Abstract
The endometrium is receptive to embryo implantation only for a short period in each reproductive cycle: development of receptivity requires alterations in endometrial gene expression. Calbindin (CaBP)-d9k and CaBP-d28k are related proteins containing EF hand motifs that have a high affinity for Ca2+. We previously demonstrated that endometrial expression of CaBP-d9k mRNA is highly regulated during implantation in the mouse. This project aimed to determine the temporal and spatial expression of both CaBP proteins during early pregnancy and to establish whether they are necessary for blastocyst implantation. CaBP-d28k protein, like CaBP-d9k, was up-regulated in the endometrial epithelium just before implantation but disappeared at implantation sites after attachment. By the judicious intrauterine injection of morpholino oligonucleotides (MO) against CaBP-d9k into WT and CaBP-d28k null mice just before implantation, we selectively eliminated one or both CaBPs from the uterine epithelium. Implantation was blocked only when both CaBP-d9k and CaBP-d28k were absent: treated WT mice and untreated CaBP-d28k null mice were fertile. Furthermore, the effect on implantation was highly dependent on the timing of injection of MO. This report examining the function of implantation-related genes in the uterus using MO demonstrates that this technique is a highly effective means to specifically target uterine proteins in vivo. This study provides evidence for an absolute requirement for CaBPs during the early phase of embryo implantation, and thus that regulation of Ca2+ availability in the uterine environment of the implanting embryo is critical for successful implantation.
Calbindin (CaBP)-d9k and CaBP-d28k are members of a large family of over 250 intracellular calcium-binding proteins, characterized structurally by EF-hand motifs, that are responsible for binding free Ca2+ (1, 2). Both are believed to act as cytosolic Ca2+ buffers in many tissues, resulting in modulation of Ca2+ adsorption (3). CaBP-d28k has additional properties as a sensor of Ca2+. Calcium buffers can modulate intracellular calcium transients, by changing their time course and spatial spreading, and can thereby modify calcium-dependent intracellular signaling (4).
CaBP-d9k is expressed in a number of tissues, including duodenal absorptive epithelium (5), kidney (6), and syncytial trophoblast (7). CaBP-d28k was originally purified from chicken intestine (8) but is also expressed in kidney and throughout the nervous system (9, 10) in species ranging from invertebrates to mammals (11). In the rat, CaBP-d9k has been demonstrated in the uterine stroma and myometrium, with uterine expression decreasing around the time of embryo implantation on day 5 (d5) of pregnancy (12).
Using RNA differential display, Nie et al. (13) identified that the CaBP-d9k gene was highly regulated in the mouse uterus in association with implantation. Its expression increases dramatically before implantation (13, 14). CaBP-d9k mRNA is localized in the luminal epithelium, and to a lesser extent in the glandular epithelium in the receptive endometrium, but is specifically down-regulated at the site of embryo attachment during implantation (13). Preliminary Northern studies have demonstrated low levels of CaBP-d28k mRNA during met-estrus, higher levels at estrus, and down-regulation of CaBP-d28k at implantation sites, indicating that CaBP-d28k may have a similar uterine expression pattern.
Given the potential importance of calcium regulation at the endometrial–embryo interface, this study examined the cellular and temporal location of both CaBPs in the mouse uterus, before and during embryo implantation, and determined the functional importance of these proteins to this process. Because CaBP-d28k null mice show no impairment in implantation and are fertile (15), we postulated that there may be functional redundancy between the two proteins. Morpholino antisense oligonucleotides (MO) are synthetic DNA analogues with highly favorable properties as in vivo gene-targeting tools (16). Because MOs have been used to effectively disrupt protein expression in developmental systems such as in zebra fish embryos (17), they seemed ideal for use in our studies due to their highly specific action and the longevity of their function (17, 18). Therefore, we developed a technique to block the production of endometrial CaBP-d9k protein using MO injected directly into the uterine lumen on different days of early pregnancy in both WT and CaBP-d28k null mice. This approach demonstrated that embryo implantation is blocked only when both CaBPs are at undetectable levels in the endometrial epithelium, and that the timing of MO injection into the uterine lumen is critical. Furthermore, the technique represents a method to manipulate endometrial proteins in vivo with temporal specificity and will have wide application in identifying markers for fertility or infertility or searching for novel targets for contraception.
