Abstract
Objectives
Fidaxomicin was non-inferior to vancomycin with respect to clinical cure rates in the treatment of Clostridium difficile infections (CDIs) in two Phase III trials, but was associated with significantly fewer recurrences than vancomycin. This economic analysis investigated the cost-effectiveness of fidaxomicin compared with vancomycin in patients with severe CDI and in patients with their first CDI recurrence.
Methods
A 1 year time horizon Markov model with seven health states was developed from the perspective of Scottish public healthcare providers. Model inputs for effectiveness, resource use, direct costs and utilities were obtained from published sources and a Scottish expert panel. The main model outcome was the incremental cost-effectiveness ratio (ICER), expressed as cost per quality-adjusted life year (QALY), for fidaxomicin versus vancomycin; ICERs were interpreted using willingness-to-pay thresholds of £20 000/QALY and £30 000/QALY. One-way and probabilistic sensitivity analyses were performed.
Results
Total costs were similar with fidaxomicin and vancomycin in patients with severe CDI (£14 515 and £14 344, respectively) and in patients with a first recurrence (£16 535 and £16 926, respectively). Improvements in clinical outcomes with fidaxomicin resulted in small QALY gains versus vancomycin (severe CDI, +0.010; patients with first recurrence, +0.019). Fidaxomicin was cost-effective in severe CDI (ICER £16 529/QALY) and dominant (i.e. more effective and less costly) in patients with a first recurrence. The probability that fidaxomicin was cost-effective at a willingness-to-pay threshold of £30 000/QALY was 60% for severe CDI and 68% in a first recurrence.
Conclusions
Fidaxomicin is cost-effective in patients with severe CDI and in patients with a first CDI recurrence versus vancomycin.
Keywords: economic, model, antibacterials
Introduction
For the past 30 years, the antibiotic treatment of Clostridium difficile infection (CDI) has depended primarily on metronidazole and vancomycin. Both agents are recommended in the current treatment guidelines issued by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID): oral metronidazole for initial non-severe episodes (evidence A-I); and oral vancomycin for initial severe episodes (A-I), non-severe episodes (B-I), first recurrence or those at risk of recurrent CDI (B-I) and multiple recurrent episodes (B-II).1 The need for new treatment options has become more pressing as the incidence and severity of CDI has increased over the last decade in Europe and North America.2,3 Additionally, the attendant economic consequences of CDI in Europe are also significant4 with hospital bed-days, the primary cost driver, accounting for up to 94% of expenditure.5 Therefore, the fiscal impact of any new therapies on healthcare resource use is also worthy of consideration.
Fidaxomicin (Dificlir™; Astellas) is a narrow-spectrum oral macrocyclic antibiotic for the treatment of CDI. It is minimally absorbed from the gastrointestinal tract and achieves mean faecal concentrations that exceed the MIC for C. difficile by >5000-fold.6 Fidaxomicin is bactericidal against C. difficile7 and inhibits spore production,8 but has minimal effect on the normal gut microflora.9 In two Phase III randomized controlled trials in patients with CDI, fidaxomicin was non-inferior to vancomycin with respect to clinical cure rates, but was associated with significantly fewer disease recurrences, which resulted in clinically meaningful improvements in sustained clinical cure (i.e. clinical cure without recurrence).10,11 Fidaxomicin is now recommended in the European treatment guidelines for use in non-severe episodes (B-I), first recurrence or those at risk of recurrent CDI (B-I) and for multiple recurrent episodes (B-II).1
It is becoming increasingly common for payers, managed care organizations and regulatory bodies to demand health economic analyses to assess the fiscal value of new pharmaceuticals. Economic analyses allow the comparison of different treatments in terms of clinical outcomes and costs and assist with decisions regarding purchasing, pricing, reimbursement and formulary acceptance. To date, the only published cost-effectiveness analyses involving fidaxomicin have been performed from a US payer perspective.12,13
The present paper describes the outcomes from a cost-effectiveness analysis of fidaxomicin versus vancomycin in patients with CDI from the perspective of the National Health Service (NHS) Scotland and Personal and Social Services in Scotland. It aims to provide a cost-effectiveness model more relevant to European healthcare systems. Given that the fidaxomicin Phase III trials10,11 suggest that patients with severe CDI and patients with a recurrence are likely to derive the greatest benefit from fidaxomicin in terms of reducing recurrences, the present analysis focused on these two patient subgroups. These subgroups are recognized as identifiable patient subgroups in the real-world clinical setting and are defined in the ESCMID guidelines.1 An indirect comparison of the clinical effectiveness of fidaxomicin, vancomycin and metronidazole will be published separately as a companion article.14
Methods
Model overview
A decision-analytical framework was used for the cost-effectiveness analyses as it provides a method for decision making when there is uncertainty. Specifically, a time-dependent Markov model, which offers tractability in the specification of the decision problem, i.e. the key health states related to the disease, transition probabilities and associated cost and health outcomes, was developed in Excel™ (Microsoft Corp., Redmond, WA, USA) (Figure 1). The Markov model consisted of seven different health states as defined in Table 1.
Figure 1.
Markov model structure. Definitions of transition states are provided in Table 1. Note: expanded model details shown for fidaxomicin arm only. tx, treatment; VAN, vancomycin.
Table 1.
Model health states
| Health state | Description |
|---|---|
| CDIa | index CDI episode and any subsequent recurrent CDI episodes; all patients enter the model in this health state |
| CDI cured | patient clinically cured after initial treatment |
| CDI cured after failure | patient clinically cured after initial treatment failure |
| CDI tx failed—VAN 250 mg | patient who failed to experience a clinical cure after fidaxomicin or vancomycin for 10 days and therefore received treatment with 250 mg of vancomycin qid for 10 days |
| CDI tx failed—VAN 500 mg | patient who failed to experience a clinical cure after 250 mg of vancomycin qid for 10 days and therefore received 500 mg of vancomycin qid for 10 days |
| CDI failed—VAN taper | patient who failed to experience a clinical cure after 500 mg of vancomycin qid for 10 days and therefore received a vancomycin taper regimen for 8 weeks |
| Death | patient who died due to either CDI or an unrelated cause |
qid, four times daily; tx, treatment; VAN, vancomycin.
aIn order to keep track of the severity and number of recurrences, this health state was split into five separate tunnel states: index CDI episode; first non-severe recurrence; first severe recurrence; second or more non-severe recurrence; and second or more severe recurrence.
The model evaluated the cost-effectiveness of fidaxomicin compared with vancomycin in adults with severe CDI and patients with their first CDI recurrence. The model was conducted from the perspective of the NHS and Personal and Social Services in Scotland and considered direct medical costs. The purpose of the model was to be submitted as part of the fidaxomicin health technology assessment to the Scottish Medicines Consortium. The model cycle length was 10 days, in line with the duration of CDI treatment in clinical trials and clinical practice. As patients with CDI can have multiple recurrences,15 it was decided to set the time horizon of the model at 1 year. No discounting was applied to costs or outcomes because of the 1 year time horizon.
Irrespective of whether the patient had severe CDI or a first recurrence, the patient entered the model in the ‘CDI’ health state and was treated either with oral fidaxomicin or oral vancomycin for 10 days. Successfully treated patients entered and remained in the ‘CDI cured’ health state unless they died due to any cause (age-matched mortality) or experienced a recurrence. If a patient had a recurrence, they then moved back to the ‘CDI’ health state and were treated again with the initial treatment.
