Abstract
Background. Prior receipt of a trivalent seasonal influenza vaccine (TIV) can affect hemagglutination inhibition (HI) antibody responses to pandemic influenza vaccines. We investigated the effect of TIV priming on humoral responses to AS03-adjuvanted and nonadjuvanted A(H1N1)pdm09 vaccines, the role of AS03 on cell-mediated immune (CMI) responses, and vaccine safety.
Methods. Healthy adults (aged 19–40 years) were randomized 1:1:1:1 to receive TIV or saline followed 4 months later by 2 doses, 3 weeks apart, of adjuvanted or nonadjuvanted A(H1N1)pdm09 vaccine and followed up to study end (day 507). Pre- and postvaccination responses of HI and neutralizing antibody, CD4+/CD8+ T cells, memory B cells, and plasmablasts were assessed.
Results. Ninety-nine of the 133 participants enrolled completed the study. No vaccine-related serious adverse events were recorded. In TIV-primed participants, A(H1N1)pdm09-specific antibody and CD4+ T-cell and memory B-cell responses to the pandemic vaccine tended to be diminished. Vaccine adjuvantation led to increased responses of vaccine-homologous and -heterologous HI and neutralizing antibodies and CD4+ T cells, homologous memory B cells, and plasmablasts.
Conclusions. In healthy adults, prior TIV administration decreased humoral and CMI responses to A(H1N1)pdm09 vaccine. Adjuvantation of A(H1N1)pdm09 antigen helped to overcome immune interference between the influenza vaccines. No safety concerns were observed.
Registration. Clinical Trials.gov identifier NCT00707967.
Keywords: A(H1N1)pdm09 vaccine, AS03, TIV, pandemic influenza, immune interference, HI antibody, neutralizing antibody, CD4+ T cells, B cells, plasmablasts
From the recognized onset of an H1N1 pandemic in 2009 until its end in August 2010, the swine-origin A/California/7/2009 [A(H1N1)pdm09] virus caused more than 600 000 laboratory-confirmed infections and 18 449 deaths [1, 2], though estimates suggest a death toll of 15 times that amount [3]. In response, a number of A(H1N1)pdm09 vaccines were developed [4–6], several of which were formulated with an adjuvant to enhance immunogenicity and reduce the antigen dose [7–9].
Influenza A(H1N1)pdm09 vaccine clinical studies have shown that the prevaccination serostatus of the studied population is an important determinant for the vaccine's immunogenicity [5, 8–19]. Specifically, results from a number of trials have suggested that prior receipt of a trivalent seasonal influenza vaccine (TIV) or another nonadjuvanted influenza vaccine can affect the hemagglutination inhibition (HI) antibody responses to adjuvanted [8, 10–14] and nonadjuvanted [5, 9, 15–19] A(H1N1)pdm09 or H5N1 [20, 21] vaccines. Several authors [16, 22] have suggested that this effect of immune interference is akin to the original antigenic sin mechanism [23], in which sequential exposure to closely related viruses can lead to diminished antibody responses to the novel antigens in the strain of the last exposure. However, the relevance of this concept for sequential influenza vaccinations is subject to debate [24, 25].
In mice, it has been shown that such immune interference effects may be circumvented by adjuvantation of the priming vaccine [26]. This is in agreement with recent clinical studies that show that adjuvants can enhance CD4+ T-cell responses [9, 14, 27]. Increased CD4+ T-cell responses, in turn, could promote B-cell adaptation to antigens that are less related to the specificities in the memory B-cell pool, such as those present in a pandemic influenza vaccine [28, 29]. Thus, CD4+ T-cell responses could play a role in shaping an immune response that is better prepared to respond to the subsequent vaccination. Indeed, clinical studies have shown that Adjuvant System 03 (AS03) [30] enhanced CD4+ T-cell and humoral responses to A(H1N1)pdm09 [9, 14] and H5N1 [27] antigens.
Our aim in this study was to investigate the effect of priming with TIV on subsequent HI and neutralizing antibody (NAb) responses to adjuvanted and nonadjuvanted A(H1N1)pdm09 vaccines. We also evaluated the role of AS03 on the frequency and/or phenotypes of cell-mediated immune (CMI) responses in terms of T cells, memory B cells, and plasmablasts, as well as vaccine safety. We selected a study population (aged 19–40 years) for whom potential immunosenescence effects would not be expected.
METHODS
Study Design
This phase 1/2 randomized observer-blind study (clinicaltrials.gov; NCT01059617) was conducted from February 2010 through January 2011 at 3 study centers in the United States, after approval by an independent local ethics committee. The study was undertaken in accordance with the Helsinki Declaration and good clinical practices. All participants provided written consent before entering the study.
Using an Internet-based algorithm, eligible participants were randomized 1:1:1:1 to 4 parallel groups to receive either TIV followed by 2 doses of adjuvanted (group A) or nonadjuvanted (group B) A(H1N1)pdm09 vaccine, or placebo (saline) followed by 2 doses of adjuvanted (group C) or nonadjuvanted (group D) A(H1N1)pdm09 vaccine (Figure 1). Vaccine doses were administered on day 0 (TIV/placebo; dose 1), followed 4 months later by 2 doses of A(H1N1)pdm09 vaccine (doses 2 and 3) given at 3-week intervals (days 122 and 143). Participants were followed for 364 days after dose 3 (day 507).
Figure 1.
CONSORT diagram of study flow. A/Cal*, A(H1N1)pdm09 vaccine. Group A received trivalent seasonal influenza vaccine (TIV) and 2 doses of A(H1N1)pdm09 (3.75 µg) /AS03. Group B received TIV and 2 doses of A(H1N1)pdm09 (15 µg). Group C received saline and 2 doses of A(H1N1)pdm09 (3.75 µg) /AS03. Group D received saline and 2 doses of A(H1N1)pdm09 (15 µg). Abbreviations: D, day; N, number of participants who received the vaccine and for whom data were available.
Blood samples for immunogenicity and safety evaluations were taken before vaccination (days 0, 122, and 143); 1, 2, and 3 weeks post dose 1 (days 7, 14, and 21); 3, 7, and 14 days post dose 2 (days 125, 129, and 136); and 1, 2, 3, and 23 weeks post dose 3 (days 150, 157, 164, and 304, respectively).
Study Objectives
The primary objective was to describe the homologous and heterologous HI antibody responses following adjuvanted or nonadjuvanted A(H1N1)pdm09 vaccination of individuals vaccinated previously with TIV or placebo. Secondary objectives were to describe the homologous and heterologous NAb and CMI responses to the pandemic vaccine virus, as well as vaccine safety across the study period.
Study Participants
Healthy participants (male and female) aged 19–40 years were enrolled. Exclusion criteria were as follows: previous administration of any influenza vaccine including any A/California/7/2009 (H1N1)v-like virus vaccine, administration of any vaccine within 30 days before first vaccination, use of investigational or nonregistered products, confirmed or suspected immunosuppression, receipt of immunoglobulins or blood products within 9 months preceding the study, and known or suspected allergy to any of the vaccine constituents.
