Abstract
Background
Cellular combinations in the same neoplasm can have intriguing physiopathological implications, which may be useful to better understand the biology of the diseases.
Main observations
Urticaria pigmentosa in association with eruptive melanocytic nevi was observed in a female patient. Maculopapular lesions extended at the base of different melanocytic nevi and the histopathological examination revealed the presence of a mast cell population in the papillary and reticular dermis combined with overlying melanocytic nevi. The re-evaluation of a melanoma removed three years before revealed the presence of the same pathological features. Immunohistochemical assays showed a strong positivity to Giemsa, Toluidine blue and CD-117 in the mast cells, while a S-100 reaction was observed in the melanocytic population.
Conclusions
We discuss possible pathogenetic linkage between cutaneous mastocytosis and melanoma.
Keywords: dermoscopy, mast cells, melanocytes, nodule, pathogenesis, skin cancer
Introduction
The presence of two associated cell lines in the same neoplasm is a possible co-occurrence. However, some cellular combinations are rather unusual and rare, resulting in many diagnostic problems for the pathologists as well as the clinicians.
Only in a few cases the association between melanocytes and mast cells in the same tumor has been so far reported in the literature.[1-5] This possible association is characterized by the presence of specific physiopathological mechanisms, which could be explained by the analysis of CD-117 (c-kit) expression, by some therapeutic treatments as well as by specific hormonal implications.
We report the case of a patient with a cutaneous maculopapular mastocytosis who developed melanoma and eruptive nevi.
Case Report
A 38-year-old Caucasian woman presented to our Institute with a hyperpigmented lesion on her right iliac crest. The patient indicated that the lesion has changed in dimension and morphology, over the last 2 months. The pigmented lesion was a dark brown macule with an irregular border, without signs of pruritus or bleeding. Dermoscopically the pigmented lesion showed a broadened network, with a central hyperpigmentation and a moth-eaten border; the lesion was also surrounded by macular-erythematous lesions, which extended towards it [Fig. 1]. The familiar history of the patient was negative for malignancies and/or other diseases, while in the personal medical history, a history of melanoma on the left shoulder.
Figure 1.
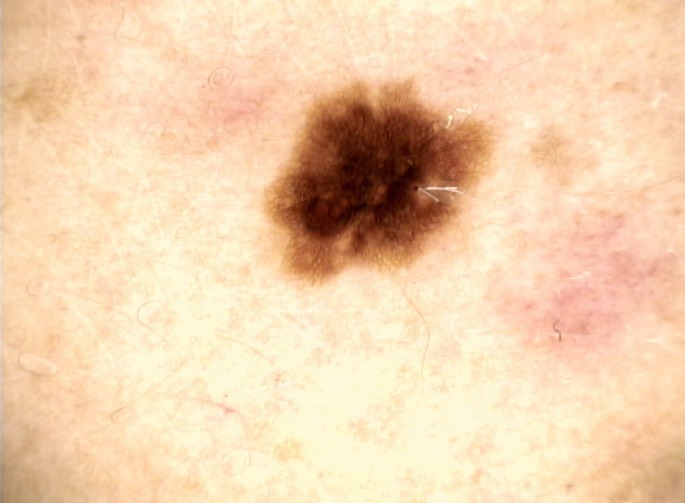
Dermoscopic image of a benign pigmented lesion with a broadened network, central hyperpigmentation and a moth-eaten border. The lesion was surrounded by macular-erythematous lesions, which extended towards it.
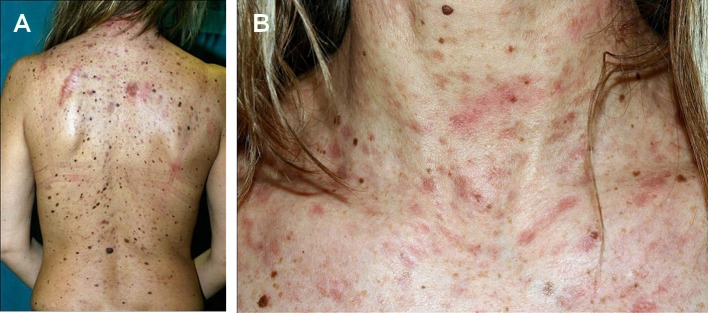
At general clinical examination we observed the presence of several and disseminated macules and papules (up to 1 cm large) in the neck and trunk regions [Fig. 2A-B]. Several of these cutaneous lesions extended at the base of different melanocytic nevi [Fig. 2A-B]. The patient reported that the maculopapular lesions were present since she was 2 years old and a vigorous rubbing of the skin induced an urticarial reaction in several of them [Fig. 2B]. She denied diarrhea, headaches and flush-like symptoms; routine laboratory investigations were all normal.
Figure 2.
