Abstract
Three sensitive methods were developed for simultaneous determination of Ezetimibe (EZB) and Atorvastatin calcium (ATVC) in binary mixtures. First derivative (D1) spectrophotometry was employed for simultaneous determination of EZB (223.8 nm) and ATVC (233.0 nm) with a mean percentage recovery of 100.23 ± 1.62 and 99.58 ± 0.84, respectively. Linearity ranges were 10.00–30.00 μg mL−1 and 10.00–35.00 μg mL−1, respectively. Isosbestic point (IS) spectrophotometry, in conjunction with second derivative (D2) spectrophotometry was employed for analysis of the same mixture. Total concentration was determined at IS, 224.6 nm and 238.6 nm over a concentration range of 10.00–35.00 μg mL−1 and 5.00–30.00 μg mL−1, respectively. ATVC concentration was determined using D2 at 313.0 nm (10.00–35.00 μg mL−1) with a mean recovery percentage of 99.72 ± 1.36, while EZB was determined mathematically at 224.6 nm (99.75 ± 1.43) and 238.6 nm (99.80 ± 0.95). TLC-densitometry was employed for the determination of the same mixture; 0.10–0.60 μg band−1 for both drugs. Separation was carried out on silica gel plates using diethyl ether–ethyl acetate (7:3 v/v). EZB and ATVC were resolved with Rf values of 0.78 and 0.13. Determination was carried out at 254.0 nm with a mean percentage recovery of 99.77 ± 1.30 and 99.86 ± 0.97, respectively. Methods were validated according to ICH guidelines and successfully applied for analysis of bulk powder and pharmaceutical formulations. Results were statistically compared to a reported method and no significant difference was noticed regarding accuracy and precision.
Keywords: Ezetimibe, Atorvastatin calcium, Derivative spectrophotometry, Isosbestic spectrophotomery, Spectrophotometry
Introduction
Ezetimibe (EZB) inhibits the absorption of cholesterol, decreasing the delivery of intestinal cholesterol to the liver. Atorvastatin calcium (ATVC) is a synthetic lipid-lowering agent that inhibits ß-hydroxy-ß-methylglutaryl-coenzyme A (HMG-CoA) reductase. Recently, a combination of EZB and ATVC has been introduced to the market. The co-administration of both drugs offers a well-tolerated and highly efficient treatment option for patients with dyslipidemia and helps in prescribing a low dose ATVC, which may reduce side effects [1]. Chemically EZB is [(3R,4S)-1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl)-2-azetidinone], and ATVC is [R-(R*,R*)]-2-(4-fluorophenyl)-β,δ-dihydroxy-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-1H-pyrrole-1 – heptanoic acid – calcium salt (2:1) trihydrate [2]. The chemical structures of ATVC and EZB are shown in Fig. 1.
Fig. 1.
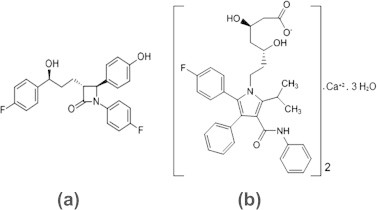
Chemical structure of Ezetimibe (a) and Atorvastatin calcium (b).
A survey of the literature revealed the following analytical techniques concerned with the determination of EZB/ATVC mixture. Reported spectrophotometric methods for the simultaneous determination of EZB/ATVC mixture include simultaneous equation method [3–5], dual wavelength measurement [6], absorbance ratio method [3,7], derivative ratio method [8,9], H-point standard addition method [9], multi-wavelength method [10] and differential spectrophotometry [9]. Other methods include; HPTLC [5,11–13], HPLC [4,5,8,14–19].
With the rapid increase in the number of generics in local markets, manufacturers tend to seek for reliable analysis protocols. Such methods should meet the strict requirements of local regulatory authorities. Unfortunately, not all published methods are reliable for this purpose. In many cases, they are not properly validated and problems arise upon method transfer to quality control labs. The aim of this work is the development of orthogonal, simple, sensitive and validated methods for the determination of EZB and ATVC in their binary mixtures and pharmaceutical preparations. Spectrophotometry and TLC-densitometry were trialled in order to provide orthogonal results via analyse of the studied mixture using different techniques.
