Abstract
Previous studies have shown that plastic compression (PC) of collagen gels allows a rapid and controlled fabrication of matrix- and cell-rich constructs in vitro that closely mimic the structure and characteristics of tissues in vivo. Microvascular endothelial cells, the major cell type making up the blood vessels in the body, were added to the PC collagen to determine whether cells attach, survive, grow, and express endothelial cell characteristics when seeded alone or in coculture with other cells. Endothelial cells seeded on the PC collagen containing human foreskin fibroblasts (HFF) or human osteoblasts (HOS) formed vessel-like structures over 3 weeks in culture without the addition of exogenous growth factors in the medium. In contrast, on the PC scaffolds without HFF or HOS, human dermal microvascular endothelial cells (HDMEC) exhibited a typical cobblestone morphology for 21 days under the same conditions. We propose that the coculture of primary endothelial cells with PC collagen constructs, containing a stromal cell population, is a valuable technique for in vitro modeling of proangiogenic responses toward such biomimetic constructs in vivo. A major observation in the cocultures was the absence of gel contraction, even after 3 weeks of fibroblast culture. This collagen form could, for example, be of great value in tissue engineering of the skin, as contractures are both aesthetically and functionally disabling.
Introduction
Tissue engineering strives to replace a diseased or damaged organ or tissue, restoring function, or to produce three-dimensional (3D) tissue models for reproducible screening of therapeutic agents. One of the challenges in scaffold-based TE is the choice of a biomaterial. Although great progress has been made in the field of synthetic materials, there are still concerns regarding their biological function, such as cell survival, attachment, and the extent to which responses reflect those seen in vivo. Naturally occurring polymers offer an attractive alternative in that, their recognition by cell receptors and susceptibility to enzyme remodeling makes them a favorable cell environment to promote tissue function. Collagen type I, a major structural protein in the mammalian body, is probably the first biomaterial proposed for tissue engineering applications. In 1962, Grillo and Gross described fabrication of reconstituted fibrillar collagen type I scaffold by drying collagen gels and reported results of implantation of such scaffolds in vivo.1 In 1979, Bell et al., described the fabrication of a collagen-based skin equivalent, where fibroblasts within the collagen gel were cultured over 10 days in vitro.2 Since then, collagen-based biomaterials have been used extensively for biomedical testing and clinical applications.3
The use of collagen-based hydrogels for tissue engineering applications is limited. Such gels are mechanically weak, difficult to handle, and degrade quickly when implanted in vivo.4,5 Although cell-mediated gel contraction improves the mechanical strength of such scaffolds, the process is lengthy, taking days to weeks, and depends strongly on the cell type and number and is therefore difficult to control.6 Moreover, contraction is an undesirable aesthetic problem in skin tissue engineering. Alternatively, mechanics of collagen scaffolds can be improved by chemical,7 photochemical,8 or enzymatic9 crosslinking, excessive heat10 or freeze drying.11,12 These strategies can potentially irreversibly change the collagen structure12 and require postassembly cell seeding, which is difficult to control.13
An alternative method for collagen scaffold preparation includes plastic compression (PC), reported by Brown et al.14 It allows fast (within 1 h) fabrication of tissue-like collagen scaffolds by expulsion of >95% of unbound water under weight and capillary actions. As a result, collagen density rapidly increases 40–100-fold, while viability of cells, seeded into the initial collagen gel, is generally not affected. Indeed, it has been shown that the viability and proliferation of fibroblasts are improved in the PC collagen constructs compared with hyperhydrated collagen gels.15,16 PC allows bottom-up engineering of native collagen biomaterial from nanometer to millimeter scale, closely mimicking the complex hierarchy of tissues in vivo.17 Since their first report, PC collagen scaffolds have been characterized extensively in terms of mechanical and structural properties18 and proposed for bone,19 skin,20 nerve,21 bladder,22 and corneal23 tissue engineering.
Microvascular endothelial cells (MEC) are a major endothelial cell type lining the luminal surface of the blood vessels and among the first cell types to come in contact with the implanted biomaterial. These cells participate in inflammatory and repair responses and are involved in the formation of new blood vessels.24 It has been shown that coculture of MEC with human osteoblasts and dermal fibroblasts leads to the formation of microcapillary-like structures by the MEC on tissue culture plastic and on a number of porous biomaterials.25–27 This effect is attributed both to the extracellular matrix produced by the osteoblasts and the molecular cross talk between the closely associated cells.25,26 However, the use of collagen type I for such cocultures has been mostly limited to hydrogels thus preventing its application for tissue engineering due to reasons outlined above. The aim of these studies was to evaluate whether endothelial cells could grow and survive in or on PC collagen, exhibit angiogenesis, and whether the inclusion of a second cell type (dermal fibroblasts and osteoblasts) in the PC collagen constructs could influence growth, survival, and angiogenic behavior of the MEC. We describe experiments here showing that only cellular, but not acellular PC collagen constructs influence the proangiogenic behavior of the endothelial cells. Ultimately, the goal was to determine how closely this in vitro 3D tissue model in PC collagen closely mimics the in vivo situation and whether it is a suitable test system for investigation of vascularization mechanisms in collagen tissues and for fine-tuning parameters (e.g., cell and collagen density) to model such biological responses.
Materials and Methods
Human dermal microvascular endothelial cells
Human dermal microvascular endothelial cells (HDMEC) were isolated from juvenile foreskin as previously described28 and were cultured in the Endothelial Basal Medium MV (PromoCell) supplemented with 15% fetal bovine serum (Invitrogen), 100 U/100 mg/mL penicillin/streptomycin (Invitrogen), sodium heparin (10 mg/mL), and basic fibroblast growth factor (2.5 ng/mL). Cells were used in passage 3. To assess the tube-forming ability of the HDMEC, cells were subjected to proangiogenic conditions.25 Cells were seeded on the gelatin-coated 24-well plate at a density of 5×104 cells/well and allowed to grow to confluency for 24–48 h. Confluent EC were then coated with 300 μL of neutralized collagen type I (First Link) and monitored using a phase-contrast microscope.
