Abstract
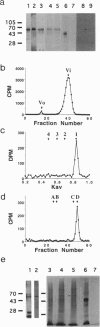
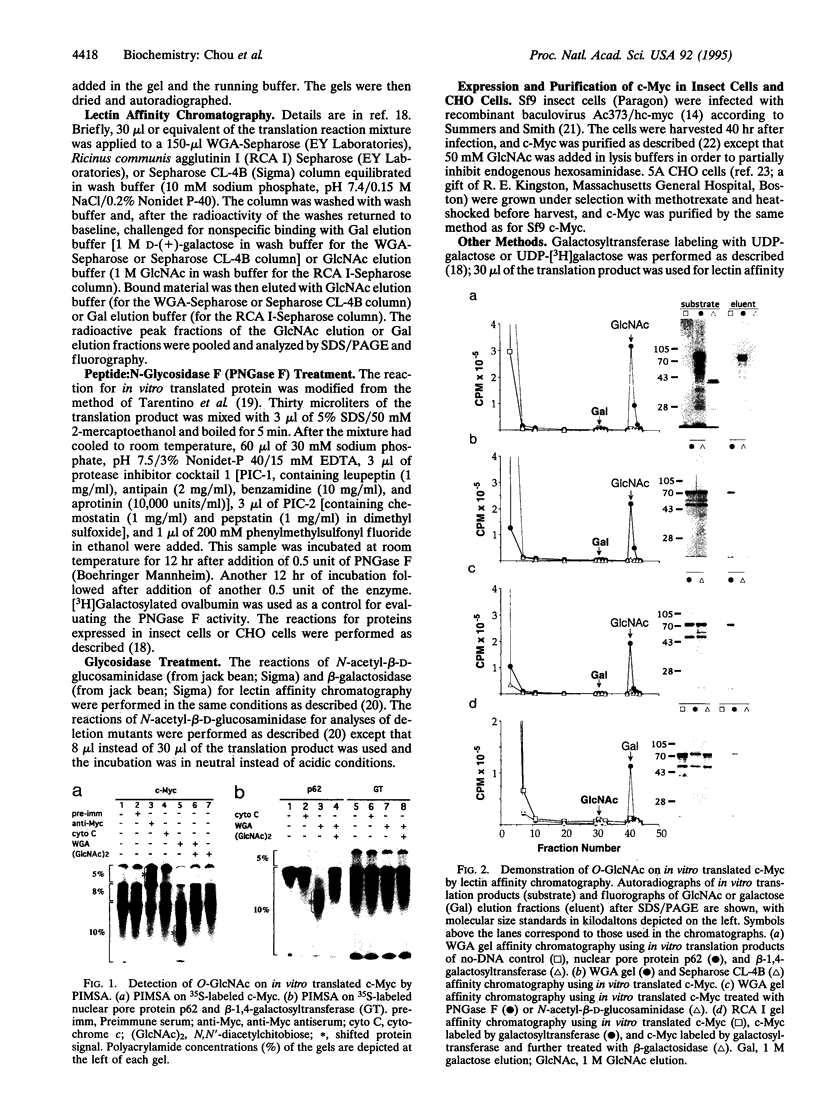
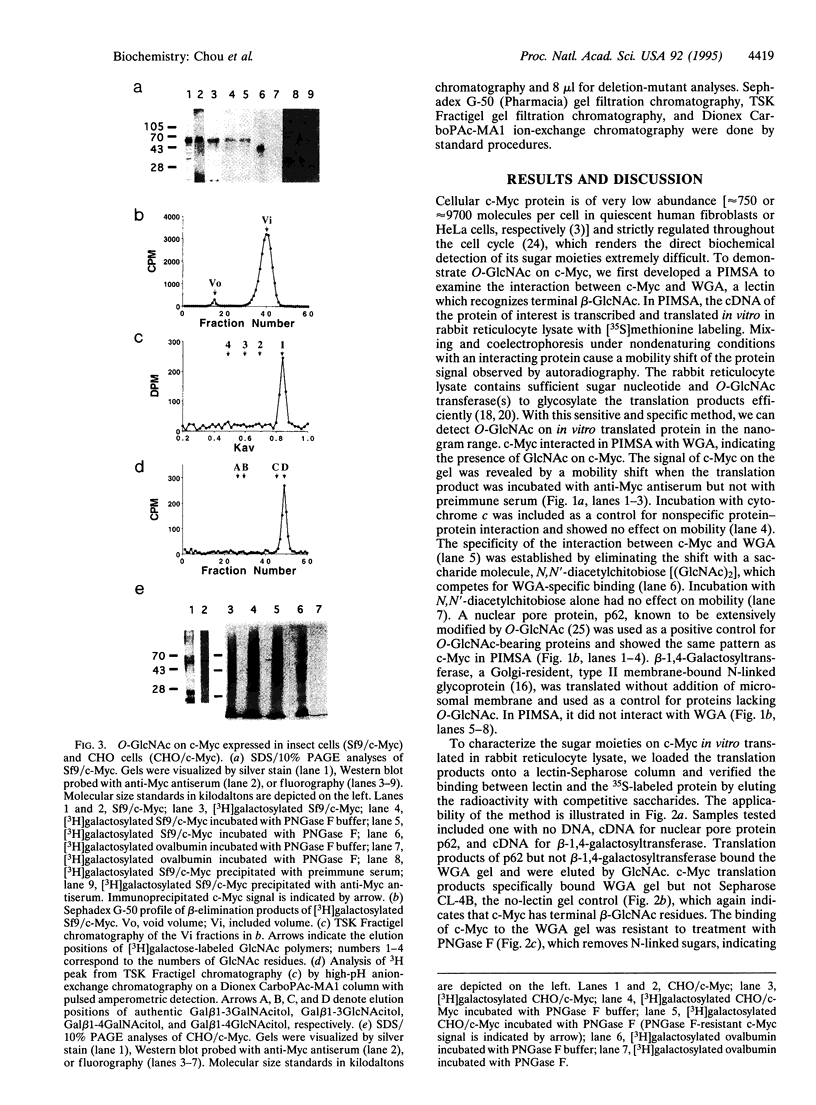
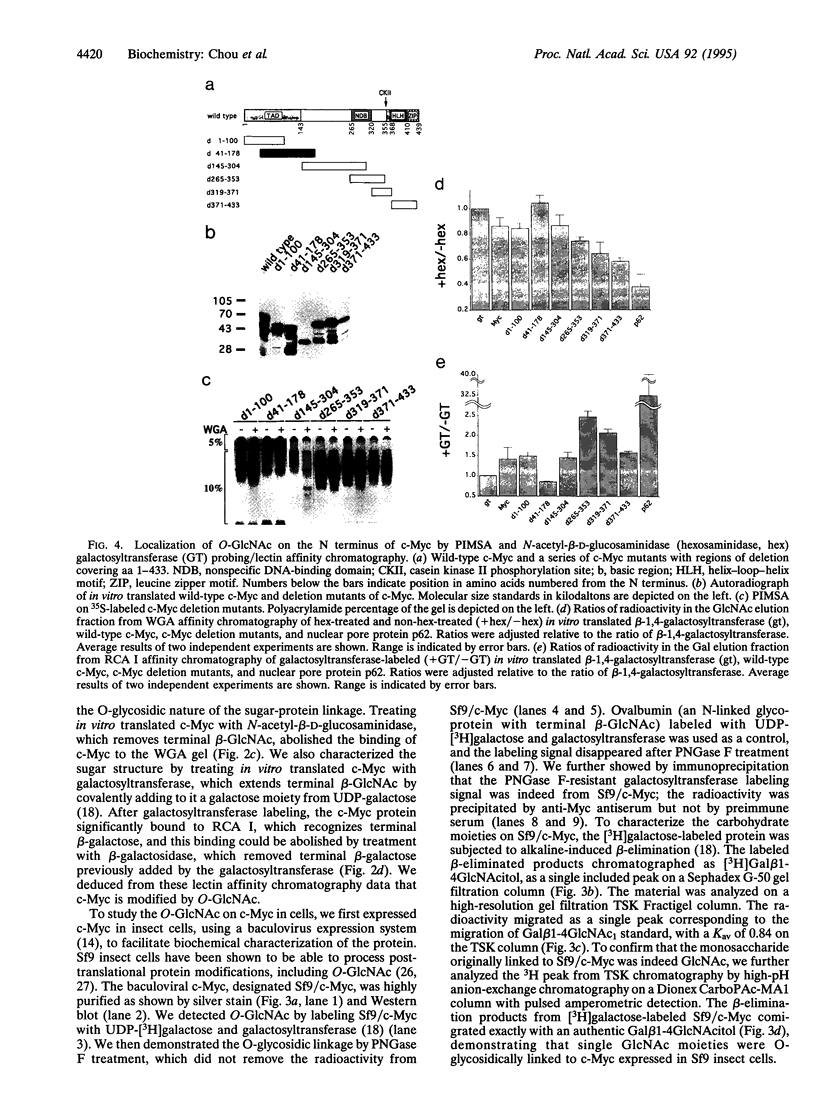
O-linked N-acetylglucosamine (O-GlcNAc) is an abundant and dynamic posttranslational modification composed of a single monosaccharide, GlcNAc, glycosidically composed of a single monosaccharide, GlcNAc, glycosidically linked to the side-chain hydroxyl of serine or threonine residues. Although O-GlcNAc occurs on a myriad of nuclear and cytoplasmic proteins, only a few have thus far been identified. These O-GlcNAc-bearing proteins are also modified by phosphorylation and form reversible multimeric complexes. Here we present evidence for O-GlcNAc glycosylation of the oncoprotein c-Myc, a helix-loop-helix/leucine zipper phosphoprotein that heterodimerizes with Max and participates in the regulation of gene transcription in normal and neoplastic cells. O-GlcNAc modification of c-Myc is shown by three different methods: (i) demonstration of lectin binding to in vitro translated protein using a protein-protein interaction mobility-shift assay; (ii) glycosidase or glycosyltransferase treatment of in vitro translated protein analyzed by lectin affinity chromatography; and (iii) direct characterization of the sugar moieties on purified recombinant protein overexpressed in either insect cells or Chinese hamster ovary cells. Analyses of serial deletion mutants of c-Myc further suggest that the O-GlcNAc site(s) are located within or near the N-terminal transcription activation/malignant transformation domain, a region where mutations of c-Myc that are frequently found in Burkitt and AIDS-related lymphomas cluster.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert T., Urlbauer B., Kohlhuber F., Hammersen B., Eick D. Ongoing mutations in the N-terminal domain of c-Myc affect transactivation in Burkitt's lymphoma cell lines. Oncogene. 1994 Mar;9(3):759–763. [PubMed] [Google Scholar]
- Amati B., Land H. Myc-Max-Mad: a transcription factor network controlling cell cycle progression, differentiation and death. Curr Opin Genet Dev. 1994 Feb;4(1):102–108. doi: 10.1016/0959-437x(94)90098-1. [DOI] [PubMed] [Google Scholar]