Experimental
Instruments
A double beam UV–visible spectrophotometer model UV-1650 PC (SHIMADZU, Japan) connected to IBM compatible computer was used for all determinations. Hardware control as well as data acquisition and treatment was carried out using UV Probe software, version 2.2.1 (SHIMADZU, Japan). An offline automatic sample applicator equipped with 100 μL syringe (Camag Linomat 5, Switzerland) and a TLC scanner (Camag, Switzerland) were employed for preparation and measurement of TLC plates, respectively. Both of the scanner and the densitometer were controlled using winCATS software. A UV lamp with short wavelength 254.0 nm (Vilber Lourmat, MARŃE LA VALLEE Cedex 1, France) was used for visualization of TLC plates.
Pure drugs and samples
EZB and ATVC pure standards were kindly supplied by Marcyrl Pharmaceutical Industries, El-Obour City, Egypt. Their purity were found to be 99.85% and 100.35%, respectively, according to the absorptivity values reported [4,5]. Samples of Atoreza® tablets (Marcyrl); batch no. 1030599, labeled to contain 10 mg Ezetimibe and 10 mg Atorvastatin, per tablet were obtained from the market.
Chemicals, reagents and standard solutions
All chemicals used throughout this work were of analytical grade, and solvents were of spectroscopic grade. TLC plates (20 × 20 cm) pre-coated with silica gel 60F254 were obtained from Merck, Germany. EZB and ATVC stock solutions (1 mg mL−1) were prepared by weighing accurately 100 mg of each powder into two separate 100-mL volumetric flasks. Methanol (50 mL) was added, shaken for a few minutes and completed to volume with the same solvent. Working solutions (100 μg mL−1 in methanol) were prepared by accurately transferring 10 mL of the stock solution of EZB and 10 mL of the stock solution of ATVC in two separate 100-mL measuring flasks and diluting to the mark with methanol. A set of laboratory prepared mixtures of different ratios (1:1, 1:1.5, 1.5:1, 1:2 and 2:1) were prepared by transferring different volumes of each of EZB and ATVC stock solutions into 10-ml volumetric flasks and diluting to volume with methanol.
Procedures
Construction of calibration curve for D1 spectrophotometric method
Different aliquots equivalent to 100.00–300.00 μg of EZB and 100.00–350.00 μg of ATVC working solutions (100 μg mL−1 in methanol) were accurately transferred into a series of 10-mL volumetric flasks then diluted to volume using methanol. D1 spectra were recorded at Δλ = 4 and scaling factor = 10 using methanol as a blank. Calibration curves were obtained by plotting the peak amplitude at 223.8 and 233.0 nm versus the corresponding concentration of EZB and ATVC, respectively.
Construction of calibration curve for D2 spectrophotometric method
Aliquots of ATVC working solution (100 μg mL−1 in methanol) equivalent to 100.00–350.00 μg were accurately transferred into a series of 10-mL volumetric flasks. The volume was completed to the mark with methanol and the D2 spectra were recorded against methanol as a blank at Δλ = 8 and scaling factor = 1000. Calibration curve was obtained by plotting the peak amplitude at 313.0 nm (corresponding to zero-crossing of EZB) versus the corresponding concentration of ATVC.
Construction of calibration curve for IS spectrophotometric method
Into two separate sets of 10-mL volumetric flasks, aliquots equivalent to 100.00–350.00 μg and 50.00–300.00 μg of EZB were transferred from their working solution (100 μg mL−1 in methanol) and the volume was completed with methanol. Calibration curves were obtained by plotting the peak amplitude at 224.6 nm and 238.6 versus the corresponding concentration of EZB.
Optimization of TLC-densitometric separation parameters
A laboratory prepared mixture of EZB and ATVC (1:1 ratio, 0.2 μg band−1) used to investigate the optimum separation conditions. Developing systems of different composition and ratios were tried: chloroform–ethyl acetate (8:2, v/v), chloroform–acetone (7:3, v/v), Toluene–methanol (6:4, v/v), and diethyl ether–acetonitrile (8:2, v/v). Various band dimensions were tested in order to obtain sharp and symmetrical peaks. Plates were scanned at different wavelengths: 232.0 nm, 246.0 nm, and 266.0 nm) and using different slit dimensions. Optimum set of instrumental parameters were employed for measurement of all plates in future experiments.