Materials and Methods
Animals. WT mice were C57BL6 strain from the Monash University Animal House. CaBP-d28k null mice were purchased from The Jackson Laboratory and were originally generated by Meyer and colleagues (15) on a C57BL6 background. CaBP-d28k deficiency does not affect development, and CaBP-d28k null mice are able to reproduce (15). All animals were housed and handled according to the Monash University animal ethics guidelines on the care and use of laboratory animals, and all experimentation was approved by the Institutional Animal Ethics Committee at the Monash Medical Centre. The phenotype of the null mutant mice was confirmed by immunohistochemical and Western blot analysis of selected tissues, including kidney, brain, and uterus.
Fertile males were mated with adult female mice (6–to 8-week-old) of the same strain to produce pregnant animals. Pregnancy d0 was defined as the morning of finding a vaginal plug.
Tissue Collection and Fixation. Uterine tissues were collected from nonpregnant (NP) mice and pregnant mice on d3.5–5.5, n = 4 per group); mouse kidney was also collected from NP mice as a control. Tissues were fixed in 10% buffered formalin (pH 7.6) for immunohistochemical analysis and processed to wax, or frozen in OCT compound (Miles). For NP, and d3.5 pregnant mice, the entire uterus was collected. For d4.5 pregnant mice, implantation sites were visualized by i.v. injection of Chicago blue dye solution (1% in saline, 0.1 ml per mouse) into the tail vein, 5 min before the animals were killed and uteri excised. Implantation sites were separated from interimplantation sites. For pregnant mice on d5.5, implantation and interimplantation sites were distinguishable without dye injection.
Immunohistochemistry. Serial 5-μm sections were mounted on poly-l-lysine-treated glass slides and dried in an incubator overnight at 37°C. Immunohistochemical analysis used rabbit anti-rat CaBP-d9k or rabbit anti-rat CaBP-d28k antibodies (both from Swant, Bellinzona, Switzerland). Lack of crossreaction between the antibodies was confirmed by Western blotting. Negative controls were performed with equivalent concentrations of nonimmune rabbit serum. Each run included a section of mouse kidney as a positive control. Sections were deparaffinized and dehydrated in ethanol, endogenous peroxidase was blocked with 0.05% H2O2 for 30 min, and nonspecific binding was blocked with TBS-Tween [20 mM Tris-buffered saline (pH 7.5)/0.1% Tween] and 10% normal goat serum (NGS) for 2 h at room temperature. The required primary antibody was applied (anti-CaBP-d9k, 1:2,000; anti-CaBP-d28k, 1:1,000) for 2 h at room temperature. The sections were rinsed twice with TBS-Tween, and biotinylated goat anti-rabbit secondary antibody (DAKO) (1:500 in TBS/Tween/10% NGS) was added for 30 min on a shaker. Sections were rinsed with TBS/Tween, and the Vectastain ABC-Elite avidin/biotin detection system (Vector Laboratories) was applied for 30 min. Sections were then rinsed in distilled H2O and treated with DAB (3,3′-diaminobenzidine tetrahydrochloride) chromogen reagent (Zymed) for 1–5 min and counterstained with 10% Harris hematoxylin for 3 min before being rehydrated and mounted.
MOs. The following MOs were used (synthesized by Gene Tools, Philomath, OR): CaBP-d9k MO, 5′-TGC AGG AGA CTT CTC AGC ACA TT-3′; and an irrelevant MO (standard control MO from Gene Tools), 5′-CCT CTT ACC TCA GTT ACA ATT TAT A-3′. FITC–labeled standard control MOs (FITC-MO) were also purchased from Gene Tools.
The MOs were prepared at a stock concentration of 2 mM. To assist delivery, partially complementary DNA molecules with a 10-base adenine 5′ overhang were used as carriers for each morpholino sequence and were provided combined with the antisense oligonucleotides (Gene Tools). For the treatment of each horn, 10 μl of the weak-base delivery reagent, ethoxylated polyethylenimine (EPEI, Gene Tools) was mixed with 15 μl of MO stock, resulting in 25 μl of solution containing 30 nmol of MO. The MO/EPEI mixture was vortexed for 30 s and incubated for 20 min at room temperature to allow the MO/EPEI delivery complexes to form before use (19).
Analysis of Oligonucleotide Penetrance. Uterine penetrance of the MOs and cross-contamination between the two horns were assessed by injecting 25 μl of FITC-MO/EPEI into one horn, with either unlabeled standard control MO or EPEI alone into the contralateral horn of an NP mouse. Forty-eight hours later, the mice were killed, and their uteri were excised and frozen in OCT compound (Tissue Tek, Elkhart, IN). Five-micrometer-thick frozen sections were then analyzed under a fluorescence microscope at 488 nm, with selected sections stained with f luorescent nuclear counterstaining reagent TOPRO-3 (DAKO).
