Abstract
Primary mucosal melanoma of the oral cavity is an exceedingly rare neoplasm which is estimated to comprise 1-2% of all oral malignancies. In contrast to cutaneous melanomas, the risk factors and pathogenesis are poorly understood. The predominate localization of primary oral melanoma is hard palate and maxillary alveolus. Dermoscopy may be utilized as an adjunctive tool in the clinical differential diagnosis of oral mucosal melanoma whenever the lesion is accessible with a dermoscope. Surgery is the mainstay of treatment, but it may be challenging depending on the location of the tumor within the oral cavity and its size. Adjuvant therapy with dacarbazine, platinum analogs, nitrosoureas and interleukin-2 have been utilized with low response rates. Imatinib may be effective for patients with with c-Kit gene mutations. Sunitinib and dasatinib have been reported effective in selected cases. Vemurafenib and dabrafenib are targeted agents for patients with BRAF mutation-positive melanoma. Ipilimumab, an anti-cytotoxic T-lymphocyte antigen 4 antibody and pembrolizumab, a monoclonal antibody targeting programmed death 1 receptor may be a feasible treatment option in patients with metastatic mucosal melanoma.
Keywords: dermoscopy, head and neck melanoma, head and neck cancer, oral cancer, mucosal melanoma
Melanoma is a malignant tumor that arises from melanocytes and is most commonly cutaneous in origin. Extracutaneous melanomas are known to be exceedingly rare and aggressive neoplasms that embrace ocular, mucosal and leptomeningeal melanomas.
Epidemiology
Mucosal melanomas are estimated to comprise 4-6.8 % of all primary melanomas.[1] The incidence rate for mucosal melanoma (MM) is 2.3 per million.[1,2] The incidence of MM increases with age. Older patients have tenfold higher incidence of MM compared to patients under the age of 60 years.[2] Primary oral mucosal melanoma (POMM) is excessively uncommon in prepubertal children.[3] Although there is a slight male preponderance (1.2:1),[1,4] some studies have been reported higher gender distribution of mucosal melanoma for women,[5,6] other indicate that there is no significant difference between sexes in the incidence of this tumor.[2,7] The incidence rate of POMM is highest among Asian men.[3,6] Among the Japanese oral melanoma accounts for 7.5% of all melanomas versus less than 1% in Caucasians.[8,9]
Pathogenesis
In contrast to cutaneous melanomas, the etiology and pathogenesis of POMM is poorly understood and no etiological and intraoral risk factors, other than preexisting pigmented nevi, have been identified.[10,11] POMM are believed to arise from pigmented nevi, pre-existing pigmented areas or de novo (30% cases) from apparently normal mucosa.[9,12] Although Kahn et al[13] described transformation of a benign oral pigmentation to primary oral melanoma, a definite precursor lesion has not been identified.[14,15] In the oral cavity, mechanical trauma including injury from ill-fitting prostheses, infection, tobacco use has been cited as possible causative factors, but its etiological role seems unlikely.[16] Ingested and inhaled environmental carcinogens at high internal body temperature may play some role.[17]
It is probable that most melanoma precursors cell originate from stem/progenitor melanocytes that have acquired cytogenetic alternation of their oncogenes, tumor-suppressor genes and DNA repair genes and have acquired a malignant phenotype.[18,19] Alternatively, precursor melanoma cells may originate as mature melanocytes staying in the submucosa, that have undergone cytogenetic alternation culminating in de-differentation. These melanoma precursor cells have an extended self-renewal capacity, that sustains the growth of melanoma.[20] There is evidence that if the process of biosynthesis of melanin by melanocytes is not regulated adequately the capacity to generate oxidative stress and metabolic by-products is increased. These byproducts may be cytotoxic, genotoxic and/or mutagenic causing DNA damage in the affected melanocytes, thus favoring initial cell transformation and later promoting the progression to cancer of initially transformed melanocytes.[21] The transformed melanocytes express abnormalities in the c-kit/stem cell factor pathway, in the endothelin receptor type B/endothelin pathway, and in the Wnt/b-catenin pathway, and also show abnormal expression of cell-adhesion molecules.[22,23] Rivera et al. indicated that increased c-kit protein expression in atypical melanocytes correlates with activating mutations suggesting a pertinent role of the proto-oncogene KIT in the development of oral mucosal melanoma.[24]
Prasad et al.[25] reported that expression of bcl-2, p53 and loss of p16 expression are frequent and early events in POMM, indicating that dysregulation of the G1/S phase and impairment of programmed cell death may play a role in tumorigenesis in head and neck mucosal tumors. The protein p16/CDKN2 is a member of the cycline-dependent kinase inhibitor protein family encoded by multiple tumor suppressor gene 1 (MTS1). Inactivation of p16 allows cells cells to pass unhindered from G1 to S phase. The wild type p53 causes G1 delay, up-regulates DNA repair genes and promotes apoptosis. The bcl-2 oncogene encodes a family of anti-apoptotic proteins that prolong cell survival.[25]
Clinical features
The predominate location of primary oral melanoma is the hard palate and maxillary alveolus.[6,26] Melanoma of the oral cavity may occur with or without a radial growth phase.[27] Several case series have demonstrated that up to a third of oral melanomas are preceded by melanosis, which is postulated to represent the radial growth phase occurring before invasion of underlying tissues (vertical growth phase).[9,16] The clinical coloration of oral melanomas has a wide range, which can appear as black, brown, white, gray, purple, or reddish.[11] The lesions are asymmetric, irregular in outline, and occasionally multiple [Fig. 1]. Satellite lesions are frequently present surrounding the initial tumor.[16] The surface architecture of oral melanomas ranges from macular to ulcerated and nodular.[28] The typical oral mucosal melanoma presents with three distinct components: a nodular component usually affecting the central part, a flat or slightly elevated, deep brownish-black pigmented plaque component and a light brown macular component.[29] Approximately a third of all oral melanomas are amelanotic.[30] Amelanotic oral melanoma is particularly difficult to diagnose. It may lack a radial growth phase and may be misdiagnosed as an benign tumor (e.g. epulis) or squamous cell carcinoma.[7,31]
Figure 1.