- Blackwood E. M., Eisenman R. N. Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc. Science. 1991 Mar 8;251(4998):1211–1217. doi: 10.1126/science.2006410. [DOI] [PubMed] [Google Scholar]
- Gu W., Bhatia K., Magrath I. T., Dang C. V., Dalla-Favera R. Binding and suppression of the Myc transcriptional activation domain by p107. Science. 1994 Apr 8;264(5156):251–254. doi: 10.1126/science.8146655. [DOI] [PubMed] [Google Scholar]
- Hann S. R., Thompson C. B., Eisenman R. N. c-myc oncogene protein synthesis is independent of the cell cycle in human and avian cells. 1985 Mar 28-Apr 3Nature. 314(6009):366–369. doi: 10.1038/314366a0. [DOI] [PubMed] [Google Scholar]
- Hart G. W., Haltiwanger R. S., Holt G. D., Kelly W. G. Glycosylation in the nucleus and cytoplasm. Annu Rev Biochem. 1989;58:841–874. doi: 10.1146/annurev.bi.58.070189.004205. [DOI] [PubMed] [Google Scholar]
- Holt G. D., Snow C. M., Senior A., Haltiwanger R. S., Gerace L., Hart G. W. Nuclear pore complex glycoproteins contain cytoplasmically disposed O-linked N-acetylglucosamine. J Cell Biol. 1987 May;104(5):1157–1164. doi: 10.1083/jcb.104.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S. P., Tjian R. O-glycosylation of eukaryotic transcription factors: implications for mechanisms of transcriptional regulation. Cell. 1988 Oct 7;55(1):125–133. doi: 10.1016/0092-8674(88)90015-3. [DOI] [PubMed] [Google Scholar]
- Kato G. J., Barrett J., Villa-Garcia M., Dang C. V. An amino-terminal c-myc domain required for neoplastic transformation activates transcription. Mol Cell Biol. 1990 Nov;10(11):5914–5920. doi: 10.1128/mcb.10.11.5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato G. J., Dang C. V. Function of the c-Myc oncoprotein. FASEB J. 1992 Sep;6(12):3065–3072. doi: 10.1096/fasebj.6.12.1521738. [DOI] [PubMed] [Google Scholar]
- Kelly W. G., Dahmus M. E., Hart G. W. RNA polymerase II is a glycoprotein. Modification of the COOH-terminal domain by O-GlcNAc. J Biol Chem. 1993 May 15;268(14):10416–10424. [PubMed] [Google Scholar]
