Abstract
Background and Aims
Dietary sodium loading has been shown to adversely impact endothelial function independently of blood pressure (BP). However, it is unknown whether dietary sodium loading impacts endothelial function differently in men as compared to women. The aim of this study was to test the hypothesis that endothelial-dependent dilation (EDD) would be lower in men as compared to women in response to a high sodium diet.
Methods and Results
Thirty subjects (14F, 31±2y; 16M, 29±2y) underwent a randomized, crossover, controlled diet study consisting of 7 days of low sodium (LS; 20 mmol/day) and 7 days of high sodium (HS; 300–350 mmol/day). Salt-resistance was determined by a change in 24-hr mean arterial pressure (MAP)≤ 5 mmHg between HS and LS as assessed on day 7 of each diet. Blood and 24-hr urine were also collected and EDD was assessed by brachial artery flow-mediated dilation(FMD). By design, MAP was not different between LS and HS conditions and urinary sodium excretion increased on HS diet (p<0.01). FMD did not differ between men and women on the LS diet (10.2±0.65 vs. 10.7±0.83; p>0.05) and declined in both men and women on HS (p<0.001). However, FMD was lower in men as compared to women on HS (5.7±0.5 vs. 8.6±0.86; p<0.01).
Conclusions
HS reduced FMD in both men and women. In response to a HS diet, FMD was lower in men compared to women suggesting a greater sensitivity of the vasculature to high sodium in men.
Keywords: sex, sodium, flow-mediated dilation
INTRODUCTION
High dietary sodium consumption is a significant risk factor for the development of hypertension. While many key dietary trials have shown the benefit of sodium reduction on blood pressure (BP) levels (1), more recent work has illustrated the detrimental effects of sodium on the vasculature prior to, or independent of, any change in blood pressure (2–4). Endothelial dysfunction is thought to be an initial event in the development of atherosclerosis and has been associated with an increased risk of cardiovascular disease (5, 6). Brachial artery flow-mediated dilation (FMD) is a non-invasive research tool used to assess endothelial function (7) and has been shown to be useful in predicting future cardiovascular events (8).
Changes in sodium consumption have been shown to alter endothelial-dependent dilation (EDD) in several populations. Reduction of sodium consumption (50 mmol/day) from the typical U.S. intake (150 mmol/day) improved EDD as assessed by FMD in healthy overweight and obese adults with a concurrent reduction in systolic BP (9). Further, a cross-sectional study on middle-aged and older adults with elevated systolic BP who followed a low sodium diet showed an improvement in FMD (10). While these studies examined changes in vascular function, BP was noted to change as well. We recently demonstrated that a high sodium diet can impair FMD in healthy salt-resistant adults independent of BP changes (2). To date, very few studies have studied the impact of salt loading on endothelial function in normotensive salt-resistant adults independent of changes in BP.
While salt loading has been shown to impair FMD, it is not clear if there are sex differences in this response. It is widely known that cardiovascular disease related morbidity and mortality are lower in women than men prior to menopause (11). He et al. (12) reported sex differences in BP in response to a 7-day high salt intervention in which women illustrated a greater BP sensitivity to the salt loading particularly with aging and in the presence of hypertension. Whether these sex differences apply to the vasculature has not been extensively studied. To date, one study has suggested that healthy young males appear to be more sensitive to sodium loading as sex differences were reported in the contribution of nitric oxide to acetylcholine induced vasodilation in the forearm however no functional difference was found(4).
Therefore, the purpose of the current study was to determine if sex differences exist in the endothelial response to dietary sodium loading in young salt-resistant men and women in a randomized crossover controlled feeding study. We hypothesized that men would have a lower FMD in response to a high sodium diet compared to women.
METHODS
Study Population
Forty-three subjects qualified for the study. Five dropped out following the screening, 5 subjects started the diet but dropped out before completion, and 3 were salt sensitive. Therefore, thirty healthy salt-resistant individuals aged 22–44 participated in this study: 14 women and 16 men. Four women and six men participated in a previous study conducted in our laboratory (2); their data were reanalyzed using the methods described in the ‘Statistical analysis' section below to address the novel hypotheses of this study. The study protocol and procedures were approved by the Institutional Review Board of the University of Delaware and conform to the provisions of the Declaration of Helsinki. Informed consent was obtained from all participants prior to enrollment in the study.
Experimental Protocol
Subjects reported to the laboratory for an initial screening visit after a 12-hour fast and completed a medical history form. Aresting 12-lead electrocardiogram, resting BP, and height and weight were determined. A venous blood sample was collected. Because the focus of this study was healthy adults, subjects with a history of cardiovascular disease, hypertension, malignancy, diabetes mellitus, or renal impairment were excluded. Subjects with a BMI of greater than 30 kg/m2, use of tobacco products, and those taking any type of medication were also excluded. Women were required to have a negative pregnancy test but menstrual cycle was not controlled for. Menopausal women were excluded from the study as salt sensitivity of BP increases with menopause and endothelial function declines(13).