(A) Several and disseminated macules-papules, distributed at the neck and trunk, extending to the base of numerous melanocytic nevi. (B) A vigorous rubbing provoked urtication in the maculopapular lesions.
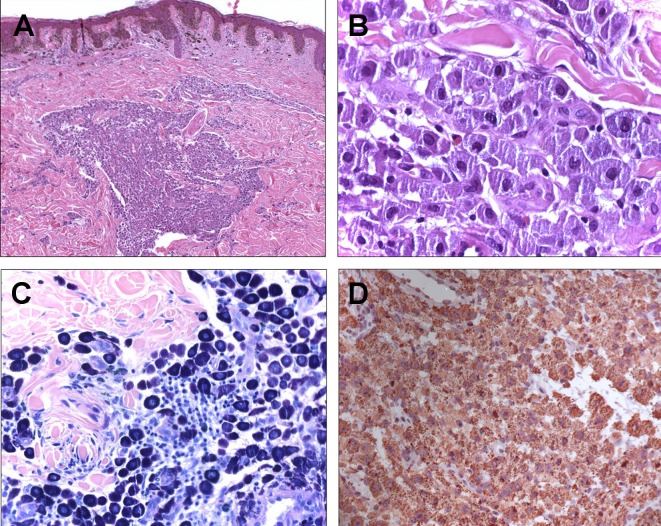
According to patient’s history and to the clinical features of the pigmented lesion, a surgical excision was performed. Histologically, at high magnification, an infiltration of a large population of mast cells in the papillary dermis, which extended through the reticular dermis, it was observed [Fig. 3A]. Basophil and polygonal mast cells showed a wide cytoplasmic rim, in which several granules were identifiable [Fig. 3B]. The nuclei presented only slight variations in size, with a general ovoid morphology. Mitotic figures were not found.
Figure 3.
(A) Dermal infiltration of a large population of mast cells with an overlying compound melanocytic nevus (H-E, 4X); (B) Mast cells exhibiting a wide cytoplasmic rim in which granules were identifiable (H-E, 20 X); (C) Metachromatic cytoplasmic granules (Giemsa, 20 X); (D) Positivity of mast cells to CD-117 stain (CD-117, 20X).
The upper part of the histological specimen showed small-to-medium size hyperpigmented nevus cell nests in the derma-epidermal junction and in the superficial papillary dermis, without significant melanocytic atypia or pagetoid spread. Melanophages and a mild perivascular lymphocytic infiltrate were also observed.
Immunohistochemical staining enabled a thorough examination of the lesion. The mast cells showed, at Giemsa [Fig. 3C] and Toluidine blue staining, purple and metachromatic cytoplasmic granules. S-100 was strongly positive in the junctional and dermal nevus nests, with small foci in the mast cells; while, CD-117 (c-kit) showed an intense reaction only among mast cells [Fig. 3D].
Based on the cutaneous biopsy and to the clinical features, a final diagnosis of compound melanocytic nevus combined with an urticaria pigmentosa was made.
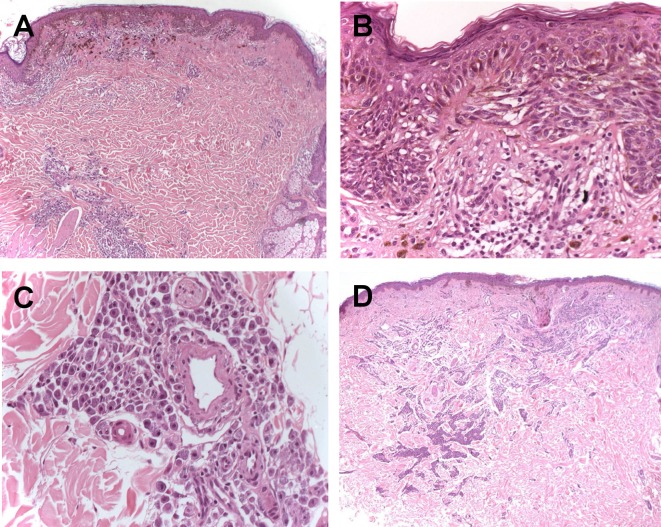
The specimen of the melanoma removed three years before was re-examined. Histological examination confirmed the presence of a melanoma (Breslow thickness: 0.5 mm), without ulceration and/or mitotic rate ≥ 1/mm2 (pT1a) [Fig. 4A-B]. Along with the malignant melanocytic component, aggregates of round and ovoid mast cells, with perivascular infiltration, were observed in the papillary and reticular dermis [Fig. 4C]. The re-excision of the melanoma scar did not show any residual neoplastic cells, while the mast cell proliferation was always present [Fig. 4D].
Figure 4.