- Ku N. O., Omary M. B. Expression, glycosylation, and phosphorylation of human keratins 8 and 18 in insect cells. Exp Cell Res. 1994 Mar;211(1):24–35. doi: 10.1006/excr.1994.1054. [DOI] [PubMed] [Google Scholar]
- Lüscher B., Kuenzel E. A., Krebs E. G., Eisenman R. N. Myc oncoproteins are phosphorylated by casein kinase II. EMBO J. 1989 Apr;8(4):1111–1119. doi: 10.1002/j.1460-2075.1989.tb03481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto C., Smith G. E., Farrell-Towt J., Chizzonite R., Summers M. D., Ju G. Production of human c-myc protein in insect cells infected with a baculovirus expression vector. Mol Cell Biol. 1985 Oct;5(10):2860–2865. doi: 10.1128/mcb.5.10.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. P., Hancock D. C., Littlewood T. D., Evan G. I. A sensitive and quantitative enzyme-linked immunosorbence assay for the c-myc and N-myc oncoproteins. Oncogene Res. 1987;2(1):65–80. [PubMed] [Google Scholar]
- Papoulas O., Williams N. G., Kingston R. E. DNA binding activities of c-Myc purified from eukaryotic cells. J Biol Chem. 1992 May 25;267(15):10470–10480. [PubMed] [Google Scholar]
- Pulverer B. J., Fisher C., Vousden K., Littlewood T., Evan G., Woodgett J. R. Site-specific modulation of c-Myc cotransformation by residues phosphorylated in vivo. Oncogene. 1994 Jan;9(1):59–70. [PubMed] [Google Scholar]
- Reason A. J., Morris H. R., Panico M., Marais R., Treisman R. H., Haltiwanger R. S., Hart G. W., Kelly W. G., Dell A. Localization of O-GlcNAc modification on the serum response transcription factor. J Biol Chem. 1992 Aug 25;267(24):16911–16921. [PubMed] [Google Scholar]
- Roquemore E. P., Chou T. Y., Hart G. W. Detection of O-linked N-acetylglucosamine (O-GlcNAc) on cytoplasmic and nuclear proteins. Methods Enzymol. 1994;230:443–460. doi: 10.1016/0076-6879(94)30028-3. [DOI] [PubMed] [Google Scholar]
- Russo R. N., Shaper N. L., Shaper J. H. Bovine beta 1----4-galactosyltransferase: two sets of mRNA transcripts encode two forms of the protein with different amino-terminal domains. In vitro translation experiments demonstrate that both the short and the long forms of the enzyme are type II membrane-bound glycoproteins. J Biol Chem. 1990 Feb 25;265(6):3324–3331. [PubMed] [Google Scholar]