Treatment of Pregnant Mice with MOs. Mice were anesthetized with xylazine (10 mg/kg, Ilium Xylazil-20)/ketamine (80 mg/kg, Ketamil, both from Troy Laboratories, Sydney, Australia) mixture on a chosen day after mating (d2.5–4.5). An incision (<1 cm) was made into the lower abdomen by means of the dorsal area at the apex of the triangle arch where the hind leg meets the abdomen. The utero-tubal junction was visualized through this incision. Injections of MOs were through a 26s gauge HPLC needle (Hamilton) inserted just below the utero-tubal junction. The MO/EPEI solution was slowly injected over a period of 20 s, and inflation of the uterine horn was observed. The needle was held in place together with the uterus at the injection site with a pair of forceps for a further 30 s after the injection to prevent reflux.
Twenty-five microliters of solution containing 30 nmol of CaBP-d9k MO was injected. The dose was determined to be sufficient to inhibit protein production in cell systems in previous studies (20). Control horns were injected with an irrelevant MO, the standard control MO available from Gene Tools, of the same concentration as the CaBP-d9k MO. In addition, some pregnant animals were injected with saline/standard MO or EPEI alone. Incisions were closed by using a surgical steel clip. The animal was injected with Yohimbine (1 mg/kg, Parnell Laboratories, New South Wales, Australia) to reverse the anesthetic effects. After the mice had recovered consciousness, they were returned to solitary habitats until d5.5 for mice injected on d2.5–3.5 or d6.5 for mice injected on d4.5. In some instances, implantation sites were visualized more clearly after tail vein injection of Chicago blue dye solution (0.1% in saline, 0.1 ml per mouse) 5 min before the animals were killed and uteri were excised. Numbers of implantation sites and numbers of corpora lutea on corresponding ovaries were recorded.
Results
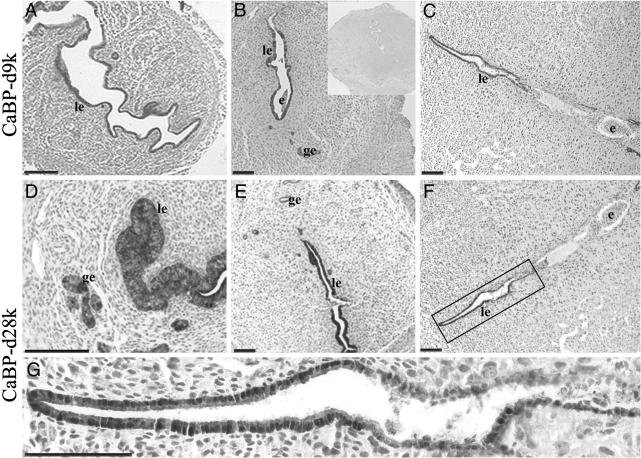
Immunohistochemical Localization of CaBP-d9k and CaBP-d28k. The cellular localization of the two CaBP proteins in the kidney (positive control) and uteri of NP, d3.5, d4.5, and d5.5 pregnant animals was determined by immunohistochemistry. In the kidney, there was high-intensity staining for CaBP-d9k protein in the distal convoluted tubules (not shown) as published (21). In NP and d3.5 uteri, the luminal epithelium was moderately stained for CaBP-d9k (Fig. 1A), and this staining increased markedly on d4.5 (Fig. 1B). Cells of all glandular epithelium also stained strongly for CaBP-d9k, but with lower intensity than that seen in luminal epithelium. On d5.5, CaBP-d9k staining was still strong in glandular and luminal epithelial cells, except those adjacent to the site of the implanting embryo, which displayed little or no CaBP-d9k immunoreactivity (Fig. 1C).
Fig. 1.
Photomicrographs showing representative immunohistochemistry for CaBP-d9k (A–C) and CaBP-d28k (D–G) in mouse uterus. (A) Nonpregnant. (B and E) Implantation and interimplantation site on d4.5 of pregnancy, respectively. (C, F, and G) D5.5 implantation site. (D) High power image of a d4.5 interimplantation site. (G) A high power view of the boxed area in F.(B Inset) Negative control. le, Luminal epithelium; ge, glandular epithelium; e, embryo. (Scale bars = 25 μm.)
