Abstract
Endostatin, a 20-kDa fragment of collagen XVIII, is a potent angiogenesis inhibitor. E-selectin, an inducible leukocyte adhesion molecule specifically expressed by endothelial cells, has also been implicated in angiogenesis. By using in vivo, ex vivo, and in vitro angiogenic assays, we investigated the functional relationship between endostatin and E-selectin. In corneal micropocket assays, recombinant endostatin administered i.p. by osmotic pump inhibited basic fibroblast growth factor-induced angiogenesis in WT, but not E-selectin-deficient, mice. Similarly, endostatin inhibited vascular endothelial growth factor-stimulated endothelial sprout formation from aortic rings dissected from WT but not from E-selectin-deficient mice. To further explore this apparent requirement for E-selectin in endostatin action, we manipulated E-selectin expression in cultured human endothelial cells. When E-selectin was induced by IL-1β, or lipopolysaccharide, human umbilical vein endothelial cells and human dermal microvascular endothelial cells each became markedly more sensitive to inhibition by endostatin in a vascular endothelial growth factor-induced cell migration assay. To dissociate E-selectin expression from other consequences of endothelial activation, human umbilical vein endothelial cells were transduced with an adenoviral human E-selectin expression construct; these cells also showed increased sensitivity to endostatin, and this effect required the E-selectin cytoplasmic domain. Taken together, these results indicate that E-selectin is required for the antiangiogenic activity of endostatin in vivo and ex vivo and confers endostatin sensitivity to nonresponsive human endothelial cells in vitro. E-selectin may be a useful predictor and modulator of endostatin efficacy in antiangiogenic therapy.
Angiogenesis, the formation of new blood vessels, is a balanced process tightly regulated by pro and antiangiogenic factors (1). Endostatin, a 20-KDa carboxyl-terminal fragment of the noncollagenous 1 domain of collagen XVIII, is a potent endogenous inhibitor of tumor growth and angiogenesis in mice (2). In vitro, endostatin has been shown to inhibit endothelial cell migration by binding to α5β1 integrin, thereby disrupting cell–matrix adhesion via focal adhesion kinase-mediated extracellular-signal-regulated kinase 1, p38 mitogen-activated protein kinase pathways, and the Src-dependent p190RhoGAP pathway (3, 4). Endostatin has also been shown to induce apoptosis through Shb adaptor protein (5) and to arrest cell cycle progression by inhibiting cyclin D1 (6). However, the relevance of these molecular interactions to endostatin action in vivo has not been explored. The fact that endostatin binds to many other cell-surface proteins, such as glypicans, heparan sulfates, and vascular endothelial growth factor (VEGF) receptor 2 (7–9), underscores the complexity of the mechanism of endostatin action.
E-selectin is an endothelial cell-specific membrane glycoprotein that mediates slow rolling and stable arrest of leukocytes on endothelium during inflammation (10–12). E-selectin consists of an amino-terminal “C type” lectin domain critical for ligand interaction, an epidermal growth factor-like domain, six complement regulatory repeats, a single transmembrane domain, and a cytoplasmic carboxyl-terminal tail (13, 14). There is evidence that E-selectin may play a role in angiogenesis. For example, we have shown that antibodies directed against E-selectin inhibited the formation of capillary-like tubes in vitro (15). E-selectin is up-regulated in proliferating endothelial cells in culture (16) and in pathologic angiogenic tissues such as human infantile hemangioma and breast carcinoma (17, 18). Koch et al. (19, 20) have reported that soluble E-selectin, which lacks the transmembrane and cytoplasmic domains, induces angiogenesis in the rat cornea and stimulates chemotaxis and tube formation of human dermal microvascular endothelial cells (HDMEC) through Src- and phosphatidylinositol 3-kinase-mediated pathways. Although E-selectin is associated with essential events of angiogenesis including endothelial cell proliferation, migration, and neovascularization, the underlying mechanisms by which E-selectin participates in the regulation of angiogenesis remain to be defined.
The crystal structure of endostatin resembles the carbohydrate recognition domain of mammalian C type lectins, and, within this family, is most similar to E-selectin (21). This finding prompted us to investigate whether E-selectin influences endostatin-mediated inhibition of angiogenesis. In this study, we demonstrated that E-selectin is required for the antiangiogenic activity of endostatin. This finding sheds new light on the mechanism of endostatin action.
Materials and Methods
Endothelial Cell Culture. HUVEC were isolated and established in culture as described in ref. 22, and maintained in 0.1% gelatin-coated dishes in Medium 199 containing 25 mM Hepes at pH 7.4 (BioWhittaker), 20% FBS (GIBCO), 25 μg/ml Endothelial Growth Supplement (Biomedical Technologies, Stoughton, MA), and 50 μg/ml porcine intestinal heparin (Sigma). HDMEC were isolated from newborn foreskin, selected with Ulex europaeus I-coated Dynabeads, and grown on 1% gelatin-coated dishes in EBM (Clonetics, San Diego), 10% FBS (HyClone), 1× glutamine/penicillin/streptomycin (Invitrogen and GIBCO), and 2 ng/ml bFGF (Scios, Mountain View, CA) as described in ref. 23. For cytokine activation, cells were induced in growth medium containing 0.1 μg/ml tumor necrosis factor α (TNFα) (R & D Systems), 10 unit/ml IL-1β (R & D Systems), or 0.1 μg/ml lipopolysaccharide (LPS) (Sigma) for 4 h. HUVEC at passage 2 isolated from seven different donor pools and HDMEC at passages 5–8 from two different donors were used for experiments.