Human osteoblasts and fibroblasts
Primary human osteoblasts (HOS) were isolated as described previously.25 HOS were harvested exclusively from excess bone tissue obtained during the contouring procedures of iliac crest bone transplant operations for use in extensive reconstruction procedures of the facial skeleton. Bone cells were processed strictly anonymously, without recording patient-related data, in accordance with the local ethical regulations. Human dermal fibroblasts (HDF) were isolated from juvenile foreskin as a negative fraction of cells following extraction of endothelial cells. Both cell types were cultured in Dulbecco's modified Eagle's medium (DMEM) 1000 mg/L d-glucose (Sigma-Aldrich), supplemented 10% fetal bovine serum (Invitrogen), 2 mM glutamax I (Life Technologies), 100 U/100 mg/mL penicillin/streptomycin, and used between passages 4 and 10. At least two donors of both cell types were used in this study.
Collagen gel preparation
Acid-soluble collagen type I (rat-tail, 2.2 mg/mL; FirstLink) was neutralized by mixing with 10×MEM and addition of 1 M NaOH dropwise until a change in color from yellow to pink. After neutralization, the mixture was allowed to rest on ice for 30 min to remove trapped air bubbles. Fibroblasts or osteoblasts were added in suspension in a serum-free DMEM; the cell-free DMEM was added to the acellular collagen solution and gently mixed to avoid bubbles.
The collagen solution was either mixed with human foreskin fibroblasts (HFF) and HOS (2.5×105 cells/mL) or cast without the cells (acellular PC constructs). Gels were cast at 1.5 mL initial volume, 7.5 mm height, and 200.96 mm2 surface area.
Plastic compression
PC was performed using a modified protocol.29 Briefly, standard tissue culture 24-well plates (well diameter 16 mm; TPP) were used as gel casting mold. The fluid-removing elements consisted of a spirally wound roll of Whatman chromatography paper (d=15 mm) and two discs of Whatman filter paper (Whatman), cut to the size of the well to rest on the collagen gel. Each roll was 87 cm in length, 4 cm in height (as supplied by the manufacturer), and 15 mm in diameter. To provide compressive load together with capillary action to drive fluid flow from the collagen gel, each gel was subjected to 29 g of initial load, resting on the surface, and compressed for 15 min (Fig. 1). After compression, liquid-containing paper rolls were removed, leaving the constructs containing HFF/HOS or acellular, in their respective wells, ready for culture.
FIG. 1.
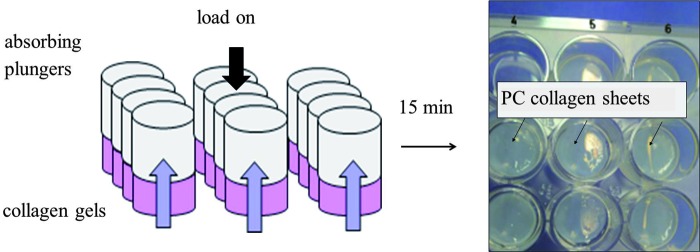
Schematic illustration of the plastic compression (PC) process. Neutralized collagen was added to wells of a standard 24-well plate and allowed to set at 37°C. Following gelation, absorbing plungers were placed on top of each gel and a load was applied for 15 min. Resulting collagen constructs were ready for culture. Color images available online at www.liebertpub.com/tea
HDMEC (5×104 cells/well) were added to the constructs in 0.5 mL of medium. After 2 h, the total medium volume was brought to 1 mL. Constructs were cultured for 3 weeks with media changes every other day. The medium was collected at various time points and used for biochemical analysis. At the end of the culture period, samples were fixed and processed for routine histology and immunofluorescent staining. All experiments were carried out with at least two donors of HDMEC, HDF, and HOS with n=3 for each combination.
2D coculture conditions
It has been shown that coculture of HOS and HDMEC results in the formation of microcapillary-like structures in 2D.25 This 2D coculture system was used to determine the effect of PC collagen on the angiogenic behavior of HDMEC. HDMEC and HOS or HFF were cocultured following an established protocol.25 Briefly, HDMEC and HOS or HFF were seeded at a 5:1 ratio in gelatin-coated 24-well plates (TPP). Cocultures were overlaid with PC collagen after 1 day. Overlay with uncompressed neutralized collagen (300 μL/well; First Link) and without overlay were used as control. The morphology of HDMEC was assessed after 3 weeks in coculture by fluorescent microscopy (Leica).
Assessment of endothelial cell viability
To assess the viability of HDMEC on PC collagen, monoculture constructs were incubated in a medium containing Calcein AM and ethidium homodimer (live/dead assay; Invitrogen) after 24 h and 7 days postassembly. After a 30-min incubation, constructs were washed in phosphate-buffered saline (PBS) and visualized using a confocal microscope (Leica).
Analysis of PECAM-1 expression by the endothelial cells
After 3 weeks in culture, samples were fixed in paraformaldehyde for 15 min at room temperature, washed three times with PBS, and then permeabilized with 0.5% Triton X-100 for 10 min.25 A primary monoclonal antibody (PECAM-1, 1:50 in 1% bovine serum albumin [BSA]/PBS) was applied overnight at 4°C. After washing with PBS, samples were incubated with Alexa Fluor 488 goat anti-mouse (1:1000 in 1% PBS/BSA) and TRITC-conjugated anti-phalloidin antibodies (1:40 in 1% PBS/BSA) for 2 h at room temperature in the dark. After washing with PBS, nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) in PBS for 5 min and samples examined by confocal microscopy (Leica).
Histological assessment of PC constructs
Fixed samples were embedded in paraffin according to standard methods and sectioned at 7 μm thickness using a microtome in the transverse plane. Samples were halved before placing into the paraffin blocks and sections were made in the middle of the constructs to avoid possible edge effects. Six consecutive sections were taken at 200 μm intervals over a 600 μm depth. Sections were stained with the PECAM-1 antibody as described above to visualize endothelial cells. Antibodies against collagen type IV (diluted 1:10; Sigma-Aldrich) and laminin (diluted 1:10; Dako) were used to assess basement membrane deposition. Appropriate antigen retrieval protocols were used as suggested by the manufacturer. Sections were incubated with the Alexa Fluor 488 goat anti-mouse (1:1000 in 1% PBS/BSA) antibody; nuclei were counterstained with DAPI in PBS for 5 min and samples examined by confocal microscopy (Leica).
SEM imaging
Samples for scanning electron microscopy (SEM) were dried after the fixation step and contrasted with osmium tetroxide (Sigma-Aldrich). The samples were transferred to a carbon-coated metal plate, sputtered with gold, and analyzed with a scanning electron microscope (DSM 962; Zeiss) using 10 kV voltage.