Dietary sodium manipulation
This experiment was a controlled feeding study with all food prepared by a registered dietitian. Participants first completed a 7-day run in diet (100 mmol sodium/day; 2300 mg/day) in order to normalize baseline dietary sodium intake. Following this, subjects were randomized to undergo 7 days of a low-sodium (LS) diet (20 mmol sodium/day; 500 mg/day) and 7 days of a high-sodium(14) diet (300–350 mmol sodium/day; ~7,000–8,000 mg/day) with no washout period in between. These sodium intakes were selected in order to allow us to accurately classify adults with salt-resistant BP and are consistent with previously published studies (2, 4). Standardized equations was used to adjust the caloric content of the diet to maintain a constant body weight. In all conditions, dietary potassium intake was held constant and averaged 75.4±1.5 mmol/day. The diet consisted of 50% carbohydrates, 30% fat, and 20% protein. Daily fluid intake was monitored and recorded and subjects were instructed to maintain normal activity levels throughout the study.
During the last 24-hour period of LS and HS diets all urine was collected and analyzed for total volume, urinary electrolytes (Easy-Electrolyte Analyzer; Medica) and urine osmolality (Advanced 3D3 Osmometer; Advanced Instruments). Free water clearance and fractional excretion of sodium and chloride were calculated using standard equations. During the same 24-hour period, subjects wore an ambulatory BP monitor (Spacelabs Medical) on their arm. BP was measured every 20 minutes while the subject was awake and every 30 minutes during sleep. Laboratory BP was also measured by an automated oscillometric sphygmomanometer (Dinamap Dash 2000; GE Medical Systems) during experimental visits.
Hemoglobin (Hb 201+ model; HemoCue), hematocrit (Readacrit Centrifuge; Becton Dickinson), serum electrolytes (EasyElectrolyte Analyzer; Medica), and plasma osmolality(Advanced 3D3 Osmometer; Advanced Instruments) were measured from a venous blood sample obtained during each experimental visit.
Salt resistance classification
Salt resistance was defined as a 5 mmHg or less change in 24-hour MAP determined while on the LS and HS diets(15) and was defined on an individual basis after completion of the entire protocol. Subjects with a greater than 5 mmHg change in MAP were classified as salt sensitive and were excluded from analysis since they could not be used to test the a priori study hypothesis regarding the BP-independent effects of dietary sodium.
Assessment of endothelium-dependent dilation
EDD was assessed by brachial artery FMD according to established guidelines(16). Each subject was assessed on the final day of the LS and HS diet. Participants were supine with the right arm supported at heart level. A BP cuff was placed on the proximal forearm approximately 3 cm below the antecubitalcrease. Longitudinal images of the brachial artery and continuous Doppler blood velocity were obtained using a 12-MHz linear phased array ultrasound transducer (GE P5; GE Healthcare). Baseline images were recorded after 15 minutes of rest. After baseline images and blood velocity were obtained, the cuff was rapidly inflated to 200 mmHg for 5 minutes. Images and blood velocity were recorded throughout the 5-minute inflation period and continued for 2 minutes following cuff release in order to determine peak diameter change and calculation of shear rate.
Ultrasound images were transmitted to a National Instruments IMAQ PCI-1411 image acquisition board at a frequency of 20 frames/s by way of an S-Video connection. Brachial artery diameter was determined using custom-designed automated edge detection software in National Instruments LabVIEW 8.0. Peak diameter was determined after applying a 3-s-wide median filter to each data point. Reproducibility in our lab for this technique is 1.3±1.1 % for baseline diameters, 1.9±1.6 % for peak diameters and 5.47% for percent FMD change (coefficient of variation)(2). FMD was expressed as a % change from baseline and Doppler blood velocity and diameter data were used to calculate shear rate area under the curve from cuff deflation to peak diameter. Shear rate AUC has been shown to best represent the stimulus for dilation (7).
Statistical analysis
The primary outcome was a difference in FMD between men and women on the HS diet. Power calculations were performed to determine the number of subjects needed to detect a 10% difference in FMD. It was determined that a total of 28 subjects or 14 in each group would be needed to provide 90% power with alpha set at 0.05. Demographic data and biochemical measurements were compared using a two-tailed unpaired t-test. Analysis of variance with repeated measures was used to compare biochemical and renal markers across the two dietary interventions as well as vascular measurements. Blood and urine markers were compared using a one-way ANOVA due to missing data. When appropriate, a Tukey’s post hoc test was performed to detect within and between group differences. Significance was set at P<0.05. Data are presented as means ± SE.