Study Vaccines
GlaxoSmithKline, Quebec, Canada, manufactured all vaccines. The TIV (FluLaval™ 2009–2010) was an inactivated split-virion influenza vaccine that contained 45 µg hemagglutinin (HA) antigen/0.5-mL dose (15 µg HA of each of 3 strains [A/Brisbane/59/2007(H1N1) IVR-148, A/Uruguay/716/2007(H3N2) NYMCX-175C, and B/Brisbane/60/2008(B)]. The placebo was phosphate-buffered saline (PBS; 0.5-mL dose).
The A(H1N1)pdm09 vaccine was a monovalent split-virion influenza vaccine that contained HA of A/California/7/2009(H1N1) NYMC X-179A mixed with either PBS to contain 15 µg HA/0.5-mL dose (groups B and D) or with AS03A to contain 3.75 µg HA/0.5-mL dose (groups A and C). AS03A (henceforth referred to as AS03) is an oil-in-water emulsion-based adjuvant system that contains 11.86 mg tocopherol/dose. Vaccines and placebo were administered intramuscularly (deltoid) in the nondominant arm (doses 1 and 2) or dominant arm (dose 3).
Immunogenicity Evaluations
Immunogenicity evaluations were performed at each blood-sampling time point with the exception of visit days 14 and 125 for NAb responses and visit day 125 for HI responses.
Assessment of Humoral Immune Responses
Neutralization and HI assays were performed as described previously [12, 31]. HI responses were assessed using seropositivity rates (percentages of participants with titer ≥1:10), seroconversion rates (SCRs; percentage of participants with prevaccination titer <1:10 and postvaccination titer ≥1:40 or prevaccination titer >1:10 and ≥4-fold increase in postvaccination titer, with day 122 considered as prevaccination for day 304), seroprotection rates (SPRs; percentage of participants with titer ≥1:40), and geometric mean fold rises (GMFRs; geometric mean of the within-participant ratios of postvaccination reciprocal titer to prevaccination reciprocal titer at days 0 or 122). Assessment of HI responses was based on the European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP) guidance targets for pandemic influenza vaccines in adults [32] (point estimates, SCR >40%; SPR >70%; GMFR >2.5) and the Center for Biologics Evaluation and Research (CBER) licensure criteria [33] [lower limits of 95% confidence interval (CI) ≥40% for SCR and ≥70% for SPR]. Seropositivity rate for NAb responses was the percentage of participants with titer ≥1:28.
Assessment of T-Cell and B-Cell Responses
T-cell frequencies were evaluated using intracellular cytokine staining (ICS) and flow cytometry, as described previously [9]. B-cell frequencies were assessed by memory B-cell enzyme-linked immunosorbent spot (ELISPOT), as described previously [27]. Frequencies of plasmablasts (CD27+/CD38+ B cells) were evaluated following a previously described method [24] and expressed as percentages of CD3−/CD20lo/CD19+ B cells.
Safety and Reactogenicity Evaluation
Solicited local and general adverse events (AEs) were recorded during the 7-day period post vaccination (days 0–6, 122–128, 143–149); unsolicited AEs during the 84-day, 42-day, and 21-day periods following doses 1, 2 and 3, respectively; and selected hematological and biochemical clinical laboratory parameter abnormalities on days 0, 21, 122, 164, and 304. The occurrence of/relationship to vaccination of medically attended adverse events (MAEs), potential immune-mediated diseases (pIMDs), serious AEs (SAEs), and withdrawals due to AEs were assessed up to day 507.
Statistical Methods
Descriptive analyses were performed. Immunogenicity analyses were performed on the according-to-protocol cohorts for immunogenicity (ATP-I) at days 164 and 304. Geometric mean titers (GMTs), SCRs, SPRs, and GMFRs for HI antibodies and GMTs and SCRs for NAb responses were tabulated with 95% CIs. Safety analysis was based on the total vaccinated cohort (TVC). Incidences of solicited and unsolicited AEs after each vaccination were tabulated with 95% CIs.
RESULTS
Population Demographics
Overall, 171 participants were screened, of whom 133 (77.8%) were vaccinated and included in the TVC. Ninety-nine patients completed the study at day 507 (Figure 1). Withdrawals from the study (24 participants) were mainly due to loss to follow-up. Demographics were comparable across groups. In the TVC, the median age was 29 years (range, 19–40 as per protocol), the male-to-female ratio was 45.9%:54.1%, and the majority of participants were of white-Caucasian/European (70.7%) or African (27.1%) heritages.
Immunogenicity
A(H1N1)pdm09-Specific HI and NAb Responses
After TIV or placebo administration (dose 1; days 0–122), GMTs of A(H1N1)pdm09-specific HI responses in both groups remained low (ranges, 12.5–19.9 and 13.3–13.9, respectively), and CHMP or CBER criteria were not met (Supplementary Table 1).
At day 122, the day of the first A(H1N1)pdm09 vaccination (dose 2), A(H1N1)pdm09-specific HI antibody responses (GMTs) ranged from 12.5 to 16.1 across groups (Table 1 and Figure 2A). Two weeks later (day 136), GMTs had increased substantially (range GMFRs, 24–40 across groups) and, when comparing the adjuvanted groups A and C as well as the nonadjuvanted groups B and D, tended to be lower after previous administration of TIV (GMTs 492, 603, 304, and 386, respectively). There was also a tendency for higher GMTs in the adjuvanted groups relative to nonadjuvanted groups. All participants were A(H1N1)pdm09 seropositive from day 136 onward (14 days following first dose of A(H1N1)pdm09 vaccine [dose 2]).
Table 1.