In order to keep track of the severity and number of recurrences, the ‘CDI’ health state was split into five separate health states: (i) index CDI episode; (ii) first non-severe recurrence; (iii) first severe recurrence; (iv) second or more non-severe recurrence; and (v) second or more severe recurrence.
In the fidaxomicin arm, the health state ‘CDI cured after failure’ for patients initially on fidaxomicin who failed with initial treatment but were cured after second- or third-line treatment was also considered.
Patients who did not respond to treatment progressed towards CDI with complications or without. The CDI complications included in the model were toxic megacolon, colonic perforation, sepsis or colectomy. Drug-related adverse events from fidaxomicin or vancomycin were not modelled as they were generally mild and would not lead to additional treatment costs or a switch in treatment.
Irrespective of whether the patient had a complication or not, all patients who failed to respond to fidaxomicin or vancomycin after 10 days were then treated with 250 mg of oral vancomycin four times daily. If the patient was still not cured after 10 days of treatment with 250 mg of vancomycin four times daily, the dose was increased to 500 mg four times daily. In the event the patient also failed to respond to 500 mg of vancomycin four times daily, they were then treated with a vancomycin taper regimen. These patients remained in the ‘CDI failed—vancomycin taper’ health state for 8 weeks, after which if the patient did not respond they then received a last-resort adjunctive treatment, which was assumed to be either an immunoglobulin infusion or a 14 day rifampicin course. Unless the patient died due to CDI, this last-resort therapy was assumed to be successful and patients moved to the ‘CDI cured’ health state after 60 days (six cycles of 10 days). No patient was assumed to undergo surgery, based on advice from Scottish experts that surgery for CDI is very rare in Scotland.
Inputs for effectiveness data, costs and utilities were extracted from published sources and are described in detail below. Additional input was sought from a group of Scottish clinical experts for parameters for which data were limited, i.e. local treatment pathway and type of hospital ward (clinician and microbiologist input), recovery of quality of life (QoL) after CDI (health economics expert). The Scottish clinical expert panel included a consultant microbiologist with NHS Greater Glasgow & Clyde, a consultant microbiologist at NHS Ayrshire & Arran, an infectious diseases consultant physician at NHS Tayside, an infectious diseases expert from a Scottish university and a health economics expert from a Scottish university. All the experts were paid a fee for service by Astellas for their time attending the advisory board and none was an employee of Astellas.
Patient groups
Two patient subgroups were considered in the model: adults (aged ≥18 years) with severe CDI and adults with a first CDI recurrence. Patients with a first recurrence (severe or non-severe) were defined as those with a recurrence treated in hospital (also see the Recurrence and reinfection section). Severe CDI was defined as in the fidaxomicin Phase III studies, i.e. >15 000 white blood cells/mm3, serum creatinine concentration >1.5 mg/dL, or body temperature >38.5°C,10 or ≥10 unformed bowel movements/day or white blood cell count ≥15 001/mm3.11
Treatment pathway
In the model, patients presenting with severe CDI or a first CDI recurrence were treated initially with 200 mg of oral fidaxomicin twice daily for 10 days or 125 mg of oral vancomycin four times daily for 10 days (Figure S1, available as Supplementary data at JAC Online). Vancomycin was selected as the comparator treatment based on current treatment guidelines in Scotland, which recommend oral vancomycin for any severe CDI episode16,17 and any non-severe recurrence.16 According to clinical expert opinion, vancomycin slurry is not prescribed in clinical practice in Scotland; therefore, only vancomycin oral capsules were considered in the model.
In order to be consistent with Scottish treatment guidelines,16,17 it was also assumed that first non-severe recurrences were treated with 450 mg of oral metronidazole three times daily for 10 days. The dose selected for oral metronidazole was the mean of the doses recommended in Scotland (40016,17 and 500 mg16).
Patients who failed to respond to fidaxomicin or vancomycin were then assumed to receive the following sequence of treatments, based on input from the Scottish clinical experts, until a response was achieved: 250 mg of oral vancomycin four times daily for 10 days; 500 mg of vancomycin four times daily for 10 days; vancomycin taper regimen for 8 weeks; and an adjunctive last-resort treatment (i.e. immunoglobulin infusion18 or 14 day rifampicin course). We did not include faecal transplantation19 as an option in our model as it is not a widely available treatment in Scotland.
Model assumptions
The assumptions made in the model and the justification for them are summarized in Table S1 (available as Supplementary data at JAC Online).1,10,11,14–17,20–24
Model input parameters
Clinical effectiveness
A summary of model effectiveness input parameters and the range of values applied in the one-way sensitivity analysis is provided in Table 2.10,14,15,24–27 Model inputs for clinical cure and recurrence rates up to day 15 from end of treatment (EOT) were based on a pooled analysis14 of the intent-to-treat populations from the two Phase III randomized controlled trials that compared fidaxomicin with vancomycin in patients with CDI. Inputs for clinical cure and recurrence rates for metronidazole were based on a randomized, double-blind comparison of metronidazole and vancomycin in patients with CDI;25 since the clinical cure and recurrence rates were not significantly different between treatments in patients with non-severe CDI,25 we assumed that the odds ratio (OR) for both outcomes was 1.0 for non-severe CDI episodes. The ranges of ORs tested in the one-way sensitivity analysis were based on the 95% CIs of these ORs.
Table 2.
Model inputs: effectiveness
| Variable description | Base-case inputa | Range for sensitivity analysisa |
Uncertainty distribution | Source(s) | |
|---|---|---|---|---|---|
| minimum | maximum | ||||
| OR for clinical cure in severe CDI treated with fidaxomicin | 1 | 0.502 | 1.465 | log normal (mean = 0.86; SD = 0.273) | 14 |
| OR for clinical cure in CDI recurrence treated with fidaxomicin | 1 | 0.415 | 2.808 | log normal (mean = 1.08; SD = 0.488) | 14 |
| OR for clinical cure in non-severe CDI (first recurrence) treated with metronidazole | 1 | 0.030 | 2.220 | log normal (mean = 0.24; SD = 1.10) | 25 |
| Clinical cure in severe CDI treated with vancomycin | 0.853 | 0.802 | 0.897 | β (n = 180; N = 211) | 14 |
| Clinical cure in CDI recurrence treated with vancomycin | 0.889 | 0.817 | 0.945 | β (n = 80; N = 90) | 10 |
| OR for recurrence in severe CDI treated with fidaxomicin | 0.456 | 0.264 | 0.788 | log normal (mean = 0.46; SD = 0.279) | 14 |
| OR for recurrence in any CDI recurrence treated with fidaxomicin | 0.528 | 0.256 | 1.086 | log normal (mean = 0.53; SD = 0.368) | 14 |
| OR for recurrence in any CDI recurrence treated with metronidazole | 1 | 0.264 | 10.37 | none | 25 |
| OR for CDI recurrence patients with ≥2 recurrences with fidaxomicin versus vancomycin | 0.528 | 0.256 | 1.00 | none | 14,15 |
| OR for recurrence in patients with ≥2 previous recurrences | 3.87 | 1.12 | 13.34 | log normal (mean = 1.35; SD = 0.63) | 15 |
| Recurrence rate in severe CDI treated with vancomycin | 0.267 (0.098) | 0.205 (0.073) | 0.333 (0.126) | β (n = 48; N = 180) | 14 |
| Recurrence rate in patients with a recurrence treated with vancomycin | 0.325 (0.123) | 0.227 (0.082) | 0.431 (0.171) | β (n = 26; N = 80) | 14 |
| Reinfection rate (recurrence 30 days after EOT) | 0.084 (0.029) | 0.059 (0.000) | 0.113 (0.392) | β (n = 33; N = 394) | Astellas, data on file |
| Probability of a complication with fidaxomicin or vancomycin (all CDI subgroups) | 0.003 | 0.001 | 0.024 | β (n = 4; N = 1147) | Astellas, data on file; 26 |
| CDI-attributable mortality (30 day) | 0.060 (0.020) | 0.056 (0.002) | 0.064b (0.002) | β (n = 657; N = 10 975) | 24 |
| Annual all-cause mortality | 0.013 (0.0004) | 0.000 (0.000) | 0.090 (0.033) | none | 27 |
n, number of patients; N, total number of patients.
