Abstract
Chronic heart failure (CHF) is a complex syndrome characterized by progressive decline in left ventricular function, low exercise tolerance and raised mortality and morbidity. Left ventricular diastolic dysfunction plays a major role in CHF and progression of most cardiac diseases. The current recommended goals can theoretically be accomplished via exercise and pharmacological therapy so the aim of the present study was to evaluate the impact of cardiac rehabilitation program on diastolic dysfunction and health related quality of life and to determine the correlation between changes in left ventricular diastolic dysfunction and domains of health-related quality of life (HRQoL). Forty patients with chronic heart failure were diagnosed as having dilated cardiomyopathy (DCM) with systolic and diastolic dysfunction. The patients were equally and randomly divided into training and control groups. Only 30 of them completed the study duration. The training group participated in rehabilitation program in the form of circuit-interval aerobic training adjusted according to 55–80% of heart rate reserve for a period of 7 months. Circuit training improved both diastolic and systolic dysfunction in the training group. On the other hand, only a significant correlation was found between improvement in diastolic dysfunction and health related quality of life measured by Kansas City Cardiomyopathy Questionnaire. It was concluded that improvement in diastolic dysfunction as a result of rehabilitation program is one of the important underlying mechanisms responsible for improvement in health-related quality of life in DCM patients.
Keywords: Cardiac rehabilitation, Dilated cardiomyopathy, Quality of life
Introduction
Chronic heart failure (CHF) is a multi system syndrome. Although initiated by a reduction in cardiac function, it is characterized by the activation of compensatory mechanisms, which involve the whole body: hemodynamic, autonomic and neuro-humoral changes may be initially beneficial, but subsequently becomes dominant and lead to perpetuation of the syndrome [1]. Idiopathic dilated cardiomyopathy (DCM) is a primary myocardial disease of unknown cause characterized by left ventricular or biventricular dilation and impaired myocardial contractility [2]. Patients with DCM have both increased left ventricular end-diastolic diameter and ejection fraction of less than 45%. By definition, diastolic dysfunction refers to abnormalities in ventricular relaxation and filling (right ventricle, left ventricle, or both) with prolonged or incomplete return to pre systolic length and force [3,4]. Three stages of diastolic dysfunction are recognized based on Echo-Doppler transmitral flow. Stage I is characterized by reduced left ventricular filling in early diastole with normal left ventricular and left atrial pressures and normal compliance (E/A ratio less than 0.8, E wave deceleration time more than 200 ms). Stage II or pseudo-normalization is characterized by a normal Doppler Echocardiography transmitral flow pattern because of an opposing increase in left atrial pressures (E/A ratio 0.8–1.5, E wave deceleration time more than 200 ms). Stage III or reversible restrictive pattern, the final and most severe stage, is characterized by severe restrictive diastolic filling with a marked decrease in left ventricular compliance (E/A ratio more than 1.5, E wave deceleration time 150–200 ms), stage IV or irreversible restrictive pattern ((E/A ratio more than 1.5, E wave deceleration time less than 150 ms) [5,6]. In patients with heart failure, the exercise capacity may be limited by the number of frequently coexisting factors such as decreased contractility, diastolic dysfunction, chronotropic incompetence, oxygen metabolism or skeletal muscle mass [7]. During peak exercise, the heart should increase the cardiac output and the diastolic mechanisms must adjust to the decrease in time to fill. Patients with heart failure may not be able to achieve this necessary increase in diastolic relaxation to accommodate the preload increase [8]. Severity of effort intolerance is linked with left ventricular filling pressure and so the strong relationship between diastolic abnormalities and exercise limitation should be not underscored [9]. Exercise training has become an accepted adjunct therapy for patients with systolic dysfunction. It is considered to be beneficial in terms of improved mortality and morbidity, quality of life and functional capacity [10–12]. Kansas City Cardiomyopathy Questionnaire (KCCQ) is a detailed, disease-specific health status measure that encompasses domains including physical limitation, symptoms, disease severity, and change in status over time, self efficacy, social interference and quality of life [13]. Although health related quality of life (HRQoL) and functional capacity may be correlated, they are not synonymous and represent different components of health status [14]. Functional status is a direct measure of the ability to carry out specific tasks with significant physical or symptom limitation. In contrast, HRQoL reflects the discrepancy between the patient’s current function and their expected health status so it increases with increasing concordance between the actual and expected health [15]. Previous studies showed a significant improvement in all aspects of HRQoL after comprehensive cardiac rehabilitation program for ischemic and non ischemic heart failure patients [10,16]. On the other hand decreased exercise capacity is a main factor restricting every day life of chronic heart failure patients, thus compromising their quality of life [17]. Exercise training could improve the exercise capacity of these patients. Although this improvement is primarily due to peripheral adaptations, and partly due to central adaptations [18], the contribution of left ventricular diastolic filling to the improved quality of life had not been well defined .As many patients with advanced heart failure give greater importance to quality of life than do to duration of life, so the purpose of this study was primarily to determine the effect of cardiac rehabilitation program on diastolic dysfunction and quality of life; an important end point in the assessment of cardiac rehabilitation program and to investigate the correlation between improvement in both measurements in DCM patients.
Subjects and method
Subjects
Forty male patients with symptomatic dilated cardiomyopathy and only 30 of them completed the study. They were diagnosed by echocardiography and coronary angiography. Patients were recruited from National Heart Institute in Imbaba, Giza, out patient clinic which accepts and follows many of chronic heart failure patients daily and had to have expertise cardiologists. Their ages ranged from 50 to 65 years old. The patients had more than an 8-month history of DCM and had been clinically stable for more than 3 months prior to the onset of study period. The patients were selected according to the following inclusion criteria: The diagnosis of DCM was made by: (i) the lack of history of typical chest pain, (ii) the absence of signs of ischemia or myocardial infarction at the electrocardiogram; (iii) global dilation of both ventricles at the echocardiogram with no regional left ventricular dyskinesia; and (iv) the presence of normal thallium scintigraphy and normal coronary angiogram, left ventricular end-diastolic dimension >5.5 cm and end-systolic diameter >4.5 cm, fractional shortening <25% and ejection fraction <45%, sinus rhythm, New York Heart Association (NYHA) class II–III, Left ventricular diastolic dysfunction in the form of reversed, pseudo normal or restrictive pattern (grades I, II, and III, respectively). The exclusion criteria included the following; significant coronary disease by history or angiography to exclude ischemic causes, evidence for secondary causes of cardiomyopathy as long standing or uncontrolled hypertension, primary valvular disease, atrial fibrillation (AF), severe functional mitral regurgitation (MR), clinical evidence of pulmonary disease (chronic obstructive lung disease, moderate to severe pulmonary hypertension).
They were on optimal medical therapy with no major changes in treatment regimen during the study. All patients in the training and control groups were medically controlled by expertise cardiologist who was blinded to grouping assignment. Patients were under chronic non-selective beta blocker; Carvedilol (Dilatriol 6.25 mg, twice daily). Also all patients were on Digitalis (0.25 mg), Furosemide (40 mg), and Angiotensin converting enzyme inhibitor. Patients were randomly assigned into training and control groups and they were informed about the nature and effects of trial. All of them were under medical treatment. For the training group, periodic adjustment of intensity throughout the training program was done according to the individual’s progression of exercise capacity. The training subjects received information regard the benefits of regular aerobic exercise and were asked to report any side effects during the treatment session. The patients in the control group remained on their individually tailored cardiac medication supervised by their physicians and to keep them motivated through the study through frequent visits each 2 weeks to receive the simple disease information sessions. The activities of the patients in the control group were checked to avoid any extra unadjusted effort by applying of the questionnaire interview every 2 weeks (physical limitation domain, question number 15 for quality of life domain which revealed their usual activities). The patients were also instructed not to perform extra unadjusted effort over the ordinary effort (some of these patients had mild limitation with the ordinary daily living activities and others had marked limitation with the ordinary daily living activities) as this may affect their results on the final assessment.