Construction of calibration curve for TLC-densitometric method
For preparation of a calibration plot, 1, 2, … , 6 μL of standard working solutions of ATVC and EZB (100 μg mL−1) were spotted as bands of 6 mm width on TLC plates (20 × 10 cm). Bands were applied at 5 mm interval and 15 mm from the bottom and sides. Linear ascending plate development to a distance of 8 cm was performed in a suitable chromatographic tank previously saturated for 1 h with the developing mobile phase (diethyl ether–ethyl acetate; 7:3, v/v) at room temperature. The peak area was recorded at a scanning wavelength of 254.0 nm. Calibration curves were constructed by plotting the integrated peak area versus the corresponding concentrations of each drug and regression equation parameters were computed.
Application to pharmaceutical formulations
A total of ten Atoreza® tablets were accurately weighed and crushed to a fine powder. An amount equivalent to one tablet (containing10 mg of EZB and 10 mg of ATVC) was taken, extracted using 30 mL of methanol using a magnetic stirrer for 30 min. The mixture was transferred into a 100 mL volumetric flask through a Whatman No. 10 filter paper (pore size = 11 μm). The residue was washed twice with methanol and the combined filtrate and washings were made up to the mark with methanol to a final concentration of 100 μg mL−1 of each drug. A suitably diluted sample was measured as mentioned under each method. The possibility of interference from dosage form additives to assay performance was investigated using the standard addition technique.
Results and discussion
Analytical methods for the determination of binary mixture without previous separation are of interest to quality control (QC) labs and national regulatory authorities (NRA) around the world. The absorption spectra of EZB and ATVC show severe overlap (Fig. 2) that makes their simultaneous determination difficult. In this work, our main task was to develop simple, sensitive and accurate analytical methods for the determination of EZB and ATVC in their binary mixture and pharmaceutical formulation with satisfactory precision for good analytical practice (GAP).
Fig. 2.
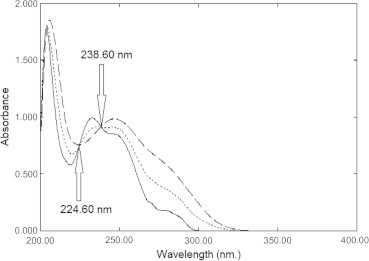
Zero order absorption spectra of 20 μg mL−1 of Ezetimibe (—), 20 μg mL−1 of Atorvastatin calcium (- - -) and a (1:1) mixture contains10 μg mL−1 of each (⋯) using methanol as a blank.
D1 spectrophotometric method
Derivative spectrophotometry offers greater selectivity than does normal spectrophotometry as it decreases spectral overlap and allows better resolution. First derivative (D1) spectrophotometric technique was used to resolve spectral overlapping of the absorption spectra of EZB and ATVC. Upon applying (D1) technique, EZB and ATVC could be determined by measuring peak amplitude of D1 spectra at 223.8 nm (corresponding to zero-crossing of ATVC) and 233.0 nm (corresponding to zero-crossing of EZB) respectively (Fig. 3). A linear correlation was obtained between peak amplitude and the corresponding concentration in the range of 10.00–30.00 μg mL−1 for EZB and in the range of 10.00–35.00 μg mL−1 for ATVC. Regression equations were computed and various regression parameters are summarized in Table 1.
where PA is peak amplitude, C is the concentration in μg mL−1 and r is the correlation coefficient. The proposed method was found valid for the simultaneous determination of EZB and ATVC in different laboratory prepared mixtures with mean percentage recoveries of 99.66 ± 1.03 and 99.39 ± 0.81, respectively, as represented in Table 2. The suggested method has been applied to assay EZB and ATVC in Atoreza® tablets and its validity was further assessed by applying the standard addition technique, Table 3.
Fig. 3.
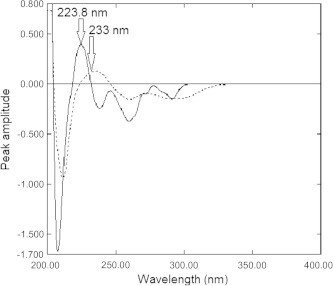
First derivative absorption spectra of 20 μg mL−1 of Ezetimibe (—) and 20 μg mL−1 of Atorvastatin calcium (⋯) using methanol as a blank.
Table 1.