Vascular endothelial growth factor quantification
The supernatants of cocultures and monocultures were collected at various time points and stored at −20°C. For each time point, three samples were taken and the vascular endothelial growth factor (VEGF) was quantified by an enzyme-linked immunosorbent assay using the human VEGF DuoSet (R&D Systems) according to the manufacturer's protocol. The culture medium was used as blank. Data are presented as mean value in pg/mL±SD.
Statistical analysis
Analysis of VEGF expression was performed using one-way ANOVA with Tukey's post hoc test (GraphPrism 5). Differences were considered significant when p<0.05. Data are presented as mean±SD.
Results
Multiwell PC resulted in fabrication of multiple constructs within 15 min of compression. The constructs remained in the corresponding wells of the plate and were easy to handle. The diameter of the cellular constructs did not change after long-term culture (3 weeks) compared with time 0 and remained 16 mm throughout the culture period (Fig. 2A). An SEM examination of the gel construct surface showed high density of interwoven collagen fibers (Fig. 2B). The average thickness of PC constructs, measured from histological images, was 122±7 μm for acellular and 136±12 for cellular (both cell types).
FIG. 2.
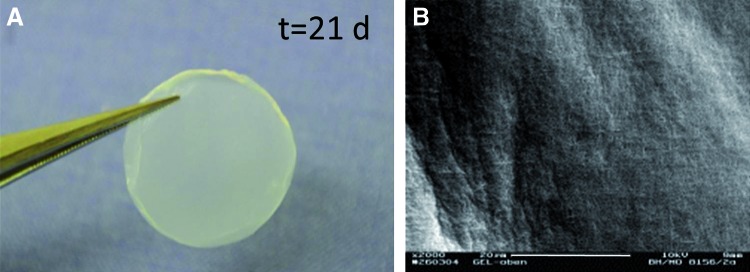
Morphology of the construct prepared using multiwell PC of collagen. (A) Gross appearance of the human foreskin fibroblast (HFF)-seeded PC construct after 3 weeks in culture. Note lack of deformation; (B) scanning electron microscopy (SEM) image of the dense, fibrillar surface of the acellular PC construct. Scale bar=20 μm. Color images available online at www.liebertpub.com/tea
To assess the effect of compressed and uncompressed collagen on the angiogenic behavior of HDMEC, a previously reported coculture system, resulting in the formation of microcapillary-like structures by endothelial cells in the presence of osteoblasts,25 was used. On plastic and other 2D and 3D biocompatible surfaces, endothelial cells in the presence of osteoblasts migrate to form capillary-like structures. A coculture of these cells on plastic, to which a PC collagen overlay was added, did not show major differences (Fig. 3A) from cultures overlaid with collagen hydrogel (noncompressed) or without overlay (Fig. 3B, C). Thus, the PC collagen had no negative effect on the ability of HDMEC to migrate to form microcapillaries.
FIG. 3.
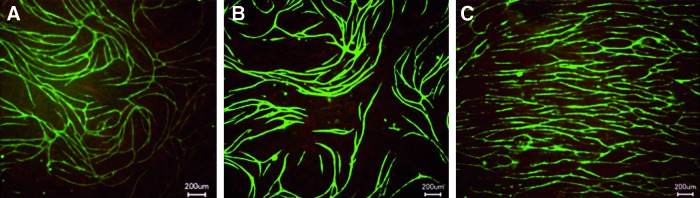
Representative images of human dermal microvascular endothelial cells (HDMEC) and human osteoblasts (HOS) cocultures on tissue culture plastic, overlaid with PC collagen (A), uncompressed collagen gel (B), or without overlay (C) after 3 weeks in culture. Addition of PC collagen to the coculture did not change proangiogenic behavior of the HDMEC. Endothelial cells were stained with endothelial cell marker PECAM-1. Scale bar=200 μm. Color images available online at www.liebertpub.com/tea
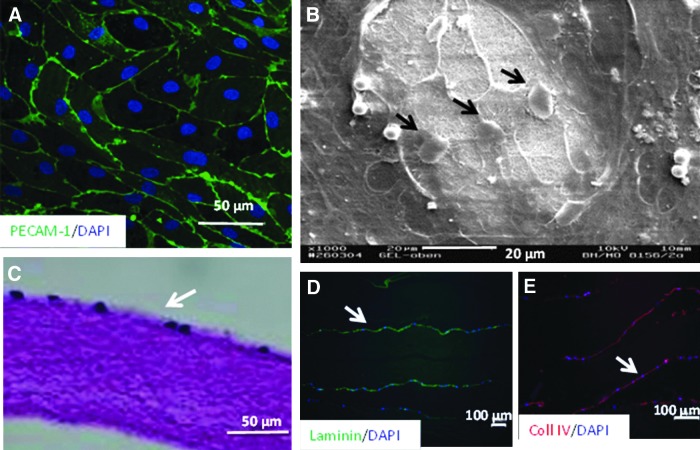
To determine the effects of compression of collagen compared with uncompressed collagen on endothelial cells, HDMEC were incorporated into the collagen before compression or added to the top of the PC collagen. Viability of the HDMEC on the surface of the PC collagen constructs after 24 h and 7 days postseeding was confirmed by live/dead staining. Cells were able to attach to the surface of the constructs and reached confluency within 24 h. HDMEC retained characteristic cobblestone morphology throughout the culture period and were present on the constructs after 21 days in culture. Immunostaining showed that HDMEC expressed a typical staining pattern for the endothelial cell marker PECAM-1 at the cell–cell borders (Fig. 4A). SEM image in Figure 4B shows endothelial cells adherent to the fibrillar surface of the PC construct. A histological evaluation confirmed the presence of a cellular monolayer on one surface of the PC collagen construct with evenly distributed cell nuclei (Fig. 4C). There were no signs of single-cell invasion of the acellular dense matrix by the HDMEC or the formation of capillary-like structures. Expression of the basal membrane proteins laminin and collagen IV was demonstrated by immunostaining (Fig. 4D, E). Interestingly, HDMEC did not survive or grow when embedded within the PC collagen (data not shown).
FIG. 4.