Patient randomization
According to the inclusion and exclusion criteria, forty patients were eligible to participate in the study. The patients were randomly assigned into two groups (training and control groups) by arrangement into numerical numbers from 1 to 40, then odd numbers were allocated as a training group and the even numbers were allocated as a control group. The training group consisted of 20 patients who received interval aerobic training program for 3 days/week (day another day) for 7 months, in addition to simple disease information sessions aimed to reinforce patient education about chronic heart failure signs and symptoms, ensure compliance with medications, identify recurrent symptoms amenable to treatment, advice on how to live with heart failure and special emphasis was given to dietary counseling (recognition and self management of fluid overload). The control group consisted of 20 patients who received only the same disease information sessions received by the training group through frequent visits every 2 weeks. The control group attended in days, other than the training days. Both groups signed an informed consent and the study was approved by Ethical Committee of National Heart Institute and represented as a controlled randomized trial.
Instrumentation
Assessment instrument
Transthoracic Doppler Echocardiography
Hewlett–Packard Sonos, USA device was used to measure changes in different diastolic dysfunction parameters. Peak transmitral flow velocity at early diastole (E wave), peak transmitral flow velocity at late diastole (A wave), and E/A ratio. It was also used to measure ejection fraction. All measurements were obtained before and after the period of the study. transmitral flow velocity is a reliable and valid tool for evaluation of diastolic dysfunction in patients with DCM [6,19–21].
Cardiopulmonary exercise testing (CPET)
Oxycon pro (Jaeger – Germany) was used to measure cardiopulmonary fitness represented as peak oxygen consumption (VO2), resting and maximal heart rate.
Kansas City Cardiomyopathy questionnaire (KCCQ)
This questionnaire was used to quantify health status using two main summary scores; functional and clinical summary scores. The questionnaire is reliable and valid tool for evaluation of health status related quality of life changes [22–24].
Procedure
Assessment procedure
Echocardiography evaluation
M-mode, two dimensional and pulsed Doppler Echocardiography examinations were performed with an ultrasound system; a two-dimensional mechanical sector scanner (2.5 MHz imaging transducer connected to Hewlett–Packard Sons Doppler flow analyzer). Each patient was examined in the supine, left lateral position, according to the standards of the American Society of Echocardiography [25]. Ejection fraction was calculated using two dimension view (2D). Pulsed Doppler mitral flow velocity analysis was obtained from the apical four chamber view. Care was taken to position the cursor line through a plane traversing the left ventricle from the apex to mitral valve annulus in order to achieve the smallest possible angle between left ventricle inflow and the orientation of the ultrasound beam. The sample volume was set in the mitral orifice on the atrial side between mitral leaflet tips during diastole. In each patient, Left ventricular diastolic flow velocity from five cardiac cycle’s waves was obtained and averaged. The duration between echocardiography examination and cardiopulmonary exercise testing was not more than 1 week. The assessment was done by a single senior member of cardiology team (consultant) who was blinded to patient allocation and the contact between him and the patients was limited to the day of evaluation procedure before and after the study period. E/A ratio was considered to be normal if it was 0.78–1.78 and E wave deceleration time 150–200 ms [6]. Peak valsalva maneuver was applied using forceful expiration against closed nose and mouth as a preload reduction maneuver to differentiate pseudo normal pattern from true normal pattern in patients with E/A ratio in the range of 0.8–1.8. The patient must generate a sufficient increase in the intrathoracic pressure. A decrease of 20 cm/s in mitral peak E wave velocity was considered an adequate effort. Using valsalva maneuver, pseudo normal pattern was reverted to stage I diastolic dysfunction (impaired relaxation phase) and this group was confirmed to be pseudo normal pattern instead of true normal.
Cardiopulmonary exercise testing (CPET)
The test was done by a single specialized physical therapist consultant, expertise in cardiopulmonary fitness assessment for cardiac patients and he was blinded to the patient allocation as the patients’ contact with the investigator was generally limited to the day of procedure before and after the study period. Before conducting the exercise tolerance test, all participants had to visit the laboratory to be familiarized with the equipment and to be cooperative during conducting the test. Brief explanation of the procedures was done, reminding the patient to wear loose-fitting comfortable clothes and suitable shoes for exercise. Patients were also instructed to avoid eating a heavy meal at least 3 h, coffee or cigarettes before testing. Pleasant environment is needed to obtain maximum confidence and performance by the patients. Patients continued to take routine medications before exercise testing.
The test was terminated in the following conditions: hypertensive blood pressure response greater than 200/110 mm Hg, failure of systolic blood pressure to rise as the intensity of the work increases, fall of diastolic blood pressure about 15 or 20 mm Hg, reached heart rate to target heart rate [(220-age) × 85%], chronotropic incompetence dizziness, unusual shortness of breath, chest pain, muscle fatigue, leg pain, pallor or cold sweating, being unable to maintain cycling revolution above 40 rpm, ECG changes: arrhythmia, (e.g. AF, premature ventricular contraction more than 10/min), deviation of ST segment.
Spirometry test was conducted to exclude patient with obstructive lung disease: FEV1/FVC ratio <70–60% of predicted and as a prerequisite for cardiopulmonary exercises testing. The patient mounted an upright electronically beaked computerized bicycle ergo meter with gas exchange analysis (breath by breath test). First, the metabolic parameters as (oxygen consumption, carbon dioxide production) and heart rate were measured every minute. Blood pressure was also measured every 2 min by cuff sphygmomanometer. The measurement was also taken at rest for 3 min. All patients were subjected to a sub maximal symptom limited exercise testing on stationary ergo meter of the cardiopulmonary exercise test unit before the beginning of training programs according to Wasserman protocol [26]. Heart rate and ECG were continuously monitored during the test. The work rate was increased by a uniform amount each minute until the patient was limited by symptoms or unable to continue safely. The patient pedaled at constant rate of 40–60 rpm, unloaded (0 W) for 3 min then an increment of 5, 10, 15 W/min for 10 min, depending on the expected performance of the patient, observing the patient’s facial expression, checking the blood pressure and ECG recording for untoward changes and verbally encouraging the patient to maximize his performance. The resistance of the cycle was removed if any of the previous contraindicated signs and symptoms occurred. Resting heart rate, maximal heart rate and oxygen consumption during the recovery phase which should be continued for about 3 min or until the values measured before were reached. Oxygen consumption at peak exercise (peak VO2) was calculated as the average of VO2 value over the final 30 s of exercise. All patients quit the test because of dyspnea or leg fatigue, and in all patients, the respiratory exchange ratio (RER) was more than or equal to 1.33 and anaerobic threshold was reached.
