Abstract
Mesenchymal stem cell (MSC) loaded bio-scaffold transplantation is a promising therapeutic approach for bone regeneration and repair. However, growing evidence shows that pro-inflammatory mediators from injured tissues suppress osteogenic differentiation and impair bone formation. To improve MSC-based bone regeneration, it is important to understand the mechanism of inflammation mediated osteogenic suppression. In the present study, we found that synovial fluid from rheumatoid arthritis patients and pro-inflammatory cytokines including interleukin-1α, interleukin-1β, and tumor necrosis factor α, stimulated intercellular adhesion molecule-1(ICAM-1) expression and impaired osteogenic differentiation of MSCs. Interestingly, overexpression of ICAM-1 in MSCs using a genetic approach also inhibited osteogenesis. In contrast, ICAM-1 knockdown significantly reversed the osteogenic suppression. In addition, after transplanting a traceable MSC-poly(lactic-co-glycolic acid) construct in rat calvarial defects, we found that ICAM-1 suppressed MSC osteogenic differentiation and matrix mineralization in vivo. Mechanistically, we found that ICAM-1 enhances MSC proliferation but causes stem cell marker loss. Furthermore, overexpression of ICAM-1 stably activated the MAPK and NF-κB pathways but suppressed the PI3K/AKT pathway in MSCs. More importantly, specific inhibition of the ERK/MAPK and NF-κB pathways or activation of the PI3K/AKT pathway partially rescued osteogenic differentiation, while inhibition of the p38/MAPK and PI3K/AKT pathway caused more serious osteogenic suppression. In summary, our findings reveal a novel function of ICAM-1 in osteogenesis and suggest a new molecular target to improve bone regeneration and repair in inflammatory microenvironments.
Introduction
Mesenchymal stem cells (MSCs) were originally identified in bone marrow and have since been found in numerous tissues including bone, fat, and muscle.1–3 Under certain conditions, MSCs can differentiate into osteoblasts, adipocytes, chondrocytes, and cells of other tissues.1–3 Thus, MSCs are considered to be the most important seed cells for the repair of injured tissue. MSC differentiation is regulated by molecular signals from the microenvironment in which they reside.4
Large bone defects caused by trauma or bone diseases have proven difficult to heal completely due to the limited regenerative capacity of bone tissue. Bio-engineered scaffolds represent an alternative to an autologous iliac crest graft. It has been shown that MSC-loaded bio-scaffold transplantation is a promising therapeutic approach for bone regeneration and repair.5–7 However, defective bones in clinical trials are frequently infected. Inflammatory mediators from injured tissues have been shown to suppress osteogenic differentiation and impair bone regeneration.8–11 Thus, to improve MSC-based bone regeneration in inflammatory microenvironments, it is necessary to understand the mechanism of inflammation-mediated osteogenic suppression.
The synovial fluid from rheumatoid arthritis patients (RASF) is a potent inflammatory mediator, which includes numerous inflammatory cytokines and has been reported to induce osteoclast formation.12,13 Our previous study revealed that RASF acts as a critical player in bone remodeling via interaction with MSCs.14 In the present study, we used RASF to mimic the inflammatory microenvironment. In addition, the inhibitory effects with pro-inflammatory cytokines including interleukin-1α (IL-1α), interleukin-1β (IL-1β), and tumor necrosis factor-α (TNF-α) on osteogenesis were also investigated by incubation of MSCs.
Intercellular adhesion molecule-1(ICAM-1) mediates cellular interactions by binding to its counter-receptors, lymphocyte function-associated antigen (LFA-1, CD11a/CD18) and Mac-1 (CD11b/CD18).15,16 Normally, ICAM-1 is not expressed on the surface of MSCs but is upregulated in the inflammatory microenvironment. Though recent studies have demonstrated that increased ICAM-1 plays an important role in the maintenance of MSC immunomodulation,17–19 the role of ICAM-1 in bone metabolism other than osteoclastogenesis has not generated much attention.
In the present study, we observed that inflammatory mediators induced expression of ICAM-1 but suppressed osteogenic differentiation of MSCs. Therefore, we hypothesize that ICAM-1 may be closely involved in bone regeneration in inflammatory microenvironments. To clarify the potential associations between ICAM-1 and bone formation, the effects of ICAM-1 on MSC osteogenic differentiation were investigated in vitro and in vivo. In addition, the proliferation and stem cell markers of MSCs were evaluated. The modulatory effects of signaling pathways, including MAPK, NF-κB, and PI3K/AKT, were also investigated in the present study. Therefore, our study uncovers a novel role of ICAM-1 on bone regeneration in inflammatory microenvironment.
Materials and Methods
RASF preparation and incubation with MSCs
RASF was obtained from 20 RA patients (2 men and 18 women) who met the 1987 revised criteria of the American College of Rheumatology and who were not receiving anti-inflammatory therapy when the samples were collected. Informed consent was obtained from all patients for research purposes. In brief, all RASF samples were aspirated from the articular cavity and subjected to gradient centrifugation at 900 g for 20 min. The supernatants were collected and filtered using a 0.4 μm pore size membrane to remove cellular components. The cell-free samples were stored at −20°C until use. To investigate the effect of RASF on osteogenesis, mouse MSCs (C3H10T1/2, purchased from ATCC) were incubated with RASF samples of different concentrations (0%, 5%, 10%, and 20%) and for different time periods (1 and 3 days) and then cultured in the presence of osteogenic induction medium.
The mouse MSCs were passaged and collected according to a previously described protocol,3 with minor modifications. In brief, the adherent cells were harvested when the MSC cultures reached 70–80% confluence. The medium was removed and 3–5 mL of 0.25% (wt/vol) trypsin/0.02% (wt/vol) EDTA was added. The cells were digested at room temperature (18–25°C) and passaged at a split ratio of 1:3. The culture medium was changed every 48 h, and the MSCs were passaged twice per week at a split ratio of 1:4 or 1:3.
Incubation of MSCs with IL-1α, IL-1β, and TNF α
To clarify the specific effect of pro-inflammatory cytokines in the synovial fluid on the osteogenic differentiation of MSCs, IL-1α, IL-1β, and TNF-α were directly added to the MSC culture medium in graded concentrations (0, 5, and 50 ng/mL) at different time periods (day 1 and 5) before osteogenic induction was performed. The expression of ICAM-1, alkaline phosphatase (ALP), osteocalcin (OCN), and Runx2 as well as mineralization and the stem cell markers were explored.
ICAM-1 plasmid construction, transfection, and identification
Mouse ICAM-1 was extracted and amplified from mouse spleenocytes. To obtain continuously expressing MSCs, full-length mouse ICAM-1 was cloned into a shuttle plasmid, MIGR1, containing an EGFP reporter gene. Sequence and restriction analysis revealed that the mouse ICAM-1 gene was cloned in frame into MIGR1. MIGR1-ICAM-1, empty plasmid MIGR1, and packaging plasmid ECOS were transfected into T293 cell lines. Next, the supernatant generated from T293 cells was used to infect mouse MSCs. The transfection efficiency of ICAM-1 in MSCs was determined by inverted fluorescence microscopy, real-time PCR, and flow cytometry. ICAM-1high MSCs (C3H10T1/2-ICAM-1) and MSCs transfected with the empty vector (C3H10T 1/2-MIGR1) were passaged and expanded in MSC culture medium.
Growth kinetics and CCK-8 assay
The growth kinetics of ICAM-1high MSCs, MSCs transfected with the empty vector, and untransfected MSCs were evaluated using the trypan blue exclusion cell count method. Briefly, all MSCs were cultured in 48-well plates at 2×104 cells/well and harvested every 2 days over a period of 16 days for hemocytometer cell counting.
Cell proliferation was also determined by Cell Counting Kit 8 (CCK-8; Dojindo) according to the manufacturer's protocol. In brief, ICAM-1high MSCs, MSCs transfected with the empty vector, and untransfected MSCs were seeded in 96-well plates (2×103cells/well), cultured in α-MEM medium with 10% FBS (five wells in each group), added to CCK-8 solution in a ratio of 100 μL/1 μL, and incubated at 37°C for 1 h. The amount of CCK-8 reagent converted to formazan by cellular dehydrogenase indicated the cell proliferation activity and the amount of the colored product formed was proportional to the number of cells and the time of incubation of the cells. Absorbance was then measured at a wavelength of 450 nm using a microplate reader. In the present study, the CCK-8 experiments were performed at different time periods of day 1, 3, 6, 9, 12, 15, 18, and 21.
Cell cycle analysis
ICAM-1high MSCs, MSCs transfected with the empty vector, and untransfected MSCs were seeded at 5×103 cells/cm2 and cultured in α-MEM medium with 10% FBS. At 80–90% confluence, the cells were harvested for cell cycle analysis. Briefly, cells were washed and fixed overnight in 70% ethanol at −20°C. Fixed cells were washed and incubated in 100 μg/mL propidium iodide (PI) (Sigma-Aldrich) and 20 ng/mL RNase (Sigma-Aldrich) in PBS for 30 min. Cell cycle analysis was performed by flow cytometry. The independent experiments were replicated at least three times.