(A) Morphology of the HDMEC cultured on PC collagen constructs after 3 weeks in culture. Endothelial cells adhere to the PC collagen and retain characteristic cobblestone morphology with expression of endothelial cell marker PECAM-1 between cell–cell contacts (nuclei counterstained with 4′,6-diamidino-2-phenylindole [DAPI]). (B) SEM imaging of HDMEC on the PC constructs showed cells (arrows) adherent to the fibrillar collagen surface. (C) Histological examination of the PC constructs revealed a monolayer of adherent endothelial cells. (D, E) HDMEC stained positively for expression of basement membrane proteins laminin (D) and collagen type IV (E). Arrows indicate endothelial cells on the images.
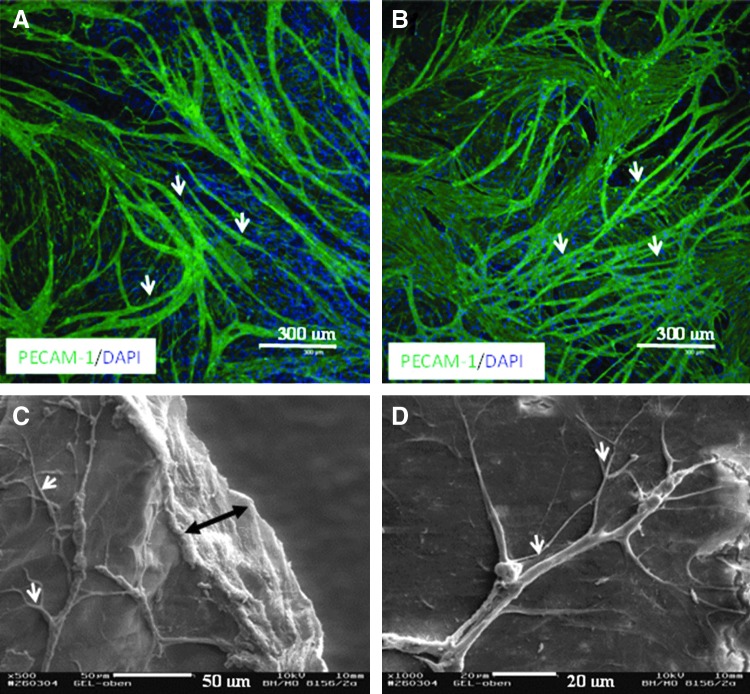
In coculture with HFF or HOS in a PC collagen construct, HDMEC again did not survive (data not shown). However, we found that HDMEC seeded onto the PC constructs containing HFF or HOS were able to attach and grow to confluency. After 3 weeks in culture, the HDMEC formed an extensive branching network of microcapillary-like structures over the surface of the constructs containing either the HFF or HOS as detected by the expression of the endothelial cell marker PECAM-1 (Fig. 5A, B). In addition, SEM images confirmed the histological findings showing complex 3D microcapillary-like structures on the surface of constructs containing HFF and HOS (Fig. 5C, D).
FIG. 5.
Morphology of the HDMEC cultured on the surface of the PC constructs that contained HFF (left panel) or HOS (right panel) after 3 weeks in culture. HDMEC cultured on the PC collagen constructs in which HFF or HOS included during gelation and compression exhibited proangiogenic behavior as visualized by expression of the endothelial cell marker PECAM-1 (A, B). SEM images showed three-dimensional microcapillary-like structures on the surface of the constructs (C, D). Double arrow indicates PC collagen scaffold. White arrows indicate microcapillary-like structures.
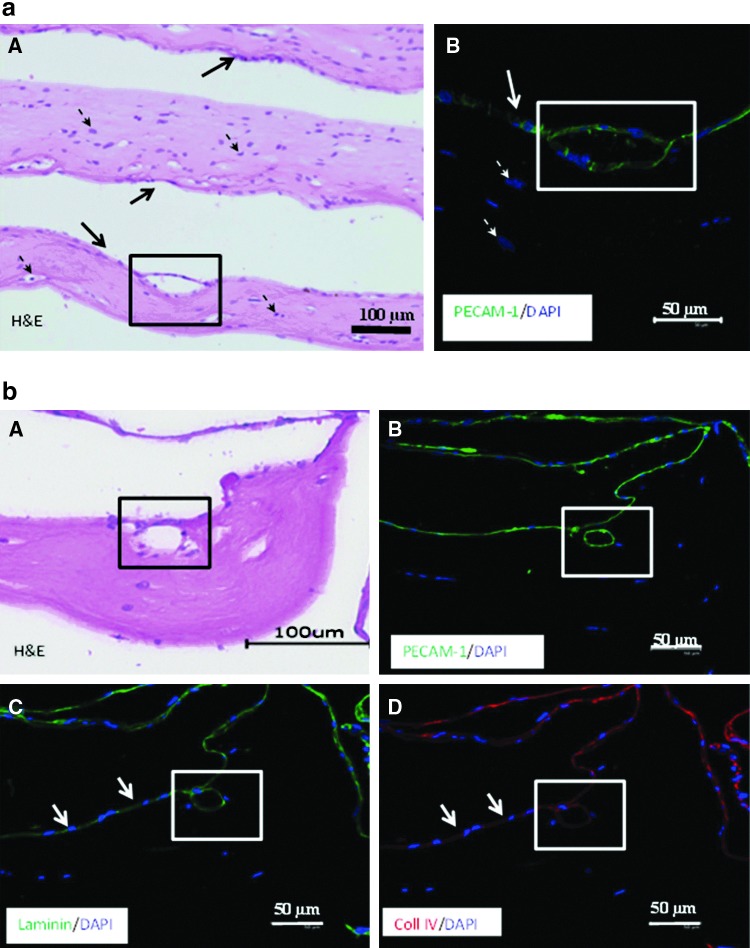
Histological evaluation revealed a typical tissue-like structure of the constructs with HFF and HOS14,30 (Fig. 6a, A). PECAM-1-positive cells were mostly confined to the surface of the cellular (for both HFF and HOS) constructs and in some cases showing luminal structures formed by multiple endothelial cells (Fig. 6a, B).
FIG. 6.
(a) Histological images of the HDMEC cultured on PC constructs containing HFF after 3 weeks in culture. Endothelial cells formed luminal structures on the surface of cell-containing PC constructs (A, black square). Endothelial origin of these structures was confirmed by expression of the endothelial cell marker PECAM-1 (B, white square). Solid arrows indicate endothelial cells, dashed arrows point at the HFF, embedded in the PC collagen. (b) Images of the HDMEC cultured on PC constructs containing HOS after 3 weeks in culture. Endothelial cells invaded the PC matrix and formed luminal structures in the cell-containing PC construct (A, black square). Endothelial origin of these structures was confirmed by expression of the endothelial cell marker PECAM-1 (B, white square). Endothelial cells expressed basement membrane proteins laminin (C) and collagen IV (D) both on the surface (arrows) and in the PC collagen (white squares) containing HOS.