Results of assay validation parameters obtained by applying the proposed methods.
| Parameter | Ezetimibe |
Atorvastatin calcium |
|||||
|---|---|---|---|---|---|---|---|
| IS |
|||||||
| D1 | 224.6 nm | 238.6 nm | TLC-densitometry | D1 | D2 | TLC-densitometry | |
| Concentration range | 10.00–30.00 (μg mL−1) | 10.00–35.00 (μg mL−1) | 5.00–30.00 (μg mL−1) | 0.10–0.60 (μg band−1) | 10.00–35.00 (μg mL−1) | 10.00–35.00 (μg mL−1) | 0.10–0.60 (μg band−1) |
| Linearity | |||||||
| Slope | 0.0170 | 0.0365 | 0.0430 | 4656.6857 | 0.0060 | 0.0349 | 5165.4857 |
| Intercept | 0.0219 | 0.0137 | 0.0316 | −190.7733 | −0.0012 | −0.0047 | 76.7133 |
| Correlation coefficient (r) | 0.9995 | 0.9995 | 0.9997 | 0.9998 | 0.9998 | 0.9994 | 0.9999 |
| Standard error of the slope | 0.0003 | 0.0006 | 0.0005 | 42.5386 | 0.00005 | 0.0006 | 33.3275 |
| Confidence limit of the slope | 0.0170 ± 0.0008 | 0.0365 ± 0.0016 | 0.0430 ± 0.0014 | 4656.6857 ± 118.1060 | 0.0060 ± 0.0002 | 0.0349 ± 0.0017 | 5165.4857 ± 92.5321 |
| Standard error of the intercept | 0.0057 | 0.0140 | 0.0095 | 16.5664 | 0.0013 | 0.0144 | 12.9792 |
| Confidence limit of the intercept | 0.0219 ± 0.0158 | 0.0137 ± 0.0389 | 0.0316 ± 0.0265 | −190.7733 ± 45.9957 | −0.0012 ± 0.0036 | −0.0047 ± 0.0399 | 76.7133 ± 36.0361 |
| Accuracy (Mean ± S.D.) | 100.23 ± 1.62 | 99.75 ± 1.43 | 99.80 ± 0.95 | 99.77 ± 1.30 | 99.58 ± 0.84 | 99.72 ± 1.36 | 99.86 ± 0.97 |
| Precision (RSD %) | |||||||
| Repeatabiltya | 0.96 | 0.45 | 0.48 | 1.00 | 1.24 | 1.14 | 0.97 |
| Intermediate precisionb | 1.16 | 1.31 | 1.10 | 1.40 | 1.32 | 1.34 | 1.29 |
| Specificity | 99.66 ± 1.03 | 100.89 ± 0.89 | 100.47 ± 0.81 | 100.25 ± 0.82 | 99.39 ± 0.81 | 100.47 ± 1.06 | 100.49 ± 0.78 |
| Limit of detection (LOD)c | 2.70 μg mL−1 | 3.10 μg mL−1 | 1.79 μg mL−1 | 0.03 μg band−1 | 1.75 μg mL−1 | 3.33 μg mL−1 | 0.02 μg band−1 |
| Limit of quantitation (LOQ)c | 8.19 μg mL−1 | 9.41 μg mL−1 | 5.43 μg mL−1 | 0.09 μg band−1 | 5.31 μg mL−1 | 10.09 μg mL−1 | 0.06 μg band−1 |
LOD = (SD of the response/slope) × 3.3; LOQ = (SD of the response/slope) × 10.
The intraday (n = 3), average of three concentrations repeated three times within day.
The interday (n = 3), average of three different concentrations repeated three times in three successive days.
Limits of detection and quantitation are determined via calculations.
Table 2.
Determination of Ezetimibe and Atorvastatin calcium in laboratory prepared mixtures by the proposed spectrophotometric methods and the reported method.
| Mixture no. | Ezetimibe recovery %a |
Atorvastatin calcium recovery %a |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Claimed taken (μg mL−1) |
IS |
||||||||
| Atorvastatin | Ezetimibe | D1 | 224.6 nm | 238.6 nm | Reported methodb | D1 | D2 | Reported methodb | |
| 1 | 10 | 10 | 98.88 | 99.67 | 100.86 | 100.85 | 100.33 | 98.77 | 100.4 |
| 2 | 10 | 15 | 100.43 | 101.82 | 99.86 | 99.91 | 98.67 | 100.77 | 100.07 |
| 3 | 10 | 20 | 98.27 | 101.7 | 101.61 | 101.68 | 98.67 | 101.06 | 98.6 |
| 4 | 15 | 10 | 100.65 | 100.7 | 100.45 | 100.1 | 99.11 | 100.23 | 99.4 |
| 5 | 20 | 10 | 100.06 | 100.58 | 99.59 | 100.57 | 100.17 | 101.53 | 98.18 |
| Mean ± S.D. | 99.66 ± 1.03 | 100.89 ± 0.89 | 100.47 ± 0.81 | 100.62 ± 0.70 | 99.39 ± 0.81 | 100.47 ± 1.06 | 99.33 ± 0.94 | ||
Average of three determinations.