Immunophenotype analysis of ICAM-1high MSCs
The cell surface antigen profile of ICAM-1high MSCs was analyzed by flow cytometry. MSCs transfected with the empty vector and untransfected MSCs served as controls. The immunophenotype of MSCs incubated with pro-inflammatory cytokines was also investigated. Phycoerythrin (PE)-conjugated monoclonal antibodies against mouse ICAM-1, CD18, CD29, CD31, CD44, CD86, CD105, CD140a (PDGF receptor α), and MHC-Ia (histocompatibility complex [MHC] class II) were used in addition to allophycocyanin-conjugated antibodies against mouse stem cell antigen-1 (Sca-1; all products were purchased from eBio-Science).
Osteogenic differentiation of MSCs in vitro
The MSCs incubated with pro-inflammatory cytokines, ICAM-1high MSCs, MSCs transfected with the empty vector, and untransfected MSCs were seeded at 5×103 cells/well in 48-well plates. Their differentiation along the osteoblastic pathway was evaluated according to the protocol described by our laboratory.3 A histochemical kit (Sigma) was used to assess ALP activity according to the manufacturer's protocol, and the mineralization capacity was evaluated using von Kossa staining.
Real-time polymerase chain reaction
MSCs incubated with pro-inflammatory cytokines, ICAM-1high MSCs, MSCs transfected with the empty vector, and untransfected MSCs were seeded in six-well plates at a density of 5×104 cells/cm2 and cultured in osteogenic differentiation medium for 10 days before the cells were harvested. The osteogenic differentiation medium contained 10−7 M dexamethasone, 10 mM β-glycerol phosphate, and 50 μM ascorbate-2-phosphate in a base medium of α-MEM supplemented with 10% FBS.3 To investigate the potential role of signaling pathways in the osteogenic suppression induced by ICAM-1, the chemical inhibitors SB203580HCL, PD98059, JNK inhibitor II, JSH-23, and LY294002 (20 μm/mL of each), which are specific inhibitors of the p38-MAPK, ERK-MAPK, JNK-MAPK, NF-κB, and PI3K/AKT pathways, respectively, were added to the osteogenic differentiation culture. Insulin-like growth factor-1(IGF-1, 100 ng/mL), an activator of the PI3K/AKT pathway, was added to the culture medium to clarify the association between PI3K/AKT pathway and ICAM-1-induced osteogenic suppression. Total RNA was extracted from MSCs with TRIzol reagent (Invitrogen) and reverse transcribed using the mRNA Selective PCR Kit (TaKaRa). Mouse ICAM-1, Runx2, OCN, Sca-1, CD105, CD140a, Nanog, Oct4, and Sox2 cDNA were also amplified by real-time PCR using the SYBR Green PCR kit (Sigma). The primer sequences used for real-time PCR are shown in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/tea).
Western blotting
ICAM-1high MSCs, MSCs transfected with the empty vector, and untransfected MSCs were plated in six-well plates at a density of 1×105 cells/cm2 and incubated overnight. The cells were starved in serum-free α-MEM medium for at least 6 h. Protein lysis buffer (Biorad) was added, and thawed lysates were vortexed and centrifuged. To detect the activity of NF-κB, nuclear proteins from MSCs were prepared using a Nuclear Protein Extraction Kit (Pierce). The proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. The membranes were blocked by incubation with 5% wt/vol nonfat dry milk. Membranes were then incubated with anti-p38, anti-phospho-p38, anti-ERK, anti-phospho-ERK, anti-JNK, anti-phospho-JNK, anti-NF-κB-p65, anti-phospho- NF-κB-p65, anti-PI3K, anti-phospho-PI3K, anti-AKT, anti-phospho-AKT, Lamin B1 (Cell Signaling Technology), and/or β-actin (Sigma) Abs at the appropriate dilutions overnight at 4°C. After incubation, the membranes were washed in Tris Buffered Saline Tween-20 (TBST). Secondary antibody conjugated to horseradish peroxidase was added to the membranes in 5% nonfat dry milk in TBST.
RNA interference of ICAM-1
The mouse ICAM-1 targeting siRNAs (ICAM-1 mimic) and negative control (NC) were obtained from Ribobio (www.ribobio.com/). The RNA interference (RNAi) experiments were performed according to the manufacturer's protocol. Briefly, ICAM-1high MSCs and MSCs incubated with inflammatory mediators were seeded in six-well plates at a density of 1×105 cells/cm2, When the cells reached 60–80% confluence, the mimic (10 nM) and NC siRNAs were each mixed with RNA transfection buffer(ribo FECT CP) before being added to the MSC culture medium. After 48–72 h, real-time PCR and FACS were performed to determine the percentage reduction of ICAM-1 expression. The efficiency of RNAi is shown in Supplementary Figure S1.
Culture of MSCs in poly(lactic-co-glycolic acid) scaffolds
The poly(lactic-co-glycolic acid) (PLGA) scaffolds were prepared according to a protocol previously described,20 with minor modifications. PLGA was fabricated into porous scaffolds (85% porosity and 200–300 μm pore diameter). PLGA scaffolds were treated with 70% alcohol for sterilization. Then, the PLGA scaffolds were prepared using a hole puncher (5 mm in diameter and 4 mm in thickness) to fit rat calvarial defects. A total of 2×106 ICAM-1high MSCs, MSCs transfected with the empty vector, or untransfected MSCs were seeded into each sterile PLGA scaffold. For in vivo cell tracking, the fluorescent carbocyanine CM-Dil (Invitrogen) was used to label the cells according to the manufacturer's protocol. The MSC-PLGA constructs were cultured in six-well plates with complete medium for 24 h.
Transplantation of the MSCs-PLGA construct in the rat calvarial defect model
The MSC-PLGA constructs were collected and gently washed in PBS for the transplantation experiment. Sixty normal inbred SD male rats were purchased from the Laboratory Animal Center of the Chinese Academy of Military Medical Science. All animal experiments were performed in accordance with the Chinese Academy of Military Medical Sciences Guide for Laboratory Animals. In brief, after general anesthesia with 7% chloral hydrate, the skin and underlying tissues of the vertex were raised to expose the calvaria. Then, full-thickness defects (5 mm in diameter) were generated using a dental bur. The animal groups are shown in Table 1. After the defects were implanted with MSC-PLGA constructs, the soft tissue and skin incision were closed with absorbable sutures. Rats without scaffold engraftment and rats transplanted with blank scaffolds (no cells) served as controls. After surgery, SD rats were kept in clean conditions with sufficient food and water.
Table 1.
Treatment Groups of Experimental Animals
| Group | Rat number | Treatment |
|---|---|---|
| A | 12 | Calvarial defects without any grafts |
| B | 12 | Calvarial defects engrafted with scaffolds without cells |
| C | 12 | Calvarial defects engrafted with scaffolds and un-transfected MSCs |
| D | 12 | Calvarial defects engrafted with scaffolds and vector-transfected MSCs |
| E | 12 | Calvarial defects engrafted with scaffolds and ICAM-1-transfected MSCs |
ICAM-1, intercellular adhesion molecule-1; MSC, mesenchymal stem cells.
Evaluation of osteogenic differentiation and the repair effects of calvarial defects in vivo
To track MSCs in vivo in scaffolds engrafted into rats, tissue samples from defect areas were harvested from five groups as shown in Table 1 (three samples per group) 10 weeks after transplantation, and prepared according to previously described protocols.21,22 In brief, the samples were decalcified in Decalcifying Fluid (Zhongshan) for 4 days at room temperature and washed in running water for approximately 2 h. Seven micrometer frozen sections were prepared and stained with DAPI for 15 min. The sections were observed using inverted fluorescence microscopy (Nikon TE2000-U).
For bone regeneration assays, five rats in each group were sacrificed by cervical dislocation at 20 weeks, and their calvariae were harvested. Hard tissue slicing was routinely performed. Briefly, tissue samples were fixed in neutral formalin and embedded in epoxide resin. Five micrometer sections were prepared and stained with hematoxylin and eosin (H&E), May-Grünwald-Giemsa, toluidine blue, and von Kossa stains.
Semi-quantitative image analysis was performed according to previously published methods.21,22 In brief, five micrometer decalcified sections of the sample were stained with H&E, and modified Masson Trichrome as previously described.21,22 The percentage of newly formed bone within the defect was estimated using the computerized image analysis software Image-Pro Plus (IPP) 6.0.
Statistical analysis
Data were represented as the mean values with standard deviations. Statistical significance was analyzed using the Student's t test. p-values less than 0.05 were considered significant.