aNumbers in brackets refer to 10 day probabilities, where applicable.
bThe maximum 30 day mortality rate for severe CDI was considered to be 0.42 (0.105).
The base-case CDI-attributable mortality rate was estimated from a literature review.24 The rate of background mortality was estimated from the Scottish Decennial Life Tables 2000–02 for an average patient in the pooled fidaxomicin studies (i.e. 62 years of age).27
Recurrence and reinfection
For the simplified purpose of the model, patients could either have a recurrence or reinfection based on the two Phase III trials.10,11 Both health outcomes incurred the same costs and if patients experienced either, they moved into the ‘CDI’ recurrence health state.
A recurrence was defined as follows: the reappearance of more than three diarrhoeal stools per 24 h period within 30 days of treatment completion; the presence of C. difficile toxin A, B or both in the stools; and the need for retreatment for CDI following resolution of diarrhoea (i.e. three or fewer diarrhoeal stools per day for 2 days consecutively, maintained for the duration of initial treatment and no further need for treatment as of the second day after the last dose of initial treatment).10,11 Since the fidaxomicin Phase III studies had a total follow-up period of 30 days and data for only one recurrence were available, data from Fekety et al.15 were used to estimate the risk of multiple recurrences. Given the substantial reduction in the risk of recurrences observed with fidaxomicin, we assumed that the treatment effect of fidaxomicin was maintained irrespective of the number of previous recurrences.
The model did not distinguish between recurrences and reinfection: any recurrence of CDI was modelled. It was assumed that 30 days after the EOT, the chance of a recurrence was reduced. CDI recurrences occurring after day 30 of EOT were assumed to be reinfections.23 At the time of model construction, there were no data available on the risk of reinfection in patients who had already experienced at least one CDI episode. It was assumed, based on the evidence from Barbut et al.,23 that CDI episodes occurring >2 weeks after successful treatment were likely to be a reinfection. The recurrence rate (8.4%) observed beyond day 15 of EOT in the fidaxomicin Phase III trials was considered to be the rate of reinfection (Astellas, data on file). It was assumed that the reinfection risk was the same for fidaxomicin and vancomycin.
As the Phase III trials only provided data up to day 30 after EOT, assumptions were made regarding reinfection after day 30. The model made a conservative assumption on reinfection after day 40, in that it was based on the recurrence rate reported in the vancomycin arm between days 15 and 30 in the trial. The reinfection rate for fidaxomicin and vancomycin was assumed to be the same and was set at 2.87% per 10 day cycle in the model. This is a conservative assumption, as it is now known that fidaxomicin has a lower reinfection rate than vancomycin.28 The model assumes that patients in the ‘CDI cured’ health state after day 40 can still have a reinfection, although in the model those patients with a reinfection go into the ‘CDI’ health state and no differentiation is made between recurrence and reinfection.
Resource use
A summary of inputs for resource use and the range of values applied in the one-way sensitivity analysis are provided in Table 3. Estimates for resource use were adjusted according to disease severity. For the base-case analysis, 12.2% of recurrences were assumed to be severe.20
Table 3.
Model inputs: resource use
| Variable description | Base-case input | Range for sensitivity analysis |
Uncertainty distribution | Source(s) | |
|---|---|---|---|---|---|
| minimum | maximum | ||||
| Excess LOS in general ward for index infection (days) | 19.32 | 16.64 | 22.20 | γ (SD = 1.42) | Hospital Episode Statistics, data on file |
| Excess LOS in general ward for a recurrence (days) | 12.24 | 9.83 | 14.91 | γ (SD = 1.30) | Hospital Episode Statistics, data on file |
| Patients hospitalized in ICU for index infection (%) | 0.077 | 0 | 0.08 | β (n = 2341; N = 30 265) | Hospital Episode Statistics, data on file; Scottish clinical expert panel |
| Patients hospitalized in ICU for a recurrence (%) | 0.077 | 0 | 0.08 | β (n = 2341; N = 30 265) | Hospital Episode Statistics, data on file; Scottish clinical expert panel |
| Patients hospitalized in isolated infectious diseases ward (%) | 0 | 0 | 0.2 | none | Scottish clinical expert panel |
| Patients hospitalized in isolated infectious diseases ward for a recurrence (%) | 0 | 0 | 0.2 | none | Scottish clinical expert panel |
| Probability that a non-severe recurrence within 30 days of the previous episode is treated in hospital | 0.67 | 0.50 | 1 | β (n = 20; N = 30) | Hospital Episode Statistics, data on file |
| Probability that a severe recurrence after 30 days of the previous episode is treated in hospital | 1 | 0.12 | 1 | β (n = 41; N = 336) | Scottish clinical expert panel |
n, number of patients; N, total number of patients.
Patients with severe CDI (index episode or recurrence) were assumed to be treated in hospital. For inpatients, the duration of hospitalization depends on the underlying disease. Therefore, only excess hospitalization due to CDI was considered in the model. Hospital Episode Statistics data (for England and two Scottish health boards) show a mean excess length of stay (LOS) of 19.3 days for an index episode of CDI and 12.2 days for a recurrence when compared with healthcare resource group-matched patients (Hospital Episode Statistics for C. difficile spells in 2010–11, data on file). Limited information is available on the ward where patients are hospitalized; however, according to Scottish clinical expert opinion, ∼70% of patients are in an isolated/side room in a general ward, 30% in an infectious diseases unit or similar isolation room and only a few patients require intensive care unit (ICU) admission. For the base-case scenario, all hospitalized patients were assumed to be in an isolated room in a general ward and the proportion of patients hospitalized in an infection isolation room was varied in the one-way sensitivity analysis (range: 0%–20%). It was assumed that 7.7% of patients stayed in an ICU/high-dependency unit for 20.4% of their excess LOS due to CDI (Hospital Episode Statistics for C. difficile spells in 2010–11, data on file). The excess stay in the ICU for these patients was estimated to be 2.2 days.29
Patients with non-severe recurrences were assumed to be treated in the community unless the recurrence occurred in hospital, in which case the patient was assumed to remain in hospital with a prolonged stay. It was estimated that two-thirds of patients with non-severe recurrences experienced their recurrence in hospital. Non-severe recurrences treated in the community were assumed to require one general practitioner (GP) visit at home and one GP visit at a primary care centre.
Costs
Direct costs only were considered in the model, which included drug acquisition and medical costs (i.e. hospitalization, complications and GP visits). All cost inputs were extracted from public data or published literature and presented in 2010/11 British pounds sterling (GBP, £). A summary of the costs used in the model is provided in Table 4.30–34
Table 4.