- Rustgi A. K., Dyson N., Bernards R. Amino-terminal domains of c-myc and N-myc proteins mediate binding to the retinoblastoma gene product. Nature. 1991 Aug 8;352(6335):541–544. doi: 10.1038/352541a0. [DOI] [PubMed] [Google Scholar]
- Seth A., Alvarez E., Gupta S., Davis R. J. A phosphorylation site located in the NH2-terminal domain of c-Myc increases transactivation of gene expression. J Biol Chem. 1991 Dec 15;266(35):23521–23524. [PubMed] [Google Scholar]
- Starr C. M., D'Onofrio M., Park M. K., Hanover J. A. Primary sequence and heterologous expression of nuclear pore glycoprotein p62. J Cell Biol. 1990 Jun;110(6):1861–1871. doi: 10.1083/jcb.110.6.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr C. M., Hanover J. A. Glycosylation of nuclear pore protein p62. Reticulocyte lysate catalyzes O-linked N-acetylglucosamine addition in vitro. J Biol Chem. 1990 Apr 25;265(12):6868–6873. [PubMed] [Google Scholar]
- Stone J., de Lange T., Ramsay G., Jakobovits E., Bishop J. M., Varmus H., Lee W. Definition of regions in human c-myc that are involved in transformation and nuclear localization. Mol Cell Biol. 1987 May;7(5):1697–1709. doi: 10.1128/mcb.7.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino A. L., Gómez C. M., Plummer T. H., Jr Deglycosylation of asparagine-linked glycans by peptide:N-glycosidase F. Biochemistry. 1985 Aug 13;24(17):4665–4671. doi: 10.1021/bi00338a028. [DOI] [PubMed] [Google Scholar]
- Whitford M., Faulkner P. A structural polypeptide of the baculovirus Autographa californica nuclear polyhedrosis virus contains O-linked N-acetylglucosamine. J Virol. 1992 Jun;66(6):3324–3329. doi: 10.1128/jvi.66.6.3324-3329.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurm F. M., Gwinn K. A., Kingston R. E. Inducible overproduction of the mouse c-myc protein in mammalian cells. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5414–5418. doi: 10.1073/pnas.83.15.5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano T., Sander C. A., Clark H. M., Dolezal M. V., Jaffe E. S., Raffeld M. Clustered mutations in the second exon of the MYC gene in sporadic Burkitt's lymphoma. Oncogene. 1993 Oct;8(10):2741–2748. [PubMed] [Google Scholar]