Absorbance ratio method (Q-analysis) at 238.6 nm (iso-absorptive point) and 232.6 nm (λmax of Ezetimibe) [3].
Table 3.
Determination of Ezetimibe and Atorvastatin calcium in Atoreza® tablets by the proposed methods and application of standard addition technique.
| D1 |
IS |
TLC-densitometry |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 224.6 nm |
238.6 nm |
|||||||||||||||
| Product | Recoverya % ±S.D. | Added μg mL−1 | Founda μg mL−1 | Recovery % | Recoverya % ±S.D. | Added μg mL−1 | Founda μg mL−1 | Recovery % | Recoverya % ±S.D. | Added μg mL−1 | Founda μg mL−1 | Recovery % | Recoverya % ±S.D. | Added μg band−1 | Founda μg band−1 | Recovery % |
| Ezetimibe in Atoreza® tablets (Batch No. 1030599). | 100.02 ± 1.29 | 101.05 ± 1.63 | 99.03 ± 0.91 | 100.44 ± 1.26 | ||||||||||||
| 5 | 5.06 | 101.2 | 8 | 8.05 | 100.63 | 8 | 8.15 | 101.88 | 0.1 | 0.102 | 102 | |||||
| 10 | 9.94 | 99.4 | 10 | 9.92 | 99.2 | 10 | 9.94 | 99.4 | 0.2 | 0.201 | 100.5 | |||||
| 15 | 15.23 | 101.53 | 12 | 12.23 | 101.92 | 12 | 11.84 | 98.67 | 0.4 | 0.407 | 101.75 | |||||
| Mean ± S.D. | 100.71 ± 1.15 | 100.58 ± 1.36 | 99.98 ± 1.68 | 101.42 ± 0.80 | ||||||||||||
|
D1 |
D2 |
TLC-densitometry |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Product | Recoverya % ±S.D. | Added μg mL−1 | Founda μg mL−1 | Recovery % | Recoverya % ±S.D. | Added μg mL−1 | Founda μg mL−1 | Recovery % | Recoverya % ±S.D. | Added μg band−1 | Founda μg band−1 | Recovery % |
| Atorvastatin calcium in Atoreza® tablets (Batch No. 1030599). | 99.48 ± 0.82 | 5.00 | 5.00 | 100.00 | 100.46 ± 0.83 | 8.00 | 7.88 | 98.50 | 100.14 ± 1.02 | 0.10 | 0.101 | 101.00 |
| 10.00 | 10.17 | 101.70 | 10.00 | 10.09 | 100.90 | 0.20 | 0.203 | 101.50 | ||||
| 15.00 | 15.00 | 100.00 | 12.00 | 11.86 | 98.83 | 0.40 | 0.400 | 100.00 | ||||
| Mean ± S.D. | 100.57 ± 0.98 | 99.41 ± 1.30 | 100.83 ± 0.76 | |||||||||
Average of three determinations
D2 spectrophotometric method
D2 spectrophotometric technique was also used to resolve spectral overlapping of the absorption spectra of EZB and ATVC, Fig. 4. Upon applying D2 technique, ATVC could be determined by measuring peak amplitude of D2 spectrum at 313.0 nm (corresponding to zero-crossing of EZB). A linear correlation was obtained between peak amplitude and the corresponding concentration in the range of 10.00–35.00 μg mL−1 for ATVC. Regression equation was computed and various regression parameters are summarized in Table 1.
Fig. 4.
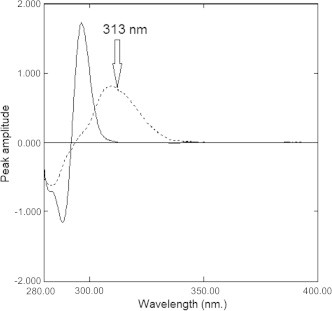
Second derivative absorption spectra of 20 μg mL−1 of Ezetimibe (—) and 20 μg mL−1 of Atorvastatin calcium (⋯) using methanol as a blank.
where PA is peak amplitude at 313.0 nm, C is the concentration in μg mL−1 and r is the correlation coefficient. The proposed method is valid for determination of ATVC in presence of EZB in different laboratory prepared mixtures with mean percentage recoveries of 100.47 ± 1.06 as represented in Table 2. The suggested method has been applied to assay ATVC in Atoreza® tablets, and its validity was further assessed by applying the standard addition technique, Table 3. The D2 method failed to determine EZB in the presence of ATVC. Thus, total concentration was determined using the below IS method. Then, EZB concentration was determined mathematically.