Results
Inflammatory mediators suppressed osteogenic activity of MSCs by inducing the expression of ICAM-1
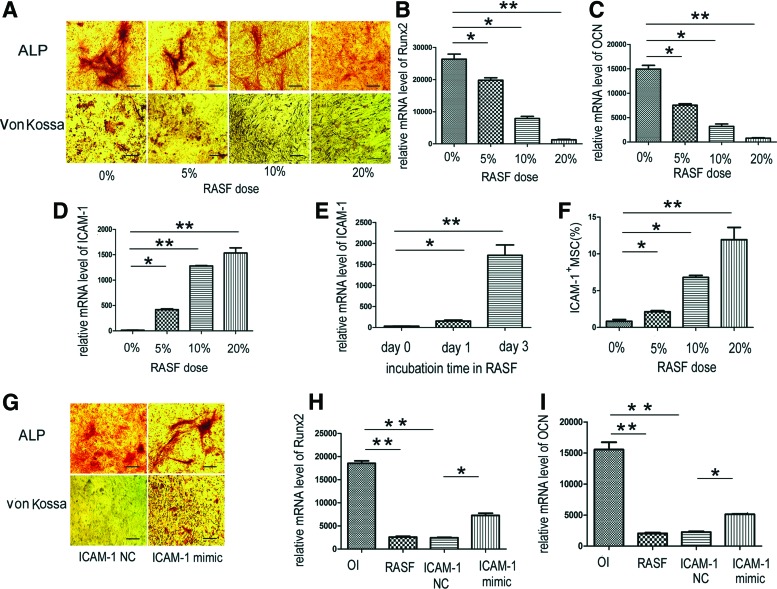
Because RASF has a crucial role in bone remodeling in the inflammatory microenvironment and MSCs are potential progenitors of osteoblasts, the influence of cell-free RASF on the osteogenic activity of MSCs was examined. In the presence of osteogenic medium, cell-free RASF incubation was able to suppress ALP activity in a dose-dependent manner (Fig. 1A). The in situ von Kossa assay showed that RASF also inhibited bone matrix mineralization (Fig. 1A). In addition, the mRNA of key bone forming markers, Runx2 and OCN, was significantly downregulated with the incubation of RASF (Fig. 1B, C) (*p<0.05; **p<0.01). ICAM-1 in MSCs was observed to be upregulated in a time- and dose-dependent manner after incubation with RASF (Fig. 1D–F) (*p<0.05; **p<0.01).
FIG. 1.
Rheumatoid arthritis synovial fluid (RASF) suppressed osteogenic activity of mesenchymal stem cells (MSCs) by inducing the expression of intercellular adhesion molecule-1 (ICAM-1). The osteogenic induction medium (OI) contained 10−7 M dexamethasone, 10 mM β-glycerol phosphate, and 50 μM ascorbate-2-phosphate in a base medium of α-MEM supplemented with 10% FBS. In the presence of OI, cell-free RASF incubation suppressed ALP activity and matrix mineralization in MSCs in a dose-dependent manner (A). The mRNA of Runx2 and OCN were downregulated with the incubation of RASF (B, C). In addition, the mRNA and protein expression of ICAM-1 in MSCs was upregulated in a time- and dose-dependent manner after incubation with RASF (D–F). Moreover, the ALP and von Kossa staining results showed that MSCs incubated with RASF partially recovered their osteogenic differentiation capacity after ICAM-1 expression was knocked down (G). In addition, the mRNA of Runx2 and OCN was upregulated when ICAM-1 was silenced (H, I). Scale bars represent 100 μm (A). (*p<0.05; **p<0.01). ALP, alkaline phosphatase; OCN, osteocalcin. Color images available online at www.liebertpub.com/tea
To clarify the potential association of ICAM-1 to MSC osteogenic differentiation, mouse ICAM-1 targeted siRNA was prepared and RNAi was performed. Interestingly, the ALP and von Kossa staining results showed that the MSCs incubated with RASF partially recovered their osteogenic differentiation capacity after ICAM-1 expression was knocked down (Fig. 1G). In addition, the mRNA of the osteogenic markers, Runx2 and OCN was upregulated when ICAM-1 was silenced (Fig. 1H, I) (*p<0.05; **p<0.01).
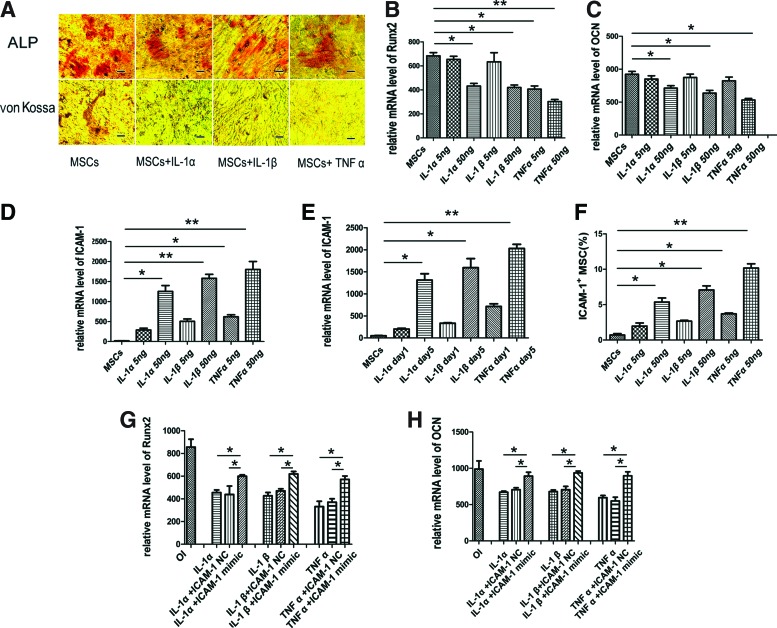
Though numerous studies have reported that pro-inflammatory cytokines in RASF are involved in bone remodeling,12–14 it is unknown whether the cytokines directly induce the expression of ICAM-1 and inhibit osteogenic differentiation by MSCs. In the presence of osteogenic medium, ALP activity and bone matrix mineralization were remarkably suppressed after MSCs were incubated with IL-1α, IL-1β, or TNF-α (50 ng/mL) for 5 days (Fig. 2A). In addition, the mRNA of key bone forming markers, Runx2 and OCN, was significantly downregulated in a dose-dependent manner (Fig. 2B, C) (*p<0.05; **p<0.01). Consistent with the data from RASF experiments, the expression of ICAM-1 in MSCs increased in a time- and dose-dependent manner after incubation with pro-inflammatory cytokines (Fig. 2D–F) (*p<0.05; **p<0.01). Importantly, the mRNA expression of Runx2 and OCN in MSCs incubated with pro-inflammatory cytokines (50 ng/mL) was partially rescued when ICAM-1 was downregulated by RNAi. (Fig. 2G, H) (*p<0.05).
FIG. 2.
IL-1α, IL-1β, and TNF-α suppressed osteogenic activity of MSCs by inducing the expression of ICAM-1. In the presence of osteogenic medium, ALP activity and bone matrix mineralization were remarkably suppressed after MSCs were incubated with IL-1α, IL-1β, or TNF-α (50 ng/mL) for 5 days (A). In addition, the mRNA of Runx2 and OCN was significantly downregulated in a dose-dependent manner (B, C) (*p<0.05; **p<0.01). Consistent with the data from RASF experiments, the expression of ICAM-1 in MSCs increased in a time- and dose-dependent manner after incubation with pro-inflammatory cytokines (D–F) (*p<0.05;**p<0.01). In addition, the mRNA expression of Runx2 and OCN in MSCs incubated with pro-inflammatory cytokines (50 ng/mL) was partially rescued when ICAM-1 was downregulated by RNAi. (G, H) (*p<0.05). Scale bars represent 100 μm (A). Color images available online at www.liebertpub.com/tea
Interestingly, though the siRNA can knock down 70% (Supplementary Fig. S1) of ICAM-1 the recovery in the expression of Runx2 and OCN is only about 40% (Fig. 1H, I), which suggest that there are some other factors in RASF such as pro-inflammatory cytokines that might also exert osteogenic inhibition independent of ICAM-1.
High expression of ICAM-1 in MSCs by gene transfection inhibited their osteogenic differentiation in vitro
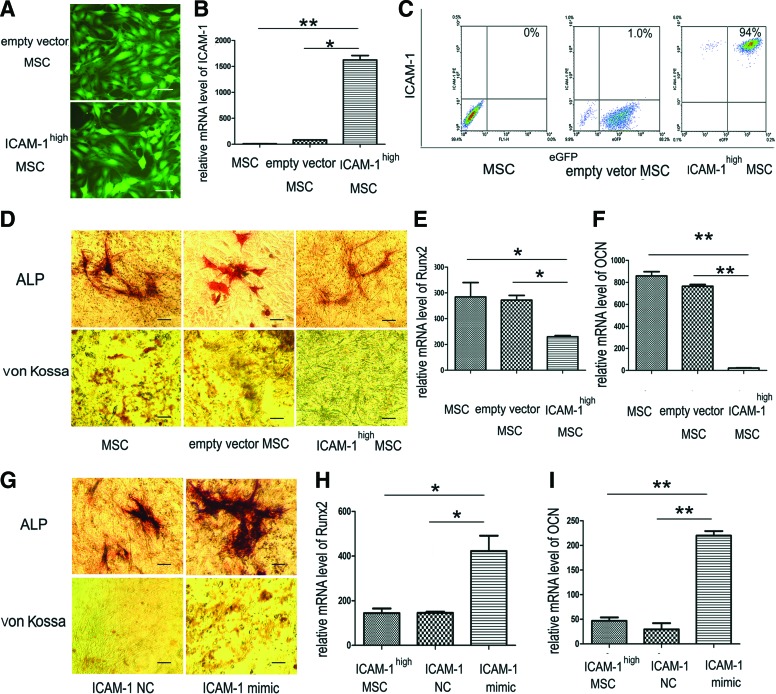
Though knockdown of ICAM-1 expression could partially rescue the osteogenic differentiation of MSCs treated with inflammatory mediators, it remains unknown whether ICAM-1 exerts an immediate regulation effect on MSCs. To explore the direct role of ICAM-1 on MSC osteogenesis, ICAM-1 was steadily overexpressed in MSCs via recombinant DNA technology.