E-Selectin-Deficient (E–/–) Mice. E–/– mice were generated by gene targeting as described in ref. 12. All E–/– and WT (E+/+) mice used in these experiments were obtained from matings between E+/– mice and were matched for gender, chronological age, and number of generations of sibling–cousin intercross. Mice used for corneal angiogenesis experiments were of a mixed C57BL/6J:129Svter genetic background. Mice used for aortic ring explants were from a colony backcrossed 11 generations into the C57BL/6J genetic background (The Jackson Laboratory) and subsequently maintained by sibling–cousin intercross of E–/– mice.
Mouse Corneal Micropocket Assay. Recombinant human endostatin (8.6 mg/ml) (EntreMed, Rockville, MD) was loaded into ALZET miniosmotic pumps (Durect, Cupertino, CA) with internal volume of 200 μl and a mean pumping rate of 1 μl/h. Mice were anesthetized with 2.5% avertin at 0.015–0.017 ml/g of body weight, and pumps were implanted i.p. (24). After the implantation, corneal micropockets were created in both eyes with a modified von Graefe cataract knife. A 0.4 × 0.4 × 0.2-mm3 sucrose aluminum sulfate pellet coated with hydron polymer type NCC (IFN Sciences, New Brunswick, NJ) containing 80 ng of bFGF was implanted 1.0–1.2 mm from the limbal vessels (25). Five days later, vessel length and clock hours were measured, and vessel area was calculated as described in ref. 26. The corneal angiogenesis induced in 7- to 13-week-old male E+/+ and E–/– littermates in the presence or absence of endostatin pumps (n = 4 mice for each experimental condition) was examined in five separate experiments.
Aortic Ring Assay. Mouse aortas were cut into 0.8-mm rings, embedded in rat collagen I gels, and cultured for 8 days in endothelial cell serum-free media (Invitrogen) (27). The presence of endothelial cells in sprouts from aortic rings was confirmed by positive staining for CD31 and von Willebrand factor antigens. Sprouting of aortas from paired E+/+ and E–/– littermates was stimulated by VEGF (R & D Systems) at doses of 0, 2, 4, and 8 ng/ml. Media containing VEGF was changed on day 3 and 6, and sprouts were counted on day 8 by an operator blinded to the genotype. In separate experiments, murine recombinant endostatin (EntreMed) was added at 0, 100, 500, and 1,000 ng/ml to the collagen gel and media to test its effect on E+/+ and E–/– aortic endothelial cell sprouting induced by 8 ng/ml VEGF. In all assays, four aortic rings were tested in each condition, and all dose–response experiments were repeated three times. A positive control for inhibition of endothelial sprouting from aortas was confirmed by adding 1 ng/ml TNP-470 (Takeda-Abbott Pharmaceuticals, Deerfield, IL).
Cell Migration Assay. Cell migration was assayed by using a modified Boyden chamber (Neuro Probe, Cabin John, MD). Polycarbonate membranes (8 μM pore) (Neuro Probe) were coated overnight with 100 μg/ml collagen type I (Collaborative Biomedical Products, Bedford, MA). VEGF was diluted to 5 ng/ml in EBM with 0.1% BSA and placed in the lower chamber. Cells were detached by trypsinization of confluent cultures and incubated for 30 min at 37°C without or with human recombinant endostatin, Fc-angiostatin (kindly provided by Kashi Javaherian, Children's Hospital, Boston) (28) or TNP-470. Cells were added to the upper chamber at 10,000 cells per well and allowed to migrate toward VEGF in the lower chamber for 4 h in a 37°C, 5% CO2 incubator. Membranes were fixed in 10% neutral buffered formalin (Fisher) and stained with Gill's Hematoxylin No. 3 (Polysciences). Cells on the upper surface of the membrane were scraped off. Stained membranes were mounted onto glass slides by using Permount (Sigma). Images of migrated cells were captured by using a 4× objective and brightfield microscopy connected to a SPOT digital camera (Diagnostic Instruments, Sterling Heights, MI). Total migrated cells per well were quantified from the captured images by using scion image software (National Institutes of Health) and manually if deemed necessary. Each condition was performed in quadruplicate.
Adenoviral E-Selectin Transduction. Recombinant adenovirus AdRSV(wt-E) for full-length E-selectin, AdRSV(ΔCyto-E) for E-selectin mutant lacking the carboxyl-terminal 31 aa of the cytoplasmic domain, and AdRSVlacZ for β-galactosidase were prepared as described in ref. 29. Two different AdRSV(wt-E) viral stocks (1.0 × 1011 and 1.9 × 1011 particles per ml, predicted particle:plaque-forming unit ratio of 102 for all stocks), two different AdRSVlacZ viral stocks (1.4 × 1011 and 7.0 × 1011 particles per ml) and a single AdRSV(ΔCyto-E) viral stock (4.2 × 1012 particles per ml) were used in the course of this study. HUVEC were plated in 60-mm dishes and reached 70% confluence (≈1.6 × 106 cells per dish) 14–18 h later. These cultures were infected with 1.5 × 1010 to 9 × 1010 viral particles in 2 ml of DMEM containing 2% FBS. After 1.5 h, 2 ml of growth medium was added, and cells were incubated for an additional 48 h before evaluation.
Flow Cytometry. Cells were trypsinized and resuspended in PBS containing 2 mM EDTA and 0.5% BSA. Single-cell suspensions were labeled with a mouse IgG1 isotype-matched control (R & D Systems) or murine anti-human E-selectin mAb, clone 5G11 (17), followed by FITC-conjugated anti-mouse IgG (Vector Laboratories). The percentage of cells expressing E-selectin was quantitated on a FACSVantage SE flow cytometer (BD Pharmingen). Ten thousand events were acquired in list mode with cellquest software (BD Pharmingen) and analyzed on a PC with winmdi 2.8 software (The Scripps Research Institute, La Jolla, CA). β-galactosidase activity in AdRSVlacZ-transduced cells was quantified by using FluoReporter lacZ Flow Cytometry Kits (Molecular Probes) according to the manufacturer's instructions.