IS spectrophotometric method
Erram and Tipnis [20] developed the isosbestic spectrophotometric method. At the isosbestic point the mixture of drugs acts as a single component and gives the same absorbance as pure drug. In this mixture, the absorbance value at the isosbestic points 224.6 nm (Aiso1) and 238.6 nm (Aiso2) was determined (Fig. 2) and the total concentration of both drugs was calculated. Since the concentration of ATVC in this mixture can be measured using D2 spectroscopy at 313.0 nm, the concentration of EZB could be calculated by subtraction. A linear correlation was obtained between the absorbance values and the corresponding drug concentrations. Regression equations were computed and various regression parameters are summarized in Table 1.
where A is the absorbance, C is the total concentration of both drugs in μg mL−1 and r is the correlation coefficient. The proposed methods were found valid for the determination of EZB in laboratory prepared mixtures with mean percentage recoveries of 100.89 ± 0.89 and 100.47 ± 0.81 as represented in Table 2. The proposed methods were successfully applied for the analysis of both drugs in pharmaceutical dosage form and the results are shown in Table 3.
TLC-densitometric method
TLC-densitometry is a useful technique for the qualitative and quantitative determination of drug mixtures. This technique offers a simple approach to quantify separated drugs directly on TLC plates via measuring band optical densities. The amount of each compound is determined by comparison to a standard curve prepared using a reference material and chromatographed under the same condition [21]. In this work, TLC-densitometric method showed low limits of detection and quantitation. To improve separation of bands, it was necessary to investigate the effect of different experimental variables.
Reported TLC-densitometric methods for the simultaneous determination of EZB/ATVC mixture employed different mobile phases [5,11–13]. Most of the reported mobile phases were of relatively complex composition. When a two-component mobile phase was employed, insufficient validation was carried out and no system suitability data was calculated [12]. Thus the aim of this TLC-densitometric work was to investigate the use of new, simple, two component only mobile phase. Different developing systems of different composition and ratios were tried for separation and results were evaluated with respect to efficiency of separation and the shape of separated bands. The optimum mobile phase composition was found to be diethyl ether–ethyl acetate (7:3, v/v). This mobile phase allowed good separation between the binary mixtures with good Rf values without tailing of the separated bands (Fig. 5). Different band dimensions were tested in order to obtain sharp, symmetrical and well resolved peaks. The optimum band width was chosen (6 mm) and the inter-space between bands was found to be 5 mm. Different scanning wavelengths were tried where 254 nm was found optimum for both drugs. Scanned peaks were sharp, symmetrical and minimum noise was noticed. Moreover, at this wavelength maximum sensitivity was obtained for both drugs. The slit dimensions of the scanning light beam should ensure complete coverage of band dimensions on the scanned track without interference of adjacent bands. Different slit dimensions were tried, where 6 mm × 0.3 mm proved to be the slit dimension of choice which provides highest sensitivity (results not shown).
Fig. 5.
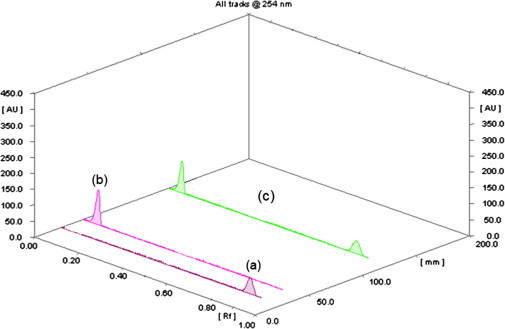
Thin layer chromatogram of separated peaks of 0.2 μg band−1 of Ezetimibe (a), 0.2 μg band−1 of Atorvastatin calcium (b), and a (1:1) mixture contains 0.2 μg band−1 of each (c) using diethyl ether: ethyl acetate (7:3, by volume) as a mobile phase.
