. Mol. Life Sci. 60, 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooper, D. N. (2002) Biochim. Biophys. Acta 1572, 209–231. [DOI] [PubMed] [Google Scholar]
- 22.Kazemi, M., Amann, J. F., Keisler, D. H., Ing, N. H., Roberts, R. M., Morgan, G. & Wooding, F. B. (1990) Biol. Reprod. 43, 80–96. [DOI] [PubMed] [Google Scholar]
- 23.Spencer, T. E., Gray, A., Johnson, G. A., Taylor, K. M., Gertler, A., Gootwine, E., Ott, T. L. & Bazer, F. W. (1999) Biol. Reprod. 61, 1409–1418. [DOI] [PubMed] [Google Scholar]
- 24.Van Heeke, G., Ott, T. L., Strauss, A., Ammaturo, D. & Bazer, F. W. (1996) J. Interferon Cytokine Res. 16, 119–126. [DOI] [PubMed] [Google Scholar]
- 25.Spencer, T. E., Becker, W. C., George, P., Mirando, M. A., Ogle, T. F. & Bazer, F. W. (1995) Biol. Reprod. 53, 732–745. [DOI] [PubMed] [Google Scholar]
- 26.Kim, S., Choi, Y., Bazer, F. W. & Spencer, T. E. (2003) Endocrinology 144, 5203–5214. [DOI] [PubMed] [Google Scholar]
- 27.Johnson, G. A., Stewart, M. D., Gray, C. A., Choi, Y., Burghardt, R. C., Yu-Lee, L. Y., Bazer, F. W. & Spencer, T. E. (2001) Biol. Reprod. 64, 1392–1399. [DOI] [PubMed] [Google Scholar]
- 28.Bazer, F. W., Roberts, R. M., Basha, S. M., Zavy, M. T., Caton, D. & Barron, D. H. (1979) J. Anim. Sci. 49, 1522–1527. [DOI] [PubMed] [Google Scholar]
- 29.Spencer, T. E., Stagg, A. G., Joyce, M. M., Jenster, G., Wood, C. G., Bazer, F. W., Wiley, A. A. & Bartol, F. F. (1999) Endocrinology 140, 4070–4080. [DOI] [PubMed] [Google Scholar]
- 30.Spencer, T. E., Bartol, F. F., Bazer, F. W., Johnson, G. A. & Joyce, M. M. (1999) Biol. Reprod. 60, 241–250. [DOI] [PubMed] [Google Scholar]
- 31.Spencer, T. E. & Bazer, F. W. (1995) Biol. Reprod. 53, 1527–1543. [DOI] [PubMed] [Google Scholar]
- 32.Dyer, K. D. & Rosenberg, H. F. (1996) Life Sci. 58, 2073–2082. [DOI] [PubMed] [Google Scholar]
- 33.Godkin, J. D., Bazer, F. W. & Roberts, R. M. (1984) Endocrinology 114, 120–130. [DOI] [PubMed] [Google Scholar]
- 34.Hirabayashi, J., Hashidate, T., Nishi, N., Nakamura, T., Hirashima, M., Urashima, T., Oka, T., Futai, M., Muller, W. E., Yagi, F. & Kasai, K. (2002) Biochim. Biophys. Acta 1572, 232–254. [DOI] [PubMed] [Google Scholar]
- 35.Oda, Y., Herrmann, J., Gitt, M. A., Turck, C. W., Burlingame, A. L., Barondes, S. H. & Leffler, H. (1993) J. Biol. Chem. 268, 5929–5939. [PubMed] [Google Scholar]
- 36.Hirabayashi, J. & Kasai, K. (1994) Glycoconj. J. 11, 437–442. [DOI] [PubMed] [Google Scholar]
- 37.Swaminathan, G. J., Leonidas, D. D., Savage, M. P., Ackerman, S. J. & Acharya, K. R. (1999) Biochemistry 38, 13837–13843. [DOI] [PubMed] [Google Scholar]
- 38.Ruoslahti, E. (1996) Annu. Rev. Cell Dev. Biol. 12, 697–715. [DOI] [PubMed] [Google Scholar]
- 39.Guillomot, M. (1995) J. Reprod. Fertil. Suppl. 49, 39–51. [PubMed] [Google Scholar]
- 40.Weller, P. F., Bach, D. & Austen, K. F. (1982) J. Immunol. 128, 1346–1349. [PubMed] [Google Scholar]
- 41.Johnson, G. A., Spencer, T. E., Burghardt, R. C., Taylor, K. M., Gray, C. A. & Bazer, F. W. (2000) Biol. Reprod. 62, 1315–1321. [DOI] [PubMed] [Google Scholar]
- 42.Spencer, T. E., Johnson, G. A., Burghardt, R. C. & Bazer, F. W. (2004) Biol. Reprod., in press. [DOI] [PubMed]
- 43.Farin, C. E., Imakawa, K. & Roberts, R. M. (1989) Mol. Endocrinol. 3, 1099–1107. [DOI] [PubMed] [Google Scholar]
- 44.Choi, Y., Johnson, G. A., Burghardt, R. C., Berghman, L. R., Joyce, M. M., Taylor, K. M., Stewart, M. D., Bazer, F. W. & Spencer, T. E. (2001) Biol. Reprod. 65, 1038–1049. [DOI] [PubMed] [Google Scholar]
- 45.Salamonsen, L. A., Doughton, B. & Findlay, J. K. (1984) in Reproduction in Sheep, eds. Lindsay, D. R. & Pearce, D. T. (Australian Academy of Science, Canberra), pp. 115–121.