The percentage of green fluorescent MSCs was observed by fluorescence microscopy (Fig. 3A). The polybrene-mediated virus infection efficiency in this study was more than 95%. Real-time PCR showed that the expression of ICAM-1 mRNA significantly increased after infection (Fig. 3B) (*p<0.05; **p<0.01). Immunophenotyping showed that the positive rate of ICAM-1 protein expression significantly increased from 0.5% before infection to 94% after infection (Fig. 3C).
FIG. 3.
Overexpression of ICAM-1 in MSCs by gene transfection inhibited their osteogenic differentiation in vitro. As shown in (A), the percentage of green fluorescent MSCs was observed by fluorescence microscopy. The polybrene-mediated virus infection efficiency was more than 95%. Real-time PCR data showed that the expression of ICAM-1 mRNA significantly increased after infection (B). Immuno-phenotyping showed that the positive rate of ICAM-1 protein expression significantly increased from 0.5% before infection to 94% after infection (C). In situ ALP and von Kossa staining showed that compared with control cells, osteogenic activity of ICAM-1high MSCs was remarkably suppressed (D). Moreover, real-time PCR results showed the Runx2 and OCN mRNA expression decreased sharply (E, F). After ICAM-1 targeted RNAi, both ALP activity and bone matrix mineralization were partially rescued (G). In addition, the mRNA expression of Runx2 and OCN recovered when ICAM-1 was knocked down (H, I). (*p<0.05, **p<0.01) Scale bars represent 100 μm (A, D, G). Color images available online at www.liebertpub.com/tea
To investigate the osteogenic differentiation capacity of ICAM-1high MSCs, the cells were maintained in osteogenic media. In situ ALP and von Kossa staining showed that the osteogenic activity of ICAM-1high MSCs was remarkably suppressed relative to that of control cells (Fig. 3D). Consistent with the histological results, real-time PCR results showed the Runx2 and OCN mRNA expression decreased sharply (Fig. 3E, F) (*p<0.05; **p<0.01).
After ICAM-1 targeted RNAi, both ALP activity and bone matrix mineralization were partially rescued (Fig. 3G). In addition, the mRNA expression of Runx2 and OCN recovered when ICAM-1 was knocked down (Fig. 3H, I) (*p<0.05; **p<0.01).
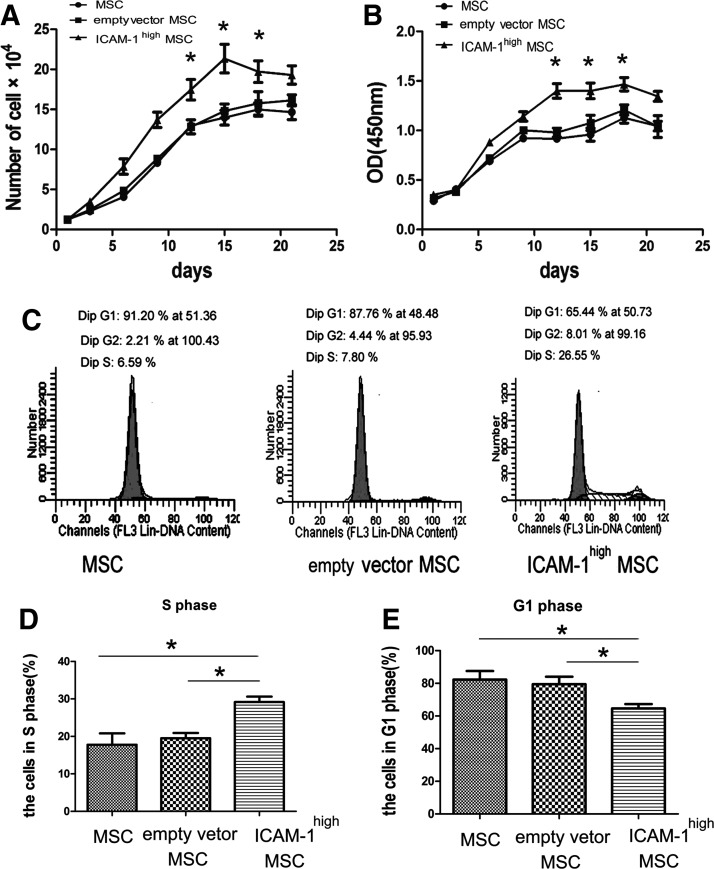
High expression of ICAM-1 in MSCs influenced cell proliferation and the cell cycle
To exclude the possibility that high expression of ICAM-1 impairs the growth of MSCs and reduces the number of osteoprogenitors, a cell proliferation assay and cell cycle analysis were performed in the present study.
Trypan blue exclusion cell counting (Fig. 4A) and a CCK-8-based cell proliferation assay (Fig. 4B) showed that ICAM-1high MSCs exert stronger proliferative effects than control treatment (*p<0.05). The data indicated that ICAM-1 enhances the repopulating ability of MSCs.
FIG. 4.
High expression of ICAM-1 on MSCs influenced cell proliferation and the cell cycle. Trypan blue exclusion cell counting (A) and a CCK-8-based cell proliferation assay (B) showed that ICAM-1high MSCs exert stronger proliferative effects than control cells (*p<0.05). Increased cell growth is also reflected by an increased number of cells in S phase and a decreased representation of cells arrested in the G0/G1 phase. Representative data from three separate experiments are shown in (C). A higher percentage of ICAM-1high MSCs (29.18%±2.47%) were in S phases than the vector-transfected control (19.5%±2.45%) and untransfected control (17.77%±5.25%) (D), indicating that an increased number of MSCs proceeded into G2/S phase after ICAM-1 overexpression (*p<0.05). In the control groups, most cells (79.47%±7.87% of the vector-transfected control and 82.30%±9.03% of the untransfected control) stayed in the G1 phase (E) while fewer cells (64.66%±4.57%) were found in the G1 phase of ICAM-1high MSCs (Fig. 4E) (*p<0.05).
Increased cell growth is also reflected by an increased number of cells in S phase and a decreased representation of cells arrested in G0/G1 phase. To show the relationship between MSC proliferation and ICAM-1 overexpression, flow cytometry was used to examine the cell cycle distribution of ICAM-1high MSCs and the control cells. Representative data from three separate experiments are shown in Figure 4C. A higher percentage of ICAM-1high MSCs (29.18%±2.47%) were in S phases compared with the vector-transfected MSCs (19.5%±2.45%) and untransfected MSCs (17.77%±5.25%) (Fig. 4D), indicating that an increased number of MSCs proceeded into G2/S phase after ICAM-1 overexpression (*p<0.05). In the control groups, most cells (79.47%±7.87% of the vector-transfected control and 82.30%±9.03% of the untransfected control) stayed in G1 phase (Fig. 4E) while fewer cells (64.66%±4.57%) were found in G1 phase of ICAM-1high MSCs (Fig. 4E) (*p<0.05).