Model inputs: costs
| Variable description | Base-case input (£) | Range for sensitivity analysis |
Source(s) | |
|---|---|---|---|---|
| minimum | maximum | |||
| Severe CDI complication | 9915 | 0 | 16 170 | 30 |
| GP visit, clinic | 53 | not varied | 31 | |
| GP visit, home | 120 | not varied | 31 | |
| General ward (per day) | 430.87 | not varied | 32 | |
| Infectious ward (per day) | 606 | not varied | Scottish clinical expert panel | |
| Intensive care (per day) | 2044 | not varied | 32 | |
| Medication costs | ||||
| fidaxomicin (200 mg bid, 10 days) | 1350 | not varied | 33 | |
| metronidazole (450 mg tid, 10 days) | 2.17 | not varied | 34 | |
| vancomycin (125 mg qid, 10 days)a | 189 | not varied | 34 | |
| vancomycin (250 mg qid, 10 days)a | 378 | not varied | 34 | |
| vancomycin (500 mg qid, 10 days)a | 757 | not varied | 34 | |
| vancomycin taper regimenb | 407 | not varied | 34 | |
| last-resort therapyc | 397 | 397 | 11 101 | 34 |
bid, twice daily; od, once daily; qid, four times daily; tid, three times daily.
aVancocin® 125 mg oral capsules.
bOral vancomycin at 125 mg qid for 14 days, then 125 mg bid for 7 days, then 125 mg od for 7 days and then 125 mg every 3 days for 28 days.
cSingle infusion of 250 mg/kg intravenous immunoglobulin or a 14 day course of 1200 mg/day oral rifampicin.
Unit costs for drugs were obtained from the British National Formulary.34
The cost of a room in a general ward was £430.87 per day.32 The cost of a visit to a GP centre was £53; this was the cost per clinic consultation lasting 17.2 min.31 The cost of a home visit was £120; this was the cost of a visit lasting 23.4 min including travel time.31
The cost of a severe CDI complication was assumed to be the average of the costs of the four complications considered in the model (i.e. toxic megacolon, colonic perforation, sepsis or colectomy) using non-elective inpatient Scottish tariffs.30 For the base case, the final cost was £9915.
Utilities
Utility estimates for CDI were based on the data from Slobogean et al.,35 which were 0.30 for the initial 7 days of CDI-attributable hospitalization, 0.34 for the following 23 days and 0.78 for the remaining model time frame following cure (i.e. remainder of year). In the current model, the duration of impact of CDI on QoL was assumed to last 10 days, i.e. duration of treatment. In agreement with Scottish clinical expert opinion, it was assumed that a patient's QoL started to improve as soon as their diarrhoea resolved. Thus, in the base case, the following utilities were applied: 0.30 for the first 3 days of diarrhoea; 0.34 for the remaining 7 days of treatment; and 0.78 for cured patients. For a severe recurrence, it was assumed that the recovery would not be as rapid as in non-severe CDI, but that this would take another 10 day cycle with a mid-point utility of 0.56 before reaching the utility of a cured patient. The utility used for CDI-related complications was 0.30. A summary of utility estimates used in the model and the range of values applied in the one-way sensitivity analysis is provided in Table 5.
Table 5.
Model inputs: utilities
| Variable description | Base-case input | Range for sensitivity analysis |
Uncertainty distribution | Source(s) | |
|---|---|---|---|---|---|
| minimum | maximum | ||||
| CDI (initial 3 days of treatment) | 0.30 | 0.10 | 0.50 | normal (SD = 0.1) | 35 |
| CDI (days 7–10 of treatment) | 0.34 | 0.14 | 0.54 | normal (SD = 0.1) | 35 |
| Cured | 0.78 | 0.58 | 0.98 | normal (SD = 0.1) | 35 |
| Decrement for patients experiencing a serious complication | 0 | 0 | 0.10 | none | assumption |
Model outputs
Model outputs included cost and effectiveness outcomes for each treatment, incremental cost and effectiveness and incremental cost-effectiveness ratios (ICERs) for fidaxomicin versus vancomycin. ICERs were expressed as cost per quality-adjusted life year (QALY) and the cost per recurrence avoided. QALYs were estimated as the sum of the weighted time spent in each cycle for the 1 year model time horizon. Willingness-to-pay thresholds of £20 000/QALY and £30 000/QALY generally applied by the UK National Institute for Health and Care Excellence36 were used to interpret ICERs.
Sensitivity analyses
Extensive one-way sensitivity analyses were performed to test the robustness of the model results using realistic ranges for each parameter derived from published sources and Scottish clinical expert opinion (Tables 2–5). For parameters with a higher impact on the ICER, we performed a threshold analysis to identify the values that would yield ICERs below the £20 000/QALY and £30 000/QALY thresholds.
A probabilistic sensitivity analysis was also performed with 10 000 Monte Carlo simulations. Input variables with uncertainties were allowed to vary simultaneously according to pre-defined distributions (Tables 2–4). The probability of fidaxomicin being cost-effective at the £30 000/QALY willingness-to-pay threshold was estimated.
Results
Base-case scenario
A summary of the total treatment costs and the results of the base-case scenario over a 1 year time horizon are presented in Table 6. According to the model, the rate of clinical cure at 1 year was slightly higher with fidaxomicin than vancomycin in patients with severe CDI (88.9% versus 86.7%, respectively) and in patients with a first recurrence (88.2% versus 84.8%, respectively). Fidaxomicin was also associated with fewer recurrences than vancomycin in patients with severe CDI (1.369 versus 1.797 per patient, respectively) and in patients with a first recurrence (1.593 versus 2.347 per patient, respectively) over the 1 year horizon.
Table 6.
Base-case results over a 1 year time horizon
| Severe CDI |
First CDI recurrence |
|||
|---|---|---|---|---|
| vancomycin | fidaxomicin | vancomycin | fidaxomicin | |
| Average costs per patient at 1 year, £ | ||||
| medication acquisition | 571 | 2567 | 800 | 3630 |
| hospitalization | 13 600 | 11 793 | 15 928 | 12 742 |
| complications | 11 | 11 | 13 | 10 |
| GP visits | 163 | 145 | 186 | 154 |
| total | 14 344 | 14 515 | 16 926 | 16 535 |
| difference | 171 | −391 | ||
| Clinical outcomes | ||||
| cure, % | 86.7 | 88.9 | 84.8 | 88.2 |
| deaths, % | 7.6 | 6.7 | 8.8 | 7.1 |
| QALYs | ||||
| mean QALYs | 0.705 | 0.715 | 0.692 | 0.711 |
| difference | 0.010 | 0.019 | ||
| Recurrences | ||||
| recurrences, n | 1.797 | 1.369 | 2.347 | 1.593 |
| recurrences avoided, n | 0.428 | 0.754 | ||
| Cost-effectiveness outcomes | ||||
| ICER, £ per QALY | 16 529 | −21 079 | ||
| cost per recurrence avoided, £ | 400 | −518 | ||
The acquisition costs of fidaxomicin were ∼4.5-fold higher than those of vancomycin, but were offset in both patient subgroups by the higher hospitalization costs of vancomycin. Overall, total costs were similar with both treatments in patients with severe CDI (fidaxomicin, £14 515; vancomycin, £14 344) and in patients with a first recurrence (fidaxomicin, £16 535; vancomycin, £16 926).