A(H1N1)pdm09-Specific Hemagglutination Inhibition Responses Before and After Vaccination
| Group | Day | N | n | Seroconversion Rate, % (95% CI) | n | Seroprotection Rate, % (95% CI) | n | Seropositivity, % (95% CI) | Geometric Mean Fold Rise Value (95% CI) | Geometric Mean Titer Value (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| A | 122 | 21 | NA | 7 | 33.3 (14.6–57.0) | 12 | 57.1 (34.0–78.2) | NA | 16.1 (8.9–29.3) | |
| 129 | 21 | 9 | 42.9 (21.8–66) | 13 | 61.9 (38.4–81.9) | 20 | 95.2 (76.2–99.9) | 4.1 (2.5–6.7) | 66.7 (35.7–124.6) | |
| 136 | 21 | 21 | 100 (83.9–100) | 21 | 100 (83.9–100) | 21 | 100 (83.9–100) | 30.5 (17.4–53.3) | 491.6 (319.3–756.9) | |
| 143 | 21 | 19 | 90.5 (69.6–98.8) | 21 | 100 (83.9–100) | 21 | 100 (83.9–100) | 24.2 (13.6–42.9) | 390.1 (266.3–571.5) | |
| 150 | 19 | 19 | 100 (82.4–100) | 19 | 100 (82.4–100) | 19 | 100 (82.4–100) | 31.4 (17.4–56.9) | 452.8 (305.6–670.9) | |
| 157 | 20 | 20 | 100 (83.2–100) | 20 | 100 (83.2–100) | 20 | 100 (83.2–100) | 43.0 (25.3–73.2) | 651.3 (487.1–870.8) | |
| 164 | 21 | 21 | 100 (83.9–100) | 21 | 100 (83.9–100) | 21 | 100 (83.9–100) | 40.3 (22.9–71.2) | 650.7 (485.5–872.1) | |
| 304 | 21 | 16 | 76.2 (52.8–91.8) | 19 | 90.5 (69.6–98.8) | 21 | 100 (83.9–100) | NA | 157.4 (98.2–252.2) | |
| B | 122 | 33 | NA | 7 | 21.2 (9.0–38.9) | 14 | 42.4 (25.5–60.8) | NA | 12.5 (7.7–20.1) | |
| 129 | 31 | 9 | 29.0 (14.2–48.0) | 17 | 54.8 (36.0–72.7) | 26 | 83.9 (66.3–94.5) | 4.2 (2.3–7.6) | 54.6 (28.7–103.9) | |
| 136 | 33 | 26 | 78.8 (61.1–91.0) | 30 | 90.9 (75.7–98.1) | 33 | 100 (89.4–100) | 24.4 (13.3–44.8) | 303.7 (191.7–481.0) | |
| 143 | 33 | 26 | 78.8 (61.1–91.0) | 31 | 93.9 (79.8–99.3) | 33 | 100 (89.4–100) | 20.6 (11.4–37.2) | 256.6 (164.9–399.3) | |
| 150 | 31 | 26 | 83.9 (66.3–94.5) | 30 | 96.8 (83.3–99.9) | 31 | 100 (88.8–100) | 22.6 (12.9–39.8) | 292.7 (198.0–432.7) | |
| 157 | 32 | 27 | 84.4 (67.2–94.7) | 32 | 100 (89.1–100) | 32 | 100 (89.1–100) | 23.6 (14.0–39.8) | 303.2 (215.1–427.4) | |
| 164 | 33 | 28 | 84.8 (68.1–94.9) | 33 | 100 (89.4–100) | 33 | 100 (89.4–100) | 20.8 (12.5–34.6) | 259.4 (186.4–361.0) | |
| 304 | 32 | 21 | 65.6 (46.8–81.4) | 28 | 87.5 (71.0–96.5) | 32 | 100 (89.1–100) | NA | 100.3 (68.0–148.2) | |
| C | 122 | 25 | NA | 6 | 24.0 (9.4–45.1) | 13 | 52.0 (31.3–72.2) | NA | 13.9 (8.1–24.0) | |
| 129 | 25 | 14 | 56.0 (34.9–75.6) | 18 | 72.0 (50.6–87.9) | 23 | 92.0 (74.0–99.0) | 6.0 (3.4–10.4) | 83.3 (44.2–157.3) | |
| 136 | 23 | 22 | 95.7 (78.1–99.9) | 23 | 100 (85.2–100) | 23 | 100 (85.2–100) | 39.6 (21.7–72.1) | 602.5 (434.1–836.4) | |
| 143 | 25 | 24 | 96.0 (79.6–99.9) | 25 | 100 (86.3–100) | 25 | 100 (86.3–100) | 40.0 (22.1–72.4) | 557.3 (426.5–728.3) | |
| 150 | 25 | 24 | 96.0 (79.6–99.9) | 25 | 100 (86.3–100) | 25 | 100 (86.3–100) | 40.6 (22.6–72.8) | 565.0 (423.3–754.1) | |
| 157 | 24 | 23 | 95.8 (78.9–99.9) | 24 | 100 (85.8–100) | 24 | 100 (85.8–100) | 56.3 (30.7–103.3) | 818.1 (634.9–1054.0) | |
| 164 | 25 | 25 | 100 (86.3–100) | 25 | 100 (86.3–100) | 25 | 100 (86.3–100) | 57.4 (31.6–104.2) | 799.0 (630.5–1012.4) | |
| 304 | 21 | 16 | 76.2 (52.8–91.8) | 21 | 100 (83.9–100) | 21 | 100 (83.9–100) | NA | 289.9 (188.2–446.6) | |
| D | 122 | 27 | NA | 4 | 14.8 (4.2–33.7) | 13 | 48.1 (28.7–68.1) | NA | 12.9 (8.1–20.7) | |
| 129 | 27 | 17 | 63.0 (42.4–80.6) | 20 | 74.1 (53.7–88.9) | 23 | 85.2 (66.3–95.8) | 5.5 (3.4–8.9) | 70.3 (38.8–127.6) | |
| 136 | 26 | 25 | 96.2 (80.4–99.9) | 26 | 100 (86.8–100) | 26 | 100 (86.8–100) | 28.8 (16.7–49.9) | 385.6 (242.9–612.1) | |
| 143 | 26 | 24 | 92.3 (74.9–99.1) | 26 | 100 (86.8–100) | 26 | 100 (86.8–100) | 27.3 (15.8–47.3) | 365.6 (241.3–553.9) | |
| 150 | 26 | 24 | 92.3 (74.9–99.1) | 26 | 100 (86.8–100) | 26 | 100 (86.8–100) | 24.9 (14.9–41.6) | 333.3 (220.8–503) | |
| 157 | 25 | 23 | 92.0 (74.0–99.0) | 25 | 100 (86.3–100) | 25 | 100 (86.3–100) | 24.3 (14.8–40.0) | 338.2 (230.7–495.7) | |
| 164 | 27 | 26 | 96.3 (81.0–99.9) | 27 | 100 (87.2–100) | 27 | 100 (87.2–100) | 25.8 (15.9–41.8) | 332.7 (222.0–498.6) | |
| 304 | 27 | 17 | 63.0 (42.4–80.6) | 23 | 85.2 (66.3–95.8) | 27 | 100 (87.2–100) | NA | 116.0 (72.7–185.2) |
Data shown are of the according-to-protocol cohort for immunogenicity (ATP-I cohort) at day 164, except for day 304, which are for the ATP-I cohort at day 304. Groups A and B received trivalent seasonal influenza vaccine at day 0 and either adjuvanted (group A) or nonadjuvanted (group B) pandemic vaccine at day 122 and day 143. Groups C and D received saline at day 0 and adjuvanted (group C) or nonadjuvanted (group D) pandemic vaccine at day 122 and day 143. Seroconversion rate: n/%, = number/percentage of seroconverted participants. Seroconversion: titer ≥40 1/DIL after vaccination (for initially seronegative participants at day 0) or a titer after vaccination ≥4-fold the prevaccination titer (for initially seropositive participants at day 0). Seropositivity: n/%, number/percentage of participants with titer within the specified range. Seroprotection rate: n/%, number/percentage of participants with titer within the specified range. Geometric mean fold rise: geometric mean of the within-participant ratios of the post-vaccination reciprocal HI titer to the day 122 reciprocal HI titer before pandemic vaccination. Abbreviations: CI, confidence interval; NA, not available.
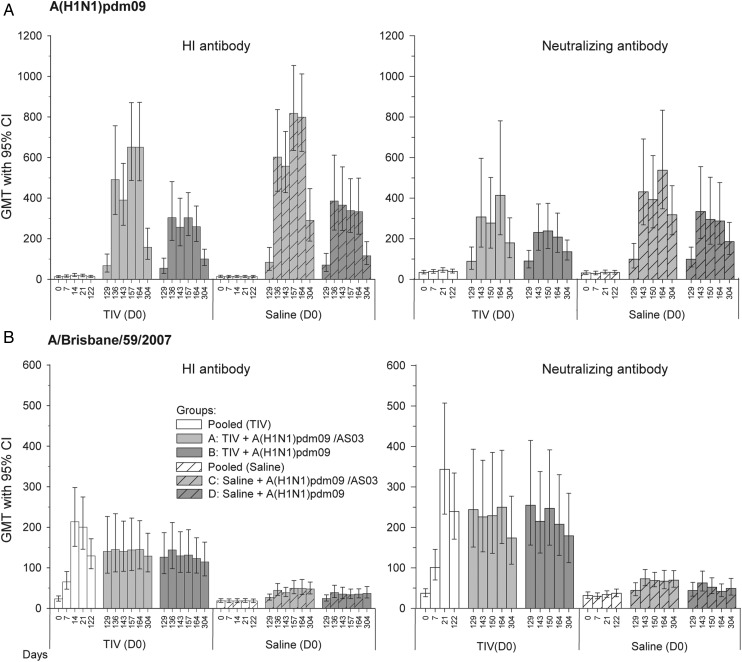
Figure 2.