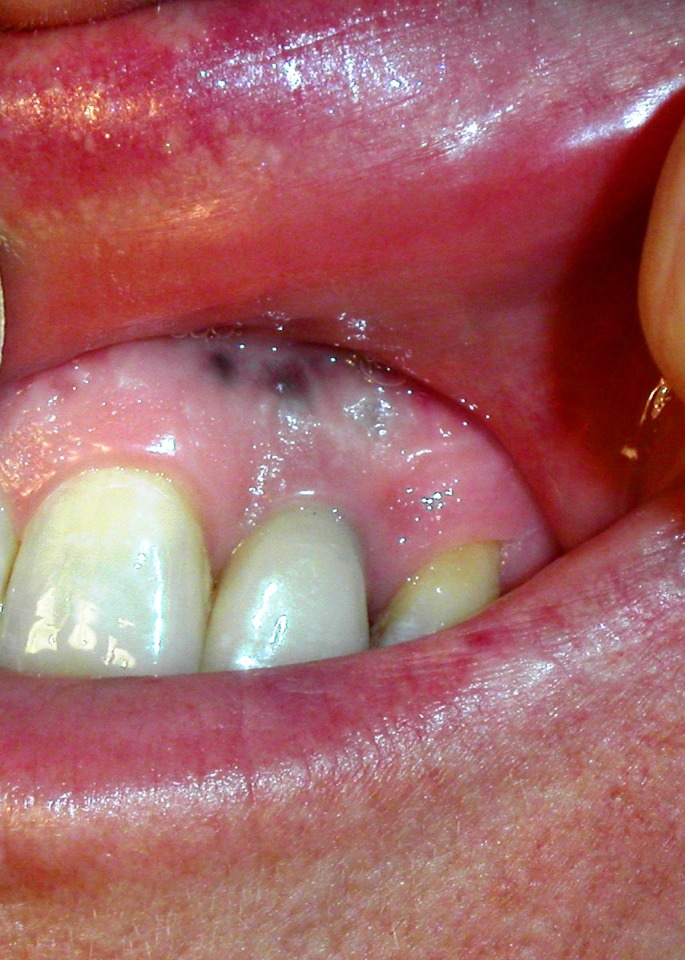
Melanoma of the oral mucous membranes.
Diagnosis and dermoscopy
Because of the anatomic localization and the lack of early signs and symptoms, the diagnosis of oral mucosal melanoma is a challenge and the tumor is usually diagnosed at an advanced stage. The diagnosis of oral mucosal melanoma can be made based on clinical and dermoscopy examination and has to be always confirmed by histopathology.
In 1953 Green et al.[32] first created criteria for diagnosis of primary oral mucosal melanoma which included:
Demonstartion of clinical and microscopic tumor in the oral mucosa
Presence of junctional activity in the oral mucosa
Inability to show any other primary site.
Dermoscopy has emerged as an effective adjunctive tool in the in vivo examination of pigmented skin lesions and in early diagnosis of cutaneous melanoma. Thus far, only limited data about dermoscopic features of mucosal lesions is available. Matsushita et al.[33] reported a case of labial melanoma, which showed dermoscopically irregular diffuse pigmentation with a pseudo-network, accompanied by regression structures and a blue-whitish veil. Olszewska et al.[34] indicated that this is the most typical pattern, not only in melanoma of the labial semi-mucosa, but also in melanoma of oral mucous membranes. This pattern allows easy differentiation of amalgam tattoos, which show a homogenous, slightly grainy, bluish pattern. According to Puig and Malvehy,[35] the dermoscopic appearance of melanoma of mucous membranes may be different in "in situ" and invasive melanoma. Common features include heterogeneity of both color and structures and abrupt cut-off of the pigment pattern at the periphery of the lesion. In "in situ" melanoma some areas show a globular aligned pattern that can resemble idiopathic melanosis, whereas other areas show large blue-grey structures or irregularly distributed dots and globules. In invasive melanoma, blue-whitish veil, ulceration and atypical vascular pattern can be present.[34] Stolz et al.[36] emphasized that irregular jagged borders, asymmetrically pigmented rete ridges and variably-sized granules are most characteristic features of oral melanoma. Lin et al.[37] performed a study, which included melanomas of the oral and genital mucous membranes and the semi-mucosa. The authors showed that 75% of these melanomas had a multicomponent pattern [Fig. 2] and 25% had a homogeneous pattern. The study also showed that the sensitivity of the ABCD rule, Menzies method, 7-point checklist, 3-point checklist and CASH algorithm in diagnosing melanoma of the semi-mucosa and mucous membranes is 100%, 100%, 63%, 88% and 100% respectively. The specificity is 100%, 94%, 100%, 94% and 100%, respectively.[37] The limitation of the study was the inclusion of Asian patients only. Further studies are needed to develop clear-cut dermoscopic criteria for melanoma of oral mucous membranes. However, lack of adequate dermoscopic equipment with flexible disposable tips inhibits progress in this regard.