HL-60 Adhesion Assay. Before the assay, near-confluent HDMEC in six-well plates were induced in fresh EBM-2 complete medium (Clonetics) containing 0.1 μg/ml LPS for 4 h. Cells were incubated with either endostatin at 0, 0.1, 1, 10, or 100 μg/ml, or mAb 7A9 (30) or mIgG1 isotype control at 1 μg/ml in EBM-2/0.01% BSA for 30 min at room temperature. HL-60 cells were maintained in RPMI medium 1640 (BioWhittaker) containing 10% FBS and 1× GPS at a density less than 1 × 106 cells per ml. The cells were washed once with RPMI medium 1640 with or without 2.5 mM EGTA, and 2 × 106 cells were added to HDMEC in 0.6 ml of medium per well. Cells were incubated at 4°C for 45 min on a rocking platform, washed 5 times with RPMI with or without EGTA, and fixed in 2.5% glutaldehyde. Bound HL-60 cells were counted in 10 randomly selected fields under a 20× objective.
Statistical Analysis. All data are expressed as mean ± SD. Statistical significance was analyzed by two-tailed Student's t test.
Results
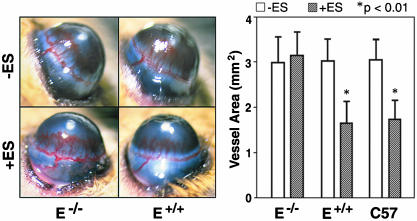
Endostatin Does Not Inhibit Corneal Angiogenesis in E–/– Mice. To assess whether the genetic absence of E-selectin affects endostatin-mediated antiangiogenesis in vivo, we conducted corneal micropocket assays in experimentally matched E–/– and E+/+ mice with a mixed C57BL/6J:129Svter genetic background and also in commercially produced inbred C57BL/6J mice. Corneal angiogenesis was induced by implanted bFGF pellets, and recombinant human endostatin (10 mg/kg/day) was administered via an i.p. osmotic pump over a 5-day period. Both control C57BL/6J and E+/+ mice showed 40% reduction in bFGF-induced vessel area upon treatment with endostatin (Fig. 1). In contrast, endostatin did not alter corneal angiogenesis stimulated by bFGF in E–/– mice, suggesting that E-selectin is required for the antiangiogenic activity of endostatin in vivo.
Fig. 1.
Endostatin does not inhibit corneal angiogenesis in E–/– mice. (Left) Corneas of E–/– and E+/+ mice 5 days after implanting hydron pellets containing bFGF. Mice were treated without (–) or with (+) 10 mg/kg/day of endostatin (ES) administered by i.p. osmotic pumps. (Right) Vessel area in the corneas of E–/–, E+/+, and C57BL/6J (C57) mice in the presence or absence of endostatin treatment. Note the statistically significant (*) inhibitory effect of endostatin in both E+/+ and C57BL/6J mice, but not in E–/– mice. Four mice were used for each experimental condition. The results are representative of five independent experiments.
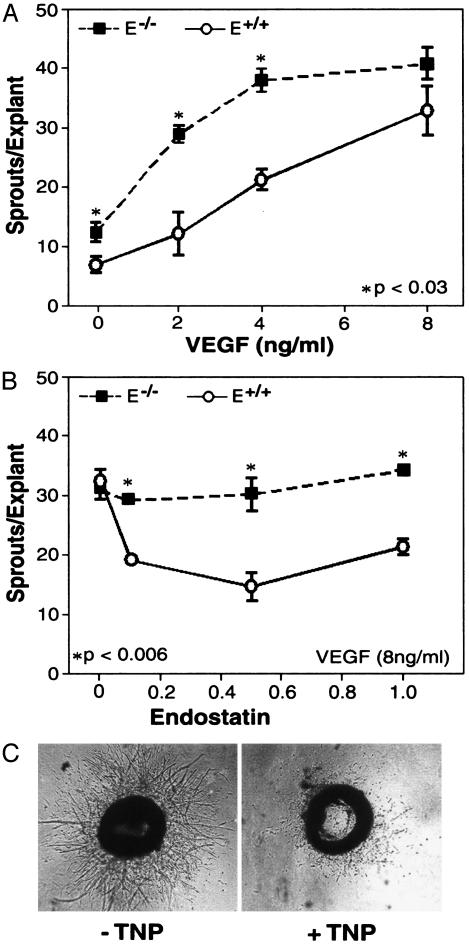
Increased VEGF Sensitivity and Resistance to Endostatin in Aortic Explants from E–/– Mice. We next performed ex vivo aortic ring assays by using another angiogenic stimulator VEGF to confirm the results observed in the corneal micropocket experiments. VEGF induced a dose-dependent increase of endothelial cell (EC) sprouting from aortic rings dissected from both E+/+ and E–/– mice (Fig. 2A). However, aortic rings from E–/– mice formed significantly more sprouts than did those from E+/+ mice at 0, 2, and 4 ng/ml of VEGF. We next examined the effect of recombinant murine endostatin on the sprout outgrowth at saturating concentrations of VEGF (Fig. 2B). Sprout formation from E+/+ explants was inhibited 40–60% by endostatin over the dose range of 0.1–1 μg/ml. In contrast, sprout formation from E–/– explants was not affected by endostatin. To determine whether E–/– explants might respond to exogenous endostatin at earlier or later time points, aortic rings from E–/– and E+/+ mice, stimulated with 8 ng/ml VEGF, were tested for response to endostatin on days 4, 6, 8, and 12. At every time point, sprout formation in E+/+ explants was inhibited by exogenous endostatin, whereas no inhibition was observed, at any time point, in E–/– explants (data not shown). To examine the specificity of E-selectin in antiangiogenesis, we tested the angiogenesis inhibitor TNP-470, an analog of fumigillin (31), for effects on sprout formation. In contrast to endostatin, TNP-470 efficiently blocked sprouting of aortic rings from both E+/+ and E–/– mice (Fig. 2C). E-selectin expression was confirmed by RT-PCR in E+/+ aortas (data not shown). Conditioned media from aortic ring assays contained a low level of exogenous endostatin (10–15 ng/ml) that is not present in basal media or conditioned media from collagen XVIII-deficient mice (data not shown). These data confirm the validity of using aortic explants to investigate the contribution of endogenous E-selectin to both endogenous and exogenous endostatin activity.