A(H1N1)pdm09-specific and A/Brisbane/59/2007-specific hemagglutination inhibition (HI) and neutralizing antibody (NAb) responses. A(H1N1)pdm09-specific (panel A) and A/Brisbane/59/2007-specific (panel B) HI and NAb responses shown are of the according-to-protocol cohort for immunogenicity (ATP-I cohort) at D164, except those for day 304 (from ATP-I cohort at day 304). Group A received trivalent seasonal influenza vaccine (TIV) at day 0 and A(H1N1)pdm09 (3.75 µg)/AS03 at day 122 and day 143. Group B received TIV at day 0 and A(H1N1)pdm09 (15 µg) at day 122 and day 143. Group C received saline at day 0 and A(H1N1)pdm09 (3.75 µg)/AS03 at day 122 and day 143. Group D received saline at day 0 and A(H1N1)pdm09 (15 µg) at day 122 and day 143. Abbreviations: CI, confidence interval; GMT, geometric mean titer.
At day 164, 3 weeks after the second A(H1N1)pdm09 vaccination (dose 3), GMTs had further increased in the adjuvanted groups and remained relatively constant in the nonadjuvanted groups. GMTs in the saline-primed groups still tended to exceed those in the TIV-primed groups for both pandemic vaccine formulations, with this trend being greater for the adjuvanted groups A and C (651 vs 799) than for the nonadjuvanted groups B and D (259 vs 333). Moreover, GMTs were higher following adjuvanted vaccine relative to nonadjuvanted vaccine, with greater differences between the saline-primed groups C and D. Similar trends were observed in SCRs, SPRs, and GMFRs. SCRs were 84.8%–100% across groups, SPRs 100% in all groups, and GMFRs ranged from 20.8 (group B) to 57.4 (group C), thus fulfilling CHMP and CBER criteria. Between day 164 and day 304, GMTs decreased 3- to 4-fold but remained, along with SCRs, SPRs, and seropositivity rates, above baseline (day 122) values in all groups; at day 304 SCRs and SPRs still met CHMP criteria.
NAb responses followed trends that were similar to trends for HI responses, with a tendency for higher responses in saline-primed groups relative to TIV-primed groups (A < C and B < D) and in adjuvanted groups relative to nonadjuvanted groups (A > B and C > D). Differences between groups were generally smaller than observed for HI responses.
A/Brisbane/59/2007-Specific HI and NAb Responses
At day 21, an A/Brisbane/59/2007-specific HI antibody response was observed only in the TIV group (GMT 200 vs 18.8 for the saline group), with SPRs meeting CHMP and CBER criteria (Supplementary Table 1).
After A(H1N1)pdm09 vaccine administration (doses 2 and 3; days 129–304), GMTs in the TIV-primed groups remained at levels comparable to prevaccination levels (day 122; Supplementary Table 2 and Figure 2B). In the saline-primed groups, GMTs increased slightly but remained lower than in the TIV-primed groups. Irrespective of the priming, GMTs in the adjuvanted groups were comparable to those in the nonadjuvanted groups. At day 164, SCRs met the CHMP criterion in both TIV-primed groups A and B but not in the saline-primed groups C and D (76.2%, 54.5%, 24%, and 7.4%, respectively), while they met the CBER criterion only in group A. SPRs met the CHMP criterion in groups A–C at day 164 and day 304 (range, 72%–95.2%) but not in group D (51.9% on both days), while the CBER criterion was only met in both TIV-primed groups. The CHMP criterion for GMFR was met at day 164 in all groups except group D.
As compared with HI responses, NAb responses followed parallel trends (Figure 2B).
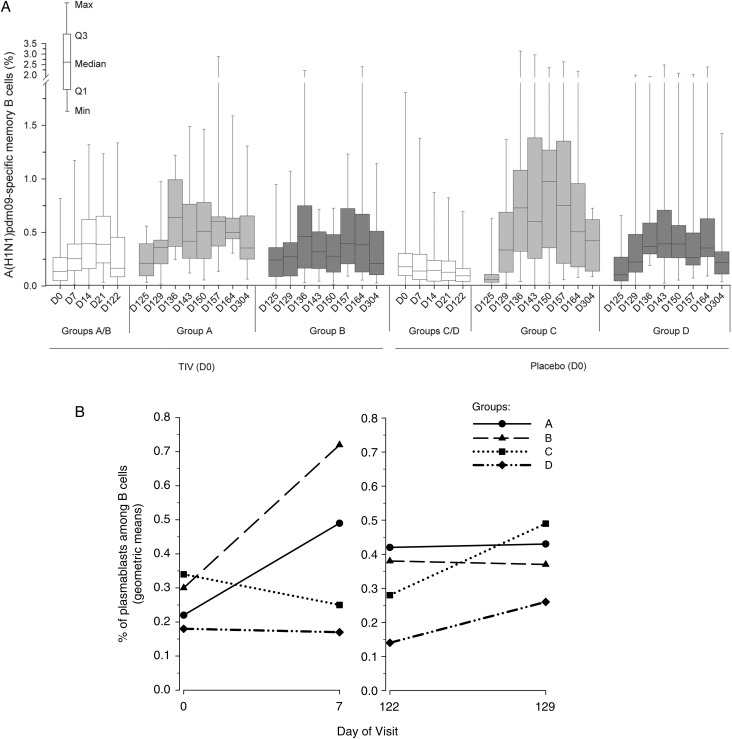
B-Cell Responses
We evaluated the roles of adjuvant and TIV priming in inducing B-cell responses. First, frequencies of A(H1N1)pdm09-specific memory B cells were measured by ELISPOT (Figure 3A). Low frequencies were observed prior to vaccination (day 0) in all groups. Some A(H1N1)pdm09-specific memory B-cell responses were induced by the TIV. Following vaccination with A(H1N1)pdm09 vaccines, stronger memory B-cell responses were observed with adjuvanted vaccine relative to nonadjuvanted vaccine (groups: A > B and C > D). Moreover, TIV vaccination prior to adjuvanted A(H1N1)pdm09 vaccination had a negative effect on the memory B-cell frequencies (group: C > A).
Figure 3.