Figure 2.
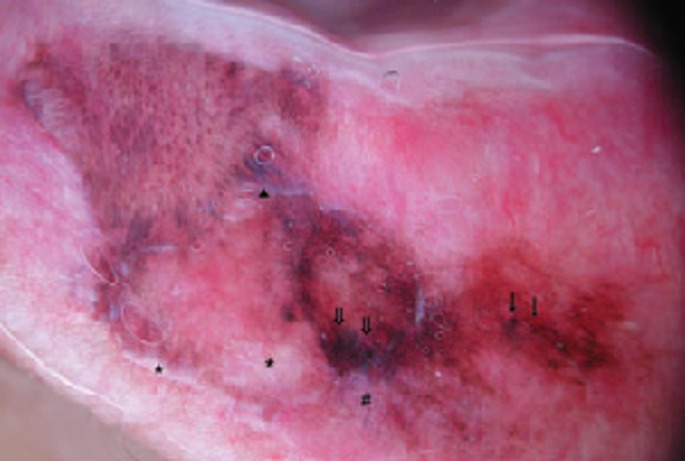
The multicomponent pattern is the most common dermoscopy pattern in mucosal melanoma. The image shows an asymmetric lesion with multiple colors (white, light brown, dark brown, black grey-blue, red), dots (arrows), blotches (double arrows), blue-whitish area (triangle), peppering (#) and areas of regression (stars). Reproduced from Lin et al.[37] with permission.
Excisional biopsy of small lesions and incisional biopsy from the thickest area of larger lesions should be performed for the final histolopathology diagnosis.[38,39]
According to Barker et al.[40] there are three histopathological patterns of melanoma which arise from melanocytes residing in the oral epithelium: an in situ pattern (melanoma is limited to the epithelium and the epithelial-connective tissue interface), a deeply invasive or nodular pattern (melanoma extends into the connective tissue), a combined lesion (nodular component occurs together with an in situ or with radially growing pattern). The phase of radial growth is characterized by proliferation of atypical melanocytes within the oral epithelium and by small breaches of the basement membrane with nests of invading cell, and with reactive inflammatory cell infiltrate in the superficial part of lamina propria. Once the basement membrane is breached, the tumor starts to invade the submucosa, characteristically in nodular aggregates of infiltrating monoclonal melanoma cells that have the capacity to metastasize. Nodular melanoma can develop without any significance preceding phase of radial growth. Mucosal melanoma may also originate from melanocyte precursors residing in the submucosa as a result of arrest in their migration from the neural crest.[12,41]
Immunoreactivity of melanoma cells to antibodies against S-100 protein, MART-1 (Melan-A) and gp100 (HMB-45) can be useful to distinguish oral melanoma from other malignancies.[42,43] Yu et al.[43] examined the expression of HMB-45, S-100 and Melana-A in primary oral and nasal melanomas and their results indicate that HMB-45 and S-100 are positive in 94% and 88% of cases respectively. Fatty acid synthase (FASN) is strongly expressed in mucosal melanomas, and it can be a helpful marker to distinguish oral melanomas from oral melanocytic nevi.[44]
The American Joint Committee on Cancer (AJCC) on Cancer Staging Manual 7th edition (2010)[45] includes a newly developed staging system for mucosal melanoma of head and neck. The new staging criteria reflect the aggressive nature of head neck mucosal melanoma.[46] The AJCC staging system for MM begins with stage T3 as the most limited form of disease [Table 1].
Table 1. AJCC-TNM classification for the mucosal melanoma of the head and neck.
| Primary tumor | |||
|---|---|---|---|
| T3 | Mucosal disease | ||
| T4a | Moderately advanced disease. Tumor involving deep soft tissue, cartilage, bone or overlying skin | ||
| T4b | Very advanced disease. Tumor involving brain, dura, skull base, lower cranial nerves (IX, X, XI, XII), masticator space, carotid artery, prevertebral space or mediastinal structures | ||
| Regional Lymph Nodes | |||
| NX | Regional lymph nodes cannot be assessed | ||
| N0 | No regional lymph node metastases | ||
| N1 | Regional lymph node metastases present | ||
| Distant Metastasis | |||
| M0 | No distant metastasis | ||
| M1 | Distant metastasis present | ||
| Clinical stages | |||
| Stage III | T3 | N0 | M0 |
| Stage IVA | T4a T3-T4a |
N0 N1 |
M0 M0 |
| Stage IVB | T4b | anyN | M0 |
| Stage IVC | anyT | anyN | M1 |
Differential diagnosis of oral mucosal melanoma[47,48] has been summarized in Table 2. The most common mimickers of mucosal melanoma of the oral cavity are amalgam tattoos [Fig. 3]. Dermoscopymay be used for differential diagnosis [Fig. 4].