Fig. 2.
Increased VEGF sensitivity and resistance to endostatin in aortic explants from E–/– mice. (A) Aortic explants from E+/+ (circles) and E–/– (squares) mice formed sprouts in response to VEGF at the indicated concentrations. The number of sprouts was higher in E–/– than in E+/+ explants before saturation by VEGF. (B) Sprout outgrowth in aortic explants from E+/+ mice, but not E–/– mice, was inhibited by endostatin at 100, 500, and 1,000 ng/ml. (C) Aortic explants from E–/– mice stimulated with 8 ng/ml VEGF in the presence and absence of TNP470 (1 ng/ml) for 8 days.
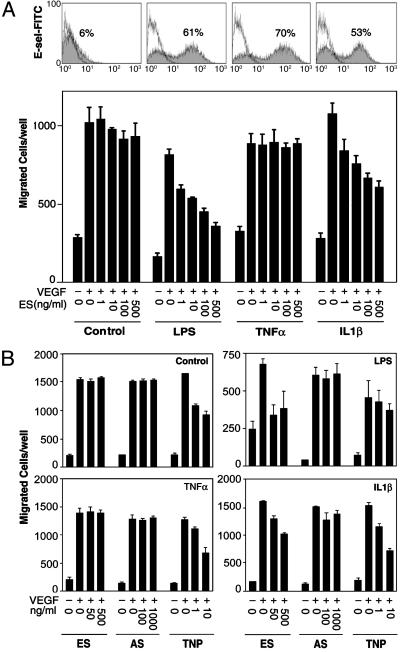
Effects of Endostatin on Cytokine Activated Human Endothelial Cells. To further elucidate the apparent requirement for E-selectin, we tested whether up-regulation of E-selectin affected endostatin activity in an in vitro VEGF-induced EC migration assay. We chose to use human EC that do not normally respond to endostatin in the migration assay (32). Cell-surface E-selectin on HDMEC was quantified by flow cytometry. Non-treated HDMEC express little E-selectin, but 4-h treatment with LPS, TNFα, or IL-1β resulted in, 57.0 ± 7.8%, 60.7 ± 12%, and 47.0 ± 8.5% E-selectin+ cells, respectively (n = 3 experiments). A representative experiment is shown in Fig. 3A. Nontreated HDMEC that expressed little or no E-selectin did not respond to endostatin at concentrations up to 500 ng/ml (Fig. 3A), a concentration that produced maximum inhibition in aortic ring assays. In contrast, upon activation by LPS or IL-1β, but not TNFα, HDMEC displayed a dose-dependent sensitivity to endostatin.
Fig. 3.
Increased responses to endostatin in LPS- and IL-1β-activated, but not TNFα-activated, human EC. (A) Migration of nontreated (Control) HDMEC and HDMEC activated by LPS (0.1 μg/ml), TNFα (0.1 μg/ml), or IL-1β (10 units/ml). E-selectin expression (shaded area) detected by flow cytometry is shown in Upper. Single line shows cells labeled with isotype-matched control mIgG1. Cell migration was stimulated by VEGF (5 ng/ml) in the presence of endostatin (ES) at the indicated concentrations. Results are representative of three independent experiments. (B) Specificity of endostatin/E-selectin interaction in VEGF-induced migration of HUVEC. Shown is the migration of nontreated control and LPS-, TNFα-, or IL-1β-activated HUVEC toward VEGF (5 ng/ml) in the presence of ES, angiostatin (AS), or TNP-470 at similar molar concentrations.
Similar to HDMEC, nontreated and TNFα-activated HUVEC did not respond to endostatin, whereas LPS- and IL-1β-treated HUVEC were endostatin-sensitive (Fig. 3B). We next assessed the specificity of endostatin action on activated EC by examining two other angiogenesis inhibitors. Fc-human angiostatin, which displayed antiangiogenic activity in vivo (data not shown), did not affect migration of control HUVEC or HUVEC activated by any of these inflammatory mediators. In contrast, TNP-470 significantly (P < 0.05) inhibited VEGF-induced migration of control HUVEC (33) and HUVEC treated with LPS, TNFα, or IL-1β (Fig. 3B), suggesting that antiangiogenic activity of TNP-470 is not dependent on E-selectin expression or other consequences of cell activation. On average, cell-surface E-selectin was detected on 67.5 ± 11.3%, 88.9 ± 6.1%, and 66.7 ± 14.0%, of HUVEC activated with LPS, TNFα, or IL-1β, respectively (n = 4 experiments).
