Abstract
Objective
To develop a short instrument to measure determinants of innovations that may affect its implementation.
Design
We pooled the original data from eight empirical studies of the implementation of evidence-based innovations. The studies used a list of 60 potentially relevant determinants based on a systematic review of empirical studies and a Delphi study among implementation experts. Each study used similar methods to measure both the implementation of the innovation and determinants. Missing values in the final data set were replaced by plausible values using multiple imputation. We assessed which determinants predicted completeness of use of the innovation (% of recommendations applied). In addition, 22 implementation experts were consulted about the results and about implications for designing a short instrument.
Setting
Eight innovations introduced in Preventive Child Health Care or schools in the Netherlands.
Participants
Doctors, nurses, doctor's assistants and teachers; 1977 respondents in total.
Results
The initial list of 60 determinants could be reduced to 29. Twenty-one determinants were based on the pooled analysis of the eight studies, seven on the theoretical expectations of the experts consulted and one new determinant was added on the basis of the experts' practical experience.
Conclusions
The instrument is promising and should be further validated. We invite researchers to use and explore the instrument in multiple settings. The instrument describes how each determinant should preferably be measured (questions and response scales). It can be used both before and after the introduction of an innovation to gain an understanding of the critical change objectives.
Keywords: implementation, preventive child healthcare, school-based health promotion
Introduction
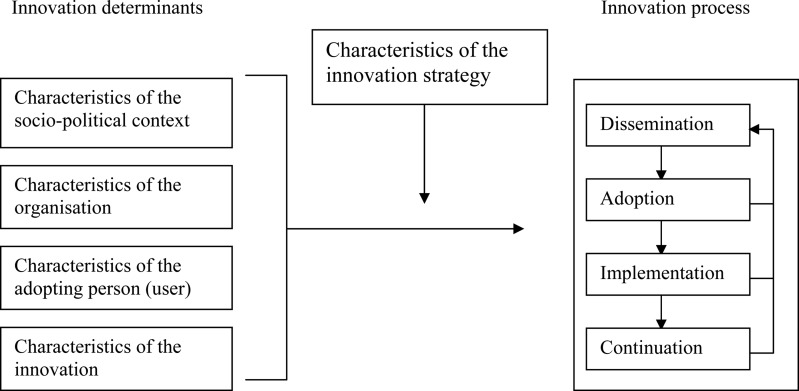
The introduction of innovations for improving—public—health outcomes is complex [1–6]. By ‘innovations’, we mean guidelines, interventions or programmes that are perceived as new by an individual or other unit of adoption [7]. Several models have been proposed that describe similar sequences for the systematic planning of innovations in general terms [1, 4, 8, 9]. Figure 1 shows a generic framework that has been used in the Netherlands and other countries since 1999 for the introduction and evaluation of innovations in healthcare and education [9]. Each of the four main stages in innovation processes (dissemination, adoption, implementation and continuation) can be thought of as critical phases where the desired change may or may not occur. Implementation differs from the preceding phase—adoption—in which people initially acquire and process information about the innovation and make their decision about using the innovation (behavioural intention). In the implementation phase, the innovation is put into daily practice by the professional (behaviour). The transition from one stage to the next can be affected, positively or negatively, by various determinants associated with characteristics of the innovation (e.g. complexity and clear procedures), the potential user of the innovation (e.g. knowledge and self-efficacy), the organisational context of the user (e.g. staff turnover and financial resources) and the socio-political context (e.g. legislation). A detailed understanding of these determinants can guide the process of designing innovation strategies that will have the potential to produce real change [1, 9, 10].
Figure 1.
Framework representing the innovation process and related categories of determinants [9].
Although most implementation experts recognize that measuring the determinants of innovation processes is essential, we are not aware of validated instruments for measuring these determinants and outcomes. In 2004, we published a list of 50 potentially relevant determinants of innovation processes in healthcare based on a systematic review of 57 empirical studies published between 1990 and 2000 and a Delphi study including 44 implementation experts [9]. More recently, ten determinants were added to the original list, mainly based on empirical studies of health promotion programmes [10]. The list is not suitable as a measurement instrument. It is too long, and the impact of specific determinants in the presence of other determinants is not clear.