RESULTS
Subjects
Thirty participants completed the study with a near equal distribution of men and women. As shown in Table 1, subjects were closely matched for age and were normotensive. Plasma osmolality was slightly lower and serum chloride was higher in women. Diets were designed to be eucaloric however weight did significantly change with both men and women gaining weight on the HS condition. However, this difference was slightly less than 1.5 kilograms and attributed to increases in extracellular volume.
TABLE 1.
Baseline subject characteristics.
| Baseline Characteristic | Men | Women |
|---|---|---|
| Demographic Data | ||
| N (M/W) | 16 | 14 |
| Age (yr) | 29 ± 2 | 31 ± 2 |
| Height (cm) | 178 ± 2 | 167 ± 2* |
| Mass (kg) | 76 ± 3 | 68 ± 2 |
| BMI (kg/m2) | 23.8 ± 0.7 | 24.5 ± 0.7 |
| SBP (mmHg) | 120 ± 3 | 114 ± 3 |
| DBP (mmHg) | 73 ± 2 | 70 ± 3 |
| MAP (mmHg) | 89 ± 2 | 85 ± 3 |
| Heart rate (bpm) | 62 ± 2 | 64 ± 3 |
| Biochemical Parameters | ||
| Hemoglobin (g/dl) | 15.2 ± 0.2 | 12.6 ± 0.3* |
| Hematocrit (%) | 45.1 ± 0.5 | 37.6 ± 0.6* |
| Serum sodium (mmol/L) | 137.2 ± 2.3 | 138.7 ± 0.6 |
| Serum potassium (mmol/L) | 4.5 ± 0.1 | 4.3 ± 0.1 |
| Serum chloride (mmol/L) | 103.5 ± 0.5 | 106.4 ± 0.7* |
| Plasma osmolality (mOsm/kg H2O) | 288.3 ± 0.9 | 284.0 ± 0.7* |
| Serum creatinine (mg/dl) | 1.0 ± 0.0 | 0.8 ± 0.0* |
| Blood urea nitrogen (mg/dl) | 15.5 ± 1.2 | 12.8 ± 0.9 |
| Fasting glucose (mg/dl) | 87.5 ± 1.5 | 87.5 ± 2.6 |
| Fasting total cholesterol (mg/dl) | 176.2 ± 6.8 | 176.5 ± 7.2 |
| Fasting HDL (mg/dl) | 55.9 ± 2.7 | 70.3 ± 5.2* |
| Fasting LDL (mg/dl) | 102.1 ± 5.4 | 90.3 ± 6.5 |
| Fasting triglycerides (mg/dl) | 90.8 ± 11.7 | 79.9 ± 8.3 |
Values are mean ± SE.
P<0.05 v. men. BP, blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; SBP, systolic blood pressure.
Dietary sodium manipulation
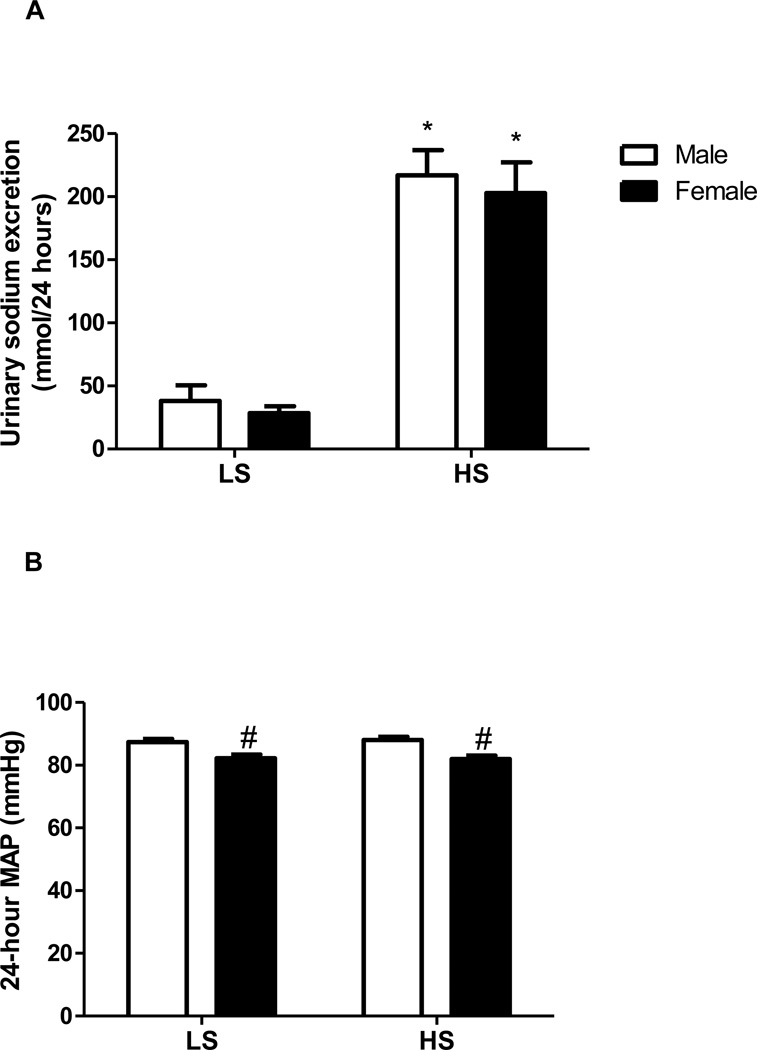
In response to the HS intervention, both men and women demonstrated a significant increase in 24-hour sodium excretion (Figure 1A) thus confirming compliance with the controlled diet. Potassium excretion was stable across the trials and no significant differences were seen among men and women or with the LS and HS diet. 24-hour urine was not available from 1 woman and 2 men for both their LS and HS trials, therefore, we were unable to calculate, urinary flow rate, and urinary sodium and potassium