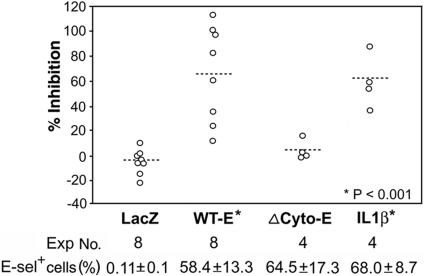
Increased Sensitivity to Endostatin in E-Selectin-Transduced HUVEC. To determine the effect of E-selectin on endostatin-mediated inhibition independent of other consequences of inflammatory activation, we transduced HUVEC with the adenoviral human E-selectin expression constructs. The overall efficiency of gene transduction was >85% as determined by flow cytometry of AdRSVlacZ-transduced cells (data not shown). E-selectin expression was comparable in HUVECs transduced with AdRSV(wt-E) expressing a full-length E-selectin or AdRSV(Δcyto-E) expressing a E-selectin mutant lacking the cytoplasmic domain, and in cells activated by IL-1β (Fig. 4). No significant difference in the number of migrated cells in the absence or presence of VEGF stimuli was found in cells transduced with AdRSVlacZ, AdRSV(wt-E), or AdRSV(Δcyto-E) (data not shown). Endostain inhibited VEGF-induced migration of AdRSV(wt-E)-transduced HUVEC to various degrees in eight experiments by using cells isolated from seven separate donor pools and virus from one of two independent viral stocks. The percentage of inhibition achieved by endostatin at 500 ng/ml in each experiment is illustrated in Fig. 4. In addition, TNP-470 inhibited AdRSV(wt-E)-transduced HUVEC, whereas Fc-human angiostatin showed no effect (data not shown). In contrast, endostatin did not alter VEGF-induced migration of AdRSV(Δcyto-E)-transduced HUVEC. The effect of endostatin on AdRSV(wt-E)-transduced HUVEC was similar to, although somewhat less consistent than, that of endostatin on IL-1β-activated HUVEC. Taken together, these results suggest that E-selectin expression is sufficient to confer endostatin responsiveness selectively and that the cytoplasmic domain of E-selectin is required for endostatin activity.
Fig. 4.
Increased sensitivity to endostatin in HUVEC expressing transduced E-selectin. HUVEC were transduced with adenoviral constructs expressing control lacZ (LacZ), WT full-length E-selectin (WT-E), or E-selectin mutant that lacks cytoplasmic tail (ΔCyto-E). The inhibitory effect of endostatin (500 ng/ml) on VEGF-induced migration of these transduced cells and IL-1β-activated control cells was quantified. Percentage of cells in each group expressing E-selectin detected by flow cytometry is shown as mean ± SD. Statistical comparison for each group with the LacZ control group was based on two-tailed Student's t test.
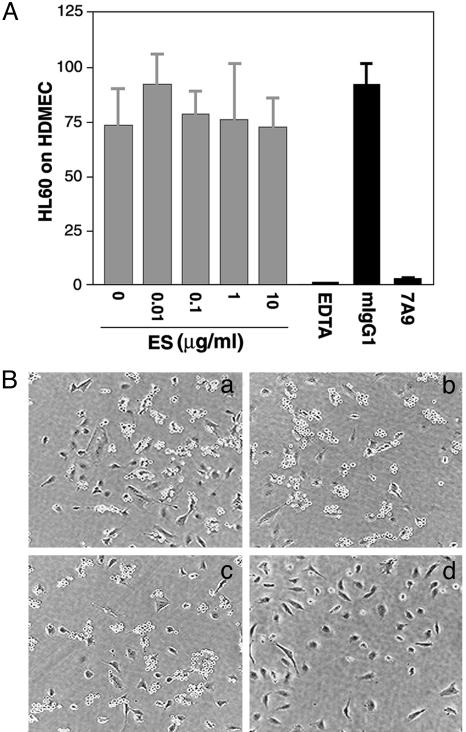
Endostatin Does Not Block E-Selectin-Mediated Leukocyte Adhesion. The structural similarity of endostatin and E-selectin calcium-dependent ligand binding domains suggests that endostatin might interrupt E-selectin-mediated cellular interactions during inflammation. To test this hypothesis, we determined the effect of endostatin on E-selectin-dependent leukocyte adhesion to endothelial cells. HDMEC activated by LPS to induce cell-surface E-selectin were incubated with leukocytes (HL-60 pro-myelocytic leukemia cells) (Fig. 5). As expected, EDTA or E-selectin neutralizing antibody 7A9 (30) efficiently blocked adhesion of HL-60 cells to the activated HDMEC. However, endostatin had no effect, suggesting that endostatin does not interfere with E-selectin binding to its ligands presented on HL-60.
Fig. 5.
Endostatin does not block E-selectin-mediated leukocyte adhesion. (A) HL-60 cell adhesion to HDMEC treated for 4 h with LPS (0.1 μg/ml) was measured in the presence of 0–10 μg/ml endostatin (ES), EDTA (2.5 mM), neutralizing antibody (7A9) to E-selectin (1 μg/ml), or isotype-matched IgG1 (1 μg/ml). Values shown are the total bound cells counted in 10 randomly selected microscopic fields by using a 20× objective. (B) Images of HL-60 (round cells) on HUVEC in the absence (a) or presence of 10 μg/ml endostatin (b), 1 μg/ml mIgG1 (c), or 1 μg/ml 7A9 (d). Results are representative of three independent experiments.