Kansas City Cardiomyopathy questionnaire (KCCQ)
It is a self-administered 23-item questionnaire measuring health-related quality of life (HRQoL). The questionnaire assess six domains of HRQoL, each item has a five, six or seven-point likert scale. Each domain’s score were calculated as the mean of its item scores. Domain scores were transformed to 0–100 (highest level of functioning scale). The domains are physical limitation (question 1), symptoms (frequency (questions 3, 5, 7 and 9), severity (questions 4, 6, 8) and change over time (question 2), self efficacy and knowledge (questions 10–12), social interference (question 16) and quality of life (questions 13–15). In addition, the KCCQ domains were aggregated into two summary scores, the functional status summary score (the mean of physical limitation and symptom domain scores excluding symptom stability) and clinical summary score (the functional summary score plus social limitation and quality of life domain scores) [13,27]. The questionnaire functional and clinical summary scores were collected before and after the period of 7-month for both training and control groups after explanation for the questionnaire and its domains for each patient. In the present study, the questionnaire was translated at first from English to Arabic. Moreover, another translator did the translation from Arabic to English. These two versions were compared with each other and the results were the same, therefore reliability of the test was ensured. The questionnaire was applied in its Arabic version through questionnaire interview before and after the study period and every 2 weeks as a follow up for both groups.
Training procedure
Training group performed a supervised training program at Physical Therapy Department of National Heart Institute based on the results of cardiopulmonary exercise testing. The training group was trained using heart rate range or reserve method (Karvonen’s method); training heart rate (THR = HRrest + (HRmax − HRrest) 55–80%) [28]. Training was applied in the form of circuit-interval aerobic training using treadmill, cycle ergo meter and stair master. The patient should not exceed his training heart rate during exercise period. For treadmill training, the speed was increased till reaching 4–5 m/h at the end of the 7th month. For cycle ergo meter training, the repetitions/minute was increased till reaching 80 repetitions per minute (rpm) at the end of the 7th month. The training heart rate increased gradually according to each patient’s response during exercise training session, starting with 55% of heart rate reserve, till reaching 80% at the end of 7th month.
Each exercise training session included three phases; warm up phase composed of an initial 5–10 min in the form pedaling on bicycle ergo meter with 60 repetitions per minute (rpm), slow walking on treadmill with 1.2 m/h or stretching exercises with breathing. The heart rate during warm-up phase reached 30–40% of the target heart rate. Aerobic phase in the form of circuit interval aerobic training exercises which were made progressively more difficult by performing the exercise in more challenging ways. The treadmill speed, inclination or bicycle resistance was set at the highest comfortable setting that was safe for the patient according to his target or training heart rate which started with a training fraction of 55% of heart rate reserve and increased to 80% of heart rate reserve at the end of the 7-month period according to the patient cardiac tolerance and adaptation with the training session. This phase also started in short bouts about 8 min for 24 min, gradually prolonged up till continuous 45 min at the end of the 7th months, finally, cool down phase for 10 min with intensity decreased gradually to resting heart rate. Exercise training done three times per week for seven months. Lead II ECG was monitored continuously throughout training sessions using ECG telemetry, blood pressure was measured at rest before training, at the middle and during recovery period.
Statistical analyses
Statistics was done using SPSS-version 14.The methods used were; percentage, mean values, standard deviation, median and inter-quartile ratios for summarizing data. Student’s t test for testing significant difference between mean values of two groups normally distributed. Mann–Whitney test was used for testing difference of two groups not normally distributed. Paired t test (for normally distributed data) and Wicoxon rank test (for not normally distributed data) were used to compare readings before and after intervention for the same group (paired data). Percent of change was calculated by using this equation: 2nd reading − 1st reading/1st reading × 100 to quantify the improvement. Chi square test, Mac Nemar’s test were used for testing significance between qualitative data between groups and within the same group respectively. Spearman’s Correlation was used for testing relation between two numeric variables for not normally distributed data. The difference was considered to be significant when p value was equal to and less than 0.05 and highly significant when it was 0.01 and less.
Calculation of sample size
Considering the prevalence of non ischemic heart failure (DCM) is 17% [29], and the worst accepted improvement in E/A ratio is 30% after intervention, the confidence level is 95%, the sample size will be 32. Adding 25% for defaulters, the sample size will be 40.
Results
Demographic and clinical characteristics of patients
The demographic and clinical characteristics of the patients are shown in Table 1. At baseline, there were no statistical significant differences between both groups as regards to age, body mass index, NYHA classification, left ventricular internal dimensions at diastole and systole (p = 0.5, 0.8, 0.1, 0.2, and 0.4, respectively).
Table 1.
Baseline clinical and demographic characteristics of patients who completed the study.
| Training group | Control group | p Value | |
|---|---|---|---|
| Number | 15 | 15 | |
| Age (years) | |||
| mean ± SD | 56.400 ± 5.829 | 54.600 ± 9.264 | |
| 25th%ile | 45 | 48 | 0.5741a |
| 50th%ile | 50 | 56 | |
| 75th%ile | 65 | 64 | |
| Body mass index (BMI) | |||
| Mean ± SD | 29.416 ± 3.932 | 29.277 ± 6.091 | 0.8843a |
| 25th%ile | 26.44 | 22.04 | |
| 50th%ile | 28.57 | 30.86 | |
| 75th%ile | 33.75 | 35.7 | |
| NYHA class | |||
| II (n) | 6 | 10 | 0.1432a |
| III (n) | 9 | 5 | |
| Left ventricular internal dimension at diastole (mm) | |||
| 25th%ile | 64.4 | 61.9 | 0.2204a |
| 50th%ile | 67.3 | 62.5 | |
| 75th%ile | 74.2 | 78 | |
| Left ventricular dimension at systole (mm) | |||
| 25th%ile | 53.4 | 47.5 | 0.4545a |
| 50th%ile | 57.2 | 52.4 | |
| 75th%ile | 62.4 | 67.8 | |
Nonsignificant. NYHA: New York Heart Association Classification.
Dropout and clinical events
There were 10 patients (25%) did not complete the 7-month study period. In the exercise training group, three patients were excluded due to worsening of heart failure symptoms, one of them developed orthopnea and progressed to cardiogenic pulmonary edema, so he was admitted to ICU to receive mechanical ventilation. The other two patients had bilateral lower limb cardiac edema, night cough, exertional dyspnea with tachycardia (decompensated heart failure) and they were admitted to the hospital. These two patients refused to continue their training program after discharge. Two patients in the training group withdrew their consent after 3-month study period as they did not have working facility. Patients who were excluded due to worsening of symptoms, all of them had grade III diastolic dysfunction (restrictive pattern). In the control group, two patients had sudden cardiac death during the study period. An additional three patients withdrew consent as they cannot attend the frequent visits each 2 weeks as they live far away from the institute, see Fig. 1.
Fig. 1.
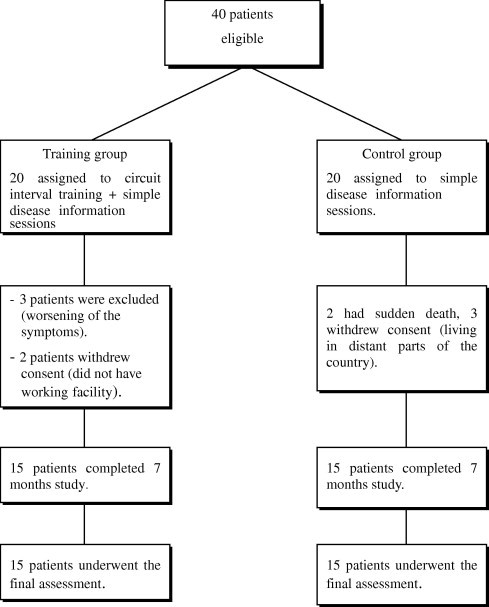
Patient randomization and study withdrawals.