excretion over the 24-hour period. By design, MAP did not change significantly from LS to HS (Figure 1B) confirming salt-resistance in these subjects. However, women had a lower MAP during both dietary conditions (P <0.05). Serum sodium increased in men alone on HS (Table 2). We were unable to draw blood from 4 women and 1 men while on the HS diet and were unable to draw blood draw blood from 5 women and 4 men on the LS diet.
FIGURE 1.
(A) Urinary sodium excretion and (B) Mean arterial pressure in 16 normotensive men (white bars) and 14 normotensive women (black bars) after a LS and HS diet, each for 7 days in randomized order. *P<0.05 compared to respective urinary sodium excretion on LS diet. #P <0.05 compared to men.
TABLE 2.
Hemodynamic and renal responses to dietary sodium perturbation.
| LS Men | LS Women | HS Men | HS Women | |
|---|---|---|---|---|
| Mass (kg) | 75 ± 3 | 67 ± 2* | 76 ± 3† | 69 ± 2*† |
| Hemoglobin (g/dl) | 15.6 ± 0.3 | 12.8 ± 0.3* | 15.0 ± 0.4† | 12.0 ± 0.3*† |
| Hematocrit (%) | 43.5 ± 0.8 | 36.7 ± 1.7* | 41.4 ± 0.7 | 36.9 ± 1.1* |
| Plasma osmolality (mOsm/kg H2O) | 285.1 ± 1.2 | 284.8 ± 1.3 | 287.5 ± 1.0 | 286.0 ± 1.4 |
| Serum sodium (mmol/L) | 137.6 ± 0.6 | 137.5 ± 0.6 | 139.8 ± 0.5† | 138.3 ± 0.7 |
| Serum potassium (mmol/L) | 4.2 ± 0.1 | 3.9 ± 0.1 | 4.1 ± 0.1 | 4.0 ± 0.1 |
| Serum chloride (mmol/L) | 100.4 ± 0.7 | 103.3 ± 0.5* | 102.9 ± 0.4† | 106.2 ± 0.6*† |
| Urine osmolality (mOsm/kg H2O) | 542.7 ± 57.6 | 389.5 ± 51.5* | 486.9 ± 46.6 | 438.4 ± 52.5 |
| Urine flow rate (mL/min) | 1.00 ± 0.14 | 1.04 ± 0.15 | 1.46 ± 0.14† | 1.28 ± 0.15† |
| Free water clearance (mL/min) | −0.70 ± 0.18 | 0.10 ± 0.21* | −0.99 ± 0.16 | −0.54 ± 0.20 |
| 24-h SBP (mm Hg) | 119.1 ± 1.3 | 110.2 ± 1.4* | 121 ± 2† | 112 ± 1* |
| 24-h DBP (mm Hg) | 71.8 ± 1.3 | 68.5 ± 1.4 | 72 ± 1 | 67 ± 1* |
| 24-h PP (mm Hg) | 47 ± 1 | 41 ± 1* | 49 ± 2† | 45 ± 1*† |
| 24-h heart rate (bpm) | 69 ± 3 | 65 ± 5 | 67 ± 3 | 67 ± 2 |
| Laboratory SBP (mm Hg) | 117 ± 3 | 109 ± 3* | 119 ± 2 | 111 ± 3* |
| Laboratory DBP (mm Hg) | 63 ± 2 | 64 ±2 | 65 ± 1 | 66 ±2 |
| Laboratory MAP (mmHg) | 81 ± 2 | 79 ± 2 | 83 ± 1 | 81 ± 2 |
| Laboratory PP (mmHg) | 54 ± 3 | 45 ± 1* | 54 ± 3 | 46 ± 2* |
Values are mean ± SE; n = 30 (14 women and 16 men). DBP, diastolic blood pressure; HS, high sodium; LS, low sodium; MAP, mean arterial pressure; PP, pulse pressure; SBP, systolic blood pressure.