Compared with vancomycin, fidaxomicin was associated with a slightly higher mean number of QALYs over the 1 year time horizon in patients with severe CDI (0.715 versus 0.705) and patients with a first recurrence (0.711 versus 0.692). Therefore, fidaxomicin was cost-effective in severe CDI with an ICER of £16 529/QALY and dominant versus vancomycin in patients with a recurrence (ICER −£21 079/QALY, i.e. more effective and less costly).
First-line administration of fidaxomicin was associated with a reduction in the number of recurrences over 1 year in patients with severe CDI (difference: −0.428) and in patients with a recurrence (difference: −0.754) compared with vancomycin. The average cost per recurrence avoided with fidaxomicin versus vancomycin was £400 for patients with severe CDI and −£518 for patients with a first recurrence.
One-way sensitivity analyses
The parameters to which the model was most sensitive were the following: OR of experiencing a recurrence with fidaxomicin in patients who had already experienced one, two or more recurrences; OR of experiencing a recurrence with fidaxomicin in patients with severe CDI; OR of having recurrent CDI; and the probability of a recurrence being treated in hospital.
The results of the threshold analysis performed to identify the values that would yield an ICER below the £20 000/QALY and £30 000/QALY thresholds are presented in Table 7. The probability of having an ICER below £20 000/QALY when varying any of the aforementioned parameters ranged between 52% and 69% in severe CDI and between 65% and 74% in patients with a first recurrence.
Table 7.
Threshold analysis for key drivers in the model
| Parameter | Base-case value | Severe CDI |
First CDI recurrence |
||
|---|---|---|---|---|---|
| ICER <£20 000/QALY | ICER <£30 000/QALY | ICER <£20 000/QALY | ICER <£30 000/QALY | ||
| OR of experiencing a recurrence with fidaxomicin in patients who have already a first or second recurrence | 0.528 | 0.540 | 0.573 | 0.606 | 0.624 |
| OR of experiencing a recurrence with fidaxomicin in patients with ≥2 recurrences | 0.528 | 0.540 | 0.576 | 0.642 | 0.665 |
| OR of having recurrent CDI | 3.87 | 3.760 | 3.450 | 2.78 | 2.59 |
| OR of experiencing a recurrence with fidaxomicin in patients with severe CDI | 0.456 | 0.474 | 0.525 | — | — |
| Probability of a recurrence being treated in hospital | 0.667 | 0.651 | 0.604 | —a | —a |
aICER remained below threshold even at the lowest range tested.
The impact of the remaining parameters on the model was small (Table S2, available as Supplementary data at JAC Online).
Probabilistic sensitivity analysis
In patients with severe CDI, the mean incremental cost for fidaxomicin versus vancomycin was −£82 [standard deviation (SD): £1325] and the incremental QALYs were 0.016 (SD: 0.008), yielding a mean ICER of −£5066/QALY. In patients with first recurrence, the mean incremental cost for fidaxomicin versus vancomycin was −£1076 (SD: £2800) and incremental QALYs were 0.019 (SD: 0.014), yielding a mean ICER of −£57 354/QALY. The effect of this uncertainty on the ICERs for fidaxomicin compared with vancomycin in both patient subgroups is shown in Figure 2. The probability that fidaxomicin was cost-effective at a willingness-to-pay threshold of £30 000/QALY was 60% for severe CDI and 68% for patients with a first recurrence. The cost-effectiveness acceptability curves for both patient subgroups are shown in Figure 3.
Figure 2.
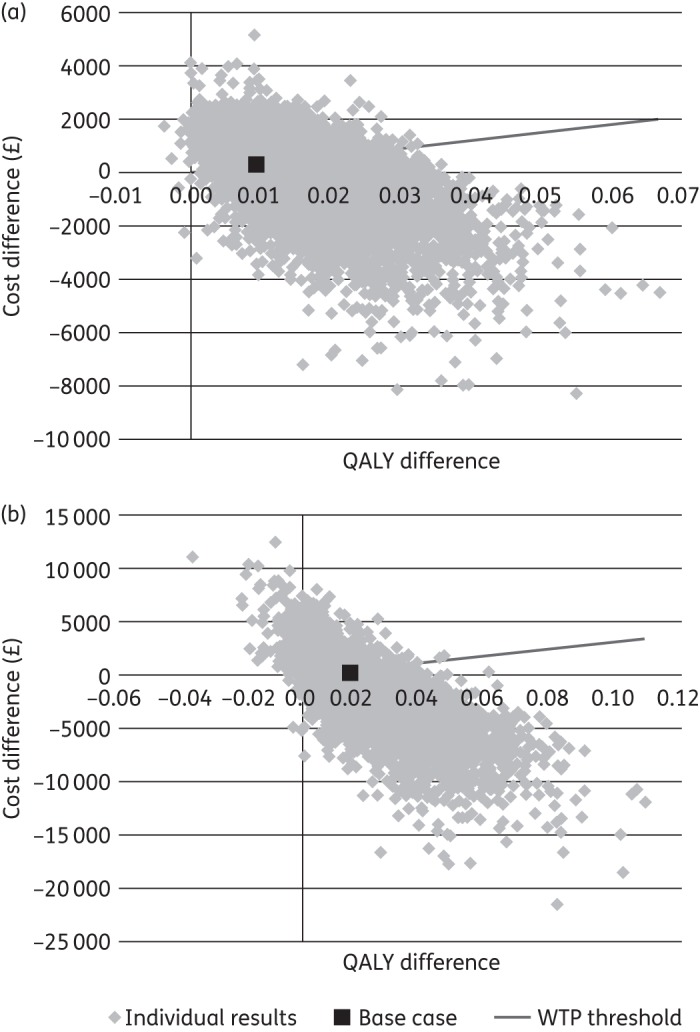
Probabilistic sensitivity analysis: ICER scatter plots for patients with (a) severe CDI and (b) first CDI recurrence. WTP, willingness to pay.
Figure 3.
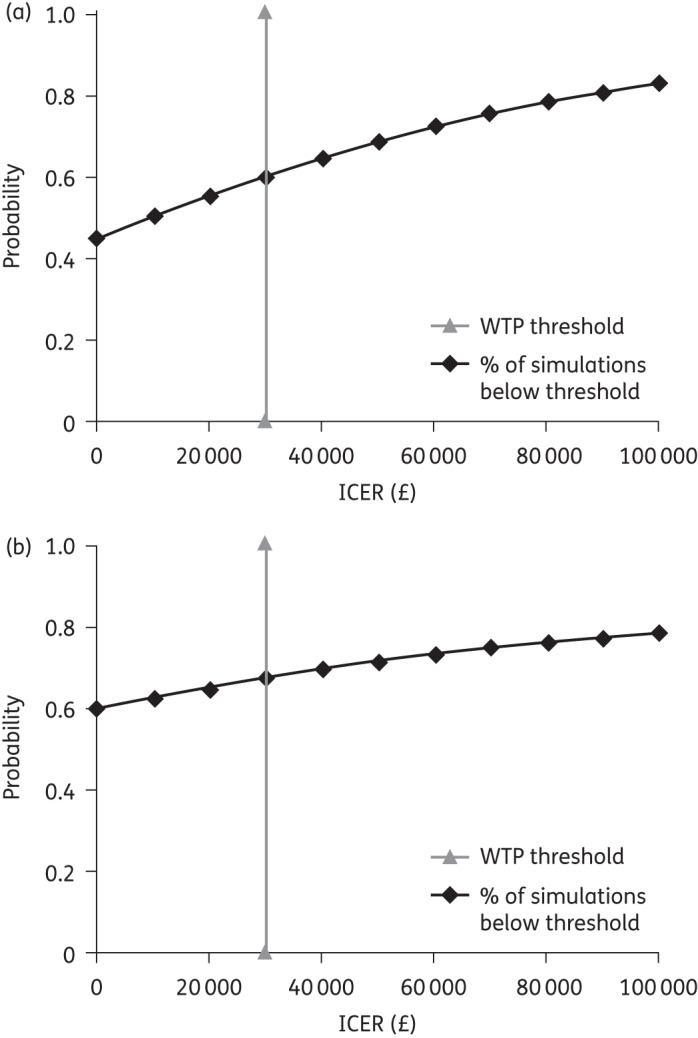
Cost-effectiveness acceptability curves for patients with (a) severe CDI and (b) first CDI recurrence. WTP, willingness to pay.