CaBP-d28k had an almost identical cellular localization to CaBP-d9k in NP and d3.5–5.5 pregnant uterus. CaBP-d28k was present with similar intensity to CaBP-d9k, in both the luminal epithelium and the functional glandular epithelium in interimplantation (Fig. 1 D and E, respectively) and implantation sites on d4.5 (not shown). The main difference identified was the absence of immunoreactive CaBP-d28k from basal glands irrespective of day of pregnancy. However, like CaBP-d9k, CaBP-d28k immunoreactivity was also lost in the luminal epithelium at the embryo attachment site on d5.5 (Fig. 1F). A higher power image (Fig. 1G) shows some interesting features of epithelial cells at the implantation sites. Most epithelial cells in close contact with the embryo have already undergone apoptosis, and many have been phagocytosed, which accounts for the loss of staining. Those cells at the tip of the lumen located away from the implantation sites are clearly intact and very positively immunoreactive. Interestingly, the area between these two distinct regions shows a lower level of staining, and in this region the epithelial integrity seems to be compromised, with some epithelial cells sloughed into the lumen. Most immunostaining is in the cytoplasm but nuclear staining is also seen.
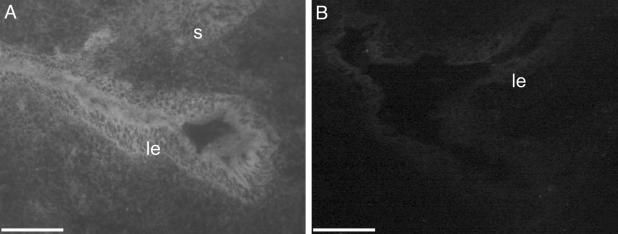
Assessment of MO Penetrance by Fluorescence Microscopy. Cross sections of mouse uteri treated with FITC-MOs were examined by fluorescence microscopy. Strong green fluorescence representing cellular uptake of FITC-MOs was observed in the luminal epithelium and to a lesser extent the underlying stroma (Fig. 2A), demonstrating that the MO had penetrated these cells in vivo. No fluorescence was observed in the contralateral horn, which had been treated with unlabeled control MO (Fig. 2B), although very low autofluorescence was present as has been described (22). Thus, there was no significant transfer of oligonucleotides between horns.
Fig. 2.
Fluorescence micrographs of mouse uteri after intrauterine administration of FITC-MO. (A) Uterine horn treated with control FITC-MO displaying high levels of fluorescence in luminal epithelial compartment and moderate fluorescence in stromal compartment. (B) Uterine horn treated with unlabeled control MO displaying only background fluorescence. (Scale bars = 25 μm.)
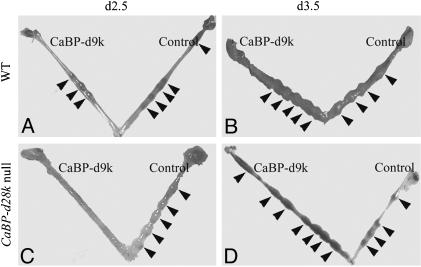
Effects of CaBP-d9k MO Treatment on Embryo Implantation in WT and CaBP-d28k Null Mice. MOs against CaBP-d9k were injected into the uterine lumen of WT or CaBP-d28k null mice on d2.5 or d3.5 of pregnancy, and their uteri were harvested on d5.5. Injection on d2.5 of pregnancy in WT mice did not affect numbers of implantation sites (Fig. 3A and Table 1). However, implantation was completely inhibited when MOs were injected into CaBP-d28k null mice on d2.5 (Fig. 3C and Table 1). In contrast, injections on d3.5 into the uterine lumen of either WT or CaBP-d28k null mice did not affect total numbers of implantation sites although slight alterations in spacing patterns were observed (Fig. 3 B and D and Table 1). Additionally, injections of CaBP-d9k MOs on d4.5 of pregnancy had no effect on implantation in either WT or CaBP-d28k null mice when their uteri were harvested on d6.5 (not shown).
Fig. 3.
Examples of uteri harvested on d5.5 from treatment groups in WT and CaBP-d28k null mice. Arrows indicate implantation sites. Horns labeled CaBP-d9k and Control were treated with antisense CaBP-d9k MO and standard control MO, respectively.