P<0.05 v. men;
P<0.05 v. respective sex’s low-sodium values
Vascular Function
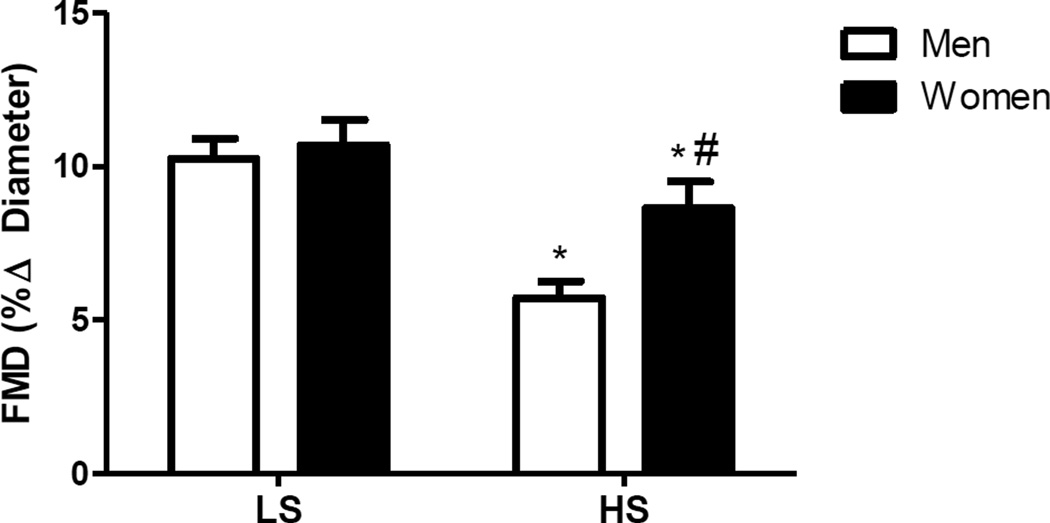
Baseline brachial artery diameters did not differ between dietary conditions for men (P=0.23) or women (P=0.14) (Table 3). Similarly, shear rate AUC, an estimate for the shear stimulus for dilation, was not different between dietary conditions for either men or women (Table 3). However, both experienced a significant decline in FMD with HS (men: 10.2±0.7 to 5.7±0.7; women 10.7±0.74 to 8.6±0.74; P<0.05) (Figure 2). Further, men demonstrated a lower FMD on HS diet compared to women.
TABLE 3.
Vascular Measurement responses to dietary sodium perturbation.
| LS Men | LS Women | HS Men | HS Women | |
|---|---|---|---|---|
| Brachial artery FMD (mm ∆) | 0.38±0.03 | 0.31±0.02 | 0.23±0.02† | 0.29±0.03*† |
| Baseline brachial artery diameter (mm) | 3.7±0.2 | 3.2±0.01* | 3.9±0.11 | 3.4±0.13* |
| Peak brachial artery diameter (mm) | 4.1±0.25 | 3.5±0.09* | 4.2±0.11 | 3.7±0.13* |
| AUC shear rate | 10276±4316 | 21702±9800 | 12596±8433 | 15039±7058 |
Values are mean ± SE; n = 30 (14 women and 16 men). FMD, flow-mediated dilation; AUC, area under the curve.
P<0.05 v. men;
P<0.05 v. respective sex’s low-sodium values
FIGURE 2.
Brachial artery flow-mediated dilation (FMD) for men (white bars) and women (black bars) after LS and HS diet as measured by brachial artery FMD. *P<0.05 compared to respective LS, # P<0.05 compared to men HS.
DISCUSSION
The primary finding of this study was that a high sodium diet resulted in a decline in FMD in both men and women. While both groups exhibited a decline in FMD on the high sodium diet, FMD was lower in men suggesting that sex differences exist in response to a HS diet. In addition, the decline in FMD that we observed occurred in normotensive, salt-resistant subjects further highlighting the detrimental effects of dietary sodium loading on the vasculature independent of BP.