ICAM-1 impaired stem cell marker expression in MSCs
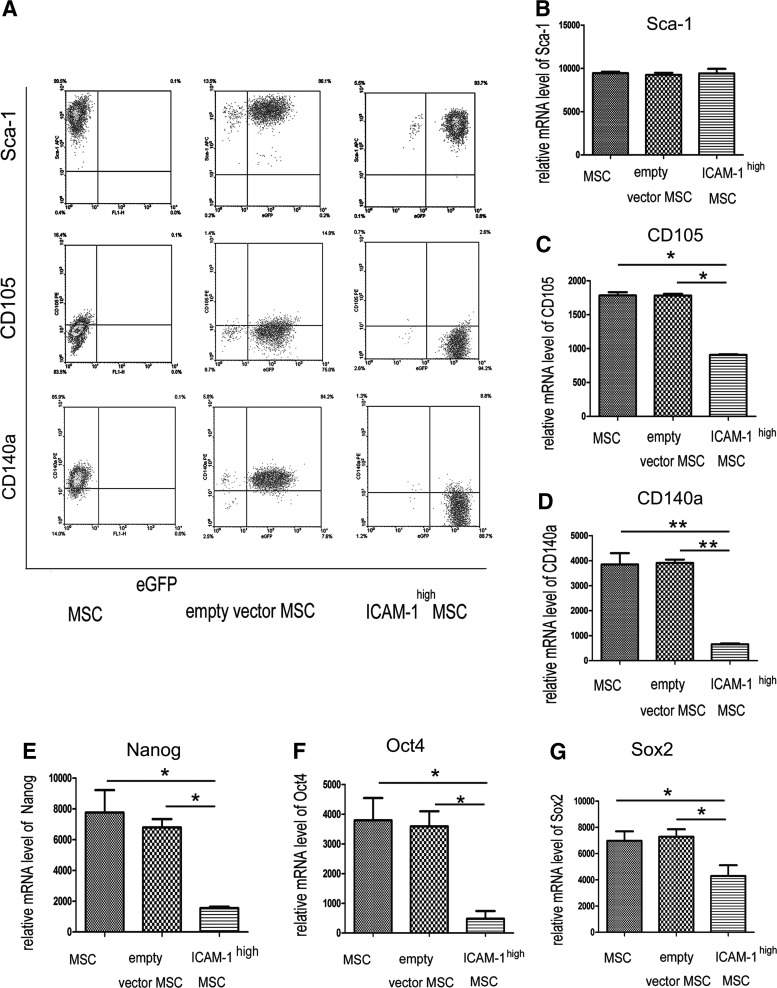
Sca-1 and PDGF receptor α (CD140a) have been regarded as markers for multipotent mesenchymal stem/progenitor cells.23,24 As shown in Figure 5A, the expression of CD140a+ cells was significantly lower in ICAM-1high cells (8.8%) than in cells transfected with the empty vector (84%), implicating ICAM-1 overexpression may be involved in the regulation of MSC stemness. However, the expression of Sca-1 was not influenced by ICAM-1 upregulation (Fig. 5A).
FIG. 5.
ICAM-1 impaired stem cell marker expression in MSCs. Representative data from three separate experiments are shown in (A). Briefly, the expression of CD140a+ cells was significantly lower in ICAM-1high cells (8.8%) than that in cells transfected with the (84%), which implicated ICAM-1 overexpression may be involved in the regulation of MSC stemness. However, the expression of Sca-1 was not influenced by ICAM-1 upregulation (A). A lower percentage of ICAM-1high MSCs (2.6%) than vector-transfected control cells (14.9%) were CD105+ (A). The mRNA levels of these markers were examined and the results of real-time PCR showed that the mRNA levels of CD105 and CD140a were lower in ICAM-1high MSCs (B–D). (E–G) showed that ICAM-1 overexpression decreased the expression of the pluripotent differentiation genes (Nanog, Oct4, and Sox2). (*p<0.05, **p<0.01).
Endoglin (CD105) was previously described as a marker of osteo-chondrogenic potential of MSCs.25 A lower percentage of CD105+ cells (2.6%) were in ICAM-1high MSCs compared with the vector-transfected control cells (14.9%) (Fig. 5A).
To further determine the effect of ICAM-1 on stem cell markers of MSCs, the mRNA levels of Sca-1, CD105, and CD140a were examined by real-time PCR. These two pluripotency-associated markers (CD105 and CD140a) were lower in ICAM-1high MSCs (Fig. 5B–D) (*p<0.05; **p<0.01).
We also observed (data not shown) that the cells highly expressed CD29 (β1- integrin) and CD44 (receptor for hyaluronate and osteopontin) and were homogenously negative for CD45 (pan-hematopoietic marker), CD31 (endothelial cell marker), MHC-Ia (MHC- II molecule), and CD86.
Nanog, Oct4, and Sox2 are known to be essential for the maintenance of the multipotent property of embryonic stem cells. MSCs also express some levels of these pluripotent genes,26–29 and their diminished expression is usually related to the loss of differentiation potential of MSCs. Figure 5E–G shows that ICAM-1 overexpression decreased the expression of these genes (*p<0.05; **p<0.01).
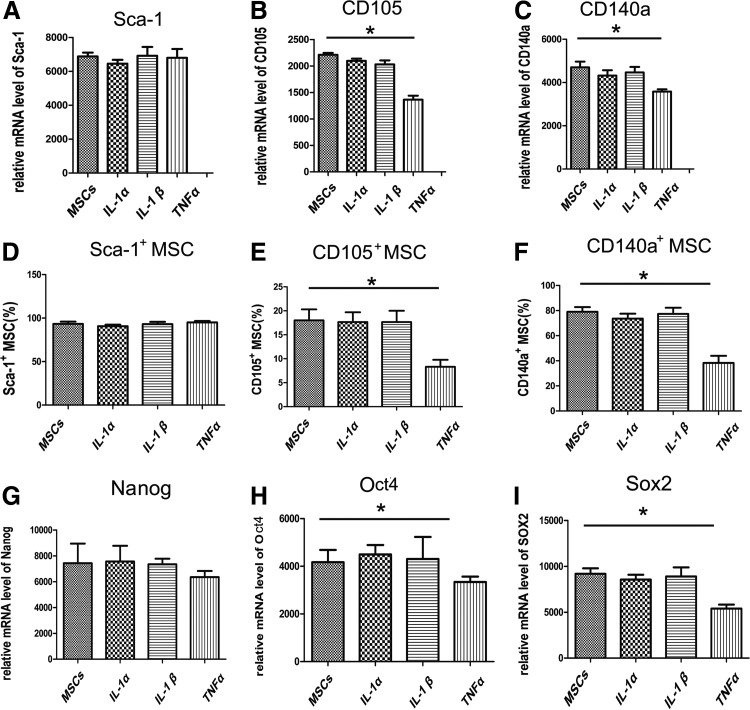
The effect of pro-inflammatory cytokines on stem cell surface markers (Sca-1, CD105, and CD140a) and stemness-associated transcription factors (Nanog, Oct4, and Sox2) was shown in Figure 6. However, only TNF-α significantly suppressed the expression of CD105 and CD140a (Fig. 6A–F) (*p<0.05), which indicates that TNF-α plays a key role in ICAM-1-induced osteogenic suppression. In addition, TNF-α inhibited the expression of Oct4 and Sox2 while IL-1α and IL-β did not (Fig. 6G–I) (*p<0.05).
FIG. 6.
IL-1α, IL-1β, and TNF-α modulate stem cell marker expression in MSCs. The effect of pro-inflammatory cytokines on the stem cell surface markers (Sca-1, CD105, and CD140a) and stemness-associated transcription factors (Nanog, Oct4, and Sox2) was shown in Figure 6. However, only TNF-α significantly suppressed the expression of CD105 and CD140a (A–F) (*p<0.05). In addition, TNF-α inhibit the expression of Oct4 and Sox2 while IL-1α or IL-β did not (G–I) (*p<0.05).
Impaired bone repair of rat calvarial defects by ICAM-1high MSCs
MSC-PLGA constructs were prepared as described previously in the “Materials and Methods section.” Rat calvarial defects (5 mm in diameter and full-thickness) were generated and transplanted with MSC-PLGA constructs of the same size as the calvarial defect (data not shown).
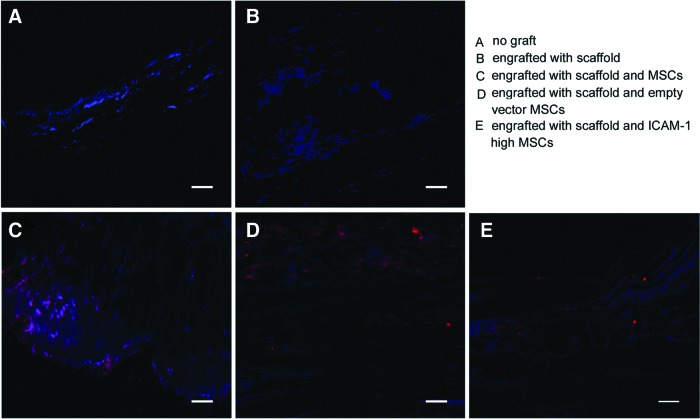
To determine the origin of the repairing tissues, three rats in each group were sacrificed at 10 weeks after MSC-PLGA constructs were transplanted, and the sections containing cells labeled with carbocyanine CM-Dil were observed under fluorescence microscopy (red staining). DAPI counterstaining was performed to identify recipient cells (blue staining). Calvarial defects that were not engrafted with scaffolds or that were engrafted with scaffolds without cells were used as negative controls. As showed in Figure 7A and B, there were no explanted cells in the area engrafted with no graft or with the blank scaffold. In contrast, the CM-Dil-labeled explanted cells can be observed in the MSC-PLGA engrafted groups (Fig. 7C–E).
FIG. 7.