A(H1N1)pdm09-specific memory B-cell responses to A(H1N1)pdm vaccine and proportion of plasmablasts (CD27+/CD38+) among B cells (CD19+/CD3−) after trivalent seasonal influenza vaccine (TIV) vaccination. A, Data shown are of the according-to-protocol cohort for immunogenicity at day 304. Group A received TIV at day 0 and A(H1N1)pdm09 (3.75 µg)/AS03 at day 122 and day 143. Group B received TIV at day 0 and A(H1N1)pdm09 (15 µg) at day 122 and day 143. Group C received saline at day 0 and A(H1N1)pdm09 (3.75 µg)/AS03 at day 122 and day 143. Group D received saline at day 0 and A(H1N1)pdm09 (15 µg) at day 122 and day 143. Data are reported as the percentages of A/California/7/2009-specific memory B cells of all B cells, with first and third quartiles, and the minimum/maximum values measured. B, Geometric means of plasmablast (CD27+/CD38+) frequencies expressed as a percentage of B cells (CD20lo/CD19+/CD3−) after administration of TIV (days 0–129). Abbreviation: D, day.
We also evaluated the frequencies of plasmablasts (defined as CD27+/CD38+ B cells) as a percentage of B cells (CD20lo/CD19+/CD3−; days 0–129; Figure 3B). It has previously been shown that plasmablasts can be detected 7 days after TIV vaccination and that a large proportion of CD27+/CD38+ plasmablasts are specific for influenza vaccine antigens [24]. Evaluation of CD27+/CD38+ plasmablast frequencies 1 week after dose 1 revealed increased frequencies in both TIV groups but not in the saline groups. One week post A(H1N1)pdm09 vaccination (day 129), plasmablast responses, defined by increased frequencies of CD27+/CD38+ B cells, were observed in both saline-primed groups but not in the TIV-primed groups, with the strongest increase in the (adjuvanted) group C.
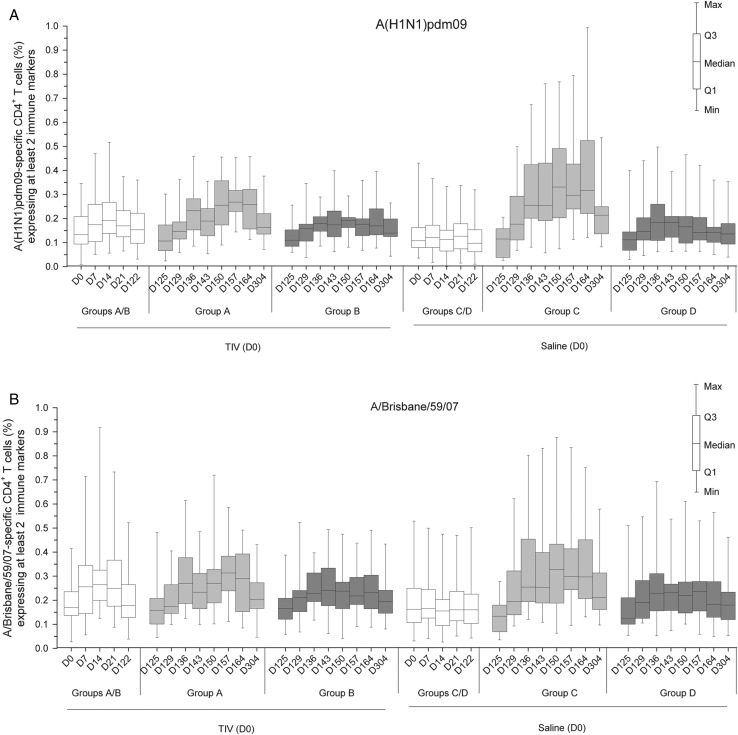
T-Cell Responses
In order to evaluate the effects of the adjuvant on the frequency and quality (polyfunctionality) of A(H1N1)pdm09-specific and TIV strain–specific T-cell responses, we assessed the vaccine-induced responses of antigen-specific CD4+ T cells expressing at least 2 immune markers (among CD40L, interleukin [IL]-2, tumor necrosis factor-alpha [TNF-α], and interferon-gamma [IFN-γ]) by ICS after in vitro stimulation of peripheral blood mononuclear cells.
Low responses of A(H1N1)pdm09-specific immune marker–expressing cells were seen after TIV administration but not after administration of saline (Figure 4A). After pandemic vaccination, A(H1N1)pdm09-specific CD4+ T-cell responses tended to be lower in the TIV-primed group A than in the saline-primed group C among the adjuvanted groups and were of comparable magnitudes for saline and TIV-primed groups among nonadjuvanted groups. Responses to the adjuvanted vaccine tended to be higher and more persistent, regardless of previous administrations of TIV or saline (groups: A > B and C > D). CD4+ T-cell responses specific for the TIV strains after pandemic vaccination followed similar patterns, although with smaller differences between groups (shown for A/Brisbane/59/2007; Figure 4B).
Figure 4.
Frequency of A(H1N1)pdm09-specific and A/Brisbane/59/2007-specific CD4+ T cells expressing at least 2 immune markers. Data shown are of the according-to-protocol cohort for immunogenicity at day 304. Group A received trivalent seasonal influenza vaccine (TIV) at day 0 and A(H1N1)pdm09 (3.75 µg)/AS03 at day 122 and day 143. Group B received TIV at day 0 and A(H1N1)pdm09 (15 µg) at day 122 and day 143. Group C received saline at day 0 and A(H1N1)pdm09 (3.75 µg)/AS03 at day 122 and day 143. Group D received saline at day 0 and A(H1N1)pdm09 (15 µg) at day 122 and day 143. Data are reported as the percentages of A(H1N1)pdm09-specific (A) or A/Brisbane/59/2007-specific (B) CD4+ T cells expressing (after in vitro stimulation) at least 2 immune markers among interferon-gamma, interleukin-2, tumor necrosis factor-alpha, and CD40L of all CD4+ T cells, with first and third quartiles, and the minimum/maximum values measured. Abbreviation: D, day.
Cytokine expression profiles of responding CD4+ T cells were assessed by evaluating the frequencies of antigen-specific cells producing at least 1 marker (Supplementary Figure 1; shown for A(H1N1)pdm09). Different cytokines were produced, with more cells expressing IL-2 and/or CD40L than TNF-α and/or IFN-γ. The strongest responses were measured in the adjuvanted groups A and C.
There were no detectable CD8+ T-cell responses to the A(H1N1)pdm09 strain or any of the TIV strains (data not shown).
Safety and Reactogenicity
Solicited AEs
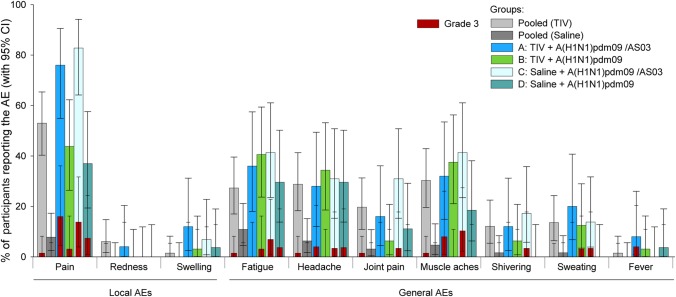
Following dose 1, 65.7% and 28.8% of the recipients of TIV and saline, respectively, reported any symptom. Among local symptoms, injection site pain was most frequently reported and was more common in the TIV group than in the saline group (53% vs 7.8%; Figure 5). Among general symptoms, headache, joint pain, and muscle aches were also more common among TIV recipients, and grade 3 AEs were only reported in the TIV group (1 each of fatigue, headache, muscle aches, and joint pain).
Figure 5.