Endothelial dysfunction is thought to precede the development of cardiovascular disease (5) and is a precursor to atherosclerosis (6). Dietary sodium has been shown to be a risk factor for hypertension (1) and other forms of CVD (17). While dietary sodium restriction continues to be important in lowering BP in hypertensive individuals and improving cardiovascular outcomes (17), its impact on the vasculature independent of BP is now being recognized. Improvements in FMD and a parallel reduction in BP was observed in overweight/obese normotensive individuals who followed a low sodium diet for two weeks (9). Dietary salt loading has been shown to increase BP in hypertensive individuals (18) as well as impair forearm blood flow responses to acetylcholine while increasing 24-hour MAP in young male normotensives (19). Recently, Jablonski et al.(20) reported improvements in brachial artery FMD in middle-aged/older adults with elevated systolic BP who followed a low sodium diet for 4 weeks compared to a diet providing the average U.S. sodium intake for 4 weeks. Systolic BP was reduced by the low sodium diet however the difference in FMD following dietary sodium restriction remained after correction for systolic BP and medication use. This suggests that the effects of low sodium on the vasculature are in addition to lowering BP. While the role of sodium loading or restriction on the vasculature has been studied in populations that are overweight/obese or have high BP, only recently have healthy, normotensive individuals and their response to sodium loading been studied (2–4, 19).
Evidence for BP-independent effects of sodium loading exists in the animal literature (21, 22). In mice and rats fed a high salt diet, acetylcholine induced arteriolar responses were impaired but MAP remain unchanged (21, 22). Translating these findings to humans, we recently reported the detrimental effect of 7 days of dietary sodium loading on FMD in healthy, salt-resistant adults (2). These changes were independent of BP and illustrate the impact of sodium on vascular function. However, it was unknown if men and women respond differently to dietary sodium loading. To date, most sex specific research has focused on sex differences related to BP. An animal study using the hypertensive Ren-2 transgenic mouse showed greater BP changes in response to salt loading in the women as compared to the male Ren-2 rats(23). This has also been seen in a human study in which 7 days of both a low sodium and high sodium diet resulted in a greater BP response in women as compared to men(12). While these sex studies on sodium and BP tend to show a greater response in women, this has not been reported in studies of vascular function. Eisenach et al., (4) demonstrated sex differences in the NO-component of acetylcholine induced vasodilation in forearm blood flow following 5 days of high sodium compared to a low sodium diet. The healthy young male subjects demonstrated a greater sensitivity to the sodium loading. While this study was novel in its findings, the forearm blood flow response to acetylcholine did not differ between low and high salt diets whereas we demonstrate a difference in FMD between low and high salt diets. Additionally, different groups of subjects underwent the high and low salt diet so salt-sensitivity of BP was not assessed as it was in the present study. In the current study, all subjects underwent both dietary perturbations allowing us to classify them as salt-resistant and we demonstrated a decline in FMD on the HS diet in both men and women. While the men and women responded similarly to the LS diet, the lower FMD in men on the HS diet suggests they are more sensitive to the sodium loading as reported by Eisenach et al (4). Because we studied salt-resistant individuals our data also indicate that this response is not due to sodium effects on BP. We did find that SBP and PP were lower in women however there was no effect of sodium on these pressures just as there wasn’t in men.
The mechanism responsible for this differential response to sodium loading in our study is unknown. Shear rate did not differ between sexes or diets indicating another mechanism(s) is responsible for differences in FMD. It is accepted that women exhibit greater cardioprotection against CVD prior to menopause (11). Indeed our woman subjects were pre-menopausal with an average age of 31 years. To date, sex differences in FMD have reported an earlier decline in endothelial function in men (24) as women appear to preserve their endothelial function due to the presence of estrogen and it’s ability to up-regulate NO synthesis(25). Further, changes in serum sodium levels can affect NO synthase (26) thereby suppressing endothelial NOS activity (27). A high sodium intake has been shown to cause a small rise in serum sodium concentrations(27). Indeed, serum sodium concentrations rose significantly in our men alone. This differential response may have contributed to a lower FMD in men on the HS diet compared to women. Women did have a lower plasma osmolality at baseline which is consistent with previous studies(28) however no differences were observed between the dietary conditions. Further, serum sodium levels did not correlate with the change in FMD for men or women on either diet.
Limitations of the current study include the short duration of the dietary perturbation, not controlling for the menstrual cycle, and the lack of assessment of endothelial-independent dilation(29). Despite the short duration of the diet, we still observed a decline in FMD on the HS diet and a lower FMD in men. Further, FMD has been show to fluctuate significantly through the course of the menstrual cycle(30) and we were not able to control for this given the study design. Despite this lack of control, we still observed significant differences between men and women on the HS diet but cannot rule out an effect of the menstrual cycle on these findings. Further, while we cannot rule out sex differences in smooth muscle responsiveness in the current study, we have previously shown that EID in response to sublingual nitroglycerin did not differ between LS and HS diets in salt-resistant individuals (2).