Engrafted MSC tracking in vivo at 10 weeks post-transplantation. The MSCs on poly(lactic-co-glycolic acid) (PLGA) scaffolds were labeled by fluorescent carbocyanine CMDil before implantation. The sections containing cells labeled with fluorescent carbocyanine CM-Dil were observed by fluorescence microscopy (red staining). DAPI counterstaining was performed to distinguish recipient cells (blue staining). Calvarial defects that were not engrafted with scaffolds or that were engrafted with scaffolds without cells were used as negative controls. As showed in (A, B), there were no explanted cells in the area engrafted with no graft or with the blank scaffold. In contrast, the CM-Dil-labeled explanted cells can be observed in the bone defects implanted with MSC-PLGA constructs (C–E). Scale bars represent 200 μm (A–E). Color images available online at www.liebertpub.com/tea
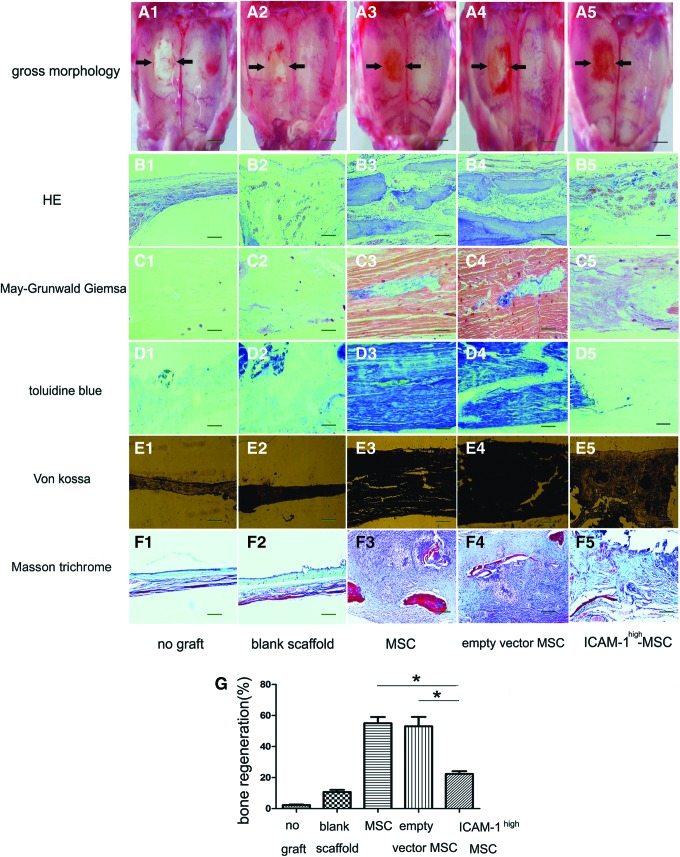
Five rats in each group were sacrificed 20 weeks post MSC-PLGA implantation to identify the bone repair efficiency. As shown in Figure 8A, it was easy to observe the bone repair effect of each group by the gross morphology of the calvariae. Calvarial defects that were left unengrafted completely failed to close (Fig. 8A1). The rats engrafted with blank PLGA scaffolds showed reduced defect size but still had visibly incomplete closure (Fig. 8A2). In contrast, calvarial defects transplanted with untransfected MSC-PLGA constructs were well repaired (Fig. 8A3). The rat calvariae engrafted with PLGA and vector-transfected MSCs exhibited a similar repair effect (Fig. 8A4). However, the calvarial defect engrafted with PLGA and ICAM-1high MSCs remained red in color though the defect closed, which may suggest the defect was covered with osteoid (Fig. 8A5).
FIG. 8.
Impaired bone repair effect of rat calvarial defects by ICAM-1high MSCs. As it was showed by arrows in (A), calvarial defects that were left unengrafted completely failed to close (A1). The rats engrafted with blank PLGA scaffolds showed a reduced defect size but still had visibly incomplete closure (A2). In contrast, calvarial defects transplanted with untransfected MSC-PLGA constructs were well repaired (A3). The rat calvariae engrafted with PLGA and vector-transfected MSCs exhibited a similar repair effect (A4). However, the calvarial defect engrafted with PLGA and ICAM-1high MSCs remained red in color though the defect closed, which may suggest the defect was covered with osteoid (A5). The histological data showed that only a thin tissue membrane cover the calvarial defect in the rats without any graft (B1, C1, D1, and E1) and a fibrous-like connective tissue filled the defect area of the rats engrafted with the blank PLGA scaffold (B2, C2, D2, and E2). Bone-like tissue filled in the defect of the rats engrafted by both untransfected MSC-PLGA constructs (B3, C3, D3, and E3) and vector-transfected MSCs-PLGA constructs (B4, C4, D4, and E4). However, only small bone nodules were observed in the calvarial defect of the rats implanted with ICAM-1high MSC-PLAG constructs (B5, C5, D5, and E5). The collagen fibers in newly formed bones were shown by Masson Trichrome staining. The regeneration of collagen fibers was also suppressed when ICAM-1 was overexpressed in MSCs (F1–F5). The calvarial defect repair percentage analysis was performed by Image-Pro Plus. Panel G shows that new born bone formation in the defect grafted by untransfected MSC-PLGA and vector-transfected MSC-PLGA were higher than those in defect grafted by the ICAM-1high MSC-PLGA (*p<0.05). Scale bars represent 500 mm (A1–A5), 200 μm (B1–F5), respectively. Color images available online at www.liebertpub.com/tea
Further histological analysis demonstrated the different bone forming activities of each group in detail. H&E and May-Grünwald Giemsa staining were employed to generally inspect the repaired tissues. Toluidine blue staining showed osteoblast differentiation and von Kossa staining revealed mineralization activity in vivo.
The histological data showed that only a thin tissue membrane covered the calvarial defect in the group without any scaffold graft (Fig. 8B1, C1, D1, E1, and F1) and a fibrous-like connective tissue filled the defect area engrafted with the blank PLGA scaffold (Fig. 8B2, C2, D2, E2, and F2). Bone-like tissue filled the defect engrafted by both untransfected MSC-PLGA constructs (Fig. 8B3, C3, D3, E3, and F3) and vector-transfected MSC-PLGA constructs (Fig. 8B4, C4, D4, E4, and F4). However, only small bone nodules were observed in the calvarial defect that was repaired with ICAM-1high MSC-PLGA constructs (Fig. 8B5, C5, D5, E5, and F5). The collagen fibers in new bones were shown by Masson Trichrome staining. The regeneration of collagen fibers was also suppressed when ICAM-1 was overexpressed in MSCs (Fig. 8F1–F5).
The new bone analysis was performed according to the results of H&E, von Kossa, and Masson Trichrome staining. The calvarial defect repair percentage was calculated by Image-Pro Plus.24,25 Figure 8G shows that new bone formation in the defect engrafted by untransfected MSC-PLGA (55.0%±7.0%) and vector-transfected MSC-PLGA (53.0%±10.58%) was higher than that in defects grafted by the ICAM-1high MSC-PLGA(22.33%±3.06%) (*p<0.05).
The MAPK, NF-κB, and PI3K/AKT pathways are involved in ICAM-1-induced osteogenic suppression
ICAM-1 mediates cellular interactions by binding its counter-receptors, lymphocyte function-associated antigen-1 (LFA-1 and CD11a/CD18) and Mac-1 (CD11b/CD18).15,16 To analyze the molecular mechanism related to ICAM-1-induced suppression of osteogenesis, the expression of CD18, the component of LFA-1 and Mac-1, was detected by real-time PCR (Supplementary Fig. S2A) (**p<0.01). FACS results of ICAM-1high MSCs and control cells are shown in Supplementary Figure S2B. However, CD18 was not expressed on the cells, which suggested that the suppressive effect did not occur via the ICAM-1/LFA-1 or ICAM-1/Mac-1 pathways (Supplementary Fig. S2A, B).
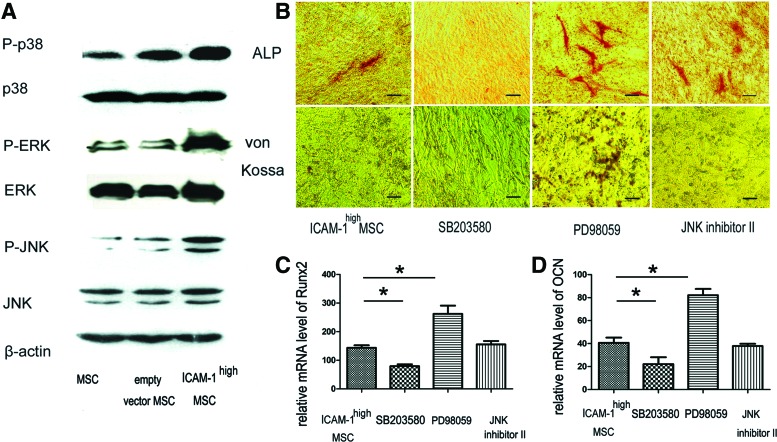
To further examine the intracellular signaling cascades, we investigated the phosphorylation of MAPK, NF-κB, and PI3K/AKT pathways in MSCs because they represent inflammatory signaling pathways that control the differentiation of MSCs.30–36 As shown in Figure 9A, ICAM-1 overexpression induced the phosphorylation of p38/MAPK, ERK/MAPK, and JNK/SAPK. We further studied the effect of the specific chemical inhibitors (SB203580HCL, PD98059, and JNK inhibitor II) on osteogenic differentiation in ICAM-1high MSCs. ICAM-1 induced osteogenic suppression was partially rescued by PD98059 (Fig. 9B). In contrast, the addition of SB23580HCL resulted in more serious inhibition of osteogenic differentiation (Fig. 9B). In addition, treatment with JNK inhibitor II did not significantly influence ICAM-1-induced inhibition (Fig. 9B).