Table 1. Mean ± SEM implantation sites and corpora lutea.
| Implantation sites
|
Corpora lutea
|
|||||
|---|---|---|---|---|---|---|
| Phenotype | n | Injection day | Control MO | CaBP-D9k MO | Control MO | CaBP-d9k MO |
| WT | 3 | 2.5 | 3.5 ± 0.2 | 2.5 ± 0.2 | 4.0 ± 0.1 | 4 ± 0.2 |
| WT | 4 | 3.5 | 3.5 ± 0.2 | 3.5 ± 0.1 | 4.5 ± 0.1 | 5.5 ± 0.3 |
| CaBP-d28k | 4 | 2.5 | 4.7 ± 0.1 | 0* | 5.0 ± 0.1 | 5 ± 0.2 |
| CaBP-d28k | 3 | 3.5 | 3.3 ± 0.1 | 4.7 ± 0.2* | 4.5 ± 0.2 | 4.7 ± 0.2 |
Numbers of implantation sites and corporal lutea (mean ± SEM) in WT and CaBP-d28k null mice on d5.5 when treated by intrauterine injection of either control MO or CaBP-d9k MO on d2.5 or d3.5 of pregnancy. *, P < 0.01 compared with control.
Table 1 shows the mean number of implantation sites (± SEM) in uterine horns injected with either CaBP-d9k MO or control MO and the mean number of corpora lutea on adjacent ovaries. The numbers of implantation sites in control and CaBP-d9k MO-treated horns, in WT mice, were not significantly altered when injections were on either d2.5 or d3.5 of pregnancy. Similarly, there was no difference between the numbers of implantation sites in control and CaBP-d9k MO-treated horns in CaBP-d28k null mice injected on d3.5. However, there was a significant difference (P < 0.01) between CaBP-d28k null animals treated on d2.5 of pregnancy, with 100% inhibition of implantation in CaBP-d9k MO-treated horns.
A number of control experiments were performed to confirm the validity of these results. These experiments included uterine horns injected with 0.9% saline, with EPEI alone, or EPEI with an irrelevant MO, at similar concentration to that used for CaBP-d9k MO. No effects on numbers of implantation sites and no significant gross morphological differences were observed in any control horns, except for minor effects upon embryo spacing.
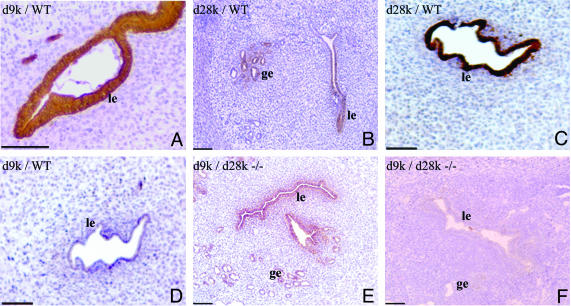
Immunohistochemical Localization of CaBP-d9k and CaBP-d28k in Uteri of WT and CaBP-d28k Null Mice Treated with CaBP-d9k MO. The efficacy of the CaBP-d9k MO in inhibiting production of CaBP-d9k protein in vivo was determined by immunohistochemistry.
In WT mice, uteri harvested on d5.5 that were treated with control MO (injected on d2.5) showed normal immunoreactivity for both CaBP-d9k and CaBP-d28k in the luminal and glandular epithelium (Fig. 4 A and B). When the uteri were treated with CaBP-d9k MO, CaBP-d28k was not affected, because normal immunoreactivity for this protein was detected (Fig. 4C), whereas CaBP-d9k protein was not detectable (Fig. 4D). This finding confirmed that the production of CaBP-d9k protein was inhibited by the specific MO.
Fig. 4.
Photomicrographs showing representative immunohistochemistry for CaBP-d9k (d9k; A and D–F) and CaBP-d28k (d28k; B and C) in d5.5 uteri of WT (A–D) and CaBP-d28k null (d28–/–; E and F). A, B, and E were treated with control MO, and C, D, and F with CaBP-d9k MO.
In untreated CaBP-d28k null mice, CaBP-d28k protein was undetectable, and expression of CaBP-d9k was the same as in WT animals (not shown). In these mice, strong uterine immunoreactive CaBP-d9k was detected after treatment with control MO (Fig. 4E). However, no CaBP-d9k protein was detected in mice treated with CaBP-d9k MO (Fig. 4F). Therefore, in CaBP-d28k null mice treated with CaBP-d9k MO, both CaBP-d28k and CaBP-d9k proteins were undetectable.
Discussion
The administration of MO directly into the uterine lumen of mice in early pregnancy, allowed us to define a critical role for CaBPs in the process of embryo implantation. Importantly, the technique allowed us to inhibit the production of CaBP-d9K protein specifically in the uterus of both WT and CaBP-d28k null mice. However, implantation was blocked only in mice lacking both CaBPs. Thus, it has been shown that these two CaBPs have overlapping functions in an in vivo situation.