Table 2. Differential diagnosis of oral mucosal melanoma.
| Differential diagnosis of oral mucosal melanoma |
|---|
| — oral melanotic macule — smoking-associated melanosis — medication-inducted melanosis (e.g. antimalarial drugs, minocycline) — melanoplakia — pituitary Cushing’s syndrome — postinflammatory pigmentation — melanoacanthoma — melanocytic nevus — blue nevus — Spitz nevus — Addison’s disease — Peutz-Jeghers syndrome — amalgam tattoo — Kaposi’s sarcoma — oral metastatic disease — monocytic leukemia — physiologic pigmentation — heavy metal intoxication |
Figure 3.
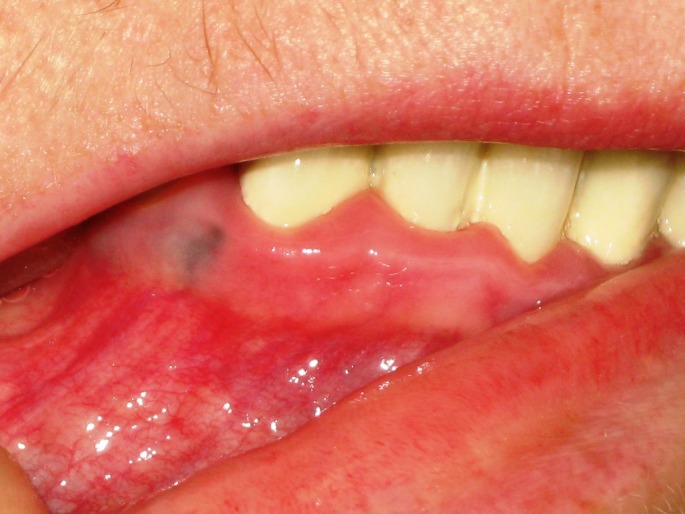
An amalgam tatoo is a frequent melanoma imitator in oral mucous membranes.
Figure 4.
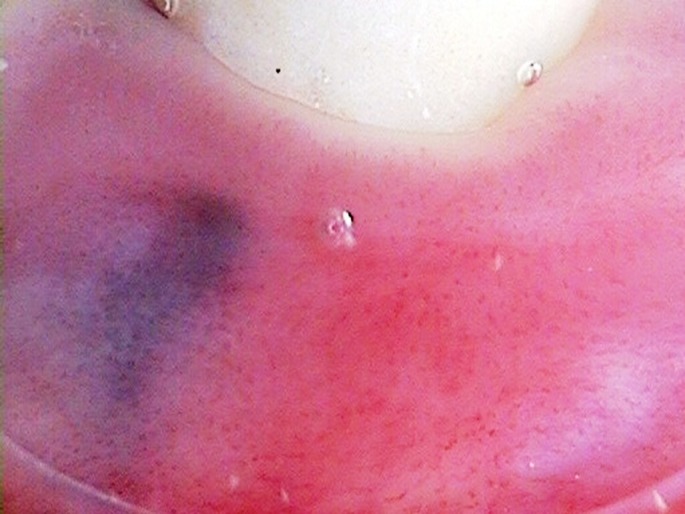
Dermoscopy of an amalgam tattoo. Dermoscopy image showing a homogenous, slightly grainy bluish lesion may allow in vivo distinction of oral an amalgam tattoo from melanoma. Nodular lesions should be excised regardless of dermoscopy results. In the case of superficial lesions dermoscopy may allow to exclude melanoma and avoid unnecessary excisional biopsies. In such cases the lesions should be monitored.