In some cases, endothelial cells exhibited signs of invasion of the matrix by the microcapillary-like structures (Fig. 6b-A, B). Moreover, the endothelial cells lining these lumina expressed both laminin and collagen type IV (Fig. 6b-C, D). No single HDMEC were found in the matrix in these cocultures.
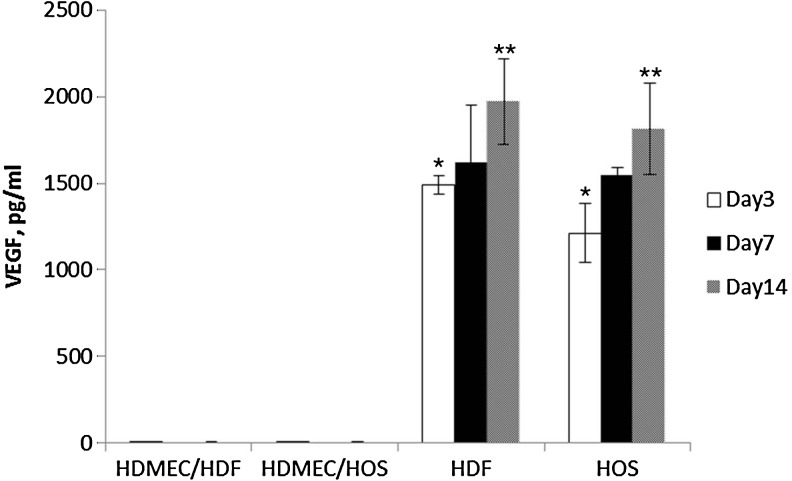
The levels of VEGF expression in the medium of cocultured cells revealed no detectable levels of the protein in the supernatant from HDMEC cocultures with either HFF or HOS in the PC collagen at the time points investigated (Fig. 7). By contrast, the medium of HFF- or HOS-containing PC constructs had clearly measurable levels of VEGF, which varied with time in culture. Thus, levels of VEGF detected in the medium increased significantly from day 3 to 14 in monocultures of both HFF and HOS.
FIG. 7.
Levels of vascular endothelial growth factor (VEGF) measured by enzyme-linked immunosorbent assay in the media of monocultures and cocultures of HDMEC, HFF, and HOS at days 3, 7, and 14. Data are presented as mean value±standard error of the mean. Asterisk indicates significant differences within the group compared to day 14; double asterisks indicate significant differences between the groups, p<0.05.
Discussion
PC of collagen gels allows a rapid and controlled fabrication of collagen constructs in vitro that closely mimic the structure and characteristics of mature tissues in vivo. These constructs have been shown to support the growth, replication, and normal phenotypic expression of a number of different human organ- or tissue-specific cell types. However, it was not known how endothelial cells that make up the microvasculature react to this material. The results from our studies have shown that endothelial cells in contrast to other cell types do not survive compression in the collagen. However, endothelial cells were able to attach and proliferate on the surface and expressed a normal cell phenotype. Alone on the PC collagen, cells did not form microcapillary-like structures, but when human fibroblasts or osteoblasts were included in the PC collagen, the endothelial cells added to the surface of the collagen migrated to form microcapillary-like structures, including a lumen, which with time began to migrate into the PC collagen.
A major advantage of this in vitro model is that it allows an analysis of EC interaction with engineered in vivo-like tissues as well as being of possible application in targeting bone and soft tissue regeneration. Importantly, attempts to make such coculture models without collagen compression are impractical and hard to interpret due to inherent cell contraction of the soft, overhydrated collagen. In vitro models are a simple and convenient way to study cell–cell and cell–biomaterial interactions under a controlled and defined environment.31 Understanding the mechanisms of these processes allows changing the design of a biomaterial so that the desired biological function can be achieved. Angiogenesis (sprouting of new blood vessels from the pre-existing microcirculation in the peri-implant tissue) represents the most central element in the process of implant integration.32 MEC are the main cell type responsible for successful vascularization and hence survival of the engineered tissue.24 Therefore, this study focused on the behavior of the MEC in contact with PC collagen and encompassed investigation of the viability, morphology, and growth factor production.
A major challenge was to design the culture system so that it reflected the in vivo situation. Previous studies investigated morphological changes of HUVEC in the PC collagen in response to coculture with human dermal fibroblasts.33 It was shown that HUVEC formed clusters and migrated toward fibroblasts over a 2-week culture period. However, in our preliminary experiments, we observed that human MEC do not form microcapillary-like structures and remained as single cells when embedded in PC collagen gels. In addition, no obvious proliferation was observable. This discrepancy may be attributed to the different origin and function of these endothelial cells in vivo and in vitro.34 Since MEC are the more biologically relevant cell type to be in contact with the biomaterial upon implantation, these cells were chosen for evaluation in the present study.
Surface seeding of the MEC on the biomaterials, including collagen, has been used and well documented in other studies.35–38 Therefore, this mode of cell seeding was evaluated in the current study on the PC collagen gels. To assess the phenotype and differentiation of endothelial cells, the expression of several markers was monitored. PECAM-1 regulates the homotypic adhesion of ECs and is involved in inflammatory processes in which ECs bind to leukocytes during emigration into the perivascular space.39 The lack of PECAM-1 expression affects endothelial cell–cell and cell–matrix interactions, thus influencing endothelial cell migration and angiogenic behavior.40 Endothelial cells in vivo reside on a basement membrane, which separates the differentiated endothelium from surrounding tissue. In the present study, we assessed the expression of collagen type IV and laminin, which are the main constituents of BM in vivo and hallmarks of mature endothelium.41
In this study, a modified method of generating PC, which is suitable for an in vitro setup, was used.29 Simultaneous compression of multiple constructs greatly reduces variability and increases the speed of construct assembly. It has been shown previously that this mode of compression does not change the morphology of the PC collagen scaffolds and they retain their main characteristics, including viability of the cells in the matrix.42
MEC seeded on the acellular PC collagen were able to survive and proliferate. After 3 weeks in culture, cells exhibited typical cobblestone morphology and expressed PECAM-1 at the cell–cell interfaces. We did not find any evidence of cell migration into the matrix under these conditions, even after long-term culture. This can be attributed to several factors such as the density of the PC collagen (12–17%) and standard culture conditions without the addition of proangiogenic factors, such as VEGF. It has been shown previously that, human umbilical vein endothelial cells seeded on the surface of 0.2% collagen type I gels invade the gels within 72 h when cultured in the presence of VEGF in the medium.43 Cross et al. demonstrated limited invasion of HUVEC into 2% collagen gels over 21 days in vitro under the same conditions.44 It has been shown that vascularization of acellular PC constructs in vivo is delayed and the number of vessels was significantly lower compared with cellular constructs.20,22,45 Since endothelial cells alone do not migrate into the matrix in our system, addition of exogenous growth factors or studies with other cell types might activate proteolytic enzymes. Further studies are needed to determine conditions for microcapillary invasion of the PC constructs.