CaBP-d9k MO injected into WT mice effectively inhibited CaBP-d9k expression in both luminal and glandular epithelium. However, there was no significant change in the number of implantation sites in the control MO treated horns regardless of the time of injection (d2.5 or d3.5). Numbers of implantation sites were also compared against numbers of corpora lutea present on adjacent ovaries, but no significant difference was found. Because of the functional similarities between CaBP-d9k and CaBP-d28k and the similar temporal expression pattern during early pregnancy, it was hypothesized there could be functional redundancy between both proteins.
CaBP-d28k null mice were reported to be fertile with no reproductive abnormalities. The only published phenotype is ataxia, with the mice unable to complete tasks requiring high levels of coordination in comparison with WT (15). This ataxia may affect their mounting success, but our studies indicated that, when mating has occurred and a vaginal plug is found, implantation proceeds normally and results in normal litter size. Injections of CaBP-d9k MO into these CaBP-d28k null mice resulted in a total inhibition of embryo implantation, but only when MO was injected on d2.5 of pregnancy. Injections of MO on subsequent days (d3.5 and d4.5) did not significantly alter the numbers of implanting embryos. Ovaries adjacent to MO-treated horns were surveyed for corpora lutea, and it was confirmed that they were releasing the normal number of ova. Furthermore, uterine horns in the CaBP-d28k null mice treated with control MO displayed normal numbers of implantation sites, supporting the hypothesis that disruption of embryo implantation is due to the specific effects of the CaBP-d9k MO.
Previously, CaBP-d9k mRNA expression was described as up-regulated around d2.5 of pregnancy (13); immunohistochemistry showed a corresponding increase in protein expression on d3.5. Additionally, CaBP-d9k mRNA was down-regulated in luminal epithelium on d4.5 of pregnancy at the embryo attachment site (13). Immunohistochemical analyses on d4.5 showed that protein persists in the epithelium adjacent to the embryo implantation site until d5.5. It is proposed, therefore, that CaBP-d9k protein is actively down-regulated in the uterus at the site of embryo attachment; however, the total disappearance of CaBP-d9k protein occurred later than its mRNA due to a longer half-life of the protein. The half-life of CaBP-d28k is reported to be between 12–24 h (23); the half-life of CaBP-d9k is not known. Our studies indicated that the cellular localization and temporal expression of CaBP-d28k mimic that of CaBP-d9k during early pregnancy. Expression patterns seem identical, except that CaBP-d28k protein is expressed only in glands proximal to the lumen but not in basal glandular epithelium.
Observations that CaBP-d9k mRNA and protein expression is tightly regulated in the mouse uterus during early pregnancy concur with previous studies in other species. In the bovine uterus, CaBP-d9k expression is higher during the luteal phase than in the follicular phase (24), and, in pigs, endometrial CaBP-d9k mRNA is high just before implantation (days 10–12). However, after attachment (d14), the levels decrease dramatically (25). In rats as in mice, endometrial expression on d1, d2, d3, and d5 of pregnancy is high, and this expression is significantly decreased immediately after initial embryo attachment (12). Thus, in all species examined, CaBP-d9k is elevated before implantation.
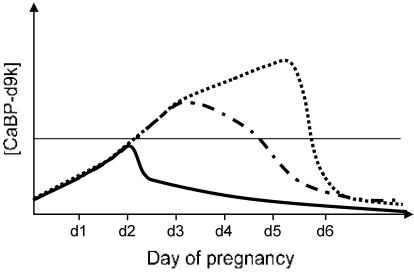
Based on this study, we have diagrammatically represented the postulated pattern of changing CaBP-d9k expression in the uterine epithelium during early pregnancy in the mouse (Fig. 5). The expression of CaBP-d28k would follow the same pattern as CaBP-d9k. We hypothesize that a threshold of CaBP concentration must be reached in the uterine epithelium for successful implantation in the mouse. This finding would explain why a total inhibition of implantation is possible in the CaBP-d28k null mouse only when the production of CaBP-d9k is inhibited because only in this situation is a minimal concentration of CaBP protein produced. This result could also explain why the timing of injection of MO is critical. Injection of MO on d3.5 is too late because the mRNA levels have already increased to a point where CaBP protein production surpasses the threshold required to sustain implantation.
Fig. 5.