Calibration curves were constructed by plotting the integrated peak area versus the corresponding concentrations in the range of 0.10–0.60 μg band−1 for both EZB and ATVC. The concentration of EZB and ATVC were calculated from the following regression equations. Regression equation parameters are summarized in Table 1.
where Y1 and Y2 are the integrated peak area of EZB and ATVC, respectively, C1 and C2 are the concentration of EZB and ATVC in μg band−1, respectively, and r1 and r2 are the correlation coefficients of EZB and ATVC, respectively. Various validation parameters are summarized in Table 1. The validity of the proposed methods was assessed by applying the standard addition technique. Results obtained were reproducible with low relative standard deviation as shown in Table 3. Various separation parameters; resolution (Rs), peak symmetry, capacity factor (K′) and selectivity factor (α) were calculated using a (1:1) mixture contains 0.2 μg band−1 of each drug and ATVC as reference. Resolution and selectivity were found to be 10.46 and 27.32, respectively. Peak symmetry factor was found to be 0.71 and 0.94 while capacity factor was 10.11 and 0.37 for ATVC and EZB, respectively.
Statistical comparison to reported method
A statistical comparison of the results obtained by the three proposed methods and the reported method [3] was carried out. The values of the calculated t and F were found smaller than the tabulated ones. This proved that there is no significant difference between the proposed methods and the reported method with respect to accuracy and precision. Results are summarized in Table 4.
Table 4.
Statistical comparison of the results obtained by applying the proposed methods and the reported reference method for the analysis of Ezetimibe and Atorvastatin calcium in pharmaceutical dosage form.
| Ezetimibe |
Atorvastatin calcium |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| IS |
Reported methodb |
||||||||
| D1 | 224.6 nm | 238.6 nm | TLC-densitometry | D1 | D2 | TLC-densitometry | Ezetimibe | Atorvastatin calcium | |
| Mean | 100.02 | 101.05 | 99.03 | 100.44 | 99.48 | 100.46 | 100.14 | 100.27 | 100.01 |
| S.D. | 1.29 | 1.63 | 0.91 | 1.26 | 0.82 | 0.83 | 1.02 | 0.87 | 0.99 |
| R.S.D. % | 1.29 | 1.61 | 0.91 | 1.25 | 0.83 | 0.83 | 1.02 | 0.87 | 0.99 |
| n | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Variance | 1.656 | 2.659 | 0.82 | 1.585 | 0.677 | 0.694 | 1.044 | 0.764 | 0.984 |
| Student’s t-test (2.31)a | 0.366 | 0.981 | 2.209 | 0.252 | 0.923 | 0.786 | 0.204 | ||
| F-value (6.39)a | 2.17 | 3.48 | 1.07 | 2.08 | 1.45 | 1.42 | 1.06 | ||
Conclusion
Three new selective and sensitive methods for the simultaneous determination of EZB and ATVC were developed. The D1, D2, IS spectrophotometric, and TLC-densitometric method were applied for the simultaneous determination of EZB and ATVC either in their bulk powder form or in their pharmaceutical formulations. Results demonstrated the lack of interference from dosage form additives and the usefulness of the methods. All methods are simple, sensitive, precise, accurate, inexpensive and non polluting to environment. Methods are suitable for routine quality control analysis of EZB and ATVC in pharmaceutical preparations.
References
- 1.Ballantyne C.M., Houri J., Notarbartolo A., Melani L., Lipka L.J., Suresh R. Effect of Ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation. 2003;107(19):2409–2415. doi: 10.1161/01.CIR.0000068312.21969.C8. [DOI] [PubMed] [Google Scholar]
- 2.O’Neil MJ, Smith A, Heckelman PE, Budavari SB. Merck Index, 14th ed. Darmstadt: Merck Sharp & Dohme Corp.; 2006.