(A) Superficial spreading melanoma with underlying infiltration of mast cells (H-E, 4 X); (B) Melanocytic atypia with pagetoid spread (H-E, 20 X); (C) Mast cells in the dermis with a particular perivascular arrangement (H-E, 20 X); (D) A rescission of the melanoma showing a scar with the simultaneous presence of a mast cell population.
Dermoscopy of the removed lesion was consistent with melanoma, showing a broadned-network, radial streaming, inverted network, moth-eaten border, central whitish-veil with a scar-like depigmentation and multiple blue-gray dots [Fig. 5].
Figure 5.
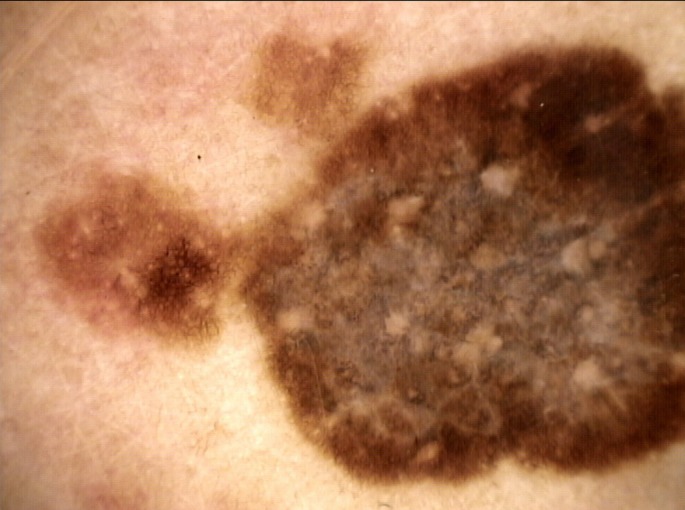
Dermoscopic image of the malignant melanoma that was removed. The lesion showed a broadned-network, radial streaming, inverted network, moth-eaten border, central whitishveil with a scar-like depigmentation and multiple blue-gray dots.
Currently the patient performs only periodic clinical and instrumental controls, without a systemic involvement neither by melanoma and nor by mastocytosis. However, due to the high number of melanocytic nevi that the patient continues to develop, she removed other benign pigmented lesions with the double cellular component.
Discussion
Cutaneous mastocytosis accounts for about 90% of cases of mastocytosis and it can be usually subdivided in mastocytoma, urticaria pigmentosa and diffuse cutaneous mastocytosis.[6] A variety of non-hematological neoplasms have been reported in patients with mastocytosis, although rarely in the same histological sample.[5] To our knowledge, only 3 cases of mastocytosis associated with benign melanocytic nevi and 2 cases of mastocytosis associated with melanoma are reported in the literature,[1-5] but never in the same patient.
In our case, consistently with the clinical and histological findings, the presence of a double cellular proliferation could have a physiopathological linkage, rather than be ascribed to a mere coincidence. Thence, the pathogenesis of the tumor studied may be multifactorial.
Melanocytes, when exposed to histamine, have been described to undergo morphological variations (becoming swollen, enlarged and dendritic), exhibiting also increased tyrosinase activity;[7-9] besides, CD-117 binding mast cells growth factor (MFG) stimulates the proliferation of mast cells and melanocytes.[1,10] All these evidences can account for the high number of eruptive melanocytic nevi in our patient, showing histologically the same double cellular component. Contrary to the literature, where CD-117 is detected in about 29% of melanomas (losing its positivity in the more invasive lesions),[11] we have not observed a diffuse and/or focal CD-117 positivity, in the component of themelanoma. Another possible linkage between mastocytosis and benign and/or malignant melanocytic tumors can be traced back to the therapeutic treatments usually performed to treat cutaneous mastocytosis. In fact, therapy for the cutaneous mastocytosis is complex, and 311 nm UVB and PUVA are currently valid therapeutic procedures.[4] At the same time, it is known that these therapies may favor the onset of skin cancers.[12] Since our patient has never been treated for the cutaneous mastocytosis, the possible role of a true physiopathological linkage is therefore emphasized.
Excluding Silvermann et al,[3] who reported a mastocytoma arising within a congenital nevocellular nevus in a 1-year-old boy, in all other cases there was a prevalence for female patients.[1-5] Some authors reported cutaneous mastocytosis complicating pregnancy and recently Zaitsu et al. found that binding estradiol to estrogen receptor-α on the membrane of mast cells supports the synthesis and release of mast cell mediators in vitro.[13,14] However, although currently there are no reliable data of a possible carcinogen role of the estrogens on the development of melanoma and/or on the exacerbation of the mastocytosis, it may be hypothesized that this is an additional linkage between the diseases, even though not yet so well explored.