Model showing the postulated concentration of CaBP-d9k protein during early pregnancy in normal mouse uterus (dotted line) and in uterus after injection of MO against CaBP-d9k on d2.5 (solid line), or d3.5 of pregnancy (dot-dash line). The likely threshold level of CaBP required for implantation is shown as a horizontal line.
How is it that inhibiting expression of these two CaBPs during early pregnancy results in implantation disruption? What is the need for high concentrations of these proteins in the luminal epithelium before implantation?
The roles established for CaBPs in other tissues emphasize their ability to enhance Ca2+ transport and increase the cells' capacity to store Ca2+. In mammalian enterocyte cells, free Ca2+ ions are bound to cytosolic CaBP-d9k and transferred across the cell by facilitated diffusion (26). This transport of Ca2+ by CaBP-d9k helps maintain homeostasis by keeping intracellular Ca2+ ion concentrations below 10–7 M, preventing premature cell death by means of apoptosis. In placenta, CaBP-d28k is proposed to enhance Ca2+ transport because syncytiotrophoblast cells expressing high levels of CaBP-d28k display higher Ca2+ uptake compared with cells with low CaBP-d28k expression (27). Thus, it can be speculated that the role of CaBP-d9k and CaBP-d28k proteins in the uterine luminal epithelium is also to enhance Ca2+ uptake by increasing the cell-buffering capacity and stimulating the calcium entry mechanism in these cells.
However, the question remains as to why it is necessary for uterine luminal epithelium to increase Ca2+ uptake during implantation. In what manner would this uptake facilitate blastocyst attachment at implantation? It has long been known that, for proper blastocyst implantation in mice and humans, there must be interaction between adhesion-competent trophoblast cells and endometrial extracellular matrix (ECM) components. The accumulation of integrin receptors for fibronectin on the blastocyst surface enables it to bind to and penetrate ECM (28), but only once the blastocyst has reached an adhesion-competent state. Recently, it has been shown that the development of an adhesion-competent embryo is facilitated by heparin-binding epidermal growth factor (EGF)-like growth factor (HB-EGF) and that this process is dependent on calcium influx from extracellular sources (29). However, this source of Ca2+ has not yet been established. We therefore suggest that a possible source of this external calcium needed for HB-EGF-mediated differentiation of the blastocyst to an adhesion-competent state is from stores in the luminal epithelium of the endometrium.
Based on the above evidence, we propose that the dynamic, changing nature of CaBP expression (up-regulation followed by down-regulation in the luminal epithelium rather than up-regulation or down-regulation alone) during early pregnancy is important for implantation. We hypothesize that this unique expression of CaBP regulates certain cellular events in these luminal epithelial cells essential for implantation. We suggest that the initial up-regulation of CaBP is to increase the storage capacity for Ca2+ in the luminal epithelial cells without any harm (Ca2+ bound to CaBP); the subsequent specific down-regulation of CaBP at implantation sites would release the bound Ca2+, leading to an increase in free Ca2+ concentration. This release may trigger apoptosis in these specific epithelial cells because high concentrations of free Ca2+ are reported to cause apoptosis in many different cell types (30) and CaBP-d28k is able to inhibit apoptosis in osteoblastic cells (31). This apoptosis in turn could destabilize the epithelial barrier at the implantation site and facilitate trophoblast invasion and implantation. In addition, the apoptotic luminal epithelial cells could be phagocytosed by the adjacent trophoblasts and the Ca2+ contained in these epithelial cells used by the implanting embryo to promote its development. Thus, we speculate that our approach disrupted the dynamic pattern of CaBP regulation by preventing the initial up-regulation and resulted in implantation failure. However, the hypothesis has yet to be substantiated.
Acknowledgments
This study was made possible by funding from the Rockefeller/World Health Organization Initiative on Implantation and by National Health and Medical Research Council of Australia Grants 143798 and 241000. K.C.L. was supported in part by a Dean's Postgraduate Scholarship from the Faculty of Medicine, Monash University.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CaBP, calbindin; MO, morpholino antisense oligonucleotide; NP, nonpregnant; EPEI, ethoxylated polyethylenimine; dn, day n of pregnancy.