In summary, we extend our previous findings of an effect of sodium loading on EDD in salt-resistant subjects. We have demonstrated here that a high sodium diet results in a decline in brachial artery FMD in both men and women. Additionally, FMD was lower on high sodium in men suggesting a greater sensitivity of the vasculature to high sodium in men. These findings support the notion that shifting the current focus away from just the effect of sodium on BP is important in light of the detrimental effect it has on vascular function.
Acknowledgments
Funding: R01 HL104106
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001 Jan 4;344(1):3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 2.DuPont JJ, Greaney JL, Wenner MM, Lennon-Edwards SL, Sanders PW, Farquhar WB, et al. High dietary sodium intake impairs endothelium-dependent dilation in healthy salt-resistant humans. J Hypertens. 2013 Mar;31(3):530–536. doi: 10.1097/HJH.0b013e32835c6ca8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greaney JL, DuPont JJ, Lennon-Edwards SL, Sanders PW, Edwards DG, Farquhar WB. Dietary sodium loading impairs microvascular function independent of blood pressure in humans: role of oxidative stress. The Journal of physiology. 2012 Nov 1;590(Pt 21):5519–5528. doi: 10.1113/jphysiol.2012.236992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisenach JH, Gullixson LR, Kost SL, Joyner MJ, Turner ST, Nicholson WT. Sex differences in salt sensitivity to nitric oxide dependent vasodilation in healthy young adults. J Appl Physiol. 2012 Mar;112(6):1049–1053. doi: 10.1152/japplphysiol.01197.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luscher TF, Tanner FC, Tschudi MR, Noll G. Endothelial dysfunction in coronary artery disease. Annual review of medicine. 1993;44:395–418. doi: 10.1146/annurev.me.44.020193.002143. [DOI] [PubMed] [Google Scholar]
- 6.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002 Mar 5;105(9):1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 7.Thijssen DH, Bullens LM, van Bemmel MM, Dawson EA, Hopkins N, Tinken TM, et al. Does arterial shear explain the magnitude of flow-mediated dilation?: a comparison between young and older humans. Am J Physiol Heart Circ Physiol. 2009 Jan;296(1):H57–H64. doi: 10.1152/ajpheart.00980.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension. 2011 Mar;57(3):363–369. doi: 10.1161/HYPERTENSIONAHA.110.167015. [DOI] [PubMed] [Google Scholar]
- 9.Dickinson KM, Keogh JB, Clifton PM. Effects of a low-salt diet on flow-mediated dilatation in humans. Am J Clin Nutr. 2009 Feb;89(2):485–490. doi: 10.3945/ajcn.2008.26856. [DOI] [PubMed] [Google Scholar]
- 10.Jablonski KL, Gates PE, Pierce GL, Seals DR. Low dietary sodium intake is associated with enhanced vascular endothelial function in middle-aged and older adults with elevated systolic blood pressure. Therapeutic advances in cardiovascular disease. 2009 Oct;3(5):347–356. doi: 10.1177/1753944709345790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eaker ED, Chesebro JH, Sacks FM, Wenger NK, Whisnant JP, Winston M. Cardiovascular disease in women. Circulation. 1993 Oct;88(4 Pt 1):1999–2009. doi: 10.1161/01.cir.88.4.1999. [DOI] [PubMed] [Google Scholar]
- 12.He J, Gu D, Chen J, Jaquish CE, Rao DC, Hixson JE, et al. Gender difference in blood pressure responses to dietary sodium intervention in the GenSalt study. J Hypertens. 2009 Jan;27(1):48–54. doi: 10.1097/hjh.0b013e328316bb87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreau KL, Hildreth KL, Meditz AL, Deane KD, Kohrt WM. Endothelial function is impaired across the stages of the menopause transition in healthy women. The Journal of clinical endocrinology and metabolism. 2012 Dec;97(12):4692–4700. doi: 10.1210/jc.2012-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. Journal of the American Society of Nephrology : JASN. 2003 Jul;14(7 Suppl 2):S148–S153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 15.Schmidlin O, Sebastian AF, Morris RC., Jr What initiates the pressor effect of salt in salt-sensitive humans? Observations in normotensive blacks. Hypertension. 2007 May;49(5):1032–1039. doi: 10.1161/HYPERTENSIONAHA.106.084640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van den Munckhof I, Riksen N, Seeger JP, Schreuder TH, Borm GF, Eijsvogels TM, et al. Aging attenuates the protective effect of ischemic preconditioning against endothelial ischemia-reperfusion injury in humans. Am J Physiol Heart Circ Physiol. 2013 Jun 15;304(12):H1727–H1732. doi: 10.1152/ajpheart.00054.2013. [DOI] [PubMed] [Google Scholar]