Conclusion
Despite few cases are reported in the literature of mast cell tumors associated with benign and/or malignant melanocytic lesions, this report suggests that such clinicopathological occurrence could be more frequent than hitherto observed. We reported the presence of these three cell lines (mast cells, melanocytic nevi, melanoma) in the same patient. This combination can have extraordinary physiopathological implications, which may prove useful in the future to better understand the biology of these diseases, apparently unconnected.
References
- Northcutt AD, Tschen JA. Combined mastocytoma-junctional nevus. Am J Dermatopathol. 2004;26:478–481. doi: 10.1097/00000372-200412000-00007. [DOI] [PubMed] [Google Scholar]
- Okun MR, Bhawan J. Combined melanocytoma-mastocytoma in a case of nodular mastocytosis. J Am Acad Dermatol. 1979;1:338–347. doi: 10.1016/s0190-9622(79)70027-2. [DOI] [PubMed] [Google Scholar]
- Silverman RA, Zaim MT. Mastocytoma arising within a congenital nevocellular nevus. Arch Dermatol. 1988;124:1016–1018. [PubMed] [Google Scholar]
- Kowalzic L, Eickenscheidt L, Seidel C, Kribus S, Ziegler H, Komar M. Telangiectasia macularis eruptiva perstans, a form of cutaneous mastocytosis, associated with malignant melanoma. J Dtsch Dermatol Ges. 2009;7:360–362. doi: 10.1111/j.1610-0387.2008.06941.x. [DOI] [PubMed] [Google Scholar]
- Todd P, Garioch J, Seywright M, Rademaker M, Thomson J. Malignant melanoma and systemic mastocytosis--a possible association? Clin Exp Dermatol. 1991;16:455–457. doi: 10.1111/j.1365-2230.1991.tb01235.x. [DOI] [PubMed] [Google Scholar]
- Wang HJ, Lin ZM, Zhang J, Yin JH, Yang Y. A new germline mutation in KIT associated with diffuse cutaneous mastocytosis in a Chinese family. Clin Exp Dermatol. 2014;39:146–149. doi: 10.1111/ced.12225. [DOI] [PubMed] [Google Scholar]
- Tomita Y, Maeda K, Tagami H. Stimulatory effect of histamine on normal human melanocytes in vitro. Tohoku J Exp Med. 1988;155:209–210. doi: 10.1620/tjem.155.209. [DOI] [PubMed] [Google Scholar]
- Tomita Y, Maeda K, Tagami H. Histamine stimulates normal human melanocytes in vitro: one of the possible inducers of hyperpigmentation in urticaria pigmentosa. J Dermatol Sci. 1993;6:146–154. doi: 10.1016/0923-1811(93)90005-a. [DOI] [PubMed] [Google Scholar]
- Tomita Y, Maeda K, Tagami H. Mechanisms for hyperpigmentation in post inflammatory pigmentation, urticaria pigmentosa and sunburn. Dermatologica. 1989;179 Suppl 1:49–53. doi: 10.1159/000248449. [DOI] [PubMed] [Google Scholar]
- Longley BJ Jr, Morganroth GS, Tyrrell L, Ding TG, Anderson DM, Williams DE, Halaban R. Altered metabolism of mast-cell growth factor (c-kit ligand) in cutaneous mastocytosis. N Engl J Med. 1993;328:1302–1307. doi: 10.1056/NEJM199305063281803. [DOI] [PubMed] [Google Scholar]
- Willmore-Payne C, Holden JA, Tripp S, Layfield LJ. Human melanoma: detection of BRAF- and c-kit-activating mutations by high-resolution amplicon melting analysis. Hum Pathol. 2005;36:486–493. doi: 10.1016/j.humpath.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Archier E, Devaux S, Castela E, Gallini A, Aubin F, Le Maître M, Aractingi S, Bachelez H, Cribier B, Joly P, Jullien D, Misery L, Paul C, Ortonne JP, Richard MA. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26 Suppl 3:22–31. doi: 10.1111/j.1468-3083.2012.04520.x. [DOI] [PubMed] [Google Scholar]
- Matito A, Álvarez-Twose I, Morgado JM, Sánchez-Muñoz L, Orfao A, Escribano L. Clinical impact of pregnancy in mastocytosis: a study of the Spanish Network on Mastocytosis (REMA) in 45 cases. Int Arch Allergy Immunol. 2011;156:104–111. doi: 10.1159/000321954. [DOI] [PubMed] [Google Scholar]
- Zaitsu M, Narita S, Lambert KC, Grady JJ, Estes DM, Curran EM, Brooks EG, Watson CS, Goldblum RM, Midoro-Horiuti T. Estradiol activates mast cells via a non-genomic estrogen receptor-alpha and calcium influx. Mol Immunol. 2007;44:1977–1985. doi: 10.1016/j.molimm.2006.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]