FIG. 9.
The MAPK pathways are involved in ICAM-1-induced osteogenic suppression. As shown in (A), ICAM-1 overexpression induced the phosphorylation of p38/MAPK, ERK/MAPK, and JNK/SAPK. ICAM-1-induced suppression of osteogenesis was partially rescued by PD98059 (B). In contrast, the addition of SB23580HCL resulted in more serious inhibition of osteogenic differentiation (B). In addition, JNK inhibitor II did not significantly influence ICAM-1-induced osteogenic inhibition (B). Moreover, PD98059 elevated the mRNA expression of Runx2 and OCN while SB203580HCL inhibited the mRNA expression of Runx2 and OCN in ICAM-1high MSCs (C, D). JNK inhibitor II exhibited little effect on these two osteogenic markers. (*p<0.05). Scale bars represent 100 μm (B). Color images available online at www.liebertpub.com/tea
PD98059 consistently elevated the mRNA expression of Runx2 (relative mRNA level, 272.43%±26.49%) and OCN (relative mRNA level, 82.1%±7.75%) while SB203580HCL inhibited the mRNA expression of Runx2 (relative mRNA level, 79.49%±9.37%) and OCN (relative mRNA level, 18.71%±3.42%) in ICAM-1high MSCs (Fig. 9C, D) (*p<0.05). JNK inhibitor II exhibited little effect on these two osteogenic markers. Therefore, the results indicate that ERK/MAPK and p38/MAPK are involved in the modulatory effects of ICAM-1 on osteogenesis.
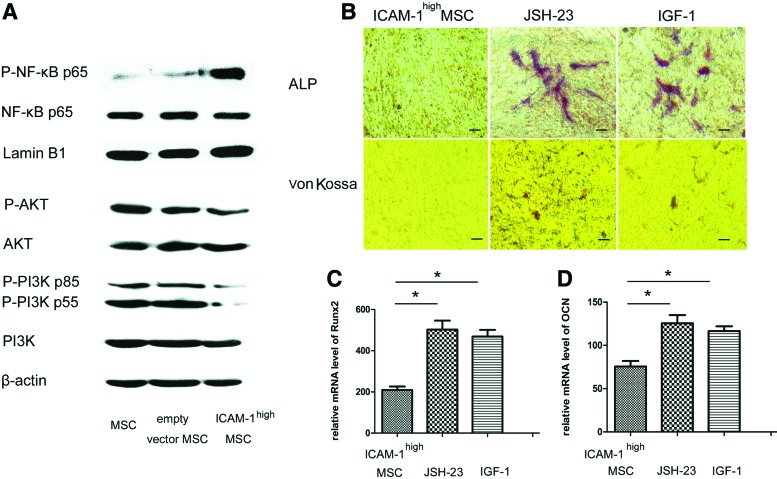
In general, pro-inflammatory cytokines utilize the NF-κB signaling pathway and/or the PI3K pathway.34–36 It is important to determine whether these other signaling pathways are involved in the ICAM-1-induced osteogenic suppression in addition to the MAPK pathways. As shown in Figure 10A, ICAM-1 induced the phosphorylation of NF-κB but suppressed the activation of PI3K/AKT in MSCs. The inhibitory effect of ICAM-1 on ALP activity and bone matrix mineralization was partially restored after the specific inhibitor JSH-23 of the NF-κB pathway or the activator IGF-1 of PI3K/AKT was added to the MSC culture (Fig. 10B). Furthermore, the mRNA expression of Runx2 and OCN in ICAM-1high MSCs was upregulated by JSH-23 or IGF-1 (Fig. 10C, D) (*p<0.05).
FIG. 10.
The NF-κB and PI3K/AKT pathways are involved in ICAM-1 induced osteogenic suppression. As shown in (A), ICAM-1 induced the phosphorylation of NF-κB but suppressed the activation of PI3K/AKT in MSCs. The inhibitory effect of ICAM-1 on ALP activity and bone matrix mineralization was partially restored after the specific inhibitor JSH-23 of the NF-κB pathway or activator IGF-1 of the PI3K/AKT was added to the MSC culture (B). Furthermore, the mRNA expression of Runx2 and OCN in ICAM-1high MSCs was upregulated by JSH-23 or IGF-1 (C, D) (*p<0.05). Scale bars represent 100 μm (B). Color images available online at www.liebertpub.com/tea
While PI3K/AKT signaling is a major signaling pathway induced by IGF-1, IGF-1 can activate other signaling pathways such as the MAPK/ERK pathway. To exclude the potential influence of that, the specific inhibitor of PI3K/AKT pathway were used to see whether there was an inhibition of osteogenic suppression. As shown in Supplementary Figure S3A, the inhibitory effect of ICAM-1 on ALP activity and bone matrix mineralization was strengthened after the specific inhibitor LY294002 of the PI3K/AKT was added to the MSC culture. Furthermore, the mRNA expression of Runx2 and OCN in ICAM-1high MSCs was downregulated by LY294002 (Supplementary Fig. S3B, C) (*p<0.05). Bars represent 100 μm (Supplementary Fig. S3A).
Discussion
MSCs are potent progenitors of osteoblasts and MSC-based therapy is a promising therapeutic approach for bone regeneration.5–7 However, bone regeneration in inflammatory microenvironments is usually repressed by inflammatory mediators. It has been demonstrated that pro-inflammatory cytokines exert direct effects on osteoprogenitors and/or osteoclast precursors by stimulating chemokines, adhesion molecules, and signaling pathways to inhibit bone formation and enhance bone destruction.8–11,14
ICAM-1 is present at the surface of osteoprogenitors at a low expression level. Its expression is upregulated in the presence of pro-inflammatory cytokines including TNF-α and IL-1β.17–19,37 Previous studies have shown that ICAM-1 regulates bone remodeling by promoting osteoclastogenesis.38,39
However, in the current study, we found that ICAM-1 was important in the osteogenic differentiation of MSCs. RASF contains numerous pro-inflammatory cytokines that promote osteoclast formation.12 We incubated MSCs with RASF to mimic bone regeneration in an inflammatory microenvironment and observed that MSCs were reluctant to differentiate into osteoblasts. Meanwhile, ICAM-1 expression in MSCs was upregulated in an RASF dose-dependent manner; thus, we explored whether ICAM-1 directly affects osteogenesis. Strikingly, we found that osteogenic differentiation recovered after ICAM-1 was knocked down. An identical effect was observed when MSCs were directly incubated with pro-inflammatory cytokines such as IL-1α, IL-1β, and TNF-α. To further confirm the association between ICAM-1 and osteogenic differentiation, ICAM-1 was steadily overexpressed in MSCs. We found that osteogenic differentiation of MSCs was significantly inhibited in vitro after high ICAM-1 expression. Consistently, ICAM-1 knockdown partially rescued the osteogenic differentiation in ICAM-1 transfected MSCs.
More importantly, the results of the cell proliferation assay and the cell cycle analysis excluded the possibility that highly expressed ICAM-1 may impair the growth of MSCs and reduce the number of osteoprogenitors. However, the stem cell markers CD140a and CD105 were remarkably downregulated by overexpressed ICAM-1. These results showed that ICAM-1 enhances MSC proliferation but impairs the multipotent capacity of MSCs. Similar effects on stem cells markers and transcription factors were observed when MSCs were stimulated by TNF-α, which suggests that TNF-α influences MSC multipotent capacity and regulates bone regeneration in inflammatory microenvironments.
Although in vitro data demonstrated that ICAM-1 inhibits osteogenesis, it remains unknown whether the adhesion molecule was potent enough to prevent bone formation in vivo. The general observation and histological analysis of the rat calvarial defects indicated that highly expressed ICAM-1 in MSCs impairs bone repair. These data prompt us to rethink the implications of ICAM-1 in inflammatory factor-mediated bone destruction. The results also suggest that strategies that target adhesion molecules in MSCs may influence bone regeneration.
ICAM-1 and its co-receptors (LFA-1 and Mac-1) are considered to be critically important in various inflammatory bone diseases.15,40 However, we failed to detect CD18 (a common component of LFA-1 and Mac-1) on untransfected MSCs, vector transfected MSCs and ICAM-1 highly expressed MSCs, which suggest that ICAM-1 inhibited osteogenic differentiation via other pathways instead of ICAM-1/LFA-1 axis.
The MAPK, NF-κB, and PI3K/AKT pathways are potent modulators of osteoblastogenesis30–36 and are closely involved with inflammatory cytokine-induced cell proliferation and differentiation. We found that ICAM-1 remarkably activated the p38/MAPK, ERK/MAPK, and JNK/SPAK pathways. Importantly, blockage of the ERK/MAPK pathways rescued osteogenic differentiation but arresting the p38/MAPK pathway resulted in more dampened osteogenic differentiation, which indicates that ICAM-1 inhibited osteogenesis via activation of the ERK/MAPK pathway. The p38/MAPK pathway appears to maintain osteogenesis in MSCs. The results suggest that specific chemical inhibitors of signaling pathways may be effective agents to improve bone repair in inflammatory environments.
