Abstract
BACKGROUND
Silent cerebral infarcts are the most common neurologic injury in children with sickle cell anemia and are associated with the recurrence of an infarct (stroke or silent cerebral infarct). We tested the hypothesis that the incidence of the recurrence of an infarct would be lower among children who underwent regular blood-transfusion therapy than among those who received standard care.
METHODS
In this randomized, single-blind clinical trial, we randomly assigned children with sickle cell anemia to receive regular blood transfusions (transfusion group) or standard care (observation group). Participants were between 5 and 15 years of age, with no history of stroke and with one or more silent cerebral infarcts on magnetic resonance imaging and a neurologic examination showing no abnormalities corresponding to these lesions. The primary end point was the recurrence of an infarct, defined as a stroke or a new or enlarged silent cerebral infarct.
RESULTS
A total of 196 children (mean age, 10 years) were randomly assigned to the observation or transfusion group and were followed for a median of 3 years. In the transfusion group, 6 of 99 children (6%) had an end-point event (1 had a stroke, and 5 had new or enlarged silent cerebral infarcts). In the observation group, 14 of 97 children (14%) had an end-point event (7 had strokes, and 7 had new or enlarged silent cerebral infarcts). The incidence of the primary end point in the transfusion and observation groups was 2.0 and 4.8 events, respectively, per 100 years at risk, corresponding to an incidence rate ratio of 0.41 (95% confidence interval, 0.12 to 0.99; P = 0.04).
CONCLUSIONS
Regular blood-transfusion therapy significantly reduced the incidence of the recurrence of cerebral infarct in children with sickle cell anemia. (Funded by the National Institute of Neurological Disorders and Stroke and others; Silent Cerebral Infarct Multi-Center Clinical Trial ClinicalTrials.gov number, NCT00072761, and Current Controlled Trials number, ISRCTN52713285.)
Sickle cell anemia affects 1 of every 3961 black newborns and approximately 100,000 persons in the United States.2,3 Among children with sickle cell anemia (defined as homozygous hemoglobin S or hemoglobin S-β0 thalassemia), silent cerebral infarcts are the most common neurologic injury.4 In contrast to overt stroke (hereinafter referred to as stroke), a silent cerebral infarct is not associated with obvious neurologic impairment and cannot be detected on neurologic examination.5 However, children with a silent cerebral infarct are at increased risk for stroke, new or enlarged silent cerebral infarcts,6 poor academic achievement,7 and lower IQ, as compared either with children with sickle cell anemia who have normal results on magnetic resonance imaging (MRI) of the brain or with siblings without sickle cell anemia.7,8
The most effective therapy for children with sickle cell anemia and silent cerebral infarcts is unknown. For the primary prevention of stroke, the Stroke Prevention Trial in Sickle Cell Anemia (STOP) showed that regular blood-transfusion therapy was efficacious.9 Given the favorable results of a single-group feasibility trial,10 coupled with the high prevalence and progressive nature of silent cerebral infarction, a critical unanswered question is whether regular blood-transfusion therapy in children with silent cerebral infarcts prevents the recurrence of an infarct (stroke or new or enlarged silent cerebral infarct). In the Silent Cerebral Infarct Multi-Center Clinical Trial (SIT), we tested the primary hypothesis that the incidence of infarct recurrence would be lower among children receiving regular blood-transfusion therapy than among children assigned to standard care.
METHODS
TRIAL OVERSIGHT
SIT was a multicenter, randomized clinical trial in which we assigned children with sickle cell anemia–related silent cerebral infarcts to receive standard care (observation group) or regular blood-transfusion therapy (transfusion group). A detailed description of the study protocol has been published previously,11 and the protocol is available with the full text of this article at NEJM.org The study was conducted at 29 clinical centers in the United States, Canada, France, and the United Kingdom. The trial was approved by the institutional review board at each participating institution. The first two authors analyzed the data and vouch for the accuracy and completeness of the data, and the first author vouches for the fidelity of the study to the protocol. A data and safety monitoring board appointed by the National Institute of Neurological Disorders and Stroke reviewed serious adverse events, study progress, and safety every 6 months. The last participant enrolled completed the exit visit on July 29, 2013. Data were adjudicated and the database was locked for this report on September 1, 2013.
PARTICIPANTS
Inclusion criteria were an age of 5 to 15 years, confirmed diagnosis of hemoglobin SS or hemoglobin Sβ0 thalassemia, and at least one infarct-like lesion on the screening MRI scan. Written informed consent was obtained from parents or legal guardians and assent from the study participants. An infarct-like lesion was defined as an MRI signal abnormality that was at least 3 mm in one dimension and that was visible in two planes on fluid-attenuated inversion recovery (FLAIR) T2-weighted images, as determined by agreement of two of the three study neuroradiologists. The members of a neurology committee adjudicated a lesion as a silent cerebral infarct if the study participant had either a normal neurologic examination or an abnormality on examination that could not be explained by the location of the brain lesion or lesions. Exclusion criteria were a history of focal neurologic deficit associated with an infarct on brain MRI, a seizure disorder, treatment with hydroxyurea in the previous 3 months, a history of regular transfusion therapy, or imaging or nonimaging transcranial Doppler measurement that was above the study-defined thresholds.11
STUDY DESIGN
Randomization assignments were provided by the statistical data coordinating center with the use of a permuted block design, with stratification according to site, age, and sex. Participants were assigned in a 1:1 ratio to the observation group or the transfusion group and were followed until the occurrence of a study end-point event or until exit from the study. At baseline and exit, participants underwent brain MRI and neurologic and cognitive examinations. If a neurologic event was suspected during the study, MRI and neurologic examination were performed.
Participants who were randomly assigned to observation received standard care (with no treatment for silent infarcts, including no hydroxyurea therapy) and were evaluated quarterly. Participants who were randomly assigned to the transfusion group received a transfusion approximately monthly to maintain a target hemoglobin concentration greater than 9.0 g per deciliter and a target hemoglobin S concentration of 30% or less of total hemoglobin. Ferritin levels were monitored before each transfusion. Site investigators were advised to initiate chelation therapy for participants who had ferritin levels greater than 1500 ng per milliliter for 2 or more consecutive months.