Treatment
The treatment options for mucosal melanomas of oral cavity include surgery, radiation and adjuvant chemotherapy and/or immunotherapy. Surgery is the mainstay of treatment, but it may be challenging, depending on the anatomic location within the oral cavity and extent of the tumor. Optional treatment of clinically negative neck nodes with neck dissection or radiotherapy was recommended because of the high risk of subclinical disease.[49] Although melanoma is classically not radiosensitive, some authors have described improved survival and local control with postoperative radiotherapy.[50] Adjuvant chemotherapy with dacarbazine, platinum analogs, nitrosoureas and immunotherapy with IL-2 have been utilized with a low response rate. Imatinib may be effective for patients with metastatic melanomas with c-Kit gene mutation.[51] Sunitinib and dasatinib have been reported effective in selected cases.[16] Vemurafenib and dabrafenib are targeted agents developed for patients with BRAF V600E mutation-positive metastatic melanoma.[52,53] Ipilimumab, an anti-cytotoxic T-lymphocyte antigen 4 antibody and monoclonal antibodies against programmed death 1 (PD1) receptor may be a feasible treatment option in patients with metastatic mucosal melanoma.[54,55]
Prognosis of mucosal melanoma
Most patients with oral mucosal melanoma have a poor prognosis[14] because they are usually diagnosed at an advantage stage with regional and distant metastases.[56] Independent risk factors in determining outcome are undifferentiated tumor cell morphology, vascular and neural invasion, tumor necrosis, thickness of the tumor, cervical lymph node metastasis and the anatomic sites.[57,58] Other factors associated with poor prognosis are loss of expression of p16 protein and aberrant expression of p53 protein.[25] Expression of bcl-2 is associated with better survival in mucosal melanoma.[25] The 5-year relative survival for mucosal melanomas of head and neck is 25,5%.[59]
Conclusion
Primary oral mucosal melanoma is a rare and biologically aggressive malignancy. In contrast to cutaneous melanomas, the etiology, risk factors and pathogenesis is poorly understood. Because of frequent delays in diagnosis, the tumors are often diagnosed when they are more advanced than the average cutaneous melanoma. Dermoscopy might be utilized as an adjunctive tool in the in vivo examination of oral mucosal melanoma.[60,61] However a limiting factor is the accessibility of the lesion with a dermoscope. In our opinion, future technology R&D should focus on developing a miniaturized, flexible dermoscope that will allow detailed examination of the whole oral cavity. Dermatologists should be aware of the risk of oral mucosal melanoma and include inspection of the oral cavity in regular melanoma check-ups.
References
- Haiducu ML, Hinek A, Astanehe A, Lee TK, Kalia S. Extracutaneous melanoma epidemiology in British Columbia. Melanoma Res. 2014;24:377–380. doi: 10.1097/CMR.0000000000000075. [DOI] [PubMed] [Google Scholar]
- Bishop KD, Olszewski AJ. Epidemiology and survival outcomes of ocular and mucosal melanomas: A population-based analysis. Int J Cancer. 2014;134:2961–2971. doi: 10.1002/ijc.28625. [DOI] [PubMed] [Google Scholar]
- Sortino-Rachou AM, Cancela Mde C, Voti L, Curado MP. Primary oral melanoma: population-based incidence. Oral Oncol. 2009;45:254–258. doi: 10.1016/j.oraloncology.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Sun CZ, Chen YF, Jiang YE, Hu ZD, Yang AK, Song M. Treatment and prognosis of oral mucosal melanoma. Oral Oncol. 2012;48:647–652. doi: 10.1016/j.oraloncology.2012.01.019. [DOI] [PubMed] [Google Scholar]
- Koomen ER, de Vries E, van Kempen LC, van Akkooi AC, Guchelaar HJ, Louwman MW, Nijsten T, Coebergh JW. Epidemiology of extracutaneous melanoma in the Netherlands. Cancer Epidemiol Biomarkers Prev. 2010;19:1453–1459. doi: 10.1158/1055-9965.EPI-09-1267. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Mimura M, Ogi K, Amagasa T. Primary malignant melanoma of the oral cavity: assessment of outcome from the clinical records of 35 patients. Int J Oral Maxillofac Surg. 2004;33:761–765. doi: 10.1016/j.ijom.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Mimura M, Kimijima Y, Amagasa T. Clinical investigation of amelanotic malignant melanoma in the oral region. J Oral Maxillofac Surg. 2004;62:933–937. doi: 10.1016/j.joms.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Takagi M, Ishikawa G, Mori W. Primary malignant melanoma of the oral cavity in Japan. With special reference to mucosal melanosis. Cancer. 