Discussion
We developed a decision-analytical model to assess the cost-effectiveness of fidaxomicin compared with vancomycin in the treatment of patients with severe CDI or a first recurrent episode from the perspective of Scottish public healthcare providers. The fidaxomicin Phase III trials indicate that 24%–39% of patients with CDI have severe episodes and 15%–17% of patients have first recurrences.10,11 Over a 1 year time horizon, the model showed that patients with severe CDI had 1.8 and 1.4 recurrences if treated with vancomycin and fidaxomicin, respectively, and that following a first CDI recurrence, patients had 2.4 and 1.6 additional recurrences with vancomycin and fidaxomicin, respectively. The model did not differentiate between relapses and reinfection and, 30 days after EOT, the probability of reinfection was assumed to be equal in both arms. This assumption was made based on the best evidence available at the time of model development, although recent clinical data suggest that this underestimates the reduction in recurrences with fidaxomicin.28 However, even with this conservative assumption, fidaxomicin is still a cost-effective treatment option for the patient subgroups analysed. The opportunity to develop the model with more accurate long-term relapse and reinfection data will be possible when long-term effectiveness data for fidaxomicin are available. The resulting downstream savings in direct medical costs meant that the acquisition cost of fidaxomicin was almost completely offset by lower hospitalization costs. The base-case analysis showed that fidaxomicin was cost-effective in patients with severe CDI (ICER: £16 529/QALY) and dominant versus vancomycin in patients with a first recurrence (−£21 079/QALY, i.e. more effective and less costly). The average cost per recurrence avoided was £400 for fidaxomicin versus vancomycin in patients with severe CDI and was cost saving (by £518) in patients with a first recurrence. These are important cost-effectiveness data for fidaxomicin in the treatment of CDI from a UK and NHS perspective. Our findings complement and support recent cost-effectiveness data from the USA12 and existing efficacy data10,11 and show that, when costs are applied to the efficacy outcomes of fidaxomicin in terms of clinical cure and recurrence rates, this agent is cost-effective versus standard therapy.
Extensive one-way and probabilistic sensitivity analyses were conducted to evaluate the input parameters and assumptions used in the model. The one-way sensitivity analysis showed that the main drivers of cost-effectiveness were inputs relating to the risk of recurrence with fidaxomicin. The larger the effect of fidaxomicin in reducing the recurrence rate, the more cost-effective it was. However, the ICERs for fidaxomicin consistently remained below the £20 000/QALY or £30 000/QALY thresholds if the various ORs for recurrence were varied over plausible ranges. These findings support the robustness of the model findings.
Our model is unique from a European healthcare perspective. By using published data and advice from Scottish clinical experts, we were able to develop a seven-state model that simulated the natural evolution of CDI. Since patients with severe CDI and most CDI recurrences are treated with oral vancomycin in clinical practice, vancomycin was selected as the comparator for the model. Metronidazole was also included for non-severe recurrent episodes in line with current Scottish guidelines,16,17 although the dose applied (450 mg three times daily) was the mean of Scottish recommendations16,17 and slightly lower than the European dose (500 mg three times daily).1 A conservative approach was taken in the model when there was uncertainty. For example, costs for disinfection and ward closures, which would have favoured fidaxomicin due to its ability to decrease the recurrence rate, were omitted. A conservative approach was also taken regarding the hospitalization of patients with CDI. In the model, only severe episodes and non-severe CDI occurring in hospitalized patients had costs for excess hospitalization. All other episodes, such as non-severe recurrences, were treated in the community. The proportion of patients requiring hospitalization in our model was driven by the proportion of episodes expected to be severe, which was estimated to be 12.2% for the base case;20 this is lower than in a recent UK study, which reported that severe disease occurred in 20% of hospitalized cases.37 We also assumed that all patients treated in hospital were in a general ward. Local clinical experts suggested that ∼30% of inpatients are treated in an infectious diseases isolation unit. In the sensitivity analysis, increasing the proportion of patients treated in such rooms decreased the ICER in favour of fidaxomicin. The model did not take into account the potential important role of transmission of infection, although recent publications38,39 suggest that onward transmission within hospitals accounts for a relatively small number of CDI cases detected. Furthermore, inclusion of these data, and estimating their impact on cost, would require a different modelling approach.
The key effectiveness inputs for the model were taken from a pooled analysis of two large randomized Phase III studies comparing fidaxomicin with vancomycin in CDI;14 however, there were some differences between the structure of the model and the design of the clinical trials. For example, in the clinical trials, patients were followed for up to 40 days if they had a successful response to initial treatment and no recurrence or until they had a recurrence. If the patient failed to respond to study treatment, data collection was stopped after 10 days of treatment. However, in clinical practice, patients who fail initial treatment or who have a recurrence continue to be treated until a satisfactory outcome is achieved. Therefore, it is not possible to ‘read across’ from the clinical trial data to the results of our model, because the cure rate in the model is based on up to four lines of treatment rather than one, as in the clinical trial. The mortality rates used in the model also differed slightly from those reported in the clinical trials. We used the 30 day CDI-attributable mortality rate (5.99%) reported by Karas et al.,24 which is a mean value from 12 studies. These changes led to a higher clinical cure rate in both arms of the economic model compared with the clinical trial data. Additionally, the model predicted a numerical advantage in the clinical cure rate for fidaxomicin over vancomycin both in severe CDI and in patients with a first recurrence. Minor differences in the recurrence rates were also noted, mainly because of differences in the mortality rates. However, the main differences between the fidaxomicin clinical trials and the model affected clinical cure rates. As clinical cure was not a major driver of the economic results, as shown by the sensitivity analysis, we believe that the implications of these differences are minimal.
Stranges et al.12 recently published a cost-effectiveness analysis of fidaxomicin [marketed in the USA as Dificid™ (Cubist)] versus vancomycin for the treatment of CDI from a US payer perspective. As in our analysis, the ICER (US$67 576/QALY) was below the accepted US willingness-to-pay threshold (US$100 000/QALY). However, there are several key differences between the two analyses. The base case of the US model considered all patients with CDI, rather than specified subgroups as in our model. The effectiveness inputs in the US model were based only on the North American Phase III study,11 whereas the present paper used a pooled analysis of both the North American and European Phase III trials.14 The US model included up to three CDI episodes and then assumed an average life expectancy of 23 years for surviving patients, whereas the present model had a 1 year time horizon, which allowed more CDI episodes to occur. These design differences should be considered when comparing these findings.