Importantly, endothelial cells were able to survive on the PC collagen for 3 weeks, whereas these cells typically detach when cultured on tissue culture plastic without passaging after 7–10 days when confluency is reached.25 It is known that endothelial cell survival is dependent on their ability to attach to the biomaterial.46 These results may have important implications for the use of PC collagen for artificial vessel engineering, where endothelial cells lining the vessel lumen need to withstand shear stress from blood flow. It is possible that PC collagen presents more cell-attachment sites compared with the hydrated gels. Further studies are needed into gene expression and morphological changes by these cells on both substrata.
Recreating tissue complexity in vitro is important for unraveling biomolecular interactions between heterotypic cell types. The development of such models is even more important for biomaterial engineering to predict the implant performance in vivo.47 An advanced model of angiogenesis has been described by our group and showed that MEC form microcapillary-like structures in coculture with osteoblasts25 in the absence of exogenously added angiogenic factors. This model system has already been used to evaluate biocompatibility, and the proangiogenic behavior of several biomaterials proposed for bone tissue engineering25,26 in vitro has been validated in in vivo studies and may be useful as a prevascularization strategy for biomaterials before implantation.48 In the present study, we were able to demonstrate that application of PC collagen to that system does not hinder microcapillary-like structure formation on tissue culture plastic.
The main hallmark of PC is compatibility with living cells included in the matrix before compression, including osteoblasts and dermal fibroblasts.14,15,28,49 It has been shown that direct coculture of both osteoblasts and fibroblasts with endothelial cells induces proangiogenic differentiation of the latter. Proangiogenic behavior of the endothelial cells has been attributed to the paracrine interactions between cocultured cell types.50,51 In this study, we show that HDMEC cocultured on PC collagen seeded with osteoblasts or fibroblasts exhibited proangiogenic behavior, forming microvessel-like structures without the addition of exogenous growth factors. These microcapillary-like structures contained lumina (confirmed by SEM) and exhibited complex, branched morphology shown by immunofluorescent staining with PECAM-1 and confirmed by SEM.
Endothelial cells in coculture were able to deposit the basement membrane proteins, laminin and collagen type IV, thus exhibiting signs of mature endothelium.
Surprisingly, PECAM-positive luminar structures were found within the dense collagen matrix, indicating cell migration toward the source of proangiogenic signaling. These structures were also surrounded by basement membrane proteins. It has been shown previously that complex cross talk takes place between osteoblasts and HDMEC cocultured on starch-based porous biomaterial,26 with changes in expression of several important genes. It is possible that similar cell–cell interactions are taking place in the model described in this study, as demonstrated by the differences in detectable VEGF level expression, possibly simulating the process of angiogenesis in vivo. In vivo, endothelial cells degrade the basement membrane in response to signaling from hypoxic tissue and migrate toward the signaling cells.41 It is known that bone marrow stromal cells and dermal fibroblasts in PC collagen upregulate gene and protein expression of VEGF in vitro52 and stimulate angiogenesis in vivo.33 Endothelial cells have also been shown to express membrane-associated type I transmembrane MMP to invade collagen hydrogels.41 Further studies are required to investigate the precise mechanisms and factors regulating such invasive behavior of HDMEC on PC. However, our initial studies with PC and cocultured cells have yielded data in vitro, which are in full agreement with the in vivo results demonstrating that cellular PC constructs on implantation led to faster vascularization of the construct with a larger number of vessels found in the collagen matrix compared with acellular controls.31
PC collagen is a versatile material in which cell type and seeding numbers, collagen density and composition can be constantly adjusted depending on the proposed application. Recently, PC collagen scaffolds have been combined with poly(ɛ-caprolactone) (PCL)-knitted mesh,53 poly(lactic acid-co-caprolactone) (PLACL) and electrospun silk fibroin,54,55 orientated focal crosslinking using riboflavin,56 and hyaluronan.57 In this study, we established a base line for future studies of endothelial cell interactions with PC collagen scaffolds. The in vitro model reported in this study could be used to assess the angiogenic potential of these composite biomaterials and to analyze complex cell culture model systems representing tissue in a defined environment. An important practical finding was the absence of contraction of the collagen gel, even after a 3-week coculture with human fibroblasts. This behavior of the compressed collagen gel could be a breakthrough for soft tissue regeneration, as conventional highly hydrated gels show marked contraction under such conditions and are therefore unsuitable for clinical translation.
Acknowledgments
BMBF (German–Chinese Young Investigator Group, Grant 0315033) for funding; Ms. Anne Sartoris for excellent technical help and advice.
Disclosure Statement
No competing financial interests exist.