Comparison of measured cardiopulmonary exercise testing parameters and left ventricular systolic function within and between both groups
There was high statistical significant increase in peak VO2 and ejection fraction after intervention only in the training group (p = 0.01 and 0.001, respectively). There was high statistical significant decrease in resting and maximal heart rates after intervention only in the training group (p = 0.01 and 0.006, respectively). There was no significant change in any parameter within the control group. As for comparison between both groups; there was high significant difference in peak VO2, resting heart rate and ejection fraction after intervention (p = 0.024, 0.004 and 0.001, respectively), see Table 2.
Table 2.
Comparison between training group and the control group in cardiopulmonary exercise testing and ejection fraction measurements before and after training.
| Variables | Control group (15) | Training group (15) | p Value |
|---|---|---|---|
| Peak VO2 ml/kg before mean ± SD | 17.17 ± 2.44 | 16.1 ± 3.65 | |
| Median | 17 | 15.7 | 0.2046a |
| Peak VO2 ml/kg after mean ± SD | 17.48 ± 2.24 | 21.08 ± 5.47 | 0.024b |
| Median | 17.9 | 19 | 0.0803a |
| Mean difference | p = 0.3594a | p = 0.01b | |
| Rest HR before mean ± SD | 87.47 ± 12.88 | 93.6 ± 7.43 | |
| Median | 87 | 95 | 0.2504a |
| Rest HR after mean ± SD | 87.33 ± 7.99 | 75 ± 8.01 | |
| Median | 85 | 80 | 0.004b |
| Mean difference | p = 0.9472a | p = 0.01b | |
| Maximal HR before mean ± SD | 133.93 ± 20.32 | 141 ± 12.41 | |
| Median | 132 | 143 | 0.2890a |
| Maximal HR after mean ± SD | 134.07 ± 14.25 | 126.8 ± 12.34 | |
| Median | 135 | 129 | 0.1002a |
| Mean difference | p = 0.9546a | p = 0.006b | |
| Ejection fraction before mean ± SD | 35.8 ± 6.87 | 33.09 ± 4.77 | |
| Median | 34 | 33 | 0.2960a |
| Ejection fraction after mean ± SD | 37.27 ± 7.82 | 48.93 ± 8.38 | |
| Median | 36 | 53 | 0.0015b |
| Mean difference | p = 0.1949a | p = 0.001b | |
Peak VO2: peak oxygen consumption in ml/kg/min, rest HR: resting heart rate in beat/minute, maximal HR: maximal heart rate in beat/minute.
Non significant.
Significant.
Comparison of diastolic dysfunction grade distribution before and after the study within and between groups
There was high statistical significant difference before and after training as regards to diastolic dysfunction pattern in the training group only (p = 0.01). The number of patients in the training group with normal diastolic pattern was zero before training, while it was 8(53.3%) after training. There was no statistical significant difference before and after intervention in diastolic dysfunction grade in the control group (p = 0.9). There was no statistical significant difference between both groups as regards to diastolic dysfunction pattern; based on E/A ratio before training (p = 0.3), while there was high statistical significant difference between both groups after training (p = 0.009) see Tables 3 and 4.
Table 3.
Distribution of training and control groups in relation to E/A ratio (diastolic grade) before and after intervention.
| E/A ratio type | Before training (15) |
After training (15) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Training group | ||||
| Normal | 0 | 0 | 8 | 53.3 |
| Grade I diastolic dysfunction | 11 | 73.4 | 5 | 33.3 |
| Grade II diastolic dysfunction | 2 | 13.3 | 1 | 6.7 |
| Grade III diastolic dysfunction | 2 | 13.3 | 1 | 6.7 |
| p Value | Mac Nemar’s X2 = 10.92, p = 0.0121b | |||
| Control group | ||||
| Normal | 0 | 0 | 0 | 0 |
| Grade I diastolic dysfunction | 7 | 46.6 | 8 | 53.3 |
| Grade II diastolic dysfunction | 4 | 22.7 | 3 | 20 |
| Grade III diastolic dysfunction | 4 | 22.7 | 4 | 22.7 |
| p Value | Mac Nemar’s X2 = 0.21, p = 0.9005a | |||
Non significant.
Significant.
Table 4.
Distribution of cases in relation to E/A ratio (diastolic grade) before and after intervention in both groups.
| E/A ratio type | Control group (15) | Training group (15) | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Before intervention | ||||
| Normal | 0 | 0 | 0 | 0 |
| Grade I diastolic dysfunction | 7 | 46.6 | 11 | 73.4 |
| Grade II diastolic dysfunction | 4 | 22.7 | 2 | 13.3 |
| Grade III diastolic dysfunction | 4 | 22.7 | 2 | 13.3 |
| p Value | X2 = 2.22, p = 0.3291a | |||
| After intervention | ||||
| Normal | 0 | 0 | 8 | 53.3 |
| Grade I diastolic dysfunction | 8 | 53.3 | 5 | 33.3 |
| Grade II diastolic dysfunction | 3 | 20 | 1 | 6.7 |
| Grade III diastolic dysfunction | 4 | 22.7 | 1 | 6.7 |
| p Value | X2 = 11.49, p = 0.0093b | |||
Nonsignificant.
Significant.
Comparison of measured diastolic parameters within group and between groups as regards to grade I (reversed pattern) diastolic dysfunction
Comparing between patients with reversed diastolic pattern (grade I) in both groups, there was no significant difference between both groups before the study (p = 0.8, 0.2 and 0.6, respectively) in E wave, A wave and E/A ratio, while there was a high statistical significant difference after the training (p = 0.003, 0.0018 and 0.0017, respectively).As regards to the percent of improvement in E wave, A wave and E/A ratio, there was a high statistical significant difference between both groups (p = 0.02,0.01 and 0.0004, respectively), see Table 5.
Table 5.
Comparison between patients with grade I diastolic dysfunction in both groups as regards to diastolic parameters before and after training.
| Variables | E/A ratio (grade I) |
p Value # | |
|---|---|---|---|
| Control group (7) | Training group (11) | ||
| E-wave(cm/s) before intervention | |||
| Median | 49 | 42.2 | 0.8914 a |
| 25%th quartile | 40 | 38.6 | |
| 75%th quartile | 53.3 | 65.1 | |
| E-wave after | |||
| Median | 36 | 62.2 | 0.0031 b |
| 25%th quartile | 35.6 | 43 | |
| 75%th quartile | 45 | 93.2 | |
| Percent of change in E wave | |||
| Median | −12.62 | 43.16 | 0.0203 b |
| 25%th quartile | −33.2 | 38.26 | |
| 75%th quartile | 12.5 | 47.85 | |
| A-wave(cm/s) before intervention | |||
| Median | 73 | 83.7 | 0.2956 a |
| 25%th quartile | 65.3 | 80 | |
| 75%th quartile | 101 | 96.4 | |
| A-wave after | |||
| Median | 73 | 56 | 0.0018 b |
| 25%th quartile | 70 | 51.5 | |
| 75%th quartile | 81 | 70 | |
| Percent of change in A wave | |||
| Median | −0.46 | −27.39 | 0.0159 b |
| 25%th quartile | −26.73 | −38.47 | |
| 75%th quartile | 0.282 | −26.49 | |
| E/A ratio before intervention | |||
| Median | 0.564 | 0.528 | 0.6823 a |
| 25%th quartile | 0.528 | 0.447 | |
| 75%th quartile | 0.685 | 0.778 | |
| E/A ratio after | |||
| Median | 0.493 | 0.982 | 0.0017 b |
| 25%th quartile | 0.481 | 0.635 | |
| 75%th quartile | 0.634 | 1.81 | |
| Percent of change in E/A ratio | |||
| Median | −8.84 | 107.69 | 0.0004 b |
| 25%th quartile | −12.5 | 40.97 | |
| 75%th quartile | 10.58 | 132.67 | |
Wicoxon rank test
# Mann–Whitney test.
Comparison between groups as regards to relative change% of functional and clinical summary scores
There was high statistical significant difference between both groups in the percent of improvement of both the functional and the clinical summary scores (p = 0.0004, 0.0001, respectively), see Table 6.
Table 6.
Comparison between patients with grade I diastolic dysfunction in both groups as regards to functional and clinical summary scores.
| Variables | E/A ratio (grade I) |
p value | |
|---|---|---|---|
| Control group (7) | Training group (11) | ||
| % of change in functional score | |||
| Median | 10.85 | 75.01 | 0.0004a |
| 25%th quartile | 0 | 54.35 | |
| 75%th quartile | 13.19 | 106.92 | |
| % of change in clinical score | |||
| Median | 7.04 | 129.28 | 0.0001a |
| 25%th quartile | 0 | 107.14 | |
| 75%th quartile | 10.55 | 162.09 | |
Significant.
Correlation coefficient between improvement percent in diastolic parameters, functional summary and clinical summary scores
The percent of change in functional summary score was negatively correlated to the percent of change in A wave (p = 0.04) and was positively correlated to E/A ratio (p = 0.02) in the training group only. The percent of change in clinical summary score was also negatively correlated to the percent of change in A wave (p = 0.04) and was positively correlated to E/A ratio (p = 0.01) in the training group only. On the other hand, there was no correlation between the percent of change in functional and clinical summary scores and percent of change in ejection fraction in the training group (p = 0.2 and 0.3, respectively) or the control group (p = 0.3 and 0.3, respectively), see Table 7.
Table 7.
Correlation between percentages of change in functional and clinical summary scores to percentages of change in diastolic functions.
| Spearman’s correlation | Control (15) |
Training (15) |
||
|---|---|---|---|---|
| r | p | r | p | |
| Percentage of change in functional score with percentage of change in A-wave | −0.05 | 0.9512a | −0.51 | 0.0457b |
| Percentage of change in functional score with percentage of change in E/A ratio | 0.16 | 0.3411a | 0.6 | 0.0291b |
| Percentage of change in functional score with percentage of change in ejection fraction | 0.2 | 0.3056a | 0.16 | 0.2998a |
| Percentage of change in clinical score with percentage of change in A-wave | 0.1 | 0.7312a | −0.54 | 0.0410b |
| Percentage of change in clinical score with percentage of change in E/A ratio | 0.17 | 0.3765a | 0.68 | 0.0124b |
| Percentage of change in clinical score with percentage of change in ejection fraction | 0.24 | 0.3158a | 0.23 | 0.3149a |
Nonsignificant.
Significant.
Discussion
It is now widely accepted that heart failure is not a disease but rather a pathophysiological syndrome that occurs when there is significant left ventricular systolic and/or diastolic dysfunction that leads to the development of heart failure signs and symptoms. Whereas systolic dysfunction can be considered a defect in the ability of myofibrils to shorten against resistance, diastolic dysfunction results from an increased resistance to left ventricular filling leading to an inappropriate upward shift of the diastolic pressure–volume relation [30].The ability to accommodate high volume loads has been demonstrated in athletes. This is done at low filling pressure; rather, the early relaxation is increased to provide for a suction force and high left ventricular compliance [31]. A study done by Hamlin et al. [5] concluded that in patients with heart failure, the decreased ability to augment the diastolic relaxation is responsible for the inability to accommodate the increase in estimated preload during exercise, resulting in higher filling pressure. Patients with heart failure have a stiffer heart with inability to relax and accept the large volume of blood in shorter period of diastole at high heart rate [32,33]. A recent meta-analysis of 14 trials that included 812 heart failure patients with reduced ejection fraction, those in exercise training groups tended to maintain their left ventricular function (ejection fraction and end-systolic and end-diastolic value) better than patients in the control arm of these studies. Interestingly, when exercise training combined with resistance training, the anti remodeling effects were no longer present. Perhaps the pressure overload associated with resistance exercise training negatively counter balances the favorable adaptations associated with exercise training [34]. The work of Belardinelli et al. [35,36] showed improvement in diastolic dysfunction represented in early and late diastolic filling after 2-month exercise training study of heart failure patients with moderate to severe systolic dysfunction. Similarly improved left ventricular stiffness [37] and reduced filling pressure [18] in heart failure patients had been reported in two other randomized, controlled trials of exercise training.
One of the earliest signs of diastolic heart failure is exercise intolerance due to exertional dyspnea [38]. In addition to that, patients who had systolic dysfunction, regardless to severity, diastolic dysfunction influences clinical signs and symptoms and the degree of exercise tolerance [39]. In an attempt to resolve this issue, the present study was conducted to evaluate the impact of cardiac rehabilitation program mainly on diastolic dysfunction and health related quality of life and to determine the correlation between changes in left ventricular functions and domains of health-related quality of life. Regarding aerobic fitness; there was statistical significant difference in peak VO2 between both groups in favor of the training group. The results also revealed a high statistical significant decrease in resting heart rate along with decrease in the maximal heart rate in the training group only with significant difference between both groups in the resting heart rate only. The results of this study were supported by numerous studies over the past two decades which have consistently demonstrated that exercise capacity of heart failure patients, best quantified by oxygen consumption at peak exercise which is 15–40% below that of age-matched healthy subjects [40]. Based on Fick equation, an appropriate increase in peak VO2 is dependent on both an increase in cardiac output (which depends on both appropriate heart rate and stroke volume responses) along with a concomitant widening of arterial-venous oxygen content difference (so increased oxygen extraction). The plethora of peripheral abnormalities in heart failure patients that limit oxygen supply and/or extraction by active skeletal tissues had been also described in these studies [41]. The results of the present study also go a head with Smart and Marwick [42] who conducted Meta-analysis on the functional capacity in heart failure patient after exercise training. His study concluded an increase by about 15–17% following exercise training and peak VO2 changes appear to be independent of the type of exercise training undertaken, for example, aerobic, intermittent or resistance training. The significant reduction in the resting heart rate showed in the present study could be related to decreased activation of adrenergic drive to the heart and vessels with augmentation for parasympathetic and decreased renin-angiotensin aldesterone system activation associated with enhanced vasodilative endothelial response [43]. For echocardiography systolic parameters, the results showed high statistical significant difference between both groups in favor of the training group. The improved ejection fraction in the present conceded with Haykowsky et al. [34] who determined the effect of exercise training and type of exercise (aerobic versus strength versus combined training) on left ventricular remodeling in heart failure. The study was represented as meta-analysis and reviewed 14 trials reported on ejection fraction (EF) data, seven trials on both end-systolic and end-diastolic volumes data. Aerobic training significantly improved EF, end systolic volume (ESV), and end diastolic volume (EDV). Combined aerobic and strength training was not associated with significant improvements in EF, EDV, or ESV. The magnitude of the improvement in EF was consistent with the magnitude of benefits seen with angiotensin-converting enzyme inhibitors or cardiac resynchronization therapy. The improved ejection fraction may be attributed to reduction in left ventricle internal dimensions, increased left ventricular wall thickness with a greater contractile reserve along with reduction in total peripheral resistance which can be inferred from the decline of resting and maximal heart rate seen in the present study. Regarding the diastolic parameters, the study showed high statistical significant difference between both training and control groups with reversed diastolic pattern (grade I) in favor of the training group as regards to the percent of change in E wave, A wave and E/A. The results of the present study go a head with that reported by Belardinelli et al.[44] who studied the effect of aerobic training on diastolic filling pattern in 55 patients with dilated cardiomyopathy (18 patients with ischemic cardiomyopathy and 37 patients with DCM). The patients were prospectively assigned to three subgroups before beginning of the training program according to the diastolic filling pattern dysfunction. Most of the idiopathic DCM in this study had a restrictive filling pattern. The training group underwent a supervised program of exercise training with the intensity adjusted to be 60% of peak VO2, three times per week for 8 weeks. The results revealed that training-induced significant improvement in exercise capacity in patients with DCM and a pattern of abnormal LV relaxation (grade I).The results of Belardinelli et al. contradicted with the present study in that, his study showed no change in left ventricular ejection fraction or chamber dimensions. The difference may be attributed to the sample in the previous study included both ischemic and dilated cardiomyopathy patients and most of the DCM patients in the previous study had a restrictive filling pattern. On the other hand, most of DCM patients in the present study had reversed and pseudo normal patterns. As diastolic dysfunction progresses, reduction in left ventricular compliance coexists with impairments in myocardial relaxation leading to large increases in the left atrial pressure. Elevations in left atrial pressure increase the pressure gradient between left atrium and left ventricle, ultimately enhancing early filling as manifested by high E wave velocity, so increase in E wave velocity alone(rapid filling phase) seen in the present study after training in patients with grade I diastolic dysfunction cannot determine improvement or worsening of the cases. Patients grade I diastolic dysfunction usually do not have symptoms at rest but may experience mild functional impairment. On the other hand, patients with grade II and III diastolic dysfunction experience moderate functional limitation and severe functional limitation, respectively. Maximal exercise capacity as well as symptoms triggered by exercise is directly related to increased pulmonary capillary pressure and therefore, to increase left ventricular filling pressure. Filling pressures so are directly related to left ventricular diastolic function [20]. Mura et al., [45] concluded that peak oxygen consumption correlated significantly with left ventricular filling pattern estimated by transmitral Echo-Doppler E/A ratio and A wave velocity. Based on the previous literature and the results of the present study which showed a significant increase in peak oxygen consumption in all patients of the training group (except for one patient in grade III) and significant increase in E/A ratio in grade I diastolic dysfunction (median value, 0.98) associated with significant decrease in A wave velocity (median value, 73), as showed in Table 5. It could be concluded that cardiac rehabilitation program caused an improvement in diastolic dysfunction, especially for patients with grade I. This improvement was also associated with improved exercise capacity. This was confirmed by the results of Belardinelli et al. who stated that dilated cardiomyopathy patients who had cardiac events had significantly higher values on E wave, rapid filling fraction, resting heart rate associated with lower values on peak VO2.
The improvement seen in the present study could by the consequence of augmented left ventricular early relaxation with an increase of suction of blood from the left atrium. This adaptation may help to accommodate the increase in the filling rate at low filling pressures at higher heart rate [8]. In patients with diastolic dysfunction, an increased heart rate and shortened diastolic time can lead to abnormal increase in left atrial filling pressure and an inability to increase forward flow [46]. Exercise training can affect diastolic function by decreasing heart rate, altering calcium uptake into the sarcoplasmic reticulum and inducing physiological hypertrophy [47]. Exercise training can also induce time-dependent reduction in sarcoplasmic-triphosphatase pump which accelerate Ca+2 uptake by sarcoplasmic reticulum along with facilitation of internal exchange of Ca+2 between sarcoplasmic reticulum and alternate Ca+2 stores [48]. All of the above findings can accelerate early ventricular relaxation. Regarding the percent of change in functional and clinical summary scores of KCCQ, there was high statistical significant difference between both training and control groups with reversed diastolic pattern (grade I) in favor of the training group (p = 0.0004, 0.0001, respectively).
Kansas City Cardiomyopathy questionnaire (KCCQ) is a recently developed disease-specific instrument for measuring health-related quality of life in patients with chronic heart failure. It reports on more dimensions and is more sensitive to change than some other questionnaires [27]. In out patients with heart failure complicating an acute myocardial infarction, KCCQ overall score was strongly associated with subsequent cardiovascular events in that those with a score ⩾75 had an 84% 1-year event free survival compared with 59% for those with a score <25 [49]. In a study designed by Heidenreich et al. and Sullivan et al. [50,51], there were significant association between KCCQ scores and a range of clinical variables; 1 year mortality was fourfold greater and hospitalization was fivefold greater, in those scoring less than 25 compared with those scoring 75 or above. This sample included broad range of heart failure etiologies, increasing the generalisability of these findings. There was a significant association between peak VO2 and KCCQ quality of life score, while ejection fraction did not have strong association with KCCQ domains, in a study conducted to evaluate association between peak VO2, clinical measures and commonly used symptom and functional tools in patients with heart failure and to determine the extent to which of these tools could be used to predict peak VO2 [33]. In the present study, there was significant proportional relationship between percent of change in E/A ratio and functional summary score along with significant inverse relationship between percent of change in A-wave velocity and functional summary score in the training group only. Also there was the same relation between percent change in clinical summary score of KCCQ, percent change in A-wave velocity and E/A ratio in the training group. On the other hand no correlation was detected between percent of change in ejection fraction and percent of change in both summary scores in both groups. Rector et al., [52] supported the results of the present study; they concluded that symptoms of heart failure explain a substantial proportion of the variation in the effects of heart failure on patient quality of life. Pathologic measures of heart failure including ejection fraction correlates with the risk of hospitalization and death but not strongly related to symptoms or quality of life. It is therefore apparent that traditional physical measures and quality of life measures assess different constructs and should not be substituted for one another [33]. Clinical outcomes focused mainly on mortality rates, but interest in HRQoL has developed as the patient have expressed preferences for quality over quantity of life. On the other hand, ejection fraction values in the present study seemed to cluster around certain values, rather than representing a smooth continuum, suggesting the possibility of clinical estimation. From the above findings, it could be concluded that improvement in diastolic dysfunction in dilated cardiomyopathy patients after cardiac rehabilitation program in the form of individualized aerobic exercise training program (circuit-interval training) may be one of the principal factors responsible for improved quality of life along with exercise capacity in chronic heart failure patients diagnosed as having dilated cardiomyopathy.
Limitations
Three noteworthy limitations exist in this study; the generalization of these results outside a clinical trial setting may be limited. It is possible that patients in this study received more attentive follow up and better treatment that would occur in other out patient clinical practice setting. Left ventricular diastolic filling patterns assessed by transmitral Echo-Doppler are influenced by a variety of factors such as valvular insufficiency, loading conditions, viscoelastic properties of the myocardium and ventricular compliance. However all patients in this study were controlled by medications that were not changed through the study period and all of them had mild degree of mitral regurge. Also the training induced reduction in the resting heart rate was evident in all DCM patients. By contract, improvement of diastolic dysfunction in grade I diastolic dysfunction was more apparent, suggesting that factors other than training-induced bradycardia can be involved in diastolic dysfunction improvement. Although the number of patients with reversed diastolic pattern (grade I) in the training group was more than that in the control group, the statistical analysis showed no significant difference in the measured diastolic parameters before the study between both groups with high statistical significant difference after the study, so it is unlikely that this could affect the results.
Conclusion
Cardiac rehabilitation program is beneficial for patients with dilated cardiomyopathy in terms of improving systolic dysfunction, diastolic dysfunction and health-related quality of life. Improved Health-related quality of life is positively correlated with improvement in diastolic dysfunction rather than improvement in systolic dysfunction.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Piepoli M.F., Guazzi M., Boriani G., Cicoira M. Exercise intolerance in chronic heart failure: mechanisms and therapies – Part 1. Eur J Cardiovasc Prve Rehabil. 2010;17:637–642. doi: 10.1097/HJR.0b013e3283361dc5. [DOI] [PubMed] [Google Scholar]
- 2.Andersson B., Caidahl K., Waagstein F. An echocardiographic evaluation of patients with idiopathic heart failure. Chest. 1995;107:680–689. doi: 10.1378/chest.107.3.680. [DOI] [PubMed] [Google Scholar]
- 3.Yu C.M., Lin H., Yang H., Kong S. Progression of systolic abnormalities in patients with isolated diastolic heart failure and diastolic dysfunction. Circulation. 2002;105:1195–1201. doi: 10.1161/hc1002.105185. [DOI] [PubMed] [Google Scholar]
- 4.Zile M., Brutsaert D. New concepts in diastolic dysfunction and diastolic heart failure, diagnosis, prognosis and measurement of diastolic function. Circulation. 2002;105:1387–1393. doi: 10.1161/hc1102.105289. [DOI] [PubMed] [Google Scholar]
- 5.Hamlin S.K., Villars P.S., Kanusky J.T., Shaw A.D. Role of diastole, in left ventricular function, II: Diagnosis and treatment. Am J Crit Care. 2004;13(6):453–468. [PubMed] [Google Scholar]
- 6.Nagueh S.F., Appleton C.P., Gillebert T.C., Marino P.N. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22(2):107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 7.Genovesi-Ebert A., Marabottic-Palombo C., Giaconi S. Echo-Doppler diastolic function and exercise tolerance. Int J Cardiol. 1994;43:67–73. doi: 10.1016/0167-5273(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 8.Rovner A., Greenberg N.L., Thomas J.D., Garcia M.J. Relationship of diastolic intraventricular pressure gradients and aerobic capacity in patients with diastolic heart failure. Am J Physiol Heart Circ Physiol. 2005;289:H2081–H2087. doi: 10.1152/ajpheart.00951.2004. [DOI] [PubMed] [Google Scholar]
- 9.Yip G.W., Frenneaux M., Sanderson J.G. Heart failure with a normal ejection fraction: new developments. Heart. 2009;95(19):1549–1552. doi: 10.1136/hrt.2009.176222. [DOI] [PubMed] [Google Scholar]
- 10.Van-Tol B.A., Huijsmans R.J., Kroon D.W., Schorthorst M. Effect of exercise training on cardiac performance, exercise capacity and quality of life in patients with heart failure “a meta analysis”. Eur J Heart Fail. 2006;8(8):841–850. doi: 10.1016/j.ejheart.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Selig S.E., Levinger I., Williams A.D., Smart N. Exercise and sports science in Australia position statement on exercise training and chronic heart failure. J Sci Med Sport. 2010;13:288–294. doi: 10.1016/j.jsams.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Smart N. Review article; Exercise training for heart failure patients with and without systolic dysfunction: an evidence based analysis of how patients benefit. Cardiol Res Pract. 2010;2011:1–7. doi: 10.4061/2011/837238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green C.P., Porter C.B., Bresena han D.R., Spertus J.A. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardio. 2000;35(5):1246–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 14.Mattera J.A., De Leon C.M., Wackers F.J., Williams C.S. Association of patients’ perception of health status and exercise electrocardiogram, myocardial imaging, and ventricular function measures. Am Heart J. 2000;140:409–418. doi: 10.1067/mhj.2000.108518. [DOI] [PubMed] [Google Scholar]
- 15.Carr A.J., Gibson B., Robinson P.G. Measuring quality of life: is quality of life determined by expectations or experience? B M J. 2001;322:1240–1243. doi: 10.1136/bmj.322.7296.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paul-Schmid J., Blatter-Buhler P., Gaillet R., Binder R.K. Impact of cardiac rehabilitation programme on exercise capacity, parameters of left ventricular function and health-related quality of life in chronic heart failure patients. Cardiovasc Med. 2010;13(3):86–91. [Google Scholar]
- 17.Maria- Sarullo F., Gristina T., Brusca I. Effect of physical training on exercise capacity, gas exchange and N-terminal pro-brain natriuretic peptide levels in patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil. 2006;13:812–817. doi: 10.1097/01.hjr.0000238396.42718.61. [DOI] [PubMed] [Google Scholar]
- 18.Wisloff U., Stoylen A., Loennechen J.P. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115(24):3086–3094. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 19.Al Jaroudi W., Alraies M.C., Halley C., Rodbriguez L., Grim R.A., Thomas J.D., Jaber W.A. Impact of progression of diastolic dysfunction on mortality in patients with normal ejection fraction. Circulation. 2012;125:782–788. doi: 10.1161/CIRCULATIONAHA.111.066423. [DOI] [PubMed] [Google Scholar]
- 20.Grewal J., McCully R.B., Kane G.C., Lam C., Pellikka P.A. Left ventricular function and exercise capacity. J Am Med Assoc. 2009;301:286–294. doi: 10.1001/jama.2008.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rossi A., Cicoira M., Golia G., Zanolla L., Franceschini L., Marino P. Amino-terminal propeptide of type III procollagen is associated with restrictive mitral filling pattern in patients with dilated cardiomyopathy: a possible link between diastolic dysfunction and prognosis. Heart. 2004;90:650–654. doi: 10.1136/hrt.2002.005371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parissi J.T., Nikolaou M., Farmakis D., Paraskevaidis L.A. Self-assessment of health status is associated with inflammatory activation and predicts long term outcomes in chronic heart failure. Eur J Heart Fail. 2009;11:163–169. doi: 10.1093/eurjhf/hfn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conard M.W., Heidenreich P., Rumsfeld J.S., Weintraub W.S., Spertus J. Patient-reported economic burden and the health status of heart failure patients. J Card Fail. 2006;12:369–374. doi: 10.1016/j.cardfail.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Feldman P.H., Murtaugh C.M., Pezzin L.E., McDonald M.V., Peng T.R. Just-in-time evidence-based e-mail ‘reminders’ in home health-care: impact on patient outcomes. Health Serv Res. 2005;40:865–885. doi: 10.1111/j.1475-6773.2005.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheitlin M.D., Armstrong W.F., Aurigemma G.P., Beller G.A. ACC/AHA/ASE guidelines update for the clinical application of echocardiography summary article. J Am Soc Echocardiogr. 2003;16:1091–1110. doi: 10.1016/S0894-7317(03)00685-0. [DOI] [PubMed] [Google Scholar]
- 26.Myers J., Wagner D., Schertler T., Beer M., Luchinger R. Effects of exercise training on left ventricular volume and function in patients with non-ischemic cardiomyopathy: application of magnetic resonance myocardial tagging. Am Heart J. 2002;144:719–725. doi: 10.1067/mhj.2002.124401. [DOI] [PubMed] [Google Scholar]
- 27.Petersen K.I., Reikvam A., Rollag A., Stavemk Reliability and validity of the Kansas City Cardiomyopathy Questionnaire in patients with previous myocardial infarction. Eur J Heart Fail. 2005;7:235–242. doi: 10.1016/j.ejheart.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Exercise prescription in coronary artery disease prevention and rehabilitation programs. In: Brubaker PH, Kaminsky LA, editors. Coronary artery disease, essentials of prevention and rehabilitation programs, 1st ed. USA: Human Kinetics; 2002. p. 201–47.
- 29.Nagib-Elkilany G.E., Al-Qbandi M.A., Sayed K.A., Kabbash I. Dilated cardiomyopathy in children and adults: what is new? Sci World J. 2008;8:762–775. doi: 10.1100/tsw.2008.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brubaker P.H., Peter H. Exercise therapy for the failing heart-harmful or helpful. ACSM Health Fit J. 2010;14(2):9–15. [Google Scholar]
- 31.McFarlane N., Northridge D.B., Wright A.R., Grant S. A comparative study of left ventricular structure and function in elite athletes. Br J Sports Med. 1991;25:45–48. doi: 10.1136/bjsm.25.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lisauskas J.B., Singh J., Bowman A.W., Kovacs S.J. Chamber properties from transmitral flow: prediction of average and passive left ventricular diastolic stiffness. J Appl Physiol. 2001;91:154–162. doi: 10.1152/jappl.2001.91.1.154. [DOI] [PubMed] [Google Scholar]
- 33.Myers J., Zaheer N., Quaglietti S., Madhavan R. Association of functional and health status measures. J Card Fail. 2006;12(6):439–445. doi: 10.1016/j.cardfail.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Haykowsky M.J., Liang Y., Pechter D. A meta-analysis of the effect of exercise training on left ventricular remodeling in heart failure patients: the benefits on the type of the training performed. J Am College Cardiol. 2007;49:2329–2336. doi: 10.1016/j.jacc.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 35.Belardinelli R., Georgious D., Cianci G., Purcaro A. Effects of exercise training on left ventricular filling at rest and during exercise in patients with ischemic cardiomyopathy and severe left ventricular systolic dysfunction. Am Heart J. 1996;132:61–70. doi: 10.1016/s0002-8703(96)90391-9. [DOI] [PubMed] [Google Scholar]
- 36.Belardinelli R., Georgious D., Cianci G., Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure. Effects on functional capacity, quality of life and clinical outcome. Circulation. 1999;99(9):1173–1182. doi: 10.1161/01.cir.99.9.1173. [DOI] [PubMed] [Google Scholar]
- 37.Malfatto G., Branzi G., Osculati G. Improvement in left ventricular diastolic stiffness induced by physical training in patients with dilated cardiomyopathy. J Card Fail. 2009;15(4):327–333. doi: 10.1016/j.cardfail.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 38.European Study Group on Diastolic Heart failure. How to diagnose diastolic heart failure. Eur Heart J 1998;19:990–1003. [DOI] [PubMed]
- 39.Nishimura R.A., Tajik A.J. Evaluation of diastolic filling of left ventricle in health and disease: Doppler echocardiography is the clinician’s Rosettastone. J Am College Cardiol. 1997;30:8–18. doi: 10.1016/s0735-1097(97)00144-7. [DOI] [PubMed] [Google Scholar]
- 40.Lejemtel T.H., Liang C.S., Stewart D.K., Kirlin P.C. Reduced Peak aerobic capacity in a symptomatic left ventricular systolic dysfunction. A substudy of the studies of left ventricular dysfunction (SOLVD). SOLVD investigator studies of left ventricular dysfunction. Circulation. 1994;90:2757–2760. doi: 10.1161/01.cir.90.6.2757. [DOI] [PubMed] [Google Scholar]
- 41.Pina I.L., Apstein C.S., Balady G.J. Exercise and heart failure: a statement from the American Heart Association Committee on exercise, rehabilitation, and Prevention. Circulation. 2003;107:1210–1225. doi: 10.1161/01.cir.0000055013.92097.40. [DOI] [PubMed] [Google Scholar]
- 42.Smart N., Marwick T.H. Exercise training for patients with heart failure: a systematic review of factors that improve mortality and morbidity. Am J Med. 2004;116(10):693–706. doi: 10.1016/j.amjmed.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 43.Teerlink J.R. Endothelins: pathophysiology and treatment implications in chronic heart failure. Curr Heart Fail Rep. 2005;2(4):191–197. doi: 10.1007/BF02696649. [DOI] [PubMed] [Google Scholar]
- 44.Belardinelli R., Georgious D., Cianc G., Berman N., Ginzton L. Exercise training improves left ventricular diastolic filling in patients with dilated cardiomyopathy. Circulation. 1995;91:2775–2784. doi: 10.1161/01.cir.91.11.2775. [DOI] [PubMed] [Google Scholar]
- 45.Mura A., Olszowska M., Tomkiewicz-Pajak L., Podolec P. Relationship between Doppler indices of diastolic function and exercise capacity in patients with congestive heart failure. Przegl Lek. 2004;61(6):660–663. [abstract] [PubMed] [Google Scholar]
- 46.Little W.C., Kitzman D.W., Cheng C.P. Diastolic dysfunction as a cause of exercise intolerance. Heart Fail Rev. 2000;5:301–306. doi: 10.1023/a:1026503028065. [DOI] [PubMed] [Google Scholar]
- 47.Libonati R. Myocardial diastolic function and exercise. Med Sci Sports Exerc. 1999;31:1741–1747. doi: 10.1097/00005768-199912000-00008. [DOI] [PubMed] [Google Scholar]
- 48.Witczak C.A., Sturek M. Training induced sarcoplasmic reticulum Ca2+ unloading occurs without Ca2+ influx. Med Sci Sports Exerc. 2005;37(7):1119–1125. doi: 10.1249/01.mss.0000170125.25749.4d. [DOI] [PubMed] [Google Scholar]
- 49.Soto G.E., Jones P., Weintraub W.S., Krumholz H.M. Prognostic value of heart status in patients with heart failure after acute myocardial infarction. Circulation. 2004;110:546–551. doi: 10.1161/01.CIR.0000136991.85540.A9. [DOI] [PubMed] [Google Scholar]
- 50.Heidenreich P.A., Spertus J.A., Jones P.G., Weintraub W.S. Health status identifies heart failure out patients at risk for hospitalization or death. J Am College Cardiol. 2006;47(4):752–756. doi: 10.1016/j.jacc.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 51.Sullivan M.D., Levy W.C., Russo J.E., Crane B. Summary health status measures in advanced heart failure; relationship to clinical variables and out comes. J Card Fail. 2007;13(7):560–568. doi: 10.1016/j.cardfail.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 52.Rector T.S., Anand I.S., Cohn J.N. Relationships between clinical assessment and patient s, perception of the effects of heart failure on their quality of life. J Card Fail. 2006;12(2):87–89. doi: 10.1016/j.cardfail.2005.10.002. [DOI] [PubMed] [Google Scholar]