- 46.Rexroad, C. E., Jr., & Powell, A. M. (1999) Theriogenology 52, 351–364. [DOI] [PubMed] [Google Scholar]
- 47.Wimsatt, W. A. (1951) J. Anat. 89, 233–282. [DOI] [PubMed] [Google Scholar]
- 48.Hoffman, L. H. & Olson, G. E. (1984) in Ultrastructure of Reproduction, eds. van Blerkom, J. & Motta, P. M. (Martinus Nijhoff, Boston), pp. 235–246.
- 49.Nakao, K., Meyer, C. J. & Noda, Y. (1971) Am. J. Obstet. Gynecol. 111, 1034–1038. [DOI] [PubMed] [Google Scholar]
- 50.Calarco, P. G. & Szollosi, D. (1973) Nat. New Biol. 243, 91–93. [PubMed] [Google Scholar]
- 51.Daniel, J. C., Jr., & Chilton, B. S. (1978) in Development in Mammals, ed. Johnson, H. (North Holland/Elsevier, Amsterdam), Vol. 3, pp. 131–187. [Google Scholar]
- 52.Daniel, J. C., Jr., & Kennedy, J. R. (1978) J. Embryol. Exp. Morphol. 44, 31–43. [PubMed] [Google Scholar]
- 53.Hernandez, J. D. & Baum, L. G. (2002) Glycobiology 12, 127R–136R. [DOI] [PubMed] [Google Scholar]
- 54.Liu, F. T., Patterson, R. J. & Wang, J. L. (2002) Biochim. Biophys. Acta 1572, 263–273. [DOI] [PubMed] [Google Scholar]
- 55.Barondes, S. H., Castronovo, V., Cooper, D. N., Cummings, R. D., Drickamer, K., Feizi, T., Gitt, M. A., Hirabayashi, J., Hughes, C., Kasai, K., et al. (1994) Cell 76, 597–598. [DOI] [PubMed] [Google Scholar]
- 56.Cho, M. & Cummings, R. D. (1995) J. Biol. Chem. 270, 5198–5206. [DOI] [PubMed] [Google Scholar]
- 57.Kimber, S. J. & Spanswick, C. (2000) Semin. Cell Dev. Biol. 11, 77–92. [DOI] [PubMed] [Google Scholar]
- 58.Wang, J. & Armant, D. R. (2002) Cells Tissues Organs 172, 190–201. [DOI] [PubMed] [Google Scholar]
- 59.Burghardt, R. C., Johnson, G. A., Jaeger, L. A., Ka, H., Garlow, J. E., Spencer, T. E. & Bazer, F. W. (2002) Cells Tissues Organs 171, 202–217. [DOI] [PubMed] [Google Scholar]
- 60.Johnson, G. A., Bazer, F. W., Jaeger, L. A., Ka, H., Garlow, J. E., Pfarrer, C., Spencer, T. E. & Burghardt, R. C. (2001) Biol. Reprod. 65, 820–828. [DOI] [PubMed] [Google Scholar]
- 61.Barondes, S. H., Cooper, D. N., Gitt, M. A. & Leffler, H. (1994) J. Biol. Chem. 269, 20807–20810. [PubMed] [Google Scholar]
- 62.Hughes, R. C. (2001) Biochimie 83, 667–676. [DOI] [PubMed] [Google Scholar]