- 17.Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP) Bmj. 2007 Apr 28;334(7599):885–888. doi: 10.1136/bmj.39147.604896.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Todd AS, Macginley RJ, Schollum JB, Johnson RJ, Williams SM, Sutherland WH, et al. Dietary salt loading impairs arterial vascular reactivity. Am J Clin Nutr. 2010 Mar;91(3):557–564. doi: 10.3945/ajcn.2009.28645. [DOI] [PubMed] [Google Scholar]
- 19.Tzemos N, Lim PO, Wong S, Struthers AD, MacDonald TM. Adverse cardiovascular effects of acute salt loading in young normotensive individuals. Hypertension. 2008 Jun;51(6):1525–1530. doi: 10.1161/HYPERTENSIONAHA.108.109868. [DOI] [PubMed] [Google Scholar]
- 20.Jablonski KL, Racine ML, Geolfos CJ, Gates PE, Chonchol M, McQueen MB, et al. Dietary sodium restriction reverses vascular endothelial dysfunction in middle-aged/older adults with moderately elevated systolic blood pressure. Journal of the American College of Cardiology. 2013 Jan 22;61(3):335–343. doi: 10.1016/j.jacc.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lenda DM, Sauls BA, Boegehold MA. Reactive oxygen species may contribute to reduced endothelium-dependent dilation in rats fed high salt. Am J Physiol Heart Circ Physiol. 2000 Jul;279(1):H7–H14. doi: 10.1152/ajpheart.2000.279.1.H7. [DOI] [PubMed] [Google Scholar]
- 22.Nurkiewicz TR, Boegehold MA. High salt intake reduces endothelium-dependent dilation of mouse arterioles via superoxide anion generated from nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2007 Apr;292(4):R1550–R1556. doi: 10.1152/ajpregu.00703.2006. [DOI] [PubMed] [Google Scholar]
- 23.Huskova Z, Kramer H, Vanourkova Z, Thumova M, Maly J, Opocensky M, et al. Effects of dietary salt load and salt depletion on the course of hypertension and angiotensin II levels in male and female heterozygous Ren-2 transgenic rats. Kidney & blood pressure research. 2007;30(1):45–55. doi: 10.1159/000099028. [DOI] [PubMed] [Google Scholar]
- 24.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. Journal of the American College of Cardiology. 1994 Aug;24(2):471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 25.Perez-Lopez FR, Larrad-Mur L, Kallen A, Chedraui P, Taylor HS. Gender differences in cardiovascular disease: hormonal and biochemical influences. Reproductive sciences. 2010 Jun;17(6):511–531. doi: 10.1177/1933719110367829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chirinos JA, Zambrano JP, Chakko S, Veerani A, Schob A, Willens HJ, et al. Aortic pressure augmentation predicts adverse cardiovascular events in patients with established coronary artery disease. Hypertension. 2005 May;45(5):980–985. doi: 10.1161/01.HYP.0000165025.16381.44. [DOI] [PubMed] [Google Scholar]
- 27.Li J, White J, Guo L, Zhao X, Wang J, Smart EJ, et al. Salt inactivates endothelial nitric oxide synthase in endothelial cells. J Nutr. 2009 Mar;139(3):447–451. doi: 10.3945/jn.108.097451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stachenfeld NS, Splenser AE, Calzone WL, Taylor MP, Keefe DL. Sex differences in osmotic regulation of AVP and renal sodium handling. J Appl Physiol. 2001 Oct;91(4):1893–1901. doi: 10.1152/jappl.2001.91.4.1893. [DOI] [PubMed] [Google Scholar]
- 29.Pryer JA, Vrijheid M, Nichols R, Kiggins M, Elliott P. Who are the 'low energy reporters' in the dietary and nutritional survey of British adults? Int J Epidemiol. 1997 Feb;26(1):146–154. doi: 10.1093/ije/26.1.146. [DOI] [PubMed] [Google Scholar]
- 30.Williams MR, Westerman RA, Kingwell BA, Paige J, Blombery PA, Sudhir K, et al. Variations in endothelial function and arterial compliance during the menstrual cycle. The Journal of clinical endocrinology and metabolism. 2001 Nov;86(11):5389–5395. doi: 10.1210/jcem.86.11.8013. [DOI] [PubMed] [Google Scholar]