In addition, ICAM-1 activated the NF-κB pathway but suppressed the PI3K/AKT pathways. “Closing” the NF-κB pathway or “opening” the PI3K/AKT pathway could improve the osteogenesis of ICAM-1high MSCs. The data suggest that ICAM-1 modulates osteogenic differentiation through multiple mechanisms, however, further investigations are needed to reinforce these conclusions.
In summary, we identified an inhibitory function of ICAM-1 on osteogenesis in vitro and in vivo. We also explored how the osteogenesis of MSCs is regulated by ICAM-1. Our findings may help in the development of more effective bone regeneration strategies in inflammatory microenvironments.
Supplementary Material
Acknowledgments
This study was supported by the National Natural Science Foundation (81371945, 81101342, 31070996, and 31171084), and Beijing Natural Sciences Grants (No. 7132133).
Disclosure Statement
The authors declare no competing financial interests.
References
- 1.Prockop D.J.Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276,71, 1997 [DOI] [PubMed] [Google Scholar]
- 2.da Silva Meirelles L., Chagastelles P.C., and Nardi N.B.Mesenchymal stem cells reside in virtually all postnatal organs and tissues. J Cell Sci 119,2204, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Zhu H., Guo Z.K., Jiang X.X., Li H., Wang X.Y., Yao H.Y., et al. . A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat Protoc 5,550, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Huebsch N., Arany P.R., Mao A.S., Shvartsman D., Ali O.A., Bencherif S.A., et al Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater 9,518, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phinney D.G., and Prockop D.J.Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair-current views. Stem Cells 25,2896, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Teitelbaum S.L.Stem cells and osteoporosis therapy. Cell Stem Cell 7,553, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Levi B., and Longaker M.T.Concise review: adipose-derived stromal cells for skeletal regenerative medicine. Stem Cells 29,576, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilbert L., et al. . Expression of the osteoblast differentiation factor RUNX2 (Cbfa1/AML3/Pebp2alpha A) is inhibited by tumor necrosis factor-alpha. J Biol Chem 277,2695, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Reikerås O., Shegarfi H., Wang J.E., and Utvåg S.E.Lipopolysaccharide impairs fracture healing: an experimental study in rats. Acta Orthop 76,749, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Bastian O., Pillay J., Alblas J., Leenen L., Koenderman L., and Blokhuis T.Systemic inflammation and fracture healing. J Leukoc Biol 89,669, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Yang N., Wang G., Hu C., Shi Y., Liao L., Shi S., et al. . Tumor necrosis factor α suppresses the mesenchymal stem cell osteogenesis promoter miR-21 in estrogen deficiency-induced osteoporosis. J Bone Miner Res 28,559, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Rivollier A., Mazzorana M., Tebib J., Piperno M., Aitsiselmi T., Rabourdin-Combe C., et al. . Immature dendritic cell transdifferentiation into osteoclasts: a novel pathway sustained by the rheumatoid arthritis microenvironment. Blood 104,4029, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Kosinska M.K., Liebisch G., Lochnit G., Wilhelm J., Klein H., Kaesser U., et al. . A Lipidomic study of phospholipid classes and species in human synovial fluid. Arthritis Rheum 65,2323, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Zhu H., Jiang X.X., Guo Z.K., Li H., Su Y.F., Yao H.Y., et al. . Tumor necrosis factor-alpha alters the modulatory effects of mesenchymal stem cells on osteoclast formation and function. Stem Cells Dev 18,1473, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Smith C.W., Marlin S.D., Rothlein R., Toman C., and Anderson D.C.Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest 83,2008, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Staunton D.E., Dustin M.L., Erickson H.P., and Springer T.A.The arrangement of the immunoglobulin-like domains of ICAM-1 and the binding sites for LFA-1 and rhinovirus. Cell 61,243, 1990 [DOI] [PubMed] [Google Scholar]
- 17.Ren G., Zhao X., Zhang L., Zhang J., L'Huillier A., Ling W., et al. . Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol 184,2321, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ren G., Roberts A.I., and Shi Y.Adhesion molecules: key players in Mesenchymal stem cell-mediated immunosuppression. Cell Adh Migr 5,20, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi Y., Su J., Roberts A.I., Shou P., Rabson A.B., and Ren G.How mesenchymal stem cells interact with tissue immune responses. Trends Immunol 33,136, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang F., Qu X., Cui W., Bei J., Yu F., Lu S., et al. . Manufacturing and morphology structure of polylactide-type microtubules orientation-structured scaffolds. Biomaterials 27,4923, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Yuan W., Zong C., Huang Y., Gao Y., Shi D., Chen C., et al. . Biological, immunological and regenerative characteristics of placenta-derived mesenchymal stem cell isolated using a time-gradient attachment method. Stem Cell Res 9,110, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Zong C., Xue D., Yuan W., Wang W., Shen D., Tong X., et al. . Reconstruction of rat calvarial defects with human mesenchymal stem cells and osteoblast-like cells in poly-lactic-co-glycolic acid scaffolds. Eur Cell Mater 1,109, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Holmes C., and Stanford W.L.Concise review: stem cell antigen-1: expression, function, and enigma. Stem Cells 25,1339, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Morikawa S., Mabuchi Y., Kubota Y., Nagai Y., Niibe K., Hiratsu E., et al. . Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med 206,2483, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang T., Liu W., Lv X., Sun H., Zhang L., Liu Y., et al. . Potent in vitro chondrogenesis of CD105 enriched human adipose-derived stem cells. Biomaterials 31,3564, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Chambers I., Silva J., Colby D., Nichols J., Nijmeijer B., Robertson M., et al. . Nanog safeguards pluripotency and mediates germline development. Nature 450,1230, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Lavial F., Acloque H., Bertocchini F., Macleod D.J., Boast S., Bachelard E., and Montillet G.The Oct4 homologue PouV and Nanog regulate pluripotency in chicken embryonic stem cells. Development 134,3549, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Basu-Roy U., Ambrosetti D., Favaro R., Nicolis S.K., Mansukhani A., et al. . The transcription factor Sox2 is required for osteoblast self-renewal. Cell Death Differ 17,1345, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seo E., Basu-Roy U., Zavadil J., Basilico C., and Mansukhani A.Distinct functions of Sox2 control self-renewal and differentiation in the osteoblast lineage. Mol Cell Biol 31,4593, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu X., Chen S., Orlando S.A., Yuan J., Kim E.T., Munugalavadla V., et al. . p85alpha regulates osteoblast differentiation by cross-talking with the MAPK pathway. J Biol Chem 286,13512, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang J., Sonoyama W., Wang Z., Jin Q., Zhang C., Krebsbach P.H., et al. . Noncanonical Wnt-4 signaling enhances bone regeneration of mesenchymal stem cells in craniofacial defects through activation of p38 MAPK. J Biol Chem 282,30938, 2007 [DOI] [PubMed] [Google Scholar]
- 32.He J., Liu Z., Zheng Y., Qian J., Li H., Lu Y., et al. . p38 MAPK in myeloma cells regulates osteoclast and osteoblast activity and induces bone destruction. Cancer Res 72,6393, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitehouse C.A., Waters S., Marchbank K., Horner A., McGowan N.W., Jovanovic J.V., et al. . Neighbor of Brca1 gene (Nbr1) functions as a negative regulator of postnatal osteoblastic bone formation and p38 MAPK activity. Proc Natl Acad Sci U S A 107,12913, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang J., Liu F., Lee M., Wu B., Ting K., Zara J.N., et al. . NF-κB inhibit osteogenic differntiation of mesenchymal stem cells by promoting β-ctenin degradation. Proc Natl Acad Sci U S A 110,9469, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao W., Guan M., Jia J., Dai W., Lay Y.A., Amugongo S., et al. . Reversing bone loss by directing mesenchymal stem cells to bone. Stem Cells 31,2003, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen L.L., Huang M., Tan J.Y., Chen X.T., Lei L.H., Wu Y.M., et al. . PI3K/AKT pathway involvement in the osteogenic effects of ostoclast culture supernatants on preosteoblast cells. Tissue Eng Part A 19,2226, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dustin M.L., Rothlein R., Bhan A.K., Dinarello C.A., and Springer T.A.Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol 137,245, 1986 [PubMed] [Google Scholar]
- 38.Tanaka Y., Morimoto I., Nakano Y., Okada Y., Hirota S., Nomura S., et al. . Osteoblasts are regulated by the cellular adhesion through ICAM-1 and VCAM-1. J Bone Miner Res 10,1462, 1995 [DOI] [PubMed] [Google Scholar]
- 39.Tanaka Y., Maruo A., Fujii K., Nomi M., Nakamura T., Eto S., et al. . Intercellular adhesion molecule 1 discriminates functionally different populations of human osteoblasts: characteristic involvement of cell cycle regulators. J Bone Miner Res 15,1912, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Bloemen V., Schoenmaker T., de Vries T.J., and Everts V.Direct cell-cell contact between periodontal ligament fibroblasts and osteoclast precursors synergistically increases the expression of genes related to osteoclastogenesis. J Cell Physiol 222,565, 2010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.