Incidence of solicited symptoms after the administration of trivalent seasonal influenza vaccine (TIV) or placebo and after each of the 2 doses of A(H1N1)pdm09 vaccine. Mean percentage of participants with 95% confidence interval (CI) experiencing solicited local (A) and general (B) adverse events (AEs) reported within 7 days postvaccination are shown for all participants for whom at least 1 administration of vaccine or placebo was documented (the total vaccinated cohort). Group A received TIV at day 0 and A(H1N1)pdm09 (3.75 µg)/AS03 at day 122 and day 143. Group B received TIV at day 0 and A(H1N1)pdm09 (15 µg) at day 122 and day 143. Group C received saline at day 0 and A(H1N1)pdm09 (3.75 µg)/AS03 at day 122 and day 143. Group D received saline at day 0 and A(H1N1)pdm09 (15 µg) at day 122 and day 143.
After A(H1N1)pdm09 vaccination (doses 2/3), injection site pain was also the most frequently reported local AE and occurred at a higher rate in the adjuvanted groups A and C than in the nonadjuvanted groups B and D (76.0%, 82.8%, 43.8%, and 37.0%, respectively), while no clear trend was observed for grade 3 pain. Fatigue, muscle aches, and headache were the most frequently reported general AEs. Grade 3 general AEs occurred at a frequency of ≤10.3% of participants’ AEs.
Unsolicited AEs
After dose 1, unsolicited AEs occurred at similar frequencies in TIV and placebo recipients; 22.4% and 21.2% of participants, respectively, reported at least 1 unsolicited AE. Grade 3 back pain was reported once by a placebo recipient. Nine percent of all participants (10.4% and 7.6% in TIV and saline groups, respectively) reported at least 1 MAE.
After A(H1N1)pdm09 vaccination (doses 2 and 3), frequencies of unsolicited AEs were comparable among groups (Table 2). Nausea and upper respiratory tract infections were the most common events (≤2 participants/group). Two grade 3 AEs (diabetes mellitus and uterine leiomyoma) were reported in group A. From the participants of groups A–D, 27.3%, 32.4%, 15.2%, and 12.1%, respectively, experienced at least 1 MAE up to day 507, none of which were considered related to vaccination, and 9.1%, 2.9%, 3.0%, and 3.0%, respectively, experienced at least 1 SAE, none of which were fatal or considered related to vaccination. SAEs (1 report each) included hepatitis C 7 days after dose 1, which was unresolved at day 507; uterine fibroid that developed within 1 month after dose 3; worsening of aortic aneurysm 37 days after dose 3 (all in group A); elevated liver-function test 6 months after dose 3 (group B); moderate Clostridium difficile infection 63 days after dose 3 (group C); and cholelithiasis 5 months after dose 3 (group D).
Table 2.
Unsolicited Adverse Events, >1% Incidence Overall, Following the 21-Day Period After Each Dose of A(H1N1)pdm09 Vaccine
| Adverse Event | Group A (N = 28) |
Group B (N = 33) |
Group C (N = 29) |
Group D (N = 28) |
||||
|---|---|---|---|---|---|---|---|---|
| n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | |
| ≥1 adverse event | 8 | 28.6 (13.2–48.7) | 6 | 18.2 (7.0–35.5) | 7 | 24.1 (10.3–43.5) | 5 | 17.9 (6.1–36.9) |
| Nausea | 1 | 3.6 (0.1–18.3) | 0 | 0.0 (0.0–10.6) | 2 | 6.9 (0.8–22.8) | 1 | 3.6 (0.1–18.3) |
| Vomiting | 0 | 0.0 (0.0–12.3) | 0 | 0.0 (0.0–10.6) | 1 | 3.4 (0.1–17.8) | 1 | 3.6 (0.1–18.3) |
| Nasopharyngitis | 1 | 3.6 (0.1–18.3) | 1 | 3.0 (0.1–15.8) | 0 | 0.0 (0.0–11.9) | 0 | 0.0 (0.0–12.3) |
| Sinusitis | 1 | 3.6 (0.1–18.3) | 1 | 3.0 (0.1–15.8) | 0 | 0.0 (0.0–11.9) | 0 | 0.0 (0.0–12.3) |
| Upper respiratory tract infection | 0 | 0.0 (0.0–12.3) | 1 | 3.0 (0.1–15.8) | 1 | 3.4 (0.1–17.8) | 1 | 3.6 (0.1–18.3) |
| Oropharyngeal pain | 1 | 3.6 (0.1–18.3) | 0 | 0.0 (0.0–10.6) | 1 | 3.4 (0.1–17.8) | 0 | 0.0 (0.0–12.3) |
| Rhinorrhea | 1 | 3.6 (0.1–18.3) | 0 | 0.0 (0.0–10.6) | 1 | 3.4 (0.1–17.8) | 0 | 0.0 (0.0–12.3) |
Data shown are of the total vaccinated cohort. Groups A and B received trivalent seasonal influenza vaccine at day 0 and either adjuvanted (group A) or nonadjuvanted (group B) pandemic vaccine at day 122 and day 143. Groups C and D received saline at day 0 and adjuvanted (group C) or nonadjuvanted (group D) pandemic vaccine at day 122 and day 143. Abbreviations: CI, confidence interval; N, number of participants with the administered dose; n/%, number/percentage of participants reporting the symptom at least once.
There were no reports of pIMDs or major clinical laboratory abnormalities, and there were no withdrawals due to SAEs or AEs.
DISCUSSION
This study assessed the effect of prior vaccination with a TIV on the immune response to the A(H1N1)pdm09 vaccine in young, healthy adult volunteers lacking a history of previous influenza vaccination. Secondary objectives were to assess the role of the adjuvant on CMI and humoral responses and to evaluate vaccine safety.
Although no formal statistical comparison between groups was made, our results indicate that there was a trend for diminished A(H1N1)pdm09-specific humoral and CD4+ T-cell responses following vaccination with the pandemic vaccine in participants who had previously received TIV. Also, results showed that adjuvantation of the A(H1N1)pdm09 vaccine led to increased responses of vaccine-homologous and -heterologous HI antibodies, NAb and CD4+ T cells, and homologous memory B cells and plasmablasts.
Receipt of TIV a few weeks to many months prior to A(H1N1)pdm09 or H5N1 vaccines has previously been shown to affect HI responses [5, 8–20], irrespective of whether these vaccines were adjuvanted or not. One explanation could be the behavior of the memory B-cell pool after vaccination. Seasonal vaccination has been shown to lead to rapid expansion of plasmablasts that produce vaccine antigen-specific antibodies [24]. The B-cell response to the TIV could be shaped by the epitopes present on the TIV strains. The resulting B-cell memory pool could limit the capacity of the B-cell compartment to adapt to antigenically more distant vaccines, such as A(H1N1)pdm09 vaccine antigens administered subsequently. Thus, a B-cell repertoire that is “fixed” by TIV could limit adaptability of this response. CD4+ T cells can have a role in helping memory B cells by stimulating somatic hypermutation, thereby facilitating adaptability of the B-cell response [34]. Indeed, not only frequencies of specific CD4+ T cells but also HI responses and memory B-cell frequencies were enhanced after the first and second doses of adjuvanted vaccine; this is in line with observations in previous H5N1 and/or A(H1N1)pdm09 vaccine studies [9, 27]. Moreover, the epitope breadth and binding affinity of the antibodies to pandemic influenza vaccines were previously shown to be improved by MF59, another oil-in-water–based adjuvant [28, 29].