Discussion
Although endostatin potently inhibits angiogenesis and tumor growth in vivo, little is known about the underlying mechanisms of endostatin action. We report that E-selectin is required for the anti-angiogenic activity of endostatin. This functional interaction was demonstrated by three independent assays. By using in vivo corneal micropocket assay, we showed that bFGF-induced corneal angiogenesis in E–/– mice was resistant to continuous systemic administration of endostatin. In ex vivo aortic ring assay, VEGF-stimulated formation of vascular sprouts from E–/– aortic explants was insensitive to exogenous endostatin. Finally, by using in vitro EC migration assay, we demonstrated that expression of E-selectin by adenoviral transduction conferred increased endostatin sensitivity to initially nonresponsive human EC.
Consistent with previous studies (34), E–/– mice did not differ from E+/+ mice in the response to angiogenic stimuli in corneal micropocket assay. However, endothelial sprout formation in aortic rings from E–/– mice showed enhanced sensitivity to VEGF compared with those from E+/+ mice. This result could reflect either an abnormally exuberant response to VEGF or insensitivity to the inhibitory effects of endogenous endostatin present in the murine aortic media. The mouse aorta is among the most abundant tissue sources of collagen XVIII and its proteolytically cleaved endostatin fragment (35). We showed that sprouting from E–/– explants was completely resistant to the continuous presence of exogenous endostatin, whereas E+/+ explants were significantly inhibited. These results are consistent with the hypothesis that enhanced VEGF-induced sprouting reflects insensitivity of E–/– aortas to endogenous endostatin.
We showed that E-selectin alone is sufficient to confer endostatin sensitivity in an in vitro EC migration assay. Expression of WT E-selectin directed by adenoviral-mediated gene transduction conferred increased endostatin responsiveness to nonresponsive human EC. Inhibition was statistically significant, varying from 13% to 110% in eight separate experiments with a mean of 65%. The wide range of inhibitory activity may reflect variations in the endothelial cells prepared from seven different donor pools, and/or in the adenoviral stocks prepared from several independent cell cultures that were used in the studies. Adenoviral gene transduction does not overtly activate HUVEC as judged by expression of other activation-responsive adhesion molecules (29). Thus, it appears that E-selectin alone was sufficient to confer endostatin sensitivity in this migration assay, and this functional change likely did not involve induction of other factors initially absent from nonresponsive EC.
Our results also reveal that the cytoplasmic domain of E-selectin is required for this cell-surface lectin to confer endostatin sensitivity in EC. HUVEC transduced with AdRSV(ΔCyto-E), which lacks the E-selectin cytoplasmic domain, remained unresponsive to endostatin in contrast with cells transduced with AdRSV(wt-E) encoding full-length E-selectin. This finding suggests that the cytoplasmic domain delivers signals to, or otherwise interacts with, intracellular elements essential for endostatin action. This important aspect of E-selectin/endostatin functional interaction can be further investigated by testing additional structural variations (29) in the E-selectin cytoplasmic domain for their effects on endostatin activity.
Although E-selectin was both required and sufficient for endostatin sensitivity in otherwise quiescent EC, additional factors were able to modulate endostatin sensitivity even when E-selectin was present on the EC surface. EC activated with the inflammatory mediators IL-1β, LPS, or TNFα each showed E-selectin expression equivalent to EC treated with AdRSV(wt-E). However, only cells treated with IL-1β or LPS, but not TNFα, became responsive to endostatin. This differential endostatin sensitivity may reflect differences in the intracellular signaling pathways activated by these distinct inflammatory mediators. For example, TNFα interacts with two different cell-surface receptors (36) and activates at least two distinct but partially overlapping signaling pathways including NFκB/IκBα and JNK/p38 MAP kinase (37). It is possible that one of these TNFα receptors generates a negative regulator and thereby interferes with endostatin activity despite the presence of E-selectin. Even though our data suggest that E-selectin induced by IL-1β or LPS enhances endostatin responsiveness of human EC, other positive regulators may also be produced as a result of cell activation. The existence of signals with such potential is suggested by the fact that IL-1β, but not TNFα, combines with activated protein kinase C to induce the expression of E-selectin itself (38). However, although such positive regulators might be absent in TNFα-stimulated EC, such a deficiency is not likely the sole explanation for differential endostatin sensitivity because our experiments with AdRSV(wt-E) demonstrate that E-selectin alone is sufficient to confer responsiveness.
The detailed mechanisms underlying functional interaction between E-selectin and endostatin remain to be elucidated. During the course of this study, we failed to detect any direct physical association between endostatin and E-selectin by solid phase binding assay, surface plasmon resonance (39), or flow cytometry (data not shown). Endostatin also did not interfere with E-selectin/ligand binding in a nonstatic leukocyte adhesion assay. It is conceivable that E-selectin may facilitate endostatin binding to a currently unidentified functional receptor. Both endostatin and E-selectin have been shown to interact with O-sulfated glycosaminoglycans (7, 8, 40). Therefore, we postulate that E-selectin presents a cell-surface glycosaminoglycan moiety in a conformation favorable for endostatin recognition and binding. The resulting ternary complex, present only on cells expressing E-selectin, may be required for endostatin to inhibit angiogenesis.
Recombinant human endostatin was not toxic in a phase I clinical trial (41). However, the lack of surrogate markers predicting endostatin efficacy has hampered these studies. The results presented here suggest that E-selectin on the endothelium of tumor blood vessels may provide a clinically useful predictor of endostatin responsiveness. Down-regulation and inherited deficiency of E-selectin expression have been described in endothelium of human melanoma and inflammatory tissues, respectively (42, 43). Hence, E-selectin expression in tumor vasculature and other clinical settings may vary significantly among patients and thereby affect responsiveness to endostatin. This conclusion suggests that determining E-selectin expression in tumors, either directly by biopsy or indirectly by less invasive methods, before therapeutic intervention may predict endostatin efficacy and, therefore, help guide selection of an effective treatment modality. It is also conceivable that E-selectin could be up-regulated, pharmacologically or by gene therapy, to improve the antiangiogenic effect of endostatin. Additional studies with well characterized animal models of tumor growth are needed to address these possibilities.
In conclusion, we demonstrated that E-selectin plays an important role in endostatin-mediated antiangiogenesis. E-selectin is required for the antiangiogenic activity of endostatin in vivo and ex vivo, and is sufficient, via a mechanism requiring the E-selectin cytoplasmic domain, to confer endostatin sensitivity in endothelial cells in vitro. Our data provide compelling evidence to suggest that E-selectin expression may predict endostatin efficacy and that modulating E-selectin may improve antiangiogenic therapy mediated by endostatin. Understanding how endothelial E-selectin, inflammatory mediators, and endostatin interact to promote and/or inhibit blood vessel formation may also provide unique insight on angiogenesis.
Acknowledgments
We thank Kaye Case and Deanna Lamont for providing HUVEC, Jeane Keily and Anthony Rosenzweig for providing initial samples and assisting with preparation of recombinant adenoviruses, and Dipak Panigrahy and Arja Kaipainen for implanting osmotic pumps. This study was supported by a grant from the Charlotte Geyer Foundation (to J.B.) and by National Institutes of Health Grants HL67255 (to K.S.M.) and HL36028 (to D.S.M.).
Abbreviations: EC, endothelial cell; HUVEC, human umbilical vein endothelial cells; HDMEC, human dermal microvascular endothelial cells; LPS, lipopolysaccharide; VEGF, vascular endothelial growth factor;
References
- 1.Hanahan, D. & Folkman, J. (1996) Cell 86, 353–364. [DOI] [PubMed] [Google Scholar]
- 2.O'Reilly, M. S., Boehm, T., Shing, Y., Fukai, N., Vasios, G., Lane, W. S., Flynn, E., Birkhead, J. R., Olsen, B. R. & Folkman, J. (1997) Cell 88, 277–285. [DOI] [PubMed] [Google Scholar]
- 3.Sudhakar, A., Sugimoto, H., Yang, C., Lively, J., Zeisberg, M. & Kalluri, R. (2003) Proc. Natl. Acad. Sci. USA 100, 4766–4771. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 4.Wickstrom, S. A., Alitalo, K. & Keski-Oja, J. (2003) J. Biol. Chem. 278, 37895–37901. [DOI] [PubMed] [Google Scholar]
- 5.Dixelius, J., Cross, M., Matsumoto, T., Sasaki, T., Timpl, R. & Claesson-Welsh, L. (2002) Cancer Res. 62, 1944–1947. [PubMed] [Google Scholar]
- 6.Hanai, J., Dhanabal, M., Karumanchi, S. A., Albanese, C., Waterman, M., Chan, B., Ramchandran, R., Pestell, R. & Sukhatme, V. P. (2002) J. Biol. Chem. 277, 16464–16469. [DOI] [PubMed] [Google Scholar]
- 7.Karumanchi, S. A., Jha, V., Ramchandran, R., Karihaloo, A., Tsiokas, L., Chan, B., Dhanabal, M., Hanai, J. I., Venkataraman, G., Shriver, Z., et al. (2001) Mol. Cell 7, 811–822. [DOI] [PubMed] [Google Scholar]
- 8.Blackhall, F. H., Merry, C. L., Lyon, M., Jayson, G. C., Folkman, J., Javaherian, K. & Gallagher, J. T. (2003) Biochem. J. 375, 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim, Y. M., Hwang, S., Pyun, B. J., Kim, T. Y., Lee, S. T., Gho, Y. S. & Kwon, Y. G. (2002) J. Biol. Chem. 277, 27872–27879. [DOI] [PubMed] [Google Scholar]
- 10.Kunkel, E. J. & Ley, K. (1996) Circ. Res. 79, 1196–1204. [DOI] [PubMed] [Google Scholar]
- 11.Ley, K., Allietta, M., Bullard, D. C. & Morgan, S. (1998) Circ. Res. 83, 287–294. [DOI] [PubMed] [Google Scholar]
- 12.Milstone, D. S., Fukumura, D., Padgett, R. C., O'Donnell, P. E., Davis, V. M., Benavidez, O. J., Monsky, W. L., Melder, R. J., Jain, R. K. & Gimbrone, M. A., Jr. (1998) Microcirculation 5, 153–171. [PubMed] [Google Scholar]
- 13.Drickamer, K. (1988) J. Biol. Chem. 263, 9557–9560. [PubMed] [Google Scholar]
- 14.Graves, B. J., Crowther, R. L., Chandran, C., Rumberger, J. M., Li, S., Huang, K. S., Presky, D. H., Familletti, P. C., Wolitzky, B. A. & Burns, D. K. (1994) Nature 367, 532–538. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen, M., Strubel, N. A. & Bischoff, J. (1993) Nature 365, 267–269. [DOI] [PubMed] [Google Scholar]
- 16.Bischoff, J., Brasel, C., Kraling, B. & Vranovska, K. (1997) Microcirculation 4, 279–287. [DOI] [PubMed] [Google Scholar]
- 17.Kraling, B. M., Razon, M. J., Boon, L. M., Zurakowski, D., Seachord, C., Darveau, R. P., Mulliken, J. B., Corless, C. L. & Bischoff, J. (1996) Am. J. Pathol. 148, 1181–1191. [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen, M., Corless, C. L., Kraling, B. M., Tran, C., Atha, T., Bischoff, J. & Barsky, S. H. (1997) Am. J. Pathol. 150, 1307–1314. [PMC free article] [PubMed] [Google Scholar]
- 19.Koch, A. E., Halloran, M. M., Haskell, C. J., Shah, M. R. & Polverini, P. J. (1995) Nature 376, 517–519. [DOI] [PubMed] [Google Scholar]
- 20.Kumar, P., Amin, M. A., Harlow, L. A., Polverini, P. J. & Koch, A. E. (2003) Blood 101, 3960–3968. [DOI] [PubMed] [Google Scholar]
- 21.Hohenester, E., Sasaki, T., Olsen, B. R. & Timpl, R. (1998) EMBO J. 17, 1656–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bevilacqua, M. P., Pober, J. S., Wheeler, M. E., Cotran, R. S. & Gimbrone, M. A., Jr. (1985) J. Clin. Invest. 76, 2003–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kraling, B. M. & Bischoff, J. (1998) In Vitro Cell Dev. Biol. Anim. 34, 308–315. [DOI] [PubMed] [Google Scholar]
- 24.Kisker, O., Becker, C. M., Prox, D., Fannon, M., D'Amato, R., Flynn, E., Fogler, W. E., Sim, B. K., Allred, E. N., Pirie-Shepherd, S. R. & Folkman, J. (2001) Cancer Res. 61, 7669–7674. [PubMed] [Google Scholar]
- 25.Kenyon, B. M., Voest, E. E., Chen, C. C., Flynn, E., Folkman, J. & D'Amato, R. J. (1996) Invest. Ophthalmol. Vis. Sci. 37, 1625–1632. [PubMed] [Google Scholar]
- 26.Kenyon, B. M., Browne, F. & D'Amato, R. J. (1997) Exp. Eye Res. 64, 971–978. [DOI] [PubMed] [Google Scholar]
- 27.Moulton, K. S., Vakili, K., Zurakowski, D., Soliman, M., Butterfield, C., Sylvin, E., Lo, K. M., Gillies, S., Javaherian, K. & Folkman, J. (2003) Proc. Natl. Acad. Sci. USA 100, 4736–4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bergers, G., Javaherian, K., Lo, K. M., Folkman, J. & Hanahan, D. (1999) Science 284, 808–812. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida, M., Szente, B. E., Kiely, J. M., Rosenzweig, A. & Gimbrone, M. A., Jr. (1998) J. Immunol. 161, 933–941. [PubMed] [Google Scholar]
- 30.Rodriguez-Romero, A., Almog, O., Tordova, M., Randhawa, Z. & Gilliland, G. L. (1998) J. Biol. Chem. 273, 11770–11775. [DOI] [PubMed] [Google Scholar]
- 31.Ingber, D., Fujita, T., Kishimoto, S., Sudo, K., Kanamaru, T., Brem, H. & Folkman, J. (1990) Nature 348, 555–557. [DOI] [PubMed] [Google Scholar]
- 32.Yamaguchi, N., Anand-Apte, B., Lee, M., Sasaki, T., Fukai, N., Shapiro, R., Que, I., Lowik, C., Timpl, R. & Olsen, B. R. (1999) EMBO J. 18, 4414–4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshida, A., Anand-Apte, B. & Zetter, B. R. (1996) Growth Factors 13, 57–64. [DOI] [PubMed] [Google Scholar]
- 34.Hartwell, D. W., Butterfield, C. E., Frenette, P. S., Kenyon, B. M., Hynes, R. O., Folkman, J. & Wagner, D. D. (1998) Microcirculation 5, 173–178. [PubMed] [Google Scholar]
- 35.Miosge, N., Sasaki, T. & Timpl, R. (1999) FASEB J. 13, 1743–1750. [DOI] [PubMed] [Google Scholar]
- 36.Aggarwal, B. B. (2003) Nat. Rev. Immunol. 3, 745–756. [DOI] [PubMed] [Google Scholar]
- 37.Read, M. A., Whitley, M. Z., Gupta, S., Pierce, J. W., Best, J., Davis, R. J. & Collins, T. (1997) J. Biol. Chem. 272, 2753–2761. [DOI] [PubMed] [Google Scholar]
- 38.Tamaru, M. & Narumi, S. (1999) J. Biol. Chem. 274, 3753–3763. [DOI] [PubMed] [Google Scholar]
- 39.Mehta, P., Cummings, R. D. & McEver, R. P. (1998) J. Biol. Chem. 273, 32506–32513. [DOI] [PubMed] [Google Scholar]
- 40.Luo, J., Kato, M., Wang, H., Bernfield, M. & Bischoff, J. (2001) J. Cell Biochem. 80, 522–531. [PubMed] [Google Scholar]
- 41.Eder, J. P., Jr., Supko, J. G., Clark, J. W., Puchalski, T. A., Garcia-Carbonero, R., Ryan, D. P., Shulman, L. N., Proper, J., Kirvan, M., Rattner, B., et al. (2002) J. Clin. Oncol. 20, 3772–3784. [DOI] [PubMed] [Google Scholar]
- 42.DeLisser, H. M., Christofidou-Solomidou, M., Sun, J., Nakada, M. T. & Sullivan, K. E. (1999) Blood 94, 884–894. [PubMed] [Google Scholar]
- 43.Berger, R., Albelda, S. M., Berd, D., Ioffreda, M., Whitaker, D. & Murphy, G. F. (1993) J. Cutan. Pathol. 20, 399–406. [DOI] [PubMed] [Google Scholar]