PRIMARY AND SECONDARY END POINTS
The primary end point was the recurrence of infarct or hemorrhage as determined by neuroimaging, clinical evidence of permanent neurologic injury, or both. A new infarct had to meet the criteria for a silent cerebral infarction; an enlarged silent cerebral infarct was defined as a previously identified silent cerebral infarct that increased by at least 3 mm along any linear dimension in any plane on MRI. A transient ischemic attack (TIA), which was included in secondary analyses of neurologic outcomes, was defined as an event that resulted in focal neurologic deficits that lasted less than 24 hours, did not result in abnormalities on T2-weighted or FLAIR images that were indicative of an acute infarct, and had no other reasonable medical explanation. Members of neuroradiology and neurology committees, who were unaware of the study-group assignments, adjudicated neurologic and MRI findings. Secondary outcomes included changes in cognition, which were assessed by measurement of IQ scores with the Wechsler Abbreviated Scale of Intelligence12 or the Wechsler Preschool and Primary Scale of Intelligence III.13 We also assessed scores on the Behavior Rating Inventory of Executive Function (BRIEF).14
STATISTICAL ANALYSIS
To test the primary hypothesis, we calculated that a sample size of 204 participants (102 in each group) would give the study 85% power to detect a decrease of at least 86% in the prevalence of the primary end point, assuming a 10% dropout rate and a crossover rate of 16% from transfusion to observation and 3% from observation to transfusion, at a two-tailed nominal alpha level of 0.05. In 2012, the data and safety monitoring board approved the use of rate ratio to test the primary hypothesis in order to adjust for variable exposure time. For the primary hypothesis, the intention-to-treat principle was used to compare incidence rates of infarct recurrence between the transfusion group and the observation group. A parallel analysis comparing the two study groups with respect to all neurologic outcomes (i.e., infarct recurrence plus TIA) was also conducted. We tested the null hypotheses (incidence ratio = 1.0) and estimated exact 95% confidence intervals by using bootstrap methods with 10,000 replications. P values were estimated with the use of a permutation test. Logistic regression models were used to adjust for an imbalance in baseline factors and to determine whether prespecified factors (as listed in the Supplementary Appendix, available at NEJM.org) were associated with infarct recurrence.
RESULTS
RECRUITMENT AND BASELINE CHARACTERISTICS
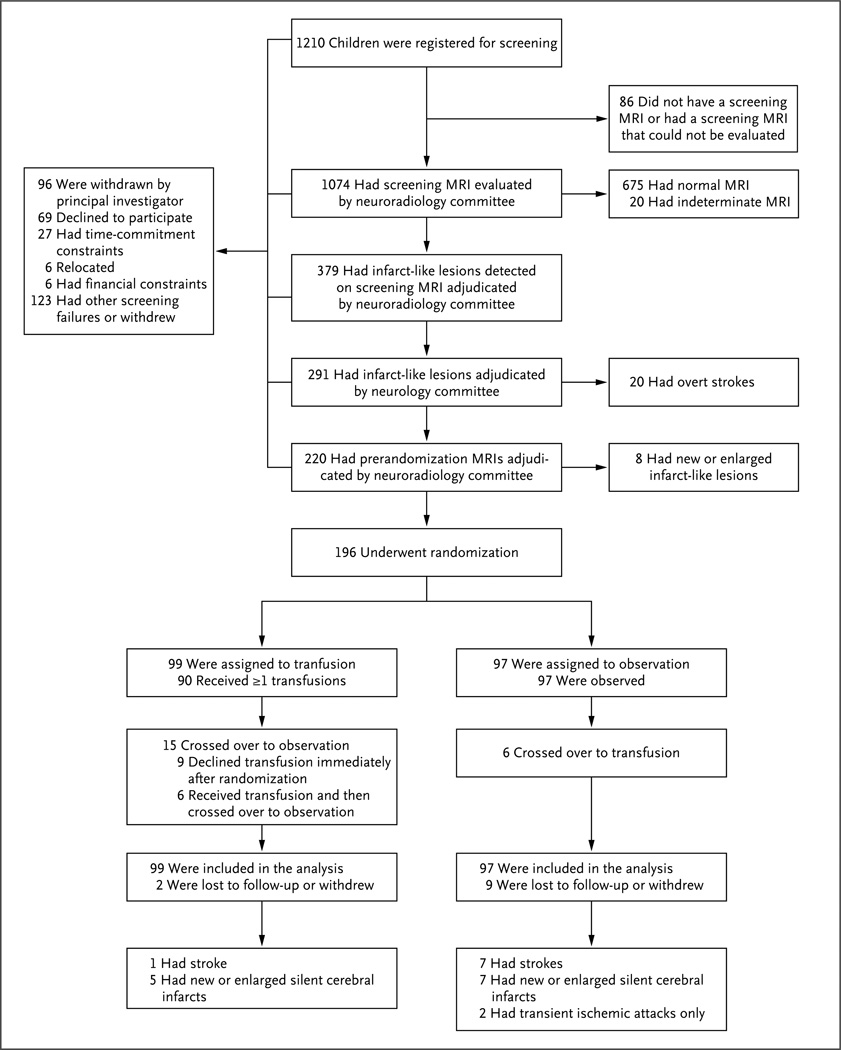
Recruitment began in December 2004 and continued through the end of May 2010. Among the 1074 children who underwent screening MRI of the brain, 20 (1.9%) had strokes and 379 (35.3%) had infarct-like lesions. Two participants with results of imaging transcranial Doppler studies of 199 cm per second and 196 cm per second, which were above the eligibility threshold, inadvertently underwent randomization because a programming error failed to distinguish between imaging and nonimaging transcranial Doppler studies. Both participants were assigned to the observation group; a stroke occurred in one of them. Table 1 shows the baseline characteristics of the participants, and Figure 1 the screening, randomization, and follow-up.
Table 1.
Baseline Characteristics of the Participants.*
| Characteristic | Observation Group (N = 97) |
Transfusion Group (N = 99) |
|---|---|---|
| Age — no. (%)† | ||
| 5–7 yr | 28 (29) | 26 (26) |
| 8–10 yr | 32 (33) | 35 (35) |
| 11–13 yr | 29 (30) | 32 (32) |
| 14–15 yr | 8(8) | 6(6) |
| Sex— no. (%) | ||
| Male | 52 (54) | 59 (60) |
| Female | 45 (46) | 40 (40) |
| Race — no. (%)‡ | ||
| Black | 90 (93) | 91 (92) |
| White | 0 | 2(2) |
| Other | 7(7) | 6(6) |
| Results of transcranial Doppler assessment — no. (%)§ | ||
| Normal | 82 (85) | 76 (77) |
| Conditional | 13 (13) | 22 (22) |
| High | 2(2) | 0 |
| Assessment attempted but unsuccessful | 0 | 1(1) |
| Parental report of recurring headaches — no. (%) | ||
| Yes | 43 (44) | 37(37) |
| No | 54 (56) | 62 (63) |
| Transcranial Doppler velocity during screening¶ | ||
| No. of patients with data | 97 | 98 |
| Median (IQR) —cm/sec | 143 (131–163) | 147 (123–168) |
| Hospital admissions for pain║ | ||
| No. of patients with data | 97 | 99 |
| Median (IQR) — rate per 100 person-yr | 33.3 (0–100.0) | 33.3 (0–66.7) |
| Hospital admissions for acute chest syndrome║ | ||
| No. of patients with data | 97 | 99 |
| Median (IQR) — rate per 100 person-yr | 0 (0–33.3) | 0 (0–33.3) |
| Steady-state hemoglobin | ||
| No. of patients with data | 97 | 99 |
| Median (IQR)—g/dl | 7.9 (7.4–8.9) | 7.7 (7.2–8.4) |
| Steady-state reticulocytes | ||
| No. of patients with data | 95 | 96 |
| Median (IQR)—% | 10.3 (7.7–13.6) | 12.3 (9.6–16.2) |
| Highest total bilirubin in previous 3 years | ||
| No. of patients with data | 88 | 92 |
| Median (IQR) —mg/dl | 2.8 (1.9–3.9) | 2.9 (1.9–4.7) |
| Hemoglobin F** | ||
| No. of patients with data | 86 | 94 |
| Median (IQR)—% | 10.0 (5.0–15.0) | 9.0 (4.0–14.0) |
| Steady-state white-cell count | ||
| No. of patients with data | 97 | 99 |
| Median (IQR) — per mm3 | 12,500 (10,000–14,700) | 12,300 (10,000–15,000) |
| Steady-state neutrophils | ||
| No. of patients with data | 92 | 94 |
| Median (IQR)—% | 47.0 (38.0–59.0) | 47.5 (42.0–57.0) |
| Steady-state platelets | ||
| No. of patients with data | 96 | 99 |
| Median (IQR) — per mm3 | 438,500 (364,000–519,500) | 423,000 (372,000–509,000) |
| Systolic blood pressure | ||
| No. of patients with data | 97 | 99 |
| Median (IQR) — mm Hg | 108.0(103.0–117.0) | 107.0(102.0–115.0) |
| Oximetry reading | ||
| No. of patients with data | 96 | 95 |
| Median (IQR)—% | 97.0 (95.0–99.0) | 97.0 (94.0–98.0) |
The baseline characteristics were determined at the time of initial screening. There were no significant differences in baseline characteristics except with respect to the reticulocyte count, for which P = 0.002. IQR denotes interquartile range.
The age listed is the age at the time of randomization rather than the age at the time of initial screening.
Race was self-reported.
For imaging transcranial Doppler assessment, 0 to less than 155 cm per second was considered to be normal, 155 to less than 185 cm per second was considered to be conditional, and 185 cm per second or higher was considered to be high. For nonimaging transcranial Doppler, 0 to less than 170 cm per second was considered to be normal, 170 to less than 200 cm per second was considered to be conditional, and 200 cm per second or higher was considered to be high.
To standardize the report to nonimaging transcranial Doppler measurements, we added 15 points to the results of imaging transcranial Doppler measurements.
The mean rate of hospital admissions for pain was 68.7 per 100 person-years in the observation group and 62.3 per 100 person-years in the transfusion group. The mean rate of hospital admissions for the acute chest syndrome was 19.6 per 100 person-years in the observation group and 16.2 per 100 person-years in the transfusion group.
Data on hemoglobin F at baseline were categorized as missing if the test was performed before the participant was 3 years of age or if the test was not performed.
Figure 1. Screening, Randomization, and Follow-up.
The screening and randomization process extended from December 2004 through May 2010. Participants may have had more than one reason for exclusion or withdrawal from the study. For participants who were lost to follow-up, data that had accrued before withdrawal were used in the analysis.
INTERVENTION
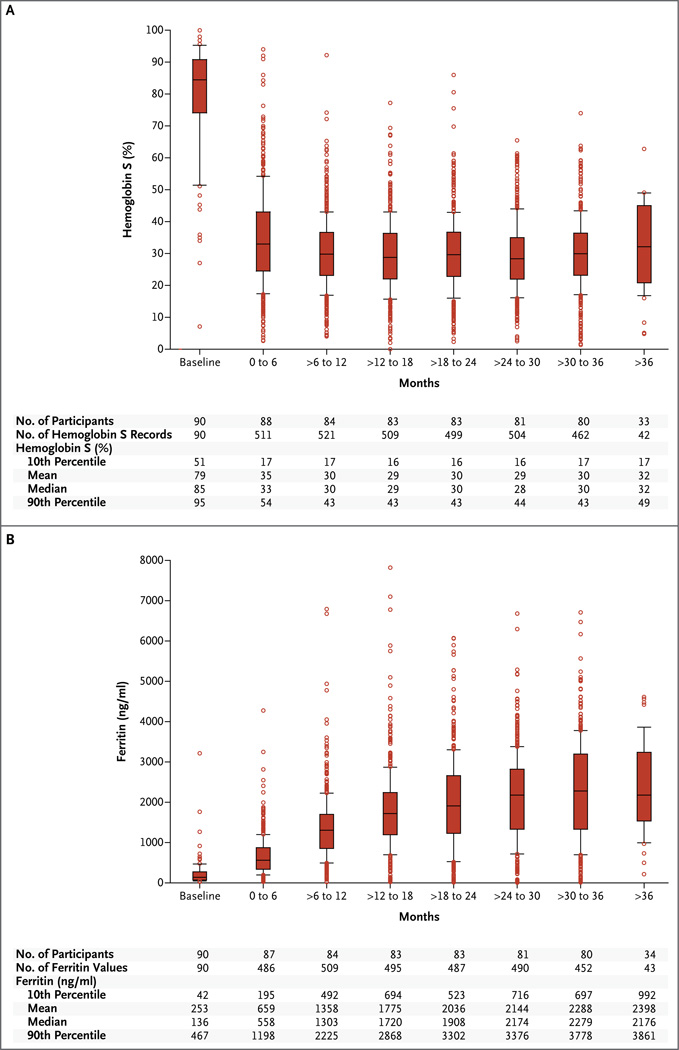
The primary end point was ascertained for 185 of the 196 participants (94%). Of the 99 participants randomly assigned to the transfusion group, 90 started receiving transfusions within 4 weeks after assignment. The crossover rate from transfusion to observation was 15% (15 of 99 participants); 9 participants declined blood transfusion, and 6 crossed over to observation at a median time of 34 days. Among the 90 participants in the transfusion group who received transfusions, the interval between transfusions was 38 days or less for 95% of the 3236 transfusions, and the median hemoglobin S level was 30.1% (Fig. 2A). Ferritin levels are shown in Figure 2B. Among participants in the observation group, 32% received transfusions (a median of three transfusions each), including 6 participants who crossed over to regular monthly transfusions at a median of 1.7 years. During the course of the trial, hydroxyurea was started in 14 of 97 participants (14%) in the observation group and in 3 of 99 (3%) in the transfusion group because of disease severity.
Figure 2. Hemoglobin S and Ferritin Levels in the Transfusion Group.
The box plots show the hemoglobin S levels (Panel A) and the ferritin levels (Panel B) in the transfusion group, in 6-month intervals. The horizontal line in the boxes represents the median, the lower and upper boundaries of the boxes represent the 25th and 75th percentiles, respectively, the I bars represent the 10th and 90th percentiles, and the circles represent values outside the 10th and 90th percentiles. The number of participants listed does not include nine participants who declined transfusion therapy immediately after they underwent randomization.
NEUROLOGIC OUTCOMES
Exit MRIs were completed for 185 of the 196 participants (94%). In the transfusion group, 99 participants accumulated 304 patient-years (median, 3.0 years per patient); in the observation group, 97 participants accumulated 289 patient-years (median, 3.0 years per patient). The prevalence of the primary end point was 6% (6 of 99 participants) in the transfusion group and 14% (14 of 97 participants) in the observation group (Table 2).
Table 2.
Neurologic Events and Baseline Characteristics, According to Patient.*
| Patient No. | Time from Randomization to End Point |
End-Point Classification |
Neuroradiology Finding† |
TIA† | Baseline Clinical Characteristics | ||||
|---|---|---|---|---|---|---|---|---|---|
| New Infarct |
Enlarged Infarct |
Age‡ | Systolic Blood Pressure |
Steady-State Hemoglobin |
Hemoglobin F§ | ||||
| mo | yr | mm Hg | g/dl | % | |||||
| Transfusion group | |||||||||
| 1 | 24 | Stroke | + | − | − | 7.9 | 107 | 6.7 | 4 |
| 2 | 35 | SCI | + | + | − | 8.5 | 85 | 9.8 | 17 |
| 3 | 36 | SCI | + | + | − | 7.5 | 101 | 10.3 | 19 |
| 4 | 36 | SCI | + | + | − | 9.3 | 107 | 7.9 | 13 |
| 5 | 39 | SCI | + | − | − | 9.1 | 92 | 7.5 | 2 |
| 6 | 36 | SCI | − | + | − | 8.3 | 107 | 7.2 | 10 |
| Observation group | |||||||||
| 7 | 6 | Stroke | + | + | − | 7.8 | 106 | 7.9 | NA |
| 8 | 19 | Stroke | + | − | − | 6.7 | 106 | 7.8 | NA |
| 9 | 30 | Stroke | + | − | − | 12.7 | 122 | 6.7 | NA |
| 10 | 36 | Stroke | + | − | − | 7.3 | 93 | 7.5 | 4 |
| 11 | 12 | Stroke | − | + | − | 6.6 | 107 | 8.0 | 9 |
| 12 | 19 | Stroke | − | − | + | 7.1 | 96 | 6.5 | 18 |
| 13 | 35 | Stroke | − | − | − | 10.3 | 100 | 6.1 | 0 |
| 14 | 16 | SCI | + | − | − | 8.0 | 110 | 7.7 | 8 |
| 15 | 27 | SCI | + | − | − | 6.3 | 103 | 8.4 | NA |
| 16 | 34 | SCI | + | − | − | 6.9 | 107 | 7.7 | 18 |
| 17 | 37 | SCI | + | − | − | 6.7 | 109 | 8.0 | 25 |
| 18 | 41 | SCI | + | − | − | 12.7 | 118 | 6.9 | 4 |
| 19 | 41 | SCI | + | − | − | 6.7 | 112 | 9.4 | 9 |
| 20 | 44 | SCI | + | − | − | 9.6 | 120 | 10.2 | 16 |
| 21 | 12 | TIA | − | − | + | 12.3 | 136 | 7.7 | 13 |
| 22 | 13 | TIA | − | − | + | 7.1 | 87 | 8.6 | 15 |
The baseline characteristics were determined at the time of initial screening. NA denotes not available, SCI silent cerebral infarct, and TIA transient ischemic attack.
A plus sign indicates that the end-point event occurred, and a minus sign that the end-point event did not occur.
The age listed is the age at randomization rather than the age at the time of initial screening.
Data on hemoglobin F at baseline were categorized as not available (NA) if the test was performed before the participant was 3 years of age or if the test was not performed.
In an intention-to-treat analysis, the incidence rate of infarct recurrence was 2.0 per 100 person-years at risk in the transfusion group and 4.8 per 100 person-years at risk in the observation group, with an incidence rate ratio of 0.41 (95% confidence interval [CI], 0.12 to 0.99; P = 0.04). The absolute risk reduction was 8 percentage points, the relative risk reduction was 58%, and the number needed to treat for 3 years to prevent one recurrence of infarct was 13.
Three TIAs occurred, all in the observation group, including a TIA in one participant who subsequently had a stroke. Adding TIA events to infarct recurrence, the incidence rate of all neurologic events in the transfusion and observation groups was 2.0 and 5.6 per 100 person-years at risk, respectively, with an incidence rate ratio of 0.36 (95% CI, 0.10 to 0.83; P = 0.02).
Fifteen postulated risk factors for infarct recurrence were evaluated, of which four were significant. The odds ratio for infarct recurrence in the transfusion group as compared with the observation group was 0.31 (95% CI, 0.10 to 0.93; P = 0.04). Baseline factors associated with infarct recurrence were younger age (odds ratio, 1.41; 95% CI, 1.12 to 1.78; P = 0.004), a history of recurring headaches (odds ratio, 4.33; 95% CI, 1.50 to 13.06; P = 0.007), and a higher steady-state reticulocyte count (odds ratio, 1.11; 95% CI, 1.01 to 1.22; P = 0.04) (Table S3 in the Supplementary Appendix). No significant changes in full-scale IQ or BRIEF measurements were observed from baseline to study exit, either within or between the observation and transfusion groups (Tables SI and S2 in the Supplementary Appendix).
ADVERSE EVENTS
Incidence rates of vaso-occlusive pain, acute chest syndrome, priapism, and new symptomatic avascular necrosis of the hip were significantly higher in the observation group than in the transfusion group (Table 3). Transfusion reactions were reported in 1 participant (1%) in the observation group and in 15 of the 90 participants (17%) in the transfusion group who actually received blood-transfusion therapy; 9 participants had one reaction, 6 had two reactions, and 1 had four reactions. Most reactions were allergic (13 of 25 [52%]) or febrile nonhemolytic (8 of 25 [32%]) reactions. A tunneled central venous catheter for vascular access was implanted in 11 participants; a catheter infection developed in 1 of these participants, and complications requiring catheter replacement developed in 2 others. A total of 3236 transfusions were administered in the transfusion group, and nine alloantibodies were detected in 4 participants — anti-C (in 2 participants), anti-V (in 2 participants), anti-FyA, anti-e, anti-S, anti-JK-b, and anti-Wra — for an alloimmunization rate of 0.278 per 100 units of red cells. No alloantibodies were detected among participants in the observation group. No deaths occurred.
Table 3.
Incidence of Adverse Events in the Observation and Transfusion Groups per 100 Person-Years.
| Adverse Event | Sample Size |
At Least 1 Adverse Event |
Total Adverse Events |
At-Risk Time |
Adverse Events/ 100 Person-Yr |
Incidence Rate Ratio (95% CI)* |
P Value |
|---|---|---|---|---|---|---|---|
| no. of participants | no. | person-yr | |||||
| Vaso-occlusive pain | 0.41 (0.20–0.75) | 0.004 | |||||
| Observation group | 97 | 56 | 295 | 289 | 102.21 | ||
| Transfusion group | 99 | 32 | 126 | 304 | 41.58 | ||
| Acute chest syndrome | 0.13 (0.04–0.28) | <0.001 | |||||
| Observation group | 97 | 24 | 41 | 289 | 14.35 | ||
| Transfusion group | 99 | 5 | 5 | 304 | 1.81 | ||
| Priapism† | 0.13 (0.03–0.55) | 0.02 | |||||
| Observation group | 52 | 7 | 10 | 158 | 6.65 | ||
| Transfusion group | 59 | 1 | 1 | 178 | 0.84 | ||
| Symptomatic avascular necrosis of the hip | 0.22 (0.05–0.85) | 0.02 | |||||
| Observation group | 97 | 6 | 6 | 289 | 2.25 | ||
| Transfusion group | 99 | 1 | 1 | 304 | 0.49 | ||
| Headache | 0.78 (0.39–1.57) | 0.40 | |||||
| Observation group | 97 | 30 | 93 | 289 | 32.34 | ||
| Transfusion group | 99 | 24 | 76 | 304 | 25.15 | ||
| Blood-transfusion reaction | 5.33 (1.67–23.52) | 0.05 | |||||
| Observation group | 31‡ | 1 | 1 | 90 | 1.66 | ||
| Transfusion group | 90§ | 15 | 24 | 277 | 8.85 | ||
| Ferritin >1500 ng/ml | 14.42 (5.41–875.17) | <0.001 | |||||
| Observation group | 31‡ | 3 | 33 | 90 | 37.07 | ||
| Transfusion group | 90§ | 76 | 1479 | 277 | 534.70 | ||
The incidence ratio was calculated as the rate of adverse events per 100 person-years in the transfusion group divided by the rate of adverse events per 100 person-years in the observation group. The 95% confidence intervals were calculated with the use of the bootstrap method with 10,000 replications.
This event was documented in male participants only.
A total of 31 participants who were randomly assigned to observation received one or more transfusions.
A total of 9 participants who declined transfusions were excluded from this analysis.
DISCUSSION
Silent cerebral infarcts have only recently been recognized as an important clinical complication of sickle cell anemia. Despite the high prevalence of silent cerebral infarcts4 and their association with lower IQ,8,15 poor academic performance,7 and increased risk for stroke,16 no evidence-based approach has been developed to systematically identify and treat children with silent cerebral infarcts. The primary results of our study indicate that children with sickle cell anemia, silent cerebral infarcts, and normal transcranial Doppler measurements will have a 58% relative risk reduction in the recurrence of infarcts while they are receiving regular blood-transfusion therapy.
The benefits of blood-transfusion therapy for the secondary prevention of infarct recurrence in SIT are substantial but are lower than those in STOP,9 which also used blood transfusion for the primary prevention of stroke. In STOP, the relative risk reduction was 92%.9 Although the benefit of blood-transfusion therapy in preventing infarct recurrence in children with silent cerebral infarcts is lower than the benefit of blood transfusions for primary stroke prevention, the prevalence of silent cerebral infarcts (which occur in approximately 33% of children with sickle cell anemia) is much higher than the prevalence of abnormal transcranial Doppler studies (approximately 10% in an unscreened population17). Thus, a greater number of children with silent cerebral infarcts are expected to benefit from blood-transfusion therapy.
Among children with preexisting silent cerebral infarcts, the precise mechanisms whereby regular blood transfusions decrease the incidence of infarct recurrence are unclear. Previously published data from SIT indicate that children in the lowest quartile of hemoglobin levels at baseline have higher odds of silent cerebral infarct than do those in the top quartile.18 Furthermore, children with sickle cell anemia, all of whom have chronic anemia, have evidence of ongoing subclinical ischemic injury.19 Acute reduction in hemoglobin concentration (<5.5 g per deciliter) in hospitalized children is temporally associated with an increase in new-onset silent cerebral infarcts, whether or not the child has sickle cell anemia.20 These findings suggest that the pathogenesis of silent cerebral infarcts could be explained in part by acute or chronic anemia with cerebral hemodynamic decompensation.21 Regular blood-transfusion therapy partially corrects the anemia and attenuates the risk of infarct recurrence, possibly by improving cerebrovascular reserve.
The timing for detecting silent cerebral infarcts is unclear. In a small study, the prevalence of silent cerebral infarcts at an average age of 13.7 months was 13%.22 In a second study, in which surveillance MRI was conducted among children up to 6 years of age, the prevalence of silent cerebral infarct was 27%.23 In a third study, the prevalence by 14 years of age was 37%.4 Thus, the majority of silent cerebral infarcts have occurred in children with sickle cell anemia by 6 years of age. However, performing MRI of the brain among children younger than 6 years of age often requires sedation. In SIT, the youngest age for evaluating silent cerebral infarcts was 5 years, and in the observation group, younger children were more likely than older children to have infarct recurrence. These findings suggest that, at a minimum, one surveillance MRI of the brain, preferably without sedation, should be performed in children with sickle cell anemia who are beginning elementary school.
Detecting silent cerebral infarcts in children is particularly important owing to the predicted effects on cognition and now the evidence from this trial that infarct recurrence can be prevented in most children. IQ scores in children with silent cerebral infarcts are 5 points lower than those in children without silent cerebral infarcts,24 which corresponds to a 5 to 9% reduction in annual income as adults.25 If silent cerebral infarcts are detected at the time children begin elementary school, cognitive difficulties may be identified and academic support initiated. Although our results did not show that infarct recurrence was associated with decreased IQ scores, the absence of a change in IQ scores must be interpreted cautiously, because infarct recurrence occurred in only 10% of our participants. In addition, specific cognitive measures such as executive function, attention, and memory might have been more sensitive than measurements of IQ to change associated with infarct recurrence.
After detection of a silent cerebral infarct, treatment options can be discussed with families, including the benefits of regular blood-transfusion therapy (decreased incidences of cerebral infarct recurrence, pain events necessitating hospitalization, priapism, avascular necrosis, and acute chest syndrome), associated risks (excessive iron stores, the need for chelation therapy, transfusion reactions, central venous catheter placement, and red-cell alloimmunization), and burdens (monthly clinic visits with associated missed school and work time). The duration of blood-transfusion therapy for the secondary prevention of silent cerebral infarcts is unknown, but results from SIT suggest that a minimum of 3 years of therapy should be considered.
To prevent alloimmunization, every effort was made to ensure that participants were matched for red-cell antigens that are most commonly associated with antibody formation in sickle cell disease.26 Alloimmunization occurred despite these efforts but was infrequent. The rate of alloimmunization was similar to that in a previous clinical trial involving participants with sickle cell disease that used identical minor red-cell antigen matching.27
The results of SIT are not directly applicable to all children with sickle cell anemia, because children who were receiving hydroxyurea therapy for severe disease, had elevated transcranial Doppler measurements, were receiving blood transfusions for primary stroke prevention, or had epilepsy were excluded. More than 15% of the children assigned to the transfusion group (15 of 99 children) never received effective therapy. Despite the fact that 9 participants who were randomly assigned to the transfusion group declined transfusion therapy immediately after assignment and 6 in that group crossed over to the observation group at a median of 34 days, the incidence rate for infarct recurrence was significantly lower among participants in the transfusion group than among participants in the observation group. A greater therapeutic effect might have been observed if treatment had been received for 36 months by all the participants who had been randomly assigned to receive blood-transfusion therapy.
In summary, blood-transfusion therapy reduced the incidence of infarct recurrence among children with sickle cell anemia who had silent cerebral infarcts. Research is needed to identify the children with silent cerebral infarcts who are at greatest risk for infarct recurrence, so that transfusion therapy can be targeted specifically to these children.
Supplementary Material
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supported by grants from the National Institute of Neurological Disorders and Stroke (5U01NS042804, 3U01NS042804 [American Recovery Reinvestment ACT supplementary grant] to Dr. DeBaun); the Institute of Clinical and Translational Sciences, National Center for Research Resources, and the National Center for Advancing Translational Sciences, Clinical and Translational Research; NIH Roadmap for Medical Research (UL1TR000448, to Washington University; UL1TR001079, to Johns Hopkins University; and UL1TR000003, to the Children’s Hospital of Philadelphia); and Research and Development in the National Health Service, United Kingdom.
Dr. McKinstry reports receiving honoraria and lecture fees from Siemens Healthcare and consulting fees from Guerbet; Dr. Woods, receiving fees for serving on a data and safety monitoring board from Mast Therapeutics and grant support from ClinDatrix and Novartis; Dr. Kwiatkowski, receiving fees for serving on an advisory board from Shire Pharmaceuticals, consulting fees from Shire Pharmaceuticals and Sideris Pharmaceuticals, and grant support from Resonance Health; Dr. Heiny, receiving lecture fees from Novartis; Dr. Redding-Lallinger, receiving grant support from Eli Lilly and Mast Therapeutics; and Dr. Casella, receiving honoraria, travel support, and consulting fees through his institution from Mast Therapeutics and being an inventor and a named party on a patent and licensing agreement for an assay panel of brain biomarkers for the detection of brain injury (PCT US2011/056338), licensed to ImmunArray with pending royalties only. No other potential conflict of interest relevant to this article was reported.
We thank the more than 1000 families of children with sickle cell disease that participated in this trial; the medical monitor, Andrew Campbell, M.D., and the neurology committee members (Rebecca Ichord, M.D., Michael Dowling, M.D., Ph.D., and E. Steve Roach, M.D.); and George Dover, M.D., for his review of and comments on an earlier version of the manuscript.
APPENDIX
The authors’ full names and academic degrees are as follows: Michael R. DeBaun, M.D., M.P.H., Mae Gordon, Ph.D., Robert C. McKinstry, M.D., Ph.D., Michael J. Noetzel, M.D., Desiree A. White, Ph.D., Sharada A. Sarnaik, M.D., Emily R. Meier, M.D., Thomas H. Howard, M.D., Suvankar Majumdar, M.D., Baba P.D. Inusa, M.D., Paul T. Telfer, M.D., Melanie Kirby-Allen, M.D., Timothy L. McCavit, M.D., Annie Kamdem, M.D., Gladstone Airewele, M.D., Gerald M. Woods, M.D., Brian Berman, M.D., Julie A. Panepinto, M.D., M.S.P.H., Beng R. Fuh, M.D., Janet L. Kwiatkowski, M.D., Allison A. King, M.D., M.P.H., Jason M. Fixler, M.D., Melissa M. Rhodes, M.D., Alexis A. Thompson, M.D., M.P.H., Mark E. Heiny, M.D., Ph.D., Rupa C. Redding-Lallinger, M.D., Fenella J. Kirkham, M.D., Natalia Dixon, M.D., Corina E. Gonzalez, M.D., Karen A. Kalinyak, M.D., Charles T. Quinn, M.D., John J. Strouse, M.D., Ph.D., J. Philip Miller, A.B., Harold Lehmann, M.D., Ph.D., Michael A. Kraut, M.D., Ph.D., William S. Ball, Jr., M.D., Deborah Hirtz, M.D., and James F. Casella, M.D.
The authors’ affiliations are as follows: Department of Pediatrics, Division of Hematology–Oncology, Vanderbilt–Meharry Center of Excellence in Sickle Cell Disease, Vanderbilt University School of Medicine, Nashville (M.R.D.); Department of Ophthalmology and Visual Sciences, Division of Biostatistics (M.G.), Departments of Radiology and Pediatrics (R.C.M.), Neurology and Pediatrics (M.J.N.), and Psychology (D.A.W.), the Program in Occupational Therapy and Department of Pediatrics Hematology–Oncology (A.A.K.), and the Division of Biostatistics and Department of Internal Medicine (J.P.M.), Washington University School of Medicine, St. Louis; Department of Pediatrics, Division of Hematology–Oncology, Wayne State University, Detroit (S.A.S.); Center for Cancer and Blood Disorders, Children’s National Medical Center, Department of Pediatrics, George Washington University Medical Center (E.R.M.), and Department of Pediatrics, Division of Hematology–Oncology, Georgetown University Hospital (C.E.G.) — all in Washington, DC; Department of Pediatrics, Division of Hematology–Oncology, University of Alabama at Birmingham, Birmingham (T.H.H.); Department of Pediatrics, Division of Hematology–Oncology, University of Mississippi Medical Center, Jackson (S.M.); Department of Paediatrics, Evelina Children’s Hospital, St. Thomas’ Hospital NHS Trust (B.P.D.I.), Department of Pediatric Hematology, Royal London Hospital, Barts Health NHS Trust (P.T.T.), and the Neurosciences Unit, Institute of Child Health, University College London (F.J.K.) — all in London; Hospital for Sick Children, Department of Paediatrics, Haematology–Oncology, University of Toronto, Toronto (M.K.-A.); Division of Hematology–Oncology, Department of Pediatrics, UT Southwestern Medical Center, Dallas (T.L.M.C.); Département Pédiatrie, Hôpital Intercommunal de Creteil, Creteil, France (A.K.); Department of Pediatrics, Division of Hematology–Oncology, Baylor College of Medicine, Houston (G.A.); Department of Pediatrics, Hematology–Oncology, University of Missouri-Kansas City, Kansas City (G.M.W.); Department of Pediatrics, Division of Hematology–Oncology, Case Western Reserve University, Cleveland (B.B.), Department of Pediatrics, Division of Hematology–Oncology, Ohio State University, Columbus (M.M.R.), and Department of Pediatrics, Division of Hematology (K.A.K., C.T.Q.), and Department of Radiology (W.S.B.), Cincinnati Children’s Hospital Medical Center, Cincinnati — all in Ohio; Department of Pediatrics, Hematology–Oncology, Medical College of Wisconsin, Milwaukee (J.A.P.); Department of Pediatrics, Hematology–Oncology, Brody School of Medicine, Greenville (B.R.F.), Department of Pediatrics, Hematology–Oncology, University of North Carolina, Chapel Hill (R.C.R.-L.), and Department of Pediatrics, Hematology–Oncology, Wake Forest University Health Sciences, Winston-Salem (N.D.) — all in North Carolina; Division of Hematology, Children’s Hospital of Philadelphia and Department of Pediatrics, Perelman School of Medicine at the University of Pennsylvania — both in Philadelphia (J.L.K.); Department of Pediatrics, Hematology–Oncology, Sinai Hospital (J.M.F.), and Department of Pediatrics and Medicine, Division of Pediatric Hematology (J.J.S.), Department of Pediatrics, Division of Hematology (J.F.C.), and Department of Radiology, Division of Neuroradiology (M.A.K.), Johns Hopkins University School of Medicine, Baltimore, the Division of Health Sciences of Informatics, Johns Hopkins Bloomberg School of Public Health, Baltimore (H.L.), and the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda (D.H.) — all in Maryland; Department of Pediatrics, Division of Hematology–Oncology, Ann and Robert H. Lurie Children’s Hospital of Chicago, Northwestern University, Chicago (A.A.T.); and Department of Pediatrics, Hematology–Oncology, Indiana University–Purdue University Indianapolis, Indianapolis (M.E.H.).
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Lorey FW, Arnopp J, Cunningham GC. Distribution of hemoglobinopathy variants by ethnicity in a multiethnic state. Genet Epidemiol. 1996;13:501–512. doi: 10.1002/(SICI)1098-2272(1996)13:5<501::AID-GEPI6>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 2.Brousseau DC, Panepinto JA, Nimmer M, Hoffmann RG. The number of people with sickle-cell disease in the United States: national and state estimates. Am J Hematol. 2010;85:77–78. doi: 10.1002/ajh.21570. [DOI] [PubMed] [Google Scholar]
- 3.Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med. 2010;38(Suppl):S512–S521. doi: 10.1016/j.amepre.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 4.Bernaudin F, Verlhac S, Arnaud C, et al. Impact of early transcranial Doppler screening and intensive therapy on cerebral vasculopathy outcome in a newborn sickle cell anemia cohort. Blood. 2011;117:1130–1140. doi: 10.1182/blood-2010-06-293514. [DOI] [PubMed] [Google Scholar]
- 5.Glauser TA, Siegel MJ, Lee BC, De-Baun MR. Accuracy of neurologic examination and history in detecting evidence of MRI-diagnosed cerebral infarctions in children with sickle cell hemoglobinopathy. J Child Neurol. 1995;10:88–92. doi: 10.1177/088307389501000203. [DOI] [PubMed] [Google Scholar]
- 6.Pegelow CH, Macklin EA, Moser FG, et al. Longitudinal changes in brain magnetic resonance imaging findings in children with sickle cell disease. Blood. 2002;99:3014–3018. doi: 10.1182/blood.v99.8.3014. [DOI] [PubMed] [Google Scholar]
- 7.Schatz J, Brown RT, Pascual JM, Hsu L, DeBaun MR. Poor school and cognitive functioning with silent cerebral infarcts and sickle cell disease. Neurology. 2001;56:1109–1111. doi: 10.1212/wnl.56.8.1109. [DOI] [PubMed] [Google Scholar]
- 8.Bernaudin F, Verlhac S, Fréard F, et al. Multicenter prospective study of children with sickle cell disease: radiographic and psychometric correlation. J Child Neurol. 2000;15:333–343. doi: 10.1177/088307380001500510. [DOI] [PubMed] [Google Scholar]
- 9.Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339:5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 10.King AA, Noetzel M, White DA, McKinstry RC, Debaun MR. Blood transfusion therapy is feasible in a clinical trial setting in children with sickle cell disease and silent cerebral infarcts. Pediatr Blood Cancer. 2008;50:599–602. doi: 10.1002/pbc.21338. [DOI] [PubMed] [Google Scholar]
- 11.Casella JF, King AA, Barton B, et al. Design of the silent cerebral infarct transfusion (SIT) trial. Pediatr Hematol Oncol. 2010;27:69–89. doi: 10.3109/08880010903360367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) manual. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- 13.Wechsler D. Manual for the Wechsler Preschool and Primary Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1967. [Google Scholar]
- 14.Gioia GA, Isquith PK, Guy SC, Ken-worthy L. Behavior rating inventory of executive function. Child Neuropsychol. 2000;6:235–238. doi: 10.1076/chin.6.3.235.3152. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong FD, Thompson RJ, Jr, Wang W, et al. Cognitive functioning and brain magnetic resonance imaging in children with sickle cell disease. Pediatrics. 1996;97:864–870. [PubMed] [Google Scholar]
- 16.Miller ST, Macklin EA, Pegelow CH, et al. Silent infarction as a risk factor for overt stroke in children with sickle cell anemia: a report from the Cooperative Study of Sickle Cell Disease. J Pediatr. 2001;139:385–390. doi: 10.1067/mpd.2001.117580. [DOI] [PubMed] [Google Scholar]
- 17.Adams RJ, Brambilla DJ, Granger S, et al. Stroke and conversion to high risk in children screened with transcranial Doppler ultrasound during the STOP study. Blood. 2004;103:3689–3694. doi: 10.1182/blood-2003-08-2733. [DOI] [PubMed] [Google Scholar]
- 18.DeBaun MR, Sarnaik SA, Rodeghier MJ, et al. Associated risk factors for silent cerebral infarcts in sickle cell anemia: low baseline hemoglobin, sex, and relative high systolic blood pressure. Blood. 2012;119:3684–3690. doi: 10.1182/blood-2011-05-349621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quinn CT, McKinstry RC, Dowling MM, et al. Acute silent cerebral ischemic events in children with sickle cell anemia. JAMA Neurol. 2013;70:58–65. doi: 10.1001/jamaneurol.2013.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dowling MM, Quinn CT, Plumb P, et al. Acute silent cerebral ischemia and infarction during acute anemia in children with and without sickle cell disease. Blood. 2012;120:3891–3897. doi: 10.1182/blood-2012-01-406314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prohovnik I, Pavlakis SG, Piomelli S, et al. Cerebral hyperemia, stroke, and transfusion in sickle cell disease. Neurology. 1989;39:344–348. doi: 10.1212/wnl.39.3.344. [DOI] [PubMed] [Google Scholar]
- 22.Wang WC, Pavlakis SG, Helton KJ, et al. MRI abnormalities of the brain in one-year-old children with sickle cell anemia. Pediatr Blood Cancer. 2008;51:643–646. doi: 10.1002/pbc.21612. [DOI] [PubMed] [Google Scholar]
- 23.Kwiatkowski JL, Zimmerman RA, Pollock AN, et al. Silent infarcts in young children with sickle cell disease. Br J Haematol. 2009;146:300–305. doi: 10.1111/j.1365-2141.2009.07753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King AA, Strouse JJ, Rodeghier MJ, et al. Parent education and biologic factors influence on cognition in sickle cell anemia. Am J Hematol. 2014;89:162–167. doi: 10.1002/ajh.23604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zagorsky JL. Do you have to be smart to be rich? The impact of IQ on wealth, income and financial distress. Intelligence. 2007;35:489–501. [Google Scholar]
- 26.Vichinsky EP, Earles A, Johnson RA, Hoag MS, Williams A, Lubin B. Alloimmunization in sickle cell anemia and transfusion of racially unmatched blood. N Engl J Med. 1990;322:1617–1621. doi: 10.1056/NEJM199006073222301. [DOI] [PubMed] [Google Scholar]
- 27.Vichinsky EP, Luban NL, Wright E, et al. Prospective RBC phenotype matching in a stroke-prevention trial in sickle cell anemia: a multicenter transfusion trial. Transfusion. 2001;41:1086–1092. doi: 10.1046/j.1537-2995.2001.41091086.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.