In a previous A(H1N1)pdm09 vaccine study, CD4+ T-cell frequencies after the first dose of pandemic vaccine correlated with HI titers measured 3 weeks later [9]. Although the presence of such correlation was not assessed in our study, we speculate that after the first dose of adjuvanted vaccine, stimulation of CD4+ T-cell responses may have resulted in increased “help” for B cells, resulting in better adaptation of B cells and, subsequently, in increased HI titers to the variant HA in the pandemic vaccine. In short, after the first dose of pandemic vaccine, the adjuvant may have promoted B-cell adaptation to the more distant A(H1N1)pdm09 antigen and helped to shape T- and B-cell pools to better respond to the subsequent vaccination.
Overall, the reactogenicity and safety data for the TIV recipients were consistent with data for those who received a comparable TIV [35]. Injection site pain was more common in the TIV group than in the placebo group after first vaccination and in recipients of adjuvanted vaccine relative to recipients of nonadjuvanted vaccine after A(H1N1)pdm09 vaccination. Consistent with earlier studies with adjuvanted and nonadjuvanted A(H1N1)pdm09 vaccines [9, 11], the current data do not suggest relevant safety concerns for any of the studied vaccines in the given study population. Several retrospective epidemiological studies have described an association between vaccination with a different A(H1N1)pdm09 vaccine (Pandemrix™) and the later onset of narcolepsy in persons aged <21 years as well as in adults [36]. Recent experiments revealed a potential molecular basis for the link, which lies in the HA amino acid sequence of H1N1 [37]. No narcolepsy cases were reported in the current study, though the current trial was not designed to detect rare events.
Trial limitations were the small sample size and relatively large number of withdrawals from the study. The study size selection was a consequence of the complexity of the laboratory assays for this descriptive study, while the number of withdrawals may have been related to the trial duration, weekly blood-sampling frequency over the course of 1 year, and/or the relatively large blood-sample volumes required to perform the detailed immunogenicity assessments.
CONCLUSION
In healthy participants aged 19–40 years, prior vaccination with TIV decreased the humoral and CMI responses to the A(H1N1)pdm09 vaccine. Adjuvantation of the pandemic vaccine helped to overcome the immune interference between influenza vaccines. No clinically relevant safety concerns were observed with either of the study vaccines.
Supplementary Data
Notes
Acknowledgments. The authors are grateful for the vital contribution of the participating study volunteers, clinicians, nurses, and laboratory technicians at the study sites. The authors are indebted to the principal investigators Jonathan Wilson and Anthony Puopolo and to all teams of the GlaxoSmithKline (GSK) group of companies for their contributions, including global study managers Wendy Talbott and Cherie Barreca, Catena Lauria for clinical operations management, and US study manager Stacy Sanders. The authors also thank Miguel Madariaga for reviewing the protocol, Karl Walravens for laboratory support, and Steven Phay Tran for CMI testing, Chuck Buscarino and Janine Linden for protocol writing assistance (all employees of GSK group of companies at the time of the study). Dorothy Slavin (GSK at the time of the study) was the clinical safety representative and Rosalia Calamera (GSK) and Veronique Grosjean (4 clinics on behalf of GSK) were responsible for database management. The authors also thank Eddy Denis and Benoit Le Pioufle (Keyrus on behalf of GSK) for their contributions to the statistical analyses and Benoit Vincart and Philippe Auquier (both GSK) for conducting the plasmablast measurements. Finally, the authors thank Ellen Oe and Shirin Khalili (both XPE Pharma&Science on behalf of GSK) for writing assistance and publication management, respectively.
Authors Contribution. All authors contributed substantially to the conception, design, analysis, and interpretation of the data presented. All authors had full access to the data and were involved in critical revision of the manuscript for important intellectual content. The corresponding author was responsible for manuscript submission.
Trademarks. Pandemrix™ and FluLaval™ are trademarks of the GlaxoSmithKline group of companies.
Financial support. The study was funded by GlaxoSmithKline Biologicals SA, which was involved in all stages of the study conduct and analysis (ClinicalTrials.gov; NCT00707967). GlaxoSmithKline Biologicals SA also paid for all costs associated with the development and publishing of this manuscript.
Potential conflicts of interest. All authors are employees of the GlaxoSmithKline group of companies. S. R.-G. holds GSK stock options and R. v.d M. and D. V. report receiving restricted shares of the company. R. v.d M. declares that US provisional patent applications have been filed in relation to some of the information discussed in this manuscript. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Jayaraman A, Pappas C, Raman R, et al. A single base-pair change in 2009 H1N1 hemagglutinin increases human receptor affinity and leads to efficient airborne viral transmission in ferrets. PLoS ONE. 2011;6:e17616. doi: 10.1371/journal.pone.0017616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Pandemic H1N1 (2009)—update 112. Global Alert and Response Weekly Update. 2010. http://www.who.int/csr/don/2010_08_06/en?/index.html . Accessed 23 May 2013.
- 3.Dawood FS, Iuliano AD, Reed C, et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis. 2012;12:687–95. doi: 10.1016/S1473-3099(12)70121-4. [DOI] [PubMed] [Google Scholar]
- 4.Greenberg ME, Lai MH, Hartel GF, et al. Response to a monovalent 2009 influenza A (H1N1) vaccine. N Engl J Med. 2009;361:2405–13. doi: 10.1056/NEJMoa0907413. [DOI] [PubMed] [Google Scholar]
- 5.Plennevaux E, Sheldon E, Blatter M, Reeves-Hoché M-K, Denis M. Immune response after a single vaccination against 2009 influenza A H1N1 in USA: a preliminary report of two randomised controlled phase 2 trials. Lancet. 2010;375:41–8. doi: 10.1016/S0140-6736(09)62026-2. [DOI] [PubMed] [Google Scholar]
- 6.Clark TW, Pareek M, Hoschler K, et al. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med. 2009;361:2424–35. doi: 10.1056/NEJMoa0907650. [DOI] [PubMed] [Google Scholar]
- 7.Jennings LC, Monto AS, Chan PK, Szucs TD, Nicholson KG. Stockpiling prepandemic influenza vaccines: a new cornerstone of pandemic preparedness plans. Lancet Infect Dis. 2008;8:650–8. doi: 10.1016/S1473-3099(08)70232-9. [DOI] [PubMed] [Google Scholar]
- 8.Roman F, Vaman T, Kafeja F, Hanon E, Van Damme P. AS03A-adjuvanted influenza A (H1N1) 2009 vaccine for adults up to 85 years of age. Clin Infect Dis. 2010;51:668–77. doi: 10.1086/655830. [DOI] [PubMed] [Google Scholar]