References
- 1.Grillo H.C., and Gross J.Thermal reconstitution of collagen from solution and the response to its heterologous implantation. J Surg Res 2,69, 1962 [DOI] [PubMed] [Google Scholar]
- 2.Bell E., Ivarsson B., and Merrill C.Production of a tissue-like structure by contraction of collagen lattices by human-fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci U S A 76,1274, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abou Neel E.A., Bozec L., Knowles J.C., Syed O., Mudera V., Day R., and Hyun J.K.Collagen—emerging collagen based therapies hit the patient. Adv Drug Deliv Rev 65,429, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Elsdale T., and Bard J.Collagen substrata for studies on cell behavior. J Cell Biol 54,626, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helary C., Bataille I., Abed A., Illoul C., Anglo A., Louedec L., Letourneur D., Meddahi-Pelle A., and Giraud-Guille M.M.Concentrated collagen hydrogels as dermal substitutes. Biomaterials 31,481, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Dallon J.C., and Ehrlich H.P.A review of fibroblast-populated collagen lattices. Wound Repair Regen 16,472, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Damink L., Dijkstra P.J., Vanluyn M.J.A., Vanwachem P.B., Nieuwenhuis P., and Feijen J.Cross-linking of dermal sheep collagen using hexamethylene diisocyanate. J Mater Sci Mater Med 6,429, 1995 [Google Scholar]
- 8.Weadock K.S., Miller E.J., Bellincampi L.D., Zawadsky J.P., and Dunn M.G.Physical cross-linking of collagen-fibers—comparison of ultraviolet-irradiation and dehydrothermal treatment. J Biomed Mater Res 29,1373, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Kagan H.M., and Li W.D.Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem 88,660, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Vernon R.B., Gooden M.D., Lara S.L., and Wight T.N.Native fibrillar collagen membranes of micron-scale and submicron thicknesses for cell support and perfusion. Biomaterials 26,1109, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Parenteau-Bareil R., Gauvin R., Cliche S., Gariepy C., Germain L., and Berthod F.Comparative study of bovine, porcine and avian collagens for the production of a tissue engineered dermis. Acta Biomater 7,3757, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Charulatha V., and Rajaram A.Influence of different crosslinking treatments on the physical properties of collagen membranes. Biomaterials 24,759, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Brown R.A.In the beginning there were soft collagen-cell gels: towards better 3D connective tissue models? Exp Cell Res 319,2460, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Brown R.A., Wiseman M., Chuo C.B., Cheema U., and Nazhat S.N.Ultrarapid engineering of biomimetic materials and tissues: fabrication of nano- and microstructures by plastic compression. Adv Funct Mater 15,1762, 2005 [Google Scholar]
- 15.Ghezzi C.E., Muja N., Marelli B., and Nazhat S.N.Real time responses of fibroblasts to plastically compressed fibrillar collagen hydrogels. Biomaterials 32,4761, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Hadjipanayi E., Mudera V., and Brown R.A.Close dependence of fibroblast proliferation on collagen scaffold matrix stiffness. J Tissue Eng Regen Med 3,77, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Kureshi A., Cheema U., Alekseeva T., Cambrey A., and Brown R.Alignment hierarchies: engineering architecture from the nanometre to the micrometre scale. J R Soc Interface 7,S707, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abou Neel E.A., Cheema U., Knowles J.C., Brown R.A., and Nazhat S.N.Use of multiple unconfined compression for control of collagen gel scaffold density and mechanical properties. Soft Matter 2,986, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Buxton P.G., Bitar M., Gellynck K., Parkar M., Brown R.A., Young A.M., Knowles J.C., and Nazhat S.N.Dense collagen matrix accelerates osteogenic differentiation and rescues the apoptotic response to MMP inhibition. Bone 43,377, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Ananta M., Brown R.A., and Mudera V.A rapid fabricated living dermal equivalent for skin tissue engineering: an in vivo evaluation in an acute wound model. Tissue Eng Part A 18,353, 2012 [DOI] [PubMed] [Google Scholar]
- 21.East E., de Oliveira D.B., Golding J.P., and Phillips J.B.Alignment of astrocytes increases neuronal growth in three-dimensional collagen gels and is maintained following plastic compression to form a spinal cord repair conduit. Tissue Eng Part A 16,3173, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Micol L.A., da Silva L.F.A., Geutjes P.J., Oosterwijk E., Hubbell J.A., Feitz W.F.J., and Frey P.In-vivo performance of high-density collagen gel tubes for urethral regeneration in a rabbit model. Biomaterials 33,7447, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Levis H.J., Brown R.A., and Daniels J.T.Plastic compressed collagen as a biomimetic substrate for human limbal epithelial cell culture. Biomaterials 31,7726, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Kirkpatrick C.J., Fuchs S., and Unger R.E.Co-culture systems for vascularization—learning from nature. Adv Drug Deliv Rev 63,291, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Unger R.E., Sartoris A., Peters K., Motta A., Migliaresi C., Kunkel M., Bulnheim U., Rychly J., and Kirkpatrick C.J.Tissue-like self-assembly in cocultures of endothelial cells and osteoblasts and the formation of microcapillary-like structures on three-dimensional porous biomaterials. Biomaterials 28,3965, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Santos M.I., Unger R.E., Sousa R.A., Reis R.L., and Kirkpatrick C.J.Crosstalk between osteoblasts and endothelial cells co-cultured on a polycaprolactone-starch scaffold and the in vitro development of vascularization. Biomaterials 30,4407, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Buschmann J., Welti M., Hemmi S., Neuenschwander P., Baltes C., Giovanoli P., Rudin M., and Calcagni M.Three-dimensional co-cultures of osteoblasts and endothelial cells in degrapol foam: histological and high-field magnetic resonance imaging analyses of pre-engineered capillary networks in bone grafts. Tissue Eng Part A 17,291, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Peters K., Schmidt H., Unger R.E., Kamp G., Prols F., Berger B.J., and Kirkpatrick C.J.Paradoxical effects of hypoxia-mimicking divalent cobalt ions in human endothelial cells in vitro. Mol Cell Biochem 270,157, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Alekseeva T., Jawad H., Purser M., and Brown R.A.New improved technique of plastic compression of collagen using upward fluid flow. In: 8th International Conference on Cell & Stem Cell Engineering (ICCE), 2011, p. 5 [Google Scholar]