References
- 1.Linse, S., Thulin, E., Gifford, L. K., Radzewsky, D., Hagan, J., Wilk, R. R. & Akerfeldt, K. S. (1997) Protein Sci. 6, 2385–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linse, S., Brodin, P., Drakenberg, T., Thulin, E., Sellers, P., Elmden, K., Grundstrom, T. & Forsen, S. (1987) Biochemistry 26, 6723–6735. [DOI] [PubMed] [Google Scholar]
- 3.Thomasset, M., Dupret, J. M., Brehier, A. & Perret, C. (1990) Adv. Exp. Med. Biol. 269, 35–36. [DOI] [PubMed] [Google Scholar]
- 4.Berggard, T., Miron, S., Onnerfjord, P., Thulin, E., Akerfeldt, K. S., Enghild, J. J., Akke, M. & Linse, S. (2002) J. Biol. Chem. 277, 16662–16672. [DOI] [PubMed] [Google Scholar]
- 5.Szabo, A., Merke, J., Thomasset, M. & Ritz, E. (1991) Eur. J. Clin. Invest. 21, 521–526. [DOI] [PubMed] [Google Scholar]
- 6.Bindels, R. J., Hartog, A., Timmermans, J. A. & van Os, C. H. (1991) Contrib. Nephrol. 91, 7–13. [DOI] [PubMed] [Google Scholar]
- 7.Glazier, J. D., Mawer, E. B. & Sibley, C. P. (1995) Pediatr. Res. 37, 720–725. [DOI] [PubMed] [Google Scholar]
- 8.Ferrari, S., Drusiani, E., Battini, R. & Fregni, M. (1988) Nucleic Acids Res. 16, 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu, L., Dunn, S. T., Christakos, S., Hanson-Painton, O. & Bourdeau, J. E. (1993) Kidney Int. 44, 322–330. [DOI] [PubMed] [Google Scholar]
- 10.Lomri, N., Perret, C., Gouhier, N. & Thomasset, M. (1989) Gene 80, 87–98. [DOI] [PubMed] [Google Scholar]
- 11.Pfyffer, G. E., Faivre-Bauman, A., Tixier-Vidal, A., Norman, A. W. & Heizmann, C. W. (1987) J. Neurochem. 49, 442–451. [DOI] [PubMed] [Google Scholar]
- 12.Krisinger, J., Setoyama, T. & Leung, P. C. (1994) Mol. Cell. Endocrinol. 102, 15–22. [DOI] [PubMed] [Google Scholar]
- 13.Nie, G. Y., Li, Y., Wang, J., Minoura, H., Findlay, J. K. & Salamonsen, L. A. (2000) Biol. Reprod. 62, 27–36. [DOI] [PubMed] [Google Scholar]
- 14.Tatsumi, K., Higuchi, T., Fujiwara, H., Nakayama, T., Itoh, K., Mori, T., Fujii, S. & Fujita, J. (1999) Mol. Hum. Reprod. 5, 153–161. [DOI] [PubMed] [Google Scholar]
- 15.Airaksinen, M. S., Eilers, J., Garaschuk, O., Thoenen, H., Konnerth, A. & Meyer, M. (1997) Proc. Natl. Acad. Sci. USA 94, 1488–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Summerton, J. (1999) Biochim. Biophys. Acta 1489, 141–158. [DOI] [PubMed] [Google Scholar]
- 17.Heasman, J. (2002) Dev. Biol. 243, 209–214. [DOI] [PubMed] [Google Scholar]
- 18.Scholpp, S. & Brand, M. (2001) Genesis 30, 129–133. [DOI] [PubMed] [Google Scholar]
- 19.Morcos, P. A. (2001) Genesis 30, 94–102. [DOI] [PubMed] [Google Scholar]
- 20.Summerton, J., Stein, D., Huang, S. B., Matthews, P., Weller, D. & Partridge, M. (1997) Antisense Nucleic Acid Drug Dev. 7, 63–70. [DOI] [PubMed] [Google Scholar]
- 21.Shamley, D. R., Opperman, L. A., Buffenstein, R. & Ross, F. P. (1992) Development (Cambridge, U.K.) 116, 491–496. [DOI] [PubMed] [Google Scholar]
- 22.Ruttner, Z., Ivanics, T., Slaaf, D. W., Reneman, R. S., Toth, A. & Ligeti, L. (2002) J. Soc. Gynecol. Invest. 9, 294–298. [DOI] [PubMed] [Google Scholar]
- 23.Wang, Y. Z. & Christakos, S. (1995) Mol. Endocrinol. 9, 1510–1521. [DOI] [PubMed] [Google Scholar]
- 24.Inpanbutr, N., Miller, E. K., Petroff, B. K. & Iacopino, A. M. (1994) Biol. Reprod. 50, 561–571. