Abstract
In the present study, the alteration in the sleep EEG in rats due to chronic exposure to low-level non-thermal electromagnetic radiation was investigated. Two types of radiation fields were used; 900 MHz unmodulated wave and 900 MHz modulated at 8 and 16 Hz waves. Animals has exposed to radiation fields for 1 month (1 h/day). EEG power spectral analyses of exposed and control animals during slow wave sleep (SWS) and rapid eye movement sleep (REM sleep) revealed that the REM sleep is more susceptible to modulated radiofrequency radiation fields (RFR) than the SWS. The latency of REM sleep increased due to radiation exposure indicating a change in the ultradian rhythm of normal sleep cycles. The cumulative and irreversible effect of radiation exposure was proposed and the interaction of the extremely low frequency radiation with the similar EEG frequencies was suggested.
Keywords: Electromagnetic radiation, Electroencephalogram, Slow wave sleep, Rapid eye movement sleep
Introduction
The widespread of radiofrequency radiation (RFR) sources in domestic use has increased over the last decades, especially in the communication field, and public concern has been raised to quantify the health hazard problems that may occur due to the exposure to such type of non-ionizing radiation.
Tissue heating is the most widely accepted mechanism of microwave radiation with biological systems. These effects can result from elevations of tissue temperature induced by radiofrequency (RF) energy deposited or absorbed in biological systems through local, partial-body or whole-body exposures. However, a large bulk of literature have evidenced that several biological effects of RF can be formed without tissue heating which are known as non-thermal biological effects of radiation [1].
EEG considered to be a sensitive tool to asses quantify and classify sleep stages as well as study their changes due to radiation interaction with the brain. In human and most animals, EEG appears as low-amplitude fast waves during awake state, high-amplitude slow waves during SWS and low amplitude fast waves during REM sleep.
It has also been repeatedly reported that exposure to low-level microwaves produces alterations in the resting or sleep EEG signal and brain physiology [2–4]. It has been demonstrated that exposure to pulse-modulated microwaves alters not only the EEG but also regional cerebral blood flow [5,6]. Furthermore, it has been reported that modulation is crucial for radiofrequency electromagnetic field-induced alterations in brain physiology [6].
Sleep function is hypothesized to be the reprocessing and consolidation of memory traces [7,8]. There is also some recent evidence suggesting that sleep may help to protect declarative memories from subsequent associative interferences [9].
Sleep is one of the biological phenomena that can be affected by RF radiation exposure. Mann and Roschke [10] reported reduction in latency to sleep onset and the percentage of REM sleep due to exposure to GSM-like signals. Loughran et al. [11] reported a decrease in REM sleep latency after 30 min of 894.6 MHz radiation exposure.
In the present study, several aims have been addressed. First, the non-thermal effect of electromagnetic radiation was studied by the application of low-level radiation (0.025 mW/cm2). Second, the differences in the effect of the continuous and the modulated wave’s electromagnetic radiation were checked out by application of these two types of radiation. The modulation frequencies were selected to be within the physiological range of the brain’s EEG signals to assess the interaction of theses similar frequencies. Finally, the chronic exposure of radiation rather than the acute exposure was used to investigate the cumulative nature of radiation effects on the biological system.
Material and methods
Experimental animals
The experimental animals used in the present study were adult male Wistar albino rats, weighing 175–250 g. The animals were obtained from the animal house of the National Research Center, Egypt. They were maintained on stock diet and kept under fixed conditions of housing and handling. They were under controlled light-dark cycle (on at 7 a.m. and off at 7 p.m.) and temperature conditions (25 ±2 °C). All experiments were carried out in accordance with the research protocols established by the Animal Care Committee of the National Research Center, Egypt which followed the recommendations of the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985).
Experimental design
A total of 40 rats were divided into four groups. Three groups were irradiated with electromagnetic radiation either 900 MHz continuous wave or frequency-modulated (8 and 16 Hz) wave on a daily basis, (1 h per day) for 1 month. The fourth group served as a control group with the same experimental conditions except radiation exposure.
The exposure setup
The radiofrequency (RF) generator (Aeroflex company, Model: 2025, UK) connected to a power amplifier (Stealth Microwave, Model: SM 0520-36, SSB Technologies, Inc., NJ, USA) was used to generate the electromagnetic radiation. The amplifier, in turn, was connected to a circular monopole antenna designed so that the reflection coefficient at its input should not more than −12 dBm and fed by a coaxial line through a Bayonet Neill-Concelman (BNC) connector. The spatial distribution of the electromagnetic radiation power density was measured with a field meter (Narda, EMR200, frequency from 0 to 4 GHz, Germany).
The specific absorption rate (SAR) distribution in the rat head was determined by using the finite different time domain (FDTD) method, with the aid of the XFDTD Bio-pro software (version: 6.3.8.4, NY, USA). Geometric/electric model was constructed for the animal’s head from the stereotaxic atlas of Paxinos and Watson [12]. An ellipsoid model with the internal anatomic layers was used. The standard dielectric properties [13] were assigned to each layer. The animal head model was subjected to RFR with the same power density as that measured by the field meter through the experimental exposure process. The FDTD algorithm was then applied to calculate the electric field distribution everywhere inside the head model. The SAR was calculated at the desired points as σDED2/2ρ, where E is the electric field peak value at the point (V/m), σ is the conductivity of the tissue at this point (S/m) and ρ is the density of the tissue (Kg/m3). The calculated spatial peak SAR averaged over 1 g was found to be 0.245 W/kg.
As shown in Fig. 2, rats were housed in a circular plastic tray (50 cm diameter) which is divided into equal sectors to ensure that all rats were equally exposed to radiation. The antenna emitting the electromagnetic radiation was fixed in the center of the tray. To avoid stress, an aperture (1.5 cm in diameter) was made in the upper lid of each sector tip toward the antenna for animal breathing and this design make the animals freely direct their heads toward the radiation antenna.
Fig. 2.
Exposure set-up of the animals with the antenna placed in the center.
EEG recording and analysis
Under Na-pentobarbital anesthesia (40 g/kg of animal), animals were positioned in the stereotaxic device (David Kopf instruments, Tujunga, California, USA) and implanted with three epidural stainless steel electrodes, of 1 mm diameter, Electrodes were implanted over the frontal cortex at 3.9 mm anterior to the Bregma and 2 mm lateral (right) to the midline, the other electrode was implanted at 6.4 mm posterior to the Bregma and 4 mm lateral (right) to the midline, whereas, the third electrode (reference electrode) was implanted over the cerebellum 1 mm posterior to Lambda, on the extension of the midline [12]. The three electrodes were connected to a multipin connector base, and the entire assembly was fixed to the skull and isolated with dental cement (zinc polycarboxylate non-irritating dental cement, purchased from Spofa-Dental-Praha, Czech Republic).
During EEG recordings, rats were housed in a sound attenuated, aerated and electrically shielded cage (25 × 25 × 30 cm). They were left 30 min prior to recording for acclimatization to the laboratory environment. EEG recordings were performed at fixed time of the day under the following conditions; 50 Hz notch filter and sampling rate of 200 sample/s.
REM sleep was characterized by low-voltage (desynchronized) EEG activity and continuous high theta power (4–8 Hz) [14,15]. SWS was characterized by high-voltage (synchronized) EEG activity and high delta power (1–4 Hz). Using both the time and frequency domains criteria, the two different sleep states were distinguished over 1 h of EEG recording session.
The Fast Fourier Transform (FFT) was used to convert data from the time domain to the frequency domain to obtain power spectra for each of the SWS and REM sleep samples. The obtained power spectrum of each sample was segmented into five frequency bands, delta (1–4 Hz); theta (4.1–8 Hz); alpha (8.1–13 Hz); beta-1 (13.1–18 Hz); beta-2 (18.1–30 Hz). The band power (BP), which is the integration of the power in certain EEG band, for SWS and REMS states were calculated, then an average was estimated over 1 h of EEG session. For comparison purpose and to overcome the inter-individual variations, a normalization of band power was achieved by dividing value of the individual band power by the total power of all bands for each animal.
The latency of REM sleep, which is the period of time between the onset of sleep and the appearance of the first REM, was measured. Statistical analysis between control and irradiated animals were determined by using student’s t-test.
Results
Identification of SWS and REM sleep patterns
The base line recording of rat’s EEG during SWS and REM sleep is illustrated in Fig. 1A and B, respectively. As shown in Fig. 1A, the pattern of the EEG recorded during SWS is generally characterized by high amplitude and slow frequency in contrast to the pattern of EEG recorded during REM sleep which is characterized by lower amplitude and higher frequency as shown in Fig. 1B. On the basis of amplitude and frequency analysis the two types of sleep (SWS and REM) were identified.
Fig. 1.
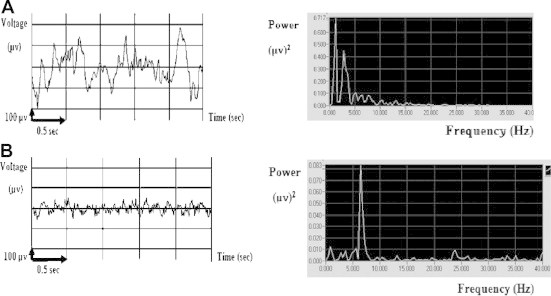
EEG time domain signals and their corresponding power spectra during: (A) SWS and (B) REM sleep in an unexposed rat.
Effect of continuous and modulated RFR on EEG bands power during SWS
The effect of RFR on the EEG band power (BP) values during SWS in adult male rats is presented in Table 1 and Fig. 3. Generally, The RFR resulted in non-significant changes in the BP values during SWS. At continuous RF, the BP values of both theta and alpha frequency bands showed increases (+7.477% and +19.093%, respectively) with respect to the control values, while the delta BP value showed a decrease of (−13.857%) below the control value. Beta-1 and beta-2 frequency bands showed nearly control-like values (+0.512% and 0.416%, respectively).
Table 1.
Effect of RFR on the EEG band power during SWS.
| SWS | EEG band | Control | 900 MHz | 900 MHz modulated at 8 Hz | 900 MHz modulated at 16 Hz |
|---|---|---|---|---|---|
| Delta | 37.84 ± 2.27 | 32.59 ± 2.39 | 40.18 ± 3.45 | 37.75 ± 3.7 | |
| Theta | 28.04 ± 0.92 | 30.14 ± 0.66 | 29.07 ± 2.30 | 27.11 ± 0.58 | |
| Alpha | 17.91 ± 1.54 | 21.33 ± 1.11 | 16.73 ± 2.55 | 20.09 ± 2.12 | |
| Beta-1 | 9.18 ± 0.99 | 9.23 ± 0.50 | 8.37 ± 1.22 | 9.62 ± 1.51 | |
| Beta-2 | 6.97 ± 0.64 | 6.99 ± 0.70 | 5.62 ± 0.61 | 5.59 ± 0.84 |
Mean ± SEM values.
*Significant P < 0.05.
Fig. 3.
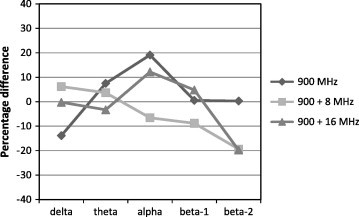
Percentage differences between control and irradiated groups of EEG bands power at 900 MHz un-modulated wave and 900 MHz modulated at 8 and 16 Hz during SWS.
At 8 Hz modulated RF, there was an increase in the band power (BP) value of the delta and theta waves (+6.205% and +3.673%, respectively). However, The BP values of alpha, beta-1 and beta-2 frequency band showed decreases with respect to the control group, the highest decrease was observed for the beta-2 (−19.351%), followed by beta-1 (−8.738%) and the least decrease (−6.315%) was recorded for the alpha band.
At 16 Hz modulated RF, The increase was detected in the alpha and beta-1 frequency band, (+12.185% and +4.859, respectively) whereas, delta, theta and beta-2 BPs showed decreases with respect to control values (−0.216%, −3.313% and −19.824% respectively).
Effect of continuous and modulated RFR on EEG bands power during REM sleep
The data showing the effect of RFR on the BP values during REM sleep of adult male rats is presented in Table 2 and Fig. 4. At continuous RF, non-significant changes were recorded, however the low frequency delta BP showed a moderate increase (+18.567%) above the control value, the theta and beta-2 BPs were recorded nearly normal-like values (+2.234% and −1.144%, respectively). Meanwhile, the BPs of alpha and beta-1 showed moderate decreases (−19.904% and −18.223%, respectively).
Table 2.
Effect of RFR on the EEG band power during REM sleep.
| REM | EEG bands | Control | 900 MHz | 900 MHz modulated at 8 Hz | 900 MHz modulated at 16 Hz |
|---|---|---|---|---|---|
| Delta | 23.36 ± 2.02 | 27.69 ± 2.86 | 26.68 ± 1.16 | 24.84 ± 3.73 | |
| Theta | 41.13 ± 2.10 | 42.05 ± 2.09 | 34.67 ± 1.53∗ | 49.14 ± 1.66∗ | |
| Alpha | 17.44 ± 2.09 | 13.97 ± 2.09 | 17.12 ± 1.82 | 12.54 ± 2.59 | |
| Beta-1 | 8.28 ± 0.56 | 6.76 ± 1.22 | 8.98 ± 1.15 | 5.98 ± 0.75∗ | |
| Beta-2 | 9.61 ± 1.32 | 9.49 ± 1.81 | 12.26 ± 1.14 | 7.47 ± 0.9 |
Mean ± SEM values.
Significant P < 0.05.
Fig. 4.
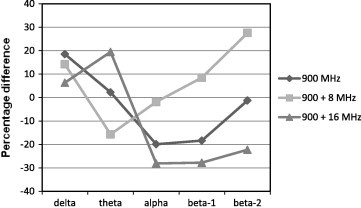
Percentage differences between control and irradiated groups of EEG bands power at 900 MHz un-modulated wave and 900 MHz modulated at 8 and 16 Hz during REM sleep.
At 8 Hz modulated RF, there was a significant decrease (−15.698%) in the BP value of the theta frequency band. In beta-2 BP value a considerable but non-significant increase (+27.646%) was recorded with respect to the control group. Moderate and slight increases in the BPs of delta and beta-1 were observed (+14.222% and 8.628%, respectively). Meanwhile, the alpha BP was recorded as nearly a control-like value (−1.834%).
At 16 Hz modulated RF, The theta BP showed a significant increase (+19.464%) and beta-1 band power showed a significant decrease (−27.794%) with respect to the control group. Considerable decreases were observed in beta-2 and alpha waves (−22.223% and −28.097%, respectively). Delta BP showed an increase by +6.349% above the control value.
Effect of continuous and modulated RFR on REM sleep latency
The effect of RFR on the REM sleep latency period (the time between the onset of the rat’s sleep and the appearance of the first REM period) during 1 h of sleep in adult male rats is presented in Table 3 and Fig. 5. The three irradiated groups showed increases in the REM sleep latency period with respect to control. At continuous RF and 8 Hz modulated waves, a considerable increase above the control value were obtained, (+28.220% and +13.794%, respectively) compared to the control value. However, at 16 Hz modulated RF a significant increase in the REM sleep latency period (+94.252%) was determined as compared to the control.
Table 3.
Effect of RFR on latency (sec) of REM sleep during 1 h of sleep.
| REM latency | Control | 900 MHz | 900 MHz modulated at 8 Hz | 900 MHz modulated at 16 Hz |
|---|---|---|---|---|
| 17.3 ± 1.11 | 22.2 ± 2.1 | 19.7 ± 2.45 | 33.6 ± 2.66∗ |
Mean ± SEM values.
Significant P < 0.05.
Fig. 5.
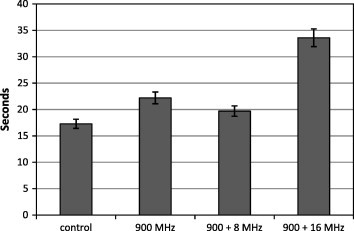
Latency in seconds of REM sleep for control, un-modulated and modulated electromagnetic radiation fields. Lines above bars represent standard deviation.
Discussion
The spectrum of rodent sleep is typically divided into two categories: slow wave sleep (SWS) and rapid-eye-movement (REM) sleep [16,17]. Both these states of sleep could be easily distinguished from each other by inspection of sleep EEG signals amplitudes and frequencies (see Material and methods section). Based upon this sleep phenomenon, the present study aimed to investigate whether these two states of sleep could be affected differently by electromagnetic radiation field’s exposure.
The current safety standards of electromagnetic radiation are based on thermal effects only and completely ignoring the non-thermal biological and health effects [18]. Several studies have showed that the low level non-ionizing radiation has adverse effects on different biological levels [19–22]. In the present study, we used low level electromagnetic radiation (0.025 mW/cm2) which resulted in low SAR value (0.245 W/Kg) to investigate the effect of such non-thermal radiation on the sleep patterns of rat. Generally, the changes induced in the sleep EEG frequency bands, either with continuous or modulated low level radiation fields in irradiated animals with respect to control animals in the present study, provide evidence about the hypothesis of non-thermal effects of electromagnetic on the brain physiology. The mechanism of non-thermal RFR on biological tissues still under investigation, however, calcium efflux and free radical production are among the candidates of the possible mechanism responsible for non-thermal effects of RFR.
In the present study the exposure of the animals to 900 MHz RFR either continuous or modulated at 8 and 16 Hz resulted in non-significant changes of all EEG bands during SWS. However, significant changes have been recorded during REM sleep especially with modulated electromagnetic radiation fields. This result denotes that the REM sleep is more sensitive to changes due to electromagnetic radiation exposure than SWS. One possible mechanism for interpretation of the sensitivity of the REM sleep for RFR is the interaction of the RFR with the central cholinergic system that known to control both REM sleep and waking state in the animal [23]. On the other hand, many studies have shown the importance of REM sleep for successful memory consolidation and learning in rats [24–27]. Therefore, the alteration in REM sleep due to RFR may compromise memory and learning process in rat.
During REM sleep, in the present study, exposure to RFR modulated at 8 Hz resulted in significant decrease in Theta BP (−15.7%) and exposure to RFR modulated at 16 Hz resulted in a significant decrease in the beta-1 BP (−27.79%). Both of these suppressed frequency bands have a frequency range which is similar to the used modulation frequency, respectively. It has earlier been reported that inhibitory as well as excitatory influences of high frequency electromagnetic fields are dependent on the kind of signal modulation [28]. Recently, Hinrikus et al. [29] have found that exposure of humans to 450 MHz microwave modulated at 14, 21, 40, 70 and 217 Hz affects the EEG frequencies lower or close to the modulation frequency and that no significant effect was detected at EEG frequencies higher than the modulation frequency. A review on animal studies suggested that pulse modulations between 8 and 16 Hz might be critical for physiological effects of GSM mobile phone signals [30]. It could be suggested that the presence of such extremely low frequencies, which are within the physiological range of the brain signals, may play a role in enhancing the interaction of RFR with the brain physiology. However, the mechanism of interaction between these frequencies and brain signals still unclear.
Using of acute rather than chronic exposure to RFR led several studies to report negative effects of exposure on the brain physiology [31–34]. In the present study; the animals were exposed to RFR for 30 consecutive days. This relatively long period of exposure allows the radiation effects to be accumulated and ends up with effects that may have not appeared in acute experiment. Furthermore, this may explain the discrepancy of results in the literature between the acute and the chronic exposure to radiation fields.
The irradiated groups, in the present study, showed a large increase in the REM sleep latency. The change in the REM sleep latency may suggest initial alterations to the ultradian rhythm of the SWS/REM sleep cycle [35]. Numerous findings confirmed that cholinergic mechanisms are essential for the generation of REM sleep and its physiologic signs [36,37]. The alterations in the cholinergic neurons or their innervations by the interaction with RFR may lead to changes in the REM latency.
Conclusions
In the present study, it can be concluded that exposure to electromagnetic radiation in awake animals can alter their subsequent sleep structure. The REM sleep considered to be more sensitive for RF radiation than SWS as indicated from sleep EEG data analyses. The using of frequencies similar or close to the biological frequencies could result in more adverse effect than other frequencies which lie far from biological frequencies. The increase in REM latency after irradiation denotes change in the sleep pattern of the exposed animals and provides evidence about the adverse effect of non-thermal electromagnetic radiation fields on brain physiology. Further studies are needed to explore the mechanism of interaction between electromagnetic radiation fields and the biological phenomena.
Acknowledgments
This study was a part of a project entitled “a study on the influence of mobile phone radiation on some function of central nervous system”. The project was granted by the sector of “International cooperation with USA”, Foreign Ministry, Egypt.
Footnotes
This work has been implemented in the neurophysiological unit at zoology department, faculty of Science, Cairo University.
Peer review under responsibility of Cairo University.
References
- 1.Giuliani L, Soffritti M. Non-thermal effects and mechanisms of interaction between electromagnetic fields and living matter. ICEMS Monograph, editors. Eur J Oncol, vol. 5; 2010 [Library].
- 2.Borbely A.A., Huber R., Graf T., Fuchs B., Gallmann E., Achermann P. Pulsed high frequency electromagnetic field affects human sleep and sleep electroencephalogram. Neurosci Lett. 1999;275:207–210. doi: 10.1016/s0304-3940(99)00770-3. [DOI] [PubMed] [Google Scholar]
- 3.Huber R., Graf T., Cote K.A., Wittmann L., Gallmann E., Matter D. Exposure to pulsed high-frequency electromagnetic field during waking affects human sleep EEG. Neuro Rep. 2000;11:3321–3325. doi: 10.1097/00001756-200010200-00012. [DOI] [PubMed] [Google Scholar]
- 4.Hinrikus H., Parts M., Lass J., Tuulik V. Changes in human EEG caused by low-level modulated electromagnetic radiation stimulation. Bioelectromagnetics. 2004;25:431–440. doi: 10.1002/bem.20010. [DOI] [PubMed] [Google Scholar]
- 5.Huber R., Treyer V., Borbely A.A., Schuderer J., Gottselig J.M., Landolt H.P. Electromagnetic fields, such as those from mobile phones, alter regional cerebral blood flow and sleep and waking EEG. J Sleep Res. 2002;11:289–295. doi: 10.1046/j.1365-2869.2002.00314.x. [DOI] [PubMed] [Google Scholar]
- 6.Huber R., Treyer V., Schuderer J., Berthold T., Buck A., Kuster N. Exposure to pulse-modulated radio frequency electromagnetic fields affects regional cerebral blood flow. Eur J Neurosci. 2005;21:1000–1006. doi: 10.1111/j.1460-9568.2005.03929.x. [DOI] [PubMed] [Google Scholar]
- 7.McGaugh J.L. Memory, a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 8.Stickgold R., James L., Hobson J.A. Visual discrimination learning requires sleep after training. Nat Neurosci. 2000;3:1237–1238. doi: 10.1038/81756. [DOI] [PubMed] [Google Scholar]
- 9.Ellenbogen J.M., Hulbert J.C., Stickgold R., Dinges D.F., Thompson-Schill S.L. Interfering with theories of sleep and memory: sleep, declarative memory, and associative interference. Curr Biol. 2006;16:1290–1294. doi: 10.1016/j.cub.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 10.Mann K., Roschke J. Effects of pulsed high-frequency electromagnetic fields on human sleep. Neuropsychobiology. 1996;33:41–47. doi: 10.1159/000119247. [DOI] [PubMed] [Google Scholar]
- 11.Loughran S.P., Wood A.W., Barton J.M., Croft R.J., Thompson B., Stough C. The effect of electromagnetic fields emitted by mobile phones on human sleep. Neuroreport. 2005;16:1973–1976. doi: 10.1097/01.wnr.0000186593.79705.3c. [DOI] [PubMed] [Google Scholar]
- 12.Paxinos G., Watson C. Academic Press; San Diego: 1986. The rat brain in stereotaxic coordinates. [DOI] [PubMed] [Google Scholar]
- 13.Gajsek P., Walters T.J., Hurt W.D., Ziriax J.M., Nelson D.A., Mason P.A. Empirical validation of SAR values predicted by FDTD modeling. Bioelectromagnetics. 2002;23(1):37–48. doi: 10.1002/bem.96. [DOI] [PubMed] [Google Scholar]
- 14.Ueta Y., Fujihara H. Short-term REM sleep deprivation and neuropeptide gene expression in the rat hypothalamus. Int Cong Ser. 2005;1278:415–418. [Google Scholar]
- 15.Ishizaki R., Shinba T., Mugishima G., Haraguchi H., Inoue M. Time-series analysis of sleep–wake stage of rat EEG using time-dependent pattern entropy. Physica A. 2008;387:3145–3154. [Google Scholar]
- 16.Neckelmann D., Olsen O.E., Fagerland S., Ursin R. The reliability and functional validity of visual and semiautomatic sleep/wake scoring in the Moll–Wistar rat. Sleep. 1994;17:120–131. doi: 10.1093/sleep/17.2.120. [DOI] [PubMed] [Google Scholar]
- 17.Robert C., Guilpin C., Limoge A. Automated sleep staging systems in rats. J Neurosci Methods. 1999;88:111–122. doi: 10.1016/s0165-0270(99)00027-8. [DOI] [PubMed] [Google Scholar]
- 18.ICNIRP statement on the guidelines for limiting exposure to time varying electric, magnetic, and electromagnetic fields (up to 300 GHz). Health Phys 2009; 97(3): 257–58. [DOI] [PubMed]
- 19.Pavacic I., Trosic I. In vitro testing of cellular response to ultra high frequency electromagnetic field radiation. Toxicol In Vitro. 2008;22(5):1344–1348. doi: 10.1016/j.tiv.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 20.Eberhardt J., Persson B.R., Brun A.E., Salford L.G., Malmgren L.O. Blood-brain barrier permeability and nerve cell damage in rat brain 14 and 28 days after exposure to microwaves from GSM mobile phones. Electromag Biol Med. 2008;27(3):215–229. doi: 10.1080/15368370802344037. [DOI] [PubMed] [Google Scholar]
- 21.Bas O., Odaci E., Kaplan S., Acer N., Ucok K., Colakoglu S. 900 MHz electromagnetic field exposure affects qualitative and quantitative features of hippocampal pyramidal cells in the adult female rat. Brain Res. 2009;1265:178–185. doi: 10.1016/j.brainres.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Vorobyov V., Janać B., Pesić V., Prolić Z. Repeated exposure to low-level extremely low frequency-modulated microwaves affects cortex–hypothalamus interplay in freely moving rats: EEG study. Int J Radiat Biol. 2010;86(5):376–383. doi: 10.3109/09553000903567938. [DOI] [PubMed] [Google Scholar]
- 23.Marrosu F., Portas C., Mascia M.S., Casu M.A., Fà M., Giagheddu M. Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep–wake cycle in freely moving cats. Brain Res. 1995;671:329–332. doi: 10.1016/0006-8993(94)01399-3. [DOI] [PubMed] [Google Scholar]
- 24.Smith C. Sleep states, memory processes and synaptic plasticity. Behav Brain Res. 1996;78:49–56E. doi: 10.1016/0166-4328(95)00218-9. [DOI] [PubMed] [Google Scholar]
- 25.Graves L.A., Heller E.A., Pack A.I., Abel T. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn Mem. 2003;10:168–176. doi: 10.1101/lm.48803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hennevin E., Huetz C., Edeline J.M. Neural representations during sleep: from sensory processing to memory traces. Neurobiol Learn Mem. 2007;87:416–440. doi: 10.1016/j.nlm.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Hagewoud R., Whitcomb S.N., Heeringa A.N., Havekes R., Koolhaas J.M., Meerlo P. A time for learning and a time for sleep: the effect of sleep deprivation on contextual fear conditioning at different times of the day. Sleep. 2010;33:1315–1322. doi: 10.1093/sleep/33.10.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arber S.L., Lin J.C. Microwave-induced changes in nerve cells: effects of modulation and temperature. Bioelectromagnetics. 1985;6:257–270. doi: 10.1002/bem.2250060306. [DOI] [PubMed] [Google Scholar]
- 29.Hinrikus H., Bachmann M., Lass J., Tuulik V. Effect of modulated at different low frequencies microwave radiation on human EEG. Environmentalist. 2009;29:215–219. [Google Scholar]
- 30.Hinrikus H., Parts M., Lass J., Tuulik V. Changes in human EEG caused by low level modulated microwave stimulation. Bioelectromagnetics. 2004;25:431–440. doi: 10.1002/bem.20010. [DOI] [PubMed] [Google Scholar]
- 31.Sienkiewicz Z.J., Blackwell R.P., Haylock R.G., Saunders R.D., Cobb B.L. Low-level exposure to pulsed 900 MHz microwave radiation does not cause deficits in the performance of a spatial learning task in mice. Bioelectromagnetics. 2000;21:151–158. doi: 10.1002/(sici)1521-186x(200004)21:3<151::aid-bem1>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 32.Lai H. Interaction of microwaves and a temporally incoherent magnetic field on spatial learning in the rat. Physiol Behav. 2004;82:785–789. doi: 10.1016/j.physbeh.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 33.Cassel J.C., Cosquer B., Galani R., Kuster N. Whole-body exposure to 2.45 GHz electromagnetic fields does not alter radial-maze performance in rats. Behav Brain Res. 2004;155:37–43. doi: 10.1016/j.bbr.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 34.Cosquer B., Kuster N., Cassel J.C. Whole-body exposure to 2.45 GHz electromagnetic fields does not alter 12-arm radial-maze with reduced access to spatial cues in rats. Behav Brain Res. 2005;161(2):331–334. doi: 10.1016/j.bbr.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 35.Roth T. Characteristics and determinants of normal sleep. J Clin Psychol. 2004;65:8–11. [PubMed] [Google Scholar]
- 36.Capece M.C., Baghdoyan H.A., Lydic R. New directions for the study of cholinergic REM sleep generation: specifying pre- and post-synaptic mechanisms. In: Mallick B.N., Inoue S., editors. Rapid eye movement sleep. Marcel Dekker; New York: 1999. pp. 123–141. [Google Scholar]
- 37.Semba K. The mesopontine cholinergic system: a dual role in REM sleep and wakefulness. In: Lydic R., Baghdoyan H.A., editors. Handbook of behavioral state control: molecular and cellular mechanisms. CRC; Boca Raton (FL): 1999. pp. 161–180. [Google Scholar]