The list of potentially relevant determinants has now been used in several empirical studies examining the implementation of evidence-based innovations in preventive child healthcare (PCHC) and in schools since 2002. While this is a substantial empirical base to build upon, the studies assessed partially overlapping sets of determinants, so the combined data set is patchy, with no single complete record that includes all the determinants. Conventional techniques are inadequate for the analysis of these missing data. Fortunately, even though the general idea of multiple imputation is already quite old [11], flexible techniques for plausible imputations in multivariate incomplete data have recently become available [12–17].
The aim of the present study is to develop a short instrument to measure determinants of innovations that may affect the stage of implementation (Fig. 1). It is based on empirical studies that used the list of potential determinants. We consulted implementation experts, asking them to comment on the results in order to facilitate consensus about the operationalization of each determinant. Our research questions were as follows:
Which determinants predict the use of innovations in PCHC and schools?
Which determinants are seen as relevant by implementation experts and what is their preference for measuring each determinant?
Methods
Databases
Original data were used from five studies in PCHC (this system is open to all children in the Netherlands from birth to the age of 19 years) and from three curriculum innovation studies in primary and secondary schools. Four of these studies examined the national implementation of PCHC guidelines, and the other four focussed on the implementation of health promotion interventions. Table 1 provides a brief description of the eight empirical studies. Different study designs were used. In order to have comparable data, only cross-sectional data were used, meaning that both determinants and the use of the innovation were measured simultaneously. In case determinants and use were measured in both the pre-test and post-test(s), we only used the pre-test measurements.
Table 1.
Description of the eight empirical studies in the meta-analysis
| Type of study | Setting | Design | Respondents | N |
|---|---|---|---|---|
| Guideline for visual disorders: levels of use [18] | PCHC | Cross sectional | Doctors, nurses, doctor's assistanta | 311 |
| Guideline for congenital heart disorders: levels of use [19] | PCHC | Cross sectional | Doctors, nursesa | 210 |
| Guideline for prevention of child abuse: effect of planned innovation strategy on levels of use [20] | PCHC | Pre-test and post-testc; experimental vs. control group | Doctors, nursesb | 302 |
| Guideline for congenital heart disorders: effect of e-learning vs. traditional learning on levels of use [21] | PCHC | Pre-test and post-testc; experimental vs. control group | Doctors, nursesb | 317 |
| Education programme for prevention of passive smoking in infants: levels of continuation of use [22] | PCHC | Cross sectional | Doctors, nursesa | 465 |
| School-wide programme for prevention of bullying: effect of planned innovation strategy on levels of used | Primary schools | Pre-test and post-testc | Teachersb | 125 |
| Mental health promotion programme: effect of planned innovation strategy on levels of used | Primary schools | Pre-test and two post-testsc | Teachersb | 188 |
| Sex education programme: effect of planned innovation strategy on levels of use [23] | Secondary schools | Pre-test and post-testc; experimental vs. control group | Teachersb | 59 |
| Total | 1977 |
PCHC, Preventive Child Health Care.
aAll PCHC organizations.
bSelected sample of PCHC organizations/schools.
cWe only used pre-test measurements.
dPublication in preparation.
Measures
Completeness of use of the innovation is the criterion variable in all eight studies. Completeness refers to the proportion of all activities prescribed by the innovation developers that are put into practice by the user. In all eight studies, the developers of the innovations selected the key activities or recommendations for the assessment of completeness. The range of key activities varied between 8 and 30. In the PCHC studies, the respondents indicated for each key activity on a Likert scale how many children they had exposed to the activity. The possible answers ranged from ‘no child at all’ to ‘all children’. Three studies used six-point Likert scales (categories ranging from 0 to 5) and two used seven-point Likert scales (categories ranging from 0 to 6). We then calculated the mean performance over all key activities and standardized this mean to a range from 0 to 100%. In case of a six-point scale, this was done by multiplying the mean score by a factor of 100/5% and in case of a seven-point scale by a factor of 100/6%. In the school studies, the respondents indicated, for each activity, whether they had applied it yes (1) or no (0). The mean performance of all activities was calculated and multiplied by 100%.
Table 2 contains the full list of 60 determinants and those determinants that were measured in the eight studies (first and second columns). The selection of determinants measured differed in the eight studies depending on the context. Sometimes there was good reason to exclude a specific determinant beforehand, for example because the determinant was considered irrelevant for that particular innovation. Furthermore, some determinants relating to the socio-political context or the organisation were excluded since these characteristics, such as formal financial arrangements or legislation, were assumed to be the same for all organisations.
Table 2.
List of the 60 original determinants (first column), determinants included in the analyses (second and third column), results of meta-analysis and experts’ opinions (forth and fifth column) and determinants in the final measurement instrument (last column)
| Original list of determinantsa | Number of empirical studies in which the determinant was measured | Determinants included (yes), excluded (no) or combined (yes, →) in meta-analysisb | Significantly associated with completeness of use in univariate analysis | Determinants including (yes), excluding (no) or combining (→) in final instrument according to the expertsb | Name of determinant in final measurement instrument |
|---|---|---|---|---|---|
| Socio-political context | |||||
| 1 Patient cooperation (measured) | 4 | Yes → 29 | – | Combining → 29 | – |
| 2 Patient awareness benefits | – | – | – | Combining → 30 | – |
| 3 Patient doubts user's expertise | – | – | – | Combining → 30 | – |
| 4 Financial burden on patient | – | – | – | Combining → 30 | – |
| 5 Patient discomfort | – | – | – | Combining → 30 | – |
| 6 Legislation and regulations | – | – | – | Yes | Legislation and regulations |
| Organisation | |||||
| 7 Decision-making process | 1 | No, only one study | – | Combining → 9 | – |
| 8 Hierarchical structure | – | – | – | No, only in adoption stage | – |
| 9 Reinforcement management | 7 | Yes | Yes | Yes | Formal ratification by management |
| 10 Organisational size | 4 | No, inconsistency | – | Combining → 45 | – |
| 11 Functional or product structure | – | – | – | Combining → 19, 56 | – |
| 12 Relationship with other organisations | 1 | No, only one study | – | No, only in adoption stage | – |
| 13 Collaboration between departments | – | – | – | Combining → 19 | – |
| 14 Staff turnover | 3 | Yes | Yes | Yes | Replacement when staff leave |
| 15 Staff capacity | 1 | No, only one study | – | Yes | Staff capacity |
| 16 Available expertise | – | – | – | Combining → 15, 26 | – |
| 17 Logistical procedures | – | – | – | Combining → 45 | – |
| 18 Number of potential users | – | – | – | Combining → 19, 23 | – |
| 43 Financial resources | 5 | Yes → 47 | – | Yes | Financial resources |
| 44 Reimbursement | – | – | – | Combining → 43 | – |
| 45 Material resources | 2 | Yes | – | Yes | Material resources and facilities |
| 46 Administrative support | 1 | Yes → 47 | – | Combining → 45 | – |
| 47 Time available | 8 | Yes | Yes | Yes | Time available |
| 48 Coordinator | 5 | Yes | Yes | Yes | Coordinator |
| 49 Users involved in development | – | – | – | No, only in adoption stage | – |
| 50 Opinion leader | – | – | – | No, only in adoption stage | – |
| 51 Feedback to user about innovation process | 7 | Yes | Yes | Yes | Feedback to user about innovation process |
| 54 Information available about use of innovation | 3 | Yes | Yes | Yes | Information accessible about use of innovation |
| 61 Unrest in organisation | – | – | – | Yes | Unrest in organisation |
| User / health professional | |||||
| 19 Social support colleagues | 5 | Yes | Yes | Yes | Social support |
| 20 Social support other professionals | 2 | Yes → 19 | – | Combining → 19 | – |
| 21 Social support supervisors | 5 | Yes → 19 | – | Combining → 19 | – |
| 22 Social support higher management | – | – | – | Combining → 19 | – |
| 23 Modelling | 3 | Yes | Yes | Yes | Descriptive norm |
| 24 Skills | 3 | Yes → 26 | – | Combining → 26 | – |
| 25 Knowledge | 1 | No, only one study | – | Yes | Knowledge |
| 26 Self-efficacy | 8 | Yes | Yes | Yes | Self-efficacy |
| 27 Ownership | – | – | – | Combining → 56 | – |
| 28 Task orientation | 3 | Yes | – | Yes | Job perception |
| 29 Expects patient cooperation | 4 | Yes | – | Yes | Client/patient cooperation |
| 30 Expects patient satisfaction | 4 | Yes | Yes | Yes | Client/patient satisfaction |
| 31 Work-related stress | 1 | No, only one study | – | Combining → 56 | – |
| 32 Contradictory goals | 1 | No, only one study | – | Combining → 56 | – |
| 33 Ethical problems | – | – | – | Combining → 56 | – |
| 52 Outcome expectations | 8 | Yes | Yes | Yes | Outcome expectations |
| 53 Subjective norm | 7 | Yes | Yes | Yes | Subjective norm |
| 55 Extent innovation is read | 5 | Yes | Yes | Yes | Awareness of content of innovation |
| 56 Personal benefits/drawbacks | 7 | Yes | Yes | Yes | Personal benefits / drawbacks |
| Innovation | |||||
| 34 Clear procedures | 8 | Yes | Yes | Yes | Procedural clarity |
| 35 Compatibility | 5 | Yes | Yes | Yes | Compatibility |
| 36 Trialability | 1 | No, only one study | – | No, relates to level of use | – |
| 37 Relative advantage | – | – | – | Combining → 52, 56 | – |
| 38 Observability | 3 | Yes | Yes | Yes | Observability |
| 39 Appealing | – | – | – | Combining → 56 | – |
| 40 Relevance for patient | 2 | Yes | Yes | Yes | Relevance for client/patient |
| 41 Risks for patient | – | – | – | Combining → 40 | – |
| 42 Frequency in the use | – | – | – | Combining → 56 | – |
| 57 Perceived prevalence of health problem | 2 | Yes | – | Combining → 52, 56 | – |
| 58 Correctness | 5 | Yes | Yes | Yes | Correctness |
| 59 Completeness | 7 | Yes | Yes | Yes | Completeness |
| 60 Complexity | 5 | Yes | Yes | Yes | Complexity |
a Numbers correspond with original list [9]; 51–60 new determinants since 2002; 61 added by experts.
b Combining because determinants belong to a single underlying construct; → refers to the number(s) of the similar determinant(s).
Concept of multiple imputation
Multiple imputation is nowadays accepted as a practical and valid way to handle incomplete data. Statistical inference through multiple imputation involves three steps: imputation, analysis and pooling. All major software packages now support multiple imputation.
Starting from the observed, incomplete data set, imputation finds replacement values for each missing entry in the data set. The special feature of multiple imputation is that several (instead of just one) replacement values are sought. The variability between the replacement values reflects our uncertainty about what value to impute. If we are almost sure what the missing value should have been, then the replacements will be very similar to each other. On the other hand, if we have little to no idea about what to impute, the variability between the replacement values will be large. In general, the replacement values are drawn from a distribution specifically modelled for each missing entry and will take all relevant information into account. In this article, we have created m = 50 completed data sets using the multiple imputation by chained equations (MICE) approach [15]. These 50 imputed sets are identical for the non-missing data entries but differ in the imputed values.
The second step is to estimate the statistic of scientific interest from each imputed data set. Typically, we apply the method we would have used had the data been complete to each imputed data set. This is now easy since all data are complete. Note that we now have 50 estimates (and not just one), which moreover differ from each other because their imputed data sets are different. It is important to realize that these differences are caused by our uncertainty about what value to impute.
In practice, we want 1 result, not 50. The last step is to pool the 50 estimates into 1 combined estimate and calculate its variance. For quantities that are approximately normally distributed, we can calculate the mean over 50 estimates and sum the within- and between-imputation variance according to the method developed by Rubin, called Rubin's rules [11]. The final estimate and its variance can be used to calculate correct P-values and 95% confidence intervals.
Data analysis
The goal of the analysis is to infer how well we can predict completeness of use on the basis of a set of determinants. The eight studies generated data about a total of 39 determinants in the list, with a mean of 20 determinants per study. Seven determinants were removed since they were measured in one study only. One determinant (size of the organisation) was removed because the different studies produced data that were found to be inconsistent. The data of ten determinants were combined to form four determinants because of their resemblance. The criteria were (i) determinants should belong to the same underlying theoretical construct as described in literature and (ii) additional factor analysis in case of doubts about the same underlying constructs. For example, four determinants related to perceived support from respectively colleagues, other healthcare professionals, supervisors and higher management. All these determinants belong to the construct of ‘social support’ and were therefore combined. The number of determinants remaining in the meta-analysis was 25. Each determinant was, on average, measured in five out of eight studies. Table 2 shows the outcomes of the reduction process (second and third columns).
The same determinant was sometimes operationalized in different ways. For example, two variants of perceived social support from colleagues were (i) ‘The support from my colleagues is….’ (four-point Likert scale ranging from ‘very insufficient’ to ‘very sufficient’) and ( ii) ‘I can always count on my colleagues when needed’ (seven-point Likert scale ranging from ‘totally disagree’ to ‘totally agree’). The 25 unique determinants were operationalized as 62 ‘variants’; 11 determinant were operationalized as 1 variant and 14 determinants consisted of more variants with a maximum of 4. There were 11 variants that had <100 (of 1977) observations; they were discarded before the analysis. In addition, data from 152 participants were discarded before the analysis because the outcome measure (completeness of use) was lacking. The final data set consisted of 1825 participants and 51 variants representing 25 unique determinants.
Multiple imputation by chained equations [14, 15] was used to produce 50 multiply-imputed data sets under the missing at random (MAR) assumption. We chose MICE since it is flexible, produces imputed data that are close to the measured data and has held up very well against alternatives in simulations [17]. The MAR assumption is plausible since nearly all missing data are created by design (i.e. the investigator did not collect the determinant) and is robust to substantial violations [24].
The imputation model was specified for each variant. Predictors were selected that had an absolute correlation with the primary outcome (‘completeness of use’) of at least 0.1 and a minimum proportion of usable observations of 0.3 [15]. Alongside the primary outcome, study identification (eight classes) was always included in the model.
Each of the 50 completed data sets was analysed by linear regression analysis, with determinants and study identification as independent variables and completeness of use as the dependent variable. The estimated regression weights for different imputations were combined using Rubin's rules [11]. Completeness of use was regressed for each potential determinant (univariate analysis). Since we wanted to avoid including different variants of the same determinant in the analysis, the variant that had the strongest association with completeness of use, as determined by the Wald statistic of the regression coefficient, was selected for further analysis. P-values of <0.05 (two-sided) were considered statistically significant.
Consultation of experts
In the next step, 22 implementation researchers, implementation advisors/consultants and policymakers commented on the results of the analyses. The majority of experts were purposely chosen from outside the field of PCHC and schools (e.g. working in mental healthcare or hospitals). Although, in the previous study [9], implementation experts reached consensus about the operationalization of each determinant, we first asked the experts whether they still agreed with the operationalization and response scales for each determinant that was significantly related to completeness of use in the meta-analysis. Second, for each determinant that was not included in the final model—because the data were insufficient or because the determinant had not been measured—or that was not significantly related to completeness of use, the experts indicated whether theoretical expectations suggested that a determinant should be retained in the final instrument and why. They also provided an operationalization when they thought the determinant should be retained. Third, they were also given the opportunity to add, on empirical or theoretical grounds, new determinants that might have been overseen in the original list.
The first two authors evaluated the answers. Consensus was considered to have been achieved if 75% of the experts agreed about whether determinants not included in the meta-analysis should be maintained or not in the final list. In all other cases, the researchers made a decision based on theoretical grounds.
Results
Pooled analysis
The model with only study identification as a predictor explained 35% of variance in completeness of use. Incorporating all 25 determinants raised this to 51%, and so the determinants explained an additional 16% of variance in completeness of use. Table 3 shows the 21 determinants that were significantly associated with completeness of use in the univariate analysis.
Table 3.
Results of the univariate regression models for predicting completeness of use
| Determinanta | Beta | SE | P-value | Number of observations |
|---|---|---|---|---|
| Formal ratification by management (9) | 9.61 | 1.08 | <0.001 | 1764 |
| Replacement when staff leave (14) | 3.33 | 0.65 | <0.001 | 914 |
| Social support (19) | 2.80 | 0.87 | 0.001 | 1362 |
| Descriptive norm (23) | 2.06 | 0.40 | <0.001 | 671 |
| Self-efficacy (26) | 19.26 | 1.14 | <0.001 | 1810 |
| Client/patient satisfaction (30) | 4.19 | 0.68 | <0.001 | 464 |
| Procedural clarity (34) | 6.01 | 1.01 | <0.001 | 1583 |
| Compatibility (35) | 4.48 | 0.68 | <0.001 | 1452 |
| Observability (38) | 3.50 | 0.66 | <0.001 | 464 |
| Relevance for client/patient (40) | 2.82 | 0.80 | 0.001 | 464 |
| Time available (47) | 3.02 | 0.63 | <0.001 | 464 |
| Coordinator (48) | 6.19 | 1.19 | <0.001 | 1396 |
| Feedback to user about innovation process (51) | 3.91 | 0.89 | <0.001 | 826 |
| Outcome expectations (52) | 0.98 | 0.24 | <0.001 | 693 |
| Subjective norm (53) | 0.92 | 0.09 | <0.001 | 1481 |
| Information accessible about use of innovation (54) | 7.11 | 2.99 | 0.02 | 637 |
| Awareness of content of innovation (55) | 7.06 | 1.01 | <0.001 | 1368 |
| Personal benefits/drawbacks (56) | 5.37 | 0.74 | <0.001 | 464 |
| Correctness (58) | 6.44 | 1.86 | 0.001 | 923 |
| Completeness (59) | 5.94 | 1.13 | <0.001 | 464 |
| Complexity (60) | 2.29 | 0.80 | 0.005 | 761 |
Consultation of experts
According to the experts, seven determinants from the original list that were not included in the analysis or were not significantly related to completeness of use should be included in a measurement instrument on the basis of theoretical expectations. These determinants are as follows: legislation and regulations, staff capacity, financial resources, material resources and facilities, knowledge, job perception and client/patient cooperation. The others could be excluded mainly because of their resemblance to other determinants (Table 2, fifth column).
The experts found that only minor changes were required to the operationalization of the ‘original’ determinants, and seven determinants were rephrased: ‘staff turnover’ as ‘replacement when staff leave’, ‘material resources’ as ‘material resources and facilities’, ‘information available’ as ‘information accessible’, ‘modelling’ as ‘descriptive norm’, ‘task orientation’ as ‘job perception’, ‘extent innovation is read’ as ‘awareness of content of innovation’ and ‘clear procedures’ as ‘procedural clarity’ (Table 2, last column).
Nine experts suggested adding a total of twelve new determinants to the list. Finally, only ‘unrest in organization’ was added since the others closely resembled determinants already on the list.
Final measurement instrument
The list of 60 potentially relevant determinants was reduced to 29. Twenty-one determinants were based on the pooled analysis of the empirical data and seven on the theoretical expectations of the implementation experts consulted; one new determinant was added on the basis of the implementation experts' practical experience. These 29 determinants have been included and described in the measurement instrument for determinants of innovations (MIDI). It describes how each determinant should preferably be measured (questions and response scales) and the syntax for analyses (Supplementary Appendix A).
Discussion
We developed the MIDI to improve our understanding of the critical determinants that may affect implementation and to target the innovation strategy better. The instrument can be used both before and after the introduction of an innovation. The instrument is intended primarily for implementation researchers, but it can also be used by implementation consultants/advisors. We expect that the instrument will help to collect information as a basis for an empirically grounded judgement of the relative importance of determinants.
In practice, the user first needs to decide which determinants should be measured. The main criterion is—given the nature of the innovation and/or the context—the anticipated impact of the determinant on possible variations in completeness of use of the innovation.
Characteristics of the socio-political context do not often result in differentiation, particularly when a study is limited to a single country. For example, legislation and regulations relating to child healthcare in the Dutch context will differentiate little, if at all, between the different organizations. However, in international research, this is likely to be a relevant factor for explaining variations in the implementation of particular innovations.
For a sound assessment of an innovation strategy, it is advisable to measure as many determinants as possible since they may all be of practical relevance for designing that strategy. Yet, it is sometimes not feasible to measure particular determinants before an innovation is implemented because the user will not yet have a clear picture of what the innovation entails. For example, a subjective assessment of certain characteristics of an innovation will not be possible if people do not have knowledge awareness of the innovation.
Our study was limited in some respects. Though MIDI is promising, it is not yet a validated instrument and further development is needed. The results in the current version are specific to preventive innovations in the context of PCHC and schools. It is not known to what extent the results can be generalized to other settings or to more technology-based innovations. On the other hand, there are some reasons suggesting that the instrument may be applicable to a broader range of settings. First, the original list of determinants was developed and used in many different healthcare settings and school settings. Second, the experts in the present study, as well as in a Delphi study performed previously [9], indicated that most determinants were generic. It should also be noted that we did not have empirical data to test all the determinants from the original list. We used self-reported adherence measures. There is sometimes a large discrepancy between self-report and objective measurements [25]. This may have affected our measures of completeness of use. As the same methods were applied in all eight studies, we expect that this will have resulted in a systematic bias (if at all). However, underestimation of completeness of use will not necessarily affect the associations with the determinants, which was the primary objective of our study.
Since our publication of the original list of determinants in 2004, several other frameworks and checklist have been published. Recently, Flottorp et al. published a nice systematic review and synthesis of frameworks and taxonomy of determinants, resulting in checklist of 57 determinants grouped in 7 domains [26]. This checklist is consensus based, and the 57 determinants closely resemble the ones in our original list. As we were able to verify determinants empirically, the present study represents a next logical step of development.
We envisage the development of MIDI into a validated instrument with sensibly chosen cut-offs for the scores for each determinant. To achieve this goal, we invite implementation researchers to use and explore MIDI in applied settings and to report and share their results. Empirical data from a broader range of innovations and settings will help to substantiate the sensitivity of the instrument in practice. As measurement in implementation research is still in its infancy, we hope that MIDI will be of interest to both implementation researchers and advisors.
Supplementary material
Funding
This project is supported by the Netherlands Organisation for Health Research and Development (ZonMw) [number 20040008]. Funding to pay the Open Access publication charges for this article was provided by The Netherlands Organisation for Scientific Research (NWO).
Supplementary Material
Acknowledgements
The authors thank Ab Rijpstra for his assistance with data management.
References
- 1.Greenhalgh T, Robert G, Macfarlane F, et al. Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Q. 2004;82:581–629. doi: 10.1111/j.0887-378X.2004.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fixsen DL, Noaam SF, Blase KA, et al. Implementation Research: A Synthesis of the Literature. Florida: University of South Florida; 2005. [Google Scholar]
- 3.Grimshaw J, Eccles M, Thomas R, et al. Toward evidence-based quality improvement. Evidence (and its limitations) of the effectiveness of guideline dissemination and implementation strategies 1966–1998. J Gen Intern Med. 2006;21(Suppl 2):S14–20. doi: 10.1111/j.1525-1497.2006.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grol R, Wensing M, Eccles M. Improving Patient Care: The Implementation of Change in Clinical Practice. Edingburgh: Elsevier; 2005. [Google Scholar]
- 5.Prior M, Guerin M, Grimmer-Somers K. The effectiveness of clinical guideline implementation strategies—a synthesis of systematic review findings. J Eval Clin Pract. 2008;14:888–97. doi: 10.1111/j.1365-2753.2008.01014.x. [DOI] [PubMed] [Google Scholar]
- 6.Gulbrandsson K. From News to Everyday Use: The Difficult Art of Implementation. Ostersund: Swedish National Institute of Public Health; 2008. [Google Scholar]
- 7.Rogers EM. Diffusion of Innovations. New York: Free press; 2003. [Google Scholar]
- 8.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89:1322–7. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleuren M, Wiefferink K, Paulussen T. Determinants of innovation within health care organizations: literature review and Delphi study. Int J Qual Health Care. 2004;16:107–23. doi: 10.1093/intqhc/mzh030. [DOI] [PubMed] [Google Scholar]
- 10.Bartholomew LK, Parcel GS, Kok G, et al. Planning Health Promotion Programs: an Intervention Mapping Approach. San Francisco: Jossey-Bass; 2006. [Google Scholar]
- 11.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: Wiley; 1987. [Google Scholar]
- 12.Buuren van S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18:681–94. doi: 10.1002/(sici)1097-0258(19990330)18:6<681::aid-sim71>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 13.Buuren van S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–42. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 14.Buuren van S, Groothuis-Oudshoorn K. MICE: multivariate imputation by chained equations in R. J Stat Software. 2011;45:1–67. [Google Scholar]
- 15.Buuren van S. Flexible Imputation of Missing Data. Boca Raton: Chapman & Hall/CRC Press; 2012. [Google Scholar]
- 16.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee KJ, Carlin JB. Mulitple imputation for missing data: fully conditional specification versus multivariate normal imputation. Am J Epidemiol. 2010;171:624–63. doi: 10.1093/aje/kwp425. [DOI] [PubMed] [Google Scholar]
- 18.Fleuren MAH, Verlaan ML, Velzen-Mol van HWM, et al. Leiden: TNO Kwaliteit van Leven; 2006. Zicht op het gebruik van de JGZ-standaard Opsporing van Visuele Stoornissen 0–19 jaar: een landelijk implementatieproject. [Looking closely at adherence to the Preventive Child Health Care guideline on early detection of visual disorders] [Google Scholar]
- 19.Fleuren MAH, Dommelen van P, Kamphuis M, et al. Leiden: TNO Kwaliteit van Leven; 2007. Landelijke Implementatie JGZ-standaard Vroegtijdige Opsporing van Aangeboren Hartafwijkingen 0–19 jaar [National implementation of the Preventive CHild Health Care guideline on early detection of congenital health disorders] [Google Scholar]
- 20.Broerse A, Fleuren MAH, Kamphuis M, et al. Leiden: TNO Kwaliteit van Leven; 2009. Effectonderzoek proefimplementatie JGZ-richtlijn secundaire preventie kindermishandeling [Evaluation of the pilot implementation of the Preventive Child Health Care guideline on secondary prevention of child abuse] [Google Scholar]
- 21.Galindo Garre F, Kamphuis M, Verheijden MW, et al. Leiden: TNO Kwaliteit van Leven; 2010. Onderzoek naar de mogelijkheden en haalbaarheid van E-learning bij de implementatie van richtlijnen in de jeugdgezondheidszorg [Feasibility and effect of e-learning on the implementation of Preventive Child Health Care guidelines] [Google Scholar]
- 22.Crone MR, Verlaan M, Willemsen MC, et al. Sustainability of the prevention of passive infant smoking within well-baby clinics. Health Educ Behav. 2006;33:178–96. doi: 10.1177/1090198105276296. [DOI] [PubMed] [Google Scholar]
- 23.Wiefferink CH, Poelman J, Linthorst M, et al. Outcomes of a systematically designed strategy for the implementation of sex education in Dutch secondary schools. Health Educ Res. 2005;20:323–33. doi: 10.1093/her/cyg120. [DOI] [PubMed] [Google Scholar]
- 24.Collins LM, Schafer JL, Kam CM. A comparison of inclusive and restrictive strategies in modern missing data procedures. Psychol Methods. 2001;6:330–51. [PubMed] [Google Scholar]
- 25.Adams AS, Soumerai SB, Lomas J, et al. Evidence of self-report bias in assessing adherence to guidelines. Int J Qual Health Care. 1999;11:187–92. doi: 10.1093/intqhc/11.3.187. [DOI] [PubMed] [Google Scholar]
- 26.Flottorp SA, Oxman AD, Krause J, et al. A checklist for identifying determinants of practice: a systematic review and synthesis of frameworks and taxonomies of factors that prevent or enable improvements in healthcare professional practice. Implement Sci. 2013;8:35. doi: 10.1186/1748-5908-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.