1974;34:358–370. doi: 10.1002/1097-0142(197408)34:2<358::aid-cncr2820340221>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Rapini RP, Golitz LE, Greer RO Jr, Krekorian EA, Poulson T. Primary malignant melanoma of the oral cavity. A review of 177 cases. Cancer. 1985;55:1543–1551. doi: 10.1002/1097-0142(19850401)55:7<1543::aid-cncr2820550722>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Meleti M, Leemans CR, Mooi WJ, Vescovi P, van der Waal I. Oral malignant melanoma: a review of the literature. Oral Oncol. 2007;43:116–121. doi: 10.1016/j.oraloncology.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Penel N, Mallet Y, Mirabel X, Van JT, Lefebvre JL. Primary mucosal melanoma of head and neck: prognostic value of clear margins. Laryngoscope. 2006;116:993–995. doi: 10.1097/01.mlg.0000217236.06585.a9. [DOI] [PubMed] [Google Scholar]
- Lourenço SV, Bologna SB, Hsieh R, Sangueza M, Fernandes JD, Nico MM. Establishment and characterization of an oral mucosal melanoma cell line (MEMO) derived from a longstanding primary oral melanoma. Am J Dermatopathol. 2013;35:248–251. doi: 10.1097/DAD.0b013e31826a9905. [DOI] [PubMed] [Google Scholar]
- Kahn MA, Weathers DR, Hoffman JG. Transformation of a benign oral pigmentation to primary oral melanoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:454–459. doi: 10.1016/j.tripleo.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Hicks MJ, Flaitz CM. Oral mucosal melanoma: epidemiology and pathobiology. Oral Oncol. 2000;36:152–169. doi: 10.1016/s1368-8375(99)00085-8. [DOI] [PubMed] [Google Scholar]
- Meleti M, Mooi WJ, Casparie MK, van der Waal I. Melanocytic nevi of the oral mucosa - no evidence of increased risk for oral malignant melanoma: an analysis of 119 cases. Oral Oncol. 2007;43:976–981. doi: 10.1016/j.oraloncology.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Tacastacas JD, Bray J, Cohen YK, Arbesman J, Kim J, Koon HB, Honda K, Cooper KD, Gerstenblith MR. Update on primary mucosal melanoma. J Am Acad Dermatol. 2014;71:366–375. doi: 10.1016/j.jaad.2014.03.031. [DOI] [PubMed] [Google Scholar]
- Prasad ML, Jungbluth AA, Patel SG, Iversen K, Hoshaw-Woodard S, Busam KJ. Expression and significance of cancer testis antigens in primary mucosal melanoma of the head and neck. Head Neck. 2004;26:1053–1057. doi: 10.1002/hed.20112. [DOI] [PubMed] [Google Scholar]
- Bandarchi B, Jabbari CA, Vedadi A, Navab R. Molecular biology of normal melanocytes and melanoma cells. J Clin Pathol. 2013;66:644–648. doi: 10.1136/jclinpath-2013-201471. [DOI] [PubMed] [Google Scholar]
- Hsieh R, Nico MM, Coutinho-Camillo CM, Buim ME, Sangueza M, Lourenço SV. The CDKN2A and MAP kinase pathways: molecular roads to primary oral mucosal melanoma. Am J Dermatopathol. 2013;35:167–175. doi: 10.1097/DAD.0b013e31825fa1f6. [DOI] [PubMed] [Google Scholar]
- Cramer SF. Stem cells for epidermal melanocytes--a challenge for students of dermatopathology. Am J Dermatopathol. 2009;31:331–341. doi: 10.1097/DAD.0b013e31819cd0cb. [DOI] [PubMed] [Google Scholar]
- Carlson JA, Murphy M, Slominski A, Evidence of skin field cancerization. In: Field cancerization: basic science and clinical applications (Dakubo GD, eds) Ontario: Nova Science Publishers; 2011. pp. 317–370. [Google Scholar]
- Lucchese A, Favia G, Maiorano E, Napoli A, Zanna P, Cicero R, Guida G. Oral malignant melanoma: immunopathological analysis of a multiphasic case. Clin Exp Dermatol. 2010;35:789–791. doi: 10.1111/j.1365-2230.2010.03801.x. [DOI] [PubMed] [Google Scholar]
- Bologna SB, Nico MM, Hsieh R, Coutinho-Camillo CM, Buim ME, Fernandes JD, Sangueza M, Soares FA, Lourenço SV. Adhesion molecules in primary oral mucosal melanoma: study of claudins, integrins and immunoglobulins in a series of 35 cases. Am J Dermatopathol. 2013;35:541–554. doi: 10.1097/DAD.0b013e318276cab3. [DOI] [PubMed] [Google Scholar]
- Rivera RS, Nagatsuka H, Gunduz M, Cengiz B, Gunduz E, Siar CH, Tsujigiwa H, Tamamura R, Han KN, Nagai N. C-kit protein expression correlated with activating mutations in KIT gene in oral mucosal melanoma. Virchows Arch. 2008;452:27–32. doi: 10.1007/s00428-007-0524-2. [DOI] [PubMed] [Google Scholar]
- Prasad ML, Patel SG, Shah JP, Hoshaw-Woodard S, Busam KJ. Prognostic significance of regulators of cell cycle and apoptosis, p16(INK4a), p53, and bcl-2 in primary mucosal melanomas of the head and neck. Head Neck Pathol. 2012;6:184–190. doi: 10.1007/s12105-011-0319-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen ZY, Liu W, Bao ZX, Zhou ZT, Wang LZ. Oral melanotic macule and primary oral malignant melanoma: epidemiology, location involved, and clinical implications. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:e21–25. doi: 10.1016/j.tripleo.2011.02.040. [DOI] [PubMed] [Google Scholar]
- Manolidis S, Donald PJ. Malignant mucosal melanoma of the head and neck: review of the literature and report of 14 patients. Cancer. 1997;80:1373–1386. doi: 10.1002/(sici)1097-0142(19971015)80:8<1373::aid-cncr3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhong Y, Li C, Song H, Guo W, Ren G. Neck dissection for oral mucosal melanoma: Caution of nodular lesion. Oral Oncol. 2014;50:319–324. doi: 10.1016/j.oraloncology.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Umeda M, Komatsubara H, Shigeta T, Ojima Y, Minamikawa T, Shibuya Y, Yokoo S, Komori T. Treatment and prognosis of malignant melanoma of the oral cavity: preoperative surgical procedure increases risk of distant metastasis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:51–57. doi: 10.1016/j.tripleo.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Tomicic J, Wanebo HJ. Mucosal melanomas. Surg Clin North Am. 2003;83:237–252. doi: 10.1016/S0039-6109(02)00100-7. [DOI] [PubMed] [Google Scholar]
- Kumar V, Shukla M, Goud U, Ravi DK, Kumar M, Pandey M. Spindle cell amelanotic lesion of the tongue: a diagnostic and therapeutic challenge. Indian J Surg. 2013;75(Suppl 1):394–397. doi: 10.1007/s12262-012-0575-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene GW, Haynes JW, Dozier M, Blumberg JM, Bernier JL. Primary malignant melanoma of the oral mucosa. Oral Surg Oral Med Oral Pathol. 1953;6:1435–1443. doi: 10.1016/0030-4220(53)90242-4. [DOI] [PubMed] [Google Scholar]
- Matsushita S, Kageshita T, Ishihara T. Comparison of dermoscopic and histopathological findings in a mucous melanoma of the lip. Br J Dermatol. 2005;152:1324–1326. doi: 10.1111/j.1365-2133.2005.06463.x. [DOI] [PubMed] [Google Scholar]
- Olszewska M, Banka A, Gorska R, Warszawik O. Dermoscopy of pigmented oral lesions. J Dermatol Case Rep. 2008;2:43–48. doi: 10.3315/jdcr.2008.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig S, Malvehy J. Dermoscopic findings of pigmented lesions of the mucosae. In: Principles of Dermoscopy (Malvehy J, Puig S, eds) Barcelona: 2002. pp. 289–289. [Google Scholar]
- Stolz W, Braun-Falco O, Bilek P, Landthaler M, Burgforf W, Cognetta AB. In: Color Atlas of Dermatoscopy. Blackwell Publishing; 2002. pp. 151–154. [Google Scholar]
- Lin J, Koga H, Takata M, Saida T. Dermoscopy of pigmented lesions on mucocutaneous junction and mucous membrane. Br J Dermatol. 2009;161:1255–1261. doi: 10.1111/j.1365-2133.2009.09251.x. [DOI] [PubMed] [Google Scholar]
- Roberts DL, Anstey AV, Barlow RJ, Cox NH, Newton Bishop JA, Corrie PG, Evans J, Gore ME, Hall PN, Kirkham N. U.K. guidelines for the management of cutaneous melanoma. Br J Dermatol. 2002;146:7–17. doi: 10.1046/j.1365-2133.2001.04614.x. [DOI] [PubMed] [Google Scholar]
- Sober AJ, Chuang TY, Duvic M, Farmer ER, Grichnik JM, Halpern AC, Ho V, Holloway V, Hood AF, Johnson TM, Lowery BJ. Guidelines of care for primary cutaneous melanoma. J Am Acad Dermatol. 2001;45:579–586. doi: 10.1067/mjd.2001.117044. [DOI] [PubMed] [Google Scholar]
- Barker BF, Carpenter WM, Daniels TE, Kahn MA, Leider AS, Lozada-Nur F, Lynch DP, Melrose R, Merrell P, Morton T, Peters E, Regezi JA, Richards SD, Rick GM, Rohrer MD, Slater L, Stewart JC, Tomich CE, Vickers RA, Wood NK, Young SK. Oral mucosal melanomas: the WESTOP Banff workshop proceedings. Western Society of Teachers of Oral Pathology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:672–679. doi: 10.1016/s1079-2104(97)90318-8. [DOI] [PubMed] [Google Scholar]
- Tlholoe MM, Khammissa RA, Bouckaert M, Altini M, Lemmer J, Feller L Oral Mucosal Melanoma: Some Pathobiological Considerations and an Illustrative Report of a Case. Head Neck Pathol. 2014. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolyer RA, Thompson JF, Stretch JR, Sharma R, McCarthy SW. Pathology of melanocytic lesions: new, controversial, and clinically important issues. J Surg Oncol. 2004;86:200–211. doi: 10.1002/jso.20083. [DOI] [PubMed] [Google Scholar]
- Yu CH, Chen HH, Liu CM, Jeng YM, Wang JT, Wang YP, Liu BY, Sun A, Chiang CP. HMB-45 may be a more sensitive maker than S-100 or Melan-A for immunohistochemical diagnosis of primary oral and nasal mucosal melanomas. J Oral Pathol Med. 2005;34:540–545. doi: 10.1111/j.1600-0714.2005.00340.x. [DOI] [PubMed] [Google Scholar]
- de Andrade BA, León JE, Carlos R, Delgado-Azañero W, Mosqueda-Taylor A, Graner E, de Almeida OP. Expression of fatty acid synthase (FASN) in oral nevi and melanoma. Oral Dis. 2011;17:808–812. doi: 10.1111/j.1601-0825.2011.01841.x. [DOI] [PubMed] [Google Scholar]