When comparing the relative value of a novel treatment with other common and effective healthcare preventative interventions, the concept of ‘number needed to treat’ (NNT) is useful. A recent analysis calculated that for every seven patients treated with fidaxomicin, one hospital readmission for CDI is prevented compared with vancomycin (i.e. NNT 7.1),40 which compares very favourably to other common preventative healthcare interventions. For example, the use of aspirin following a myocardial infarction has an NNT of ∼40 to prevent one death within 5 weeks.41 As such, judicious use of fidaxomicin in a specific population at risk of recurrence is cost-effective and compares favourably with many treatments currently regarded as standards of care.
Our model was designed to reflect the clinical situation in Scotland using relevant treatment guidelines and information sources where available. The findings reported in this paper are, therefore, directly relevant to the healthcare environment in Scotland alone. However, as the Scottish treatment guidelines for CDI16,17 are consistent with European guidelines (2009 version),42 it is anticipated that the model structure will be broadly applicable to other European countries, although adjustment of relevant costs and resource use data will be required for each country.
To put the value of this analysis in a broader context, we need to consider the emphasis currently placed within European healthcare systems to reduce hospital admissions, readmissions and overall LOS.43 Indeed, the cost to England of readmissions has been estimated to be £2.3 billion and a range of measures are being implemented to reduce these.44 For adults hospitalized with CDI, 41% are readmitted within 90 days and 13% of these patients develop recurrent CDI during readmission;45 therefore, treatments that reduce recurrences, and thereby readmissions, are welcomed.
In conclusion, this economic model suggests that fidaxomicin is cost-effective in the treatment of patients with severe CDI and in patients with a first recurrence of CDI when compared with vancomycin. First-line administration of fidaxomicin in these two patient subgroups may reduce the fiscal impact of CDI. Our findings are likely to be of broader applicability to European healthcare systems.
Funding
This work was supported by Astellas Pharma Europe Ltd. Writing and editorial assistance in the preparation of this manuscript, which was provided by Bioscript Medical, was funded by Astellas. The work of Quintiles Consulting was also funded by Astellas Pharma Europe Ltd.
Transparency declarations
D. N. has received lecture or advisory board honoraria or research grants from Astellas, AstraZeneca, Bayer, Basilea, Cubist, Durata and Pfizer. He has received no remuneration for this manuscript. The views expressed and findings presented by D. N. are personal and not the views of the Scottish Antimicrobial Prescribing Group that he chairs. O. A. C. is supported by the German Federal Ministry of Research and Education (BMBF grant 01KN1106) and has received research grants from, is an advisor to or has received lecture honoraria from 3M, Actelion, Astellas, Basilea, Bayer, Celgene, Cubist, F2G, Genzyme, Gilead, GSK, Merck/Schering, Miltenyi, Optimer, Pfizer, Quintiles Consulting, Sanofi Pasteur, Summit and Viropharma. A. K. v. E. is an employee of Quintiles Consulting. O. O.-S. and I. A. O. O. are employees of Astellas Pharma Europe Ltd, and do not own stocks or options in Astellas Pharma Ltd. P. R. was employed by Astellas Pharma Europe Ltd at the time of the analysis, and is now employed by Merck.
The development of the manuscript, editing and submission assistance for this manuscript was provided by Harriet Lamb of Bioscript Medical.
Supplementary data
References
- 1.Debast SB, Bauer MP, Kuijper EJ on behalf of the Committee. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014;20(Suppl S2):1–26. doi: 10.1111/1469-0691.12418. [DOI] [PubMed] [Google Scholar]
- 2.Bobo LD, Dubberke ER, Kollef M. Clostridium difficile in the ICU: the struggle continues. Chest. 2011;140:1643–53. doi: 10.1378/chest.11-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones AM, Kuijper EJ, Wilcox MH. Clostridium difficile: a European perspective. J Infect. 2013;66:115–28. doi: 10.1016/j.jinf.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 4.Wiegand PN, Nathwani D, Wilcox MH, et al. Clinical and economic burden of Clostridium difficile infection in Europe: a systematic review of healthcare-facility-acquired infection. J Hosp Infect. 2012;81:1–14. doi: 10.1016/j.jhin.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Al-Eidan FA, McElnay JC, Scott MG, et al. Clostridium difficile-associated diarrhoea in hospitalised patients. J Clin Pharm Ther. 2000;25:101–9. doi: 10.1046/j.1365-2710.2000.00266.x. [DOI] [PubMed] [Google Scholar]
- 6.Sears P, Crook DW, Louie TJ, et al. Fidaxomicin attains high fecal concentrations with minimal plasma concentrations following oral administration in patients with Clostridium difficile infection. Clin Infect Dis. 2012;55(Suppl 2):S116–20. doi: 10.1093/cid/cis337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Babakhani F, Gomez A, Robert N, et al. Killing kinetics of fidaxomicin and its major metabolite, OP-1118, against Clostridium difficile. J Med Microbiol. 2011;60:1213–7. doi: 10.1099/jmm.0.029470-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Babakhani F, Bouillaut L, Gomez A, et al. Fidaxomicin inhibits spore production in Clostridium difficile. Clin Infect Dis. 2012;55(Suppl 2):S162–9. doi: 10.1093/cid/cis453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tannock GW, Munro K, Taylor C, et al. A new macrocyclic antibiotic, fidaxomicin (OPT-80), causes less alteration to the bowel microbiota of Clostridium difficile-infected patients than does vancomycin. Microbiology. 2010;156:3354–9. doi: 10.1099/mic.0.042010-0. [DOI] [PubMed] [Google Scholar]
- 10.Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12:281–9. doi: 10.1016/S1473-3099(11)70374-7. [DOI] [PubMed] [Google Scholar]
- 11.Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422–31. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- 12.Stranges PM, Hutton DW, Collins CD. Cost-effectiveness analysis evaluating fidaxomicin versus oral vancomycin for the treatment of Clostridium difficile infection in the United States. Value Health. 2013;16:297–304. doi: 10.1016/j.jval.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Bartsch SM, Umscheid CA, Fishman N, et al. Is fidaxomicin worth the cost? An economic analysis. Clin Infect Dis. 2013;57:555–61. doi: 10.1093/cid/cit346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornely OA, Nathwani D, Ivanescu C, et al. Clinical efficacy of fidaxomicin compared with vancomycin and metronidazole in Clostridium difficile infections: a meta-analysis and indirect treatment comparison. J Antimicrob Chemother. 2014;69:2892–900. doi: 10.1093/jac/dku261. [DOI] [PubMed] [Google Scholar]
- 15.Fekety R, McFarland LV, Surawicz CM, et al. Recurrent Clostridium difficile diarrhea: characteristics of and risk factors for patients enrolled in a prospective, randomized, double-blinded trial. Clin Infect Dis. 1997;24:324–33. doi: 10.1093/clinids/24.3.324. [DOI] [PubMed] [Google Scholar]