- 9.Roman F, Clément F, Dewé W, et al. Effect on cellular and humoral immune responses of the AS03 Adjuvant System in an A/H1N1/2009 influenza virus vaccine administered to adults during two randomized controlled trials. Clin Vaccine Immunol. 2011;18:835–43. doi: 10.1128/CVI.00480-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrews NJ, Walker WT, Finn A, et al. Predictors of immune response and reactogenicity to AS03B-adjuvanted split virion and non-adjuvanted whole virion H1N1 (2009) pandemic influenza vaccines. Vaccine. 2011;29:7913–9. doi: 10.1016/j.vaccine.2011.08.076. [DOI] [PubMed] [Google Scholar]
- 11.Roman F, Vaman T, Gerlach B, Markendorf A, Gillard P, Devaster J-M. Immunogenicity and safety in adults of one dose of influenza A H1N1v 2009 vaccine formulated with and without AS03A-adjuvant: preliminary report of an observer-blind, randomised trial. Vaccine. 2010;28:1740–5. doi: 10.1016/j.vaccine.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Peeters M, Regner S, Vaman T, Devaster J-M, Rombo L. Safety and immunogenicity of an AS03-adjuvanted A(H1N1)pmd09 vaccine administered simultaneously or sequentially with a seasonal trivalent vaccine in adults 61 years or older: data from two multicentre randomised trials. Vaccine. 2012;30:6483–91. doi: 10.1016/j.vaccine.2012.07.081. [DOI] [PubMed] [Google Scholar]
- 13.Huijskens E, Rossen J, Mulder P, et al. Immunogenicity, boostability, and sustainability of the immune response after vaccination against influenza A virus (H1N1) 2009 in a healthy population. Clin Vaccine Immunol. 2011;18:1401–5. doi: 10.1128/CVI.05046-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Damme P, Kafeja F, Bambure V, et al. Long-term persistence of humoral and cellular immune responses induced by an AS03A-adjuvanted H1N1 2009 influenza vaccine: an open-label, randomized study in adults aged 18–60 years and older. Hum Vaccin Immunother. 2013;9:1512–22. doi: 10.4161/hv.24504. [DOI] [PubMed] [Google Scholar]
- 15.Uno S, Kimachi K, Kei J, et al. Effect of prior vaccination with a seasonal trivalent influenza vaccine on the antibody response to the influenza pandemic H1N1 2009 vaccine: a randomized controlled trial. Microbiol Immunol. 2011;55:783–9. doi: 10.1111/j.1348-0421.2011.00381.x. [DOI] [PubMed] [Google Scholar]
- 16.Wu J, Zhong X, Li CK, et al. Optimal vaccination strategies for 2009 pandemic H1N1 and seasonal influenza vaccines in humans. Vaccine. 2011;29:1009–16. doi: 10.1016/j.vaccine.2010.11.058. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Y, Ng DM, Seto WH, et al. Seroprevalence of pandemic H1N1 antibody among health care workers in Hong Kong following receipt of monovalent 2009 H1N1 influenza vaccine. PLoS One. 2011;6:e27169. doi: 10.1371/journal.pone.0027169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi YS, Baek YH, Kang W, et al. Reduced antibody responses to the pandemic (H1N1) 2009 vaccine after recent seasonal influenza vaccination. Clin Vaccine Immunol. 2011;18:1519–23. doi: 10.1128/CVI.05053-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nolan T, McVernon J, Skeljo M, et al. Immunogenicity of a monovalent 2009 influenza A(H1N1) vaccine in infants and children: a randomized trial. JAMA. 2010;303:37–46. doi: 10.1001/jama.2009.1911. [DOI] [PubMed] [Google Scholar]
- 20.Nolan T, Richmond PC, Formica NT, et al. Safety and immunogenicity of a prototype adjuvanted inactivated split-virus influenza A (H5N1) vaccine in infants and children. Vaccine. 2008;26:6383–91. doi: 10.1016/j.vaccine.2008.08.046. [DOI] [PubMed] [Google Scholar]
- 21.Leroux-Roels I, Roman F, Forgus S, et al. Priming with AS03A-adjuvanted H5N1 influenza vaccine improves the kinetics, magnitude and durability of the immune response after a heterologous booster vaccination: an open non-randomised extension of a double-blind randomised primary study. Vaccine. 2010;28:849–57. doi: 10.1016/j.vaccine.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 22.Kim JH, Skountzou I, Compans R, Jacob J. Original antigenic sin responses to influenza viruses. J Immunol. 2009;183:3294–301. doi: 10.4049/jimmunol.0900398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fazekas de St Groth S, Webster RG. Disquisitions on original Antigenic Sin. I. Evidence in man. J Exp Med. 1966;124:331–45. doi: 10.1084/jem.124.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wrammert J, Smith K, Miller J, et al. Rapid cloning of high affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–71. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gulati U, Kumari K, Wu W, Keitel WA, Air GM. Amount and avidity of serum antibodies against native glycoproteins and denatured virus after repeated influenza whole-virus vaccination. Vaccine. 2005;23:1414–25. doi: 10.1016/j.vaccine.2004.08.053. [DOI] [PubMed] [Google Scholar]
- 26.Kim JH, Davis WG, Sambhara S, Jacob J. Strategies to alleviate original antigenic sin responses to influenza viruses. Proc Natl Acad Sci U S A. 2012;109:13751–6. doi: 10.1073/pnas.0912458109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moris P, van der Most R, Leroux-Roels I, et al. H5N1 influenza vaccine formulated with AS03A induces strong cross-reactive and polyfunctional CD4 T-cell responses. J Clin Immunol. 2011;31:443–54. doi: 10.1007/s10875-010-9490-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khurana S, Chearwae W, Castellino F, et al. Vaccines with MF59 adjuvant expand the antibody repertoire to target protective sites of pandemic avian H5N1 influenza virus. Sci Transl Med. 2010;2 doi: 10.1126/scitranslmed.3000624. 15ra5. [DOI] [PubMed] [Google Scholar]
- 29.Khurana S, Verma N, Yewdell JW, et al. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3002336. 85ra48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morel S, Didierlaurent A, Bourguignon P, et al. Adjuvant System AS03 containing α-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine. 2011;29:2461–73. doi: 10.1016/j.vaccine.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Hehme NW, Künzel W, Petschke F, et al. Ten years of experience with the trivalent split-influenza vaccine, Fluarix™. Clin Drug Invest. 2002;22:751–69. [Google Scholar]
- 32.European Agency for Proprietary Medicinal Products. European Agency for the Evaluation of Medicinal Products (CHMP); 2007. Guideline on influenza vaccine prepared from viruses with the potential to cause a pandemic and intended for use outside of the core dossier context (EMEA/CHMP/VWP/263499/2006) [Google Scholar]
- 33.US Food and Drug Administration. Guidance for Industry: Clinical Data Needed to Support the Licensure of Pandemic Influenza Vaccines. 2007.
- 34.Deenick EK, Ma CS. The regulation and role of T follicular helper cells in immunity. Immunology. 2011;134:361–7. doi: 10.1111/j.1365-2567.2011.03487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Treanor JJ, Campbell JD, Brady RC, et al. Rapid licensure of a new, inactivated influenza vaccine in the United States. Hum Vaccin. 2005;1:239–44. doi: 10.4161/hv.1.6.2376. [DOI] [PubMed] [Google Scholar]
- 36.Barker CI, Snape MD. Pandemic influenza A H1N1 vaccines and narcolepsy: vaccine safety surveillance in action. Lancet Infect Dis. 2014;14:227–38. doi: 10.1016/S1473-3099(13)70238-X. [DOI] [PubMed] [Google Scholar]
- 37.De la Herrán-Arita AK, Kornum BR, Mahlios J, et al. CD4+ T cell autoimmunity to hypocretin/orexin and cross-reactivity to a 2009 H1N1 influenza A epitope in narcolepsy. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3007762. 216ra176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.