- Edge S, Byrd D, Compton C, AJCC Cancer Staging Manual, 7th ed. New York: Springer; 2010. [Google Scholar]
- Brandwein-Gensler M, Smith RV. Prognostic indicators in head and neck oncology including the new 7th edition of the AJCC staging system. Head Neck Pathol. 2010;4:53–61. doi: 10.1007/s12105-010-0161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen D, Voorhees JJ. Oral melanoma and other pigmented lesions of the oral cavity. J Am Acad Dermatol. 1991;24:527–537. doi: 10.1016/0190-9622(91)70077-f. [DOI] [PubMed] [Google Scholar]
- Rudnicka L, Olszewska M, Słowinska M. Early diagnosis of malignant melanoma of the skin and of the oral mucous membrane. Wsp Onkol. 2003;8:556–563. [Google Scholar]
- Pfister DG, Ang KK, Brizel DM, Burtness BA, Busse PM, Caudell JJ, Cmelak AJ, Colevas AD, Dunphy F, Eisele DW, Gilbert J, Gillison ML, Haddad RI, Haughey BH, Hicks WL Jr, Hitchcock YJ, Kies MS, Lydiatt WM, Maghami E, Martins R, McCaffrey T, Mittal BB, Pinto HA, Ridge JA, Samant S, Schuller DE, Shah JP, Spencer S, Weber RS, Wolf GT, Worden F, Yom SS, McMillian NR, Hughes M. Head and neck cancers, version 2.2013. Featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2013;11:917–923. doi: 10.6004/jnccn.2013.0113. [DOI] [PubMed] [Google Scholar]
- Kingdom TT, Kaplan MJ. Mucosal melanoma of the nasal cavity and paranasal sinuses. Head Neck. 1995;17:184–189. doi: 10.1002/hed.2880170303. [DOI] [PubMed] [Google Scholar]
- Guo J, Si L, Kong Y, Flaherty KT, Xu X, Zhu Y, Corless CL, Li L, Li H, Sheng X, Cui C, Chi Z, Li S, Han M, Mao L, Lin X, Du N, Zhang X, Li J, Wang B, Qin S. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol. 2011;29:2904–2909. doi: 10.1200/JCO.2010.33.9275. [DOI] [PubMed] [Google Scholar]
- Young K, Minchom A, Larkin J. BRIM-1, -2 and -3 trials: improved survival with vemurafenib in metastatic melanoma patients with a BRAF(V600E) mutation. Future Oncol. 2012;8:499–507. doi: 10.2217/fon.12.43. [DOI] [PubMed] [Google Scholar]
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, Hogg D, Lorigan P, Lebbe C, Jouary T, Schadendorf D, Ribas A, O'Day SJ, Sosman JA, Kirkwood JM, Eggermont AM, Dreno B, Nolop K, Li J, Nelson B, Hou J, Lee RJ, Flaherty KT, McArthur GA. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Vecchio M, Di Guardo L, Ascierto PA, Grimaldi AM, Sileni VC, Pigozzo J, Ferraresi V, Nuzzo C, Rinaldi G, Testori A, Ferrucci PF, Marchetti P, De Galitiis F, Queirolo P, Tornari E, Marconcini R, Calabrò L, Maio M. Efficacy and safety of ipilimumab 3mg/kg in patients with pretreated, metastatic, mucosal melanoma. Eur J Cancer. 2014;50:121–127. doi: 10.1016/j.ejca.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Min L, Hodi FS. Anti-PD1 following ipilimumab for mucosal melanoma: durable tumor response associated with severe hypothyroidism and rhabdomyolysis. Cancer Immunol Res. 2014;2:15–18. doi: 10.1158/2326-6066.CIR-13-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp TK, Fu YS, Calcaterra TC. Melanoma of the nasal and paranasal sinus mucosa. Arch Otolaryngol Head Neck Surg. 1987;113:1086–1089. doi: 10.1001/archotol.1987.01860100064023. [DOI] [PubMed] [Google Scholar]
- Liu YS, Yang CH, Sun YF, Geng B, Yuan KF. [Multivariate analysis of the prognostic factors in 230 surgically treated oral mucosal malignant melanomas] Shanghai Kou Qiang Yi Xue. 2005;14:466–471. [PubMed] [Google Scholar]
- Keller DS, Thomay AA, Gaughan J, Olszanski A, Wu H, Berger AC, Farma JM. Outcomes in patients with mucosal melanomas. J Surg Oncol. 2013;108:516–520. doi: 10.1002/jso.23445. [DOI] [PubMed] [Google Scholar]
- Mallone S, De Vries E, Guzzo M, Midena E, Verne J, Coebergh JW, Marcos-Gragera R, Ardanaz E, Martinez R, Chirlaque MD, Navarro C, Virgili G. Descriptive epidemiology of malignant mucosal and uveal melanomas and adnexal skin carcinomas in Europe. Eur J Cancer. 2012;48:1167–1175. doi: 10.1016/j.ejca.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Kardynal A, Olszewska M. Modern non-invasive diagnostic techniques in the detection of early cutaneous melanoma. J Dermatol Case Rep. 2014;8:1–8. doi: 10.3315/jdcr.2014.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajar-Serviansky T, Gutierrez-Mendoza D, Galvan IL, Lammoglia-Ordiales L, Mosqueda-Taylor A, Hernandez-Cázares Mde L, Toussaint-Caire S. A case of oral mucosal melanoma. Clinical and dermoscopic correlation. J Dermatol Case Rep. 2012;6:1–4. doi: 10.3315/jdcr.2012.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]