- 16.Health Protection Network. Guidance on Prevention and Control of Clostridium difficile Infection (CDI) in Healthcare Settings in Scotland. Health Protection Network Scottish Guidance 6. UK: Health Protection Scotland; 2009. http://www.his.org.uk/files/4213/7483/8488/Guidance_on_Prevention_and_Control_of_Clostridium_difficile_Infection_CDI_in_Healthcare_Settings_in_Scotland_Health_Protection_Network_September_2009.pdf. (31 July 2011, date last accessed) [Google Scholar]
- 17.Scottish Antimicrobial Prescribing Group. 2008. Guidance to Optimise Antimicrobial Use and Reduce Clostridium difficile Associated Disease in Scottish Hospitals Position Paper. http://www.scottishmedicines.org.uk/files/sapg/Guidance_to_Optimise_Antimicrobial_use_and_Reduce_Clostridium_difficile_Associated_disese_in_Scottish_Hospitals.pdf. (30 April 2012, date last accessed)
- 18.Kyne L, Kelly CP. Recurrent Clostridium difficile diarrhoea. Gut. 2001;49:152–3. doi: 10.1136/gut.49.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–15. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 20.Henrich TJ, Krakower D, Bitton A, et al. Clinical risk factors for severe Clostridium difficile-associated disease. Emerg Infect Dis. 2009;15:415–22. doi: 10.3201/eid1503.080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–55. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 22.Cheng A, Ferguson J, Richards M, et al. Australasian Society for Infectious Diseases guidelines for the diagnosis and treatment of Clostridium difficile infection. Med J Aust. 2011;194:353–8. doi: 10.5694/j.1326-5377.2011.tb03006.x. [DOI] [PubMed] [Google Scholar]
- 23.Barbut F, Richard A, Hamadi K, et al. Epidemiology of recurrences or reinfections of Clostridium difficile-associated diarrhea. J Clin Microbiol. 2000;38:2386–8. doi: 10.1093/gao/9781884446054.article.t031141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karas JA, Enoch DA, Alay SH. A review of mortality due to Clostridium difficile infection. J Infect. 2010;61:1–8. doi: 10.1016/j.jinf.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 25.Zar FA, Bakkanagari SR, Moorthi KM, et al. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302–7. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- 26.Perras C, Tsakonas E, Ndegwa S, et al. Vancomycin or Metronidazole for Treatment of Clostridium difficile Infection: Clinical and Economic Analyses. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2011. (Technology report no. 136). http://www.cadth.ca/preview.php/en/hta/reports-publications/search/publication/2775. (30 April 2012, date last accessed) [PubMed] [Google Scholar]
- 27.General Register Office for Scotland. Scottish Decennial Life Tables 2000–02. http://www.gro-scotland.gov.uk/files2/stats/life-expectancy-at-scotland-level/table4-scot-dlt-latest00-02.pdf. (30 April 2012, date last accessed)
- 28.Eyre DW, Babakhani F, Griffiths D, et al. Whole-genome sequencing demonstrates that fidaxomicin is superior to vancomycin for preventing reinfection and relapse of infection with Clostridium difficile. J Infect Dis. 2014;209:1446–51. doi: 10.1093/infdis/jit598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kenneally C, Rosini JM, Skrupky LP, et al. Analysis of 30-day mortality for Clostridium difficile-associated disease in the ICU setting. Chest. 2007;132:418–24. doi: 10.1378/chest.07-0202. [DOI] [PubMed] [Google Scholar]
- 30.ISD Scotland. 2011/12 Scottish Tariffs for Cross Boundary Flow Costing. https://isdscotland.scot.nhs.uk/Health-Topics/Finance/Publications/2011-10-25/1112ScotTariffs.xls?78246552. (30 April 2012, date last accessed)
- 31.Personal Social Services Research Unit. Curtis L. Unit Costs of Health and Social Care 2010. http://www.pssru.ac.uk/project-pages/unit-costs/ (31 December 2011, date last accessed)
- 32.Information Services Division Scotland. R040: Specialty Group Costs—Inpatients in All Specialties (Excluding Long Stay) http://www.isdscotland.org/Health-Topics/Finance/Costs/File-Listings-2011.asp. (30 April 2012, date last accessed)
- 33.National Institute for Health and Care Excellence. Evidence Summary: New Medicine: Clostridium difficile Infection: Fidaxomicin. 13 July 2012. http://publications.nice.org.uk/clostridium-difficile-infection-fidaxomicin-esnm1/overview. (28 May 2014, date last accessed)
- 34.Joint Formulary Committee. British National Formulary. 61st edn. London: BMJ Group and Pharmaceutical Press; 2011. [Google Scholar]
- 35.Slobogean GP, O'Brien PJ, Brauer CA. Single-dose versus multiple-dose antibiotic prophylaxis for the surgical treatment of closed fractures. Acta Orthop. 2010;81:256–62. doi: 10.3109/17453671003587119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Devlin N, Parkin D. Does NICE have a cost-effectiveness threshold and what other factors influence its decisions? A binary choice analysis. Health Econ. 2004;13:437–52. doi: 10.1002/hec.864. [DOI] [PubMed] [Google Scholar]
- 37.Walker AS, Eyre DW, Wyllie DH, et al. Characterisation of Clostridium difficile hospital ward-based transmission using extensive epidemiological data and molecular typing. PLoS Med. 2012;9:e1001172. doi: 10.1371/journal.pmed.1001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harbarth S, Samore MH. Clostridium: transmission difficile? PLoS Med. 2012;9:e1001171. doi: 10.1371/journal.pmed.1001171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morgan OW, Rodrigues B, Elston T, et al. Clinical severity of Clostridium difficile PCR ribotype 027: a case–case study. PLoS One. 2008;3:e1812. doi: 10.1371/journal.pone.0001812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sclar DA, Robison LM, Oganov AM, et al. Fidaxomicin for Clostridium difficile-associated diarrhoea: epidemiological method for estimation of warranted price. Clin Drug Investig. 2012;32:e17–24. doi: 10.1007/BF03261906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farkouh ME, Lang JD, Sackett DL. Thrombolytic agents: the science of the art of choosing the better treatment. Ann Intern Med. 1994;120:886–8. doi: 10.7326/0003-4819-120-10-199405150-00011. [DOI] [PubMed] [Google Scholar]
- 42.Bauer MP, Kuijper EJ, van Dissel JT. European Society of Clinical Microbiology and Infectious Diseases (ESCMID): treatment guidance document for Clostridium difficile infection (CDI) Clin Microbiol Infect. 2009;15:1067–79. doi: 10.1111/j.1469-0691.2009.03099.x. [DOI] [PubMed] [Google Scholar]
- 43.European Hospital and Healthcare Federation. Hospitals in Europe Healthcare Data 2011. http://www.hope.be/03activities/quality_eu-hospitals/eu_country_profiles/00-hospitals_in_europe-synthesis_vs2011-06.pdf. (5 March 2013, date last accessed)
- 44.Northamptonshire Observatory. 2011. Joint Strategic Needs Assessment 2011 and Beyond. Chapter 2, Hospital Activity. http://www.northamptonshireobservatory.org.uk/projects/projectdetail.asp?projectid=145. (5 March 2013, date last accessed)
- 45.Wayne E, Grein J, Murthy R. Evaluation of hospital readmissions following Clostridium difficile infection (CDI) and patient characteristics associated with CDI recurrence during hospital readmission; Abstracts of IDWeek, San Francisco, CA, 2013. Abstract 1400. https://idsa.confex.com/idsa/2013/webprogram/Paper41261.html. (29 April 2014, date last accessed) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