- 3.Godse V.P., Deodhar M.N., Bhosale A.V., Sonawane R.A., Sakpal P.S., Borkar D.D. Simultaneous spectrophotometric estimation of Ezetimibe and atorvastatin in pharmaceutical dosage form. Asian J Res Chem. 2009;2(1):86–89. [Google Scholar]
- 4.Sonawane S.S., Shirkhedkar A.A., Fursule R.A., Surana S.J. Application of UV-Spectrophotometry and RP-HPLC for simultaneous determination of atorvastatin calcium and Ezetimibe in pharmaceutical dosage form. Eurasian J Anal Chem. 2006;1(1):31–41. [Google Scholar]
- 5.Rajamanickam V., Rajasekaran A., Rathinaraj B.S., Anandarajagopal K. Development and validation of analytical methods for simultaneous estimation of atorvastatin calcium and Ezetimibe in combined dosage form. World Appl Sci J. 2010;9(12):1424–1429. [Google Scholar]
- 6.Baldha RG., Patel Vandana B., Mayank B. Simultaneous spectrophotometric determination of atorvastatin calcium and Ezetimibe in tablet dosage form. Int J ChemTech Res. 2009;1(2):233–236. [Google Scholar]
- 7.Sonawane S.S., Shirkhedkar A.A., Fursule R.A., Surana S.J. Simultaneous spectrophotometric estimation of atorvastatin calcium and Ezetimibe in tablets. Indian J Pharm Sci. 2007;69(5):683–684. [Google Scholar]
- 8.Patel V., Baldha R., Patel D. Simultaneous determination of atorvastatin calcium and Ezetimibe by ratio spectra derivative spectrophotometry and reverse phase-high performance liquid chromatography. Asian J Chem. 2010;22(4):2507–2511. [Google Scholar]
- 9.Maher H.M., Youssef R.M., Hassan E.M., El Kimary E.I., Barary M.A. Enhanced spectrophotometric determination of two antihyperlipidemic mixtures containing Ezetimibe in pharmaceutical preparations. Drug Test Anal. 2010;3:97–105. doi: 10.1002/dta.165. [DOI] [PubMed] [Google Scholar]
- 10.Deshmukh D.D., Bhatia N.M., More H.N., Bhatia M.S. Colorimetric estimation of Ezetimibe and simultaneous spectrophotometric estimation of Ezetimibe with atorvastatin calcium in tablet formulation. Asian J Chem. 2008;20:155–160. [Google Scholar]
- 11.Aiyalu R., Mani K. HPTLC method development, validation, and stress degradation studies for atorvastatin and Ezetimibe in multicomponent tablet dosage form. Med Chem Res. 2012;21(7):1297–1301. [Google Scholar]
- 12.Dhaneshwar S.S., Dhaneshwar S.R., Deshpande P., Patil M. Development and validation of a method for simultaneous densitometric estimation of atorvastatin calcium and Ezetimibe as the bulk drug and in tablet dosage forms. Acta Chromatogr. 2007;19:141. [Google Scholar]
- 13.Chaudhari B.G., Patel N.M., Shah P.B., Modi K.P. Development and validation of a HPTLC method for the simultaneous estimation of atorvastatin calcium and Ezetimibe. Indian J Pharm Sci. 2006;68(6):793–796. [Google Scholar]
- 14.Sama J.R., Kalakuntla R.R., Rao V.S.N., Reddanna P. Simultaneous estimation of atorvastatin and Ezetimibe in pharmaceutical formulations by RP-HPLC method. Der Pharmacia Lettre. 2010;2(1):427–436. [Google Scholar]
- 15.Bhatt K.K., Shankar M.B., Patel J.B., Christian M.C. Simultaneous estimation of atorvastatin calcium and Ezetimibe in tablet by RP-HPLC method. Int J Pharm Appl Sci. 2010;1(1):114–117. [Google Scholar]
- 16.Chaudhari B.G., Patel N.M., Shah P.B., Patel L.J., Patel V.P. Stability-indicating reversed-phase liquid chromatographic method for simultaneous determination of atorvastatin and Ezetimibe from their combination drug. J AOAC Int. 2007;90(6):1539–1546. [PubMed] [Google Scholar]
- 17.Seshachalam U., Kothapally C.B. HPLC analysis for simultaneous determination of atorvastatin and Ezetimibe in pharmaceutical formulations. J Liq Chromatogr Relat Technol. 2008;31(5):714–721. [Google Scholar]
- 18.Qutab S.S., Razzaq S.N., Khan I.U., Ashfaq M., Shuja Z.A. Simultaneous determination of atorvastatin calcium and Ezetimibe in pharmaceutical formulations by liquid chromatography. J Food Drug Anal. 2007;15(2):139–144. [Google Scholar]
- 19.Choudhari VP., Nikalje AP. Simultaneous estimation of atorvastatin, Ezetimibe and fenofibrate in pharmaceutical formulation by RP-LC-PDA. Pharm Anal Acta. 2010;1(111) [open access] [Google Scholar]
- 20.Erram S.V., Tipnis H.P. Simple spectrometric analysis of propranolol hydrochloride and hydrochlorothiazide from combined pharmaceutical dosages. Indian Drugs. 1994;31:65–68. [Google Scholar]
- 21.Grinberg N. Chromatographic science series. Modern thin-layer chromatography, vol. 52. Marcel Dekker; 1990.