- 30.Bitar M., Brown R.A., Salih V., Kidane A.G., Knowles J.C., and Nazhat S.N.Effect of cell density on osteoblastic differentiation and matrix degradation of biomimetic dense collagen scaffolds. Biomacromolecules 9,129, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Kirkpatrick C.J., Krump-Konvalinkova V., Unger R.E., Bittinger F., Otto M., and Peters K.Tissue response and biomaterial integration: the efficacy of in vitro methods. Biomol Eng 19,211, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Kirkpatrick C.J., Unger R.E., Krump-Konvalinkova V., Peters K., Schmidt H., and Kamp G.Experimental approaches to study vascularization in tissue engineering and biomaterial applications. J Mater Sci Mater Med 14,677, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Hadjipanayi E., Brown R.A., Mudera V., Deng D., Liu W., and Cheema U.Controlling physiological angiogenesis by hypoxia-induced signaling. J Control Release 146,309, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Swerlick R.A., Lee K.H., Wick T.M., and Lawley T.J.Human dermal microvascular endothelial but not human umbilical vein endothelial-cells express cd36 in vivo and in vitro. J Immunol 148,78, 1992 [PubMed] [Google Scholar]
- 35.Burrows M.C., Zamarion V.M., Filippin-Monteiro F.B., Schuck D.C., Toma H.E., Campa A., Garcia C.R.S., and Catalani L.H.Hybrid scaffolds built from PET and collagen as a model for vascular graft architecture. Macromol Biosci 12,1660, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Oh J.H., Lee J.S., Park K.M., Moon H.T., and Park K.D.Tyrosinase-mediated surface grafting of cell adhesion peptide onto micro-fibrous polyurethane for improved endothelialization. Macromol Res 20,1150, 2012 [Google Scholar]
- 37.Bayramoglu G., Kayaman-Apohan N., Kahraman M.V., Karadenizli S., Kuruca S.E., and Gungor A.Preparation of bow tie-type methacrylated poly(caprolactone-co-lactic acid) scaffolds: effect of collagen modification on cell growth. Polym Adv Technol 23,1403, 2012 [Google Scholar]
- 38.Sgarioto M., Vigneron P., Patterson J., Malherbe F., Nagel M.D., and Egles C.Collagen type I together with fibronectin provide a better support for endothelialization. C R Biol 335,520, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Albelda S.M., Muller W.A., Buck C.A., and Newman P.J.Molecular and cellular properties of pecam-1 (endocam/cd31)—a novel vascular cell cell-adhesion molecule. J Cell Biol 114,1059, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park S., DiMaio T.A., Scheef E.A., Sorenson C.M., and Sheibani N.PECAM-1 regulates proangiogenic properties of endothelial cells through modulation of cell-cell and cell-matrix interactions. Am J Physiol Cell Physiol 299,C1468, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davis G.E., and Senger D.R.Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res 97,1093, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Levis H.J., Menzel-Severing J., Drake R.A.L., and Daniels J.T.Plastic compressed collagen constructs for ocular cell culture and transplantation: a new and improved technique of confined fluid loss. Curr Eye Res 38,41, 2013 [DOI] [PubMed] [Google Scholar]
- 43.Davis G.E., Black S.M., and Bayless K.J.Capillary morphogenesis during human endothelial cell invasion of three-dimensional collagen matrices. In Vitro Cell Dev Biol Anim 36,513, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Cross V.L., Zheng Y., Choi N.W., Verbridge S.S., Sutermaster B.A., Bonassar L.J., Fischbach C., and Stroock A.D.Dense type I collagen matrices that support cellular remodeling and microfabrication for studies of tumor angiogenesis and vasculogenesis in vitro. Biomaterials 31,8596, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mudera V., Morgan M., Cheema U., Nazhat S., and Brown R.Ultra-rapid engineered collagen constructs tested in an in vivo nursery site. J Tissue Eng Regen Med 1,192, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Kemeny S.F., Cicalese S., Figueroa D.S., and Clyne A.M.Glycated collagen and altered glucose increase endothelial cell adhesion strength. J Cell Physiol 228,1727, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Kirkpatrick C.J., Fuchs S., Hermanns M.I., Peters K., and Unger R.E.Cell culture models of higher complexity in tissue engineering and regenerative medicine. Biomaterials 28,5193, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Unger R.E., Ghanaati S., Orth C., Sartoris A., Barbeck M., Halstenberg S., Motta A., Migliaresi C., and Kirkpatrick C.J.The rapid anastomosis between prevascularized networks on silk fibroin scaffolds generated in vitro with cocultures of human microvascular endothelial and osteoblast cells and the host vasculature. Biomaterials 31,6959, 2010 [DOI] [PubMed] [Google Scholar]
- 49.Bitar M., Salih V., Brown R.A., and Nazhat S.N.Effect of multiple unconfined compression on cellular dense collagen scaffolds for bone tissue engineering. J Mater Sci Mater Med 18,237, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Stahl A., Wenger A., Weber H., Stark G.B., Augustin H.G., and Finkenzeller G.Bi-directional cell contact-dependent regulation of gene expression between endothelial cells and osteoblasts in a three-dimensional spheroidal coculture model. Biochem Biophys Res Commun 322,684, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Stahl A., Wu X., Wenger A., Klagsburn M., and Kurschat P.Endothelial progenitor cell sprouting in spheroid cultures is resistant to inhibition by osteoblasts: a model for bone replacement grafts. FEBS Lett 579,5338, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Hadjipanayi E., Cheema U., Mudera V., Deng D., Liu W., and Brown R.A.First implantable device for hypoxia-mediated angiogenic induction. J Control Release 153,217, 2011 [DOI] [PubMed] [Google Scholar]
- 53.Ajalloueian F., Zeiai S., Rojas R., Fossum M., and Hilborn J.One-stage tissue engineering of bladder wall patches for an easy-to-use approach at the surgical table. Tissue Eng Part C Methods 19,688, 2013 [DOI] [PubMed] [Google Scholar]
- 54.Ananta M., Aulin C.E., Hilborn J., Aibibu D., Houis S., Brown R.A., and Mudera V.A poly(lactic acid-co-caprolactone)-collagen hybrid for tissue engineering applications. Tissue Eng Part A 15,1667, 2009 [DOI] [PubMed] [Google Scholar]
- 55.Ghezzi C.E., Marelli B., Muja N., Hirota N., Martin J.G., Barralet J.E., Alessandrino A., Freddi G., and Nazhat S.N.Mesenchymal stem cell-seeded multilayered dense collagen-silk fibroin hybrid for tissue engineering applications. Biotechnol J 6,1198, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Wong J.P.F., MacRobert A.J., Cheema U., and Brown R.A.Mechanical anisotropy in compressed collagen produced by localised photodynamic cross-linking. J Mech Behav Biomed Mater 18,132, 2013 [DOI] [PubMed] [Google Scholar]
- 57.Anandagoda N., Ezra D.G., Cheema U., Bailly M., and Brown R.A.Hyaluronan hydration generates three-dimensional meso-scale structure in engineered collagen tissues. J R Soc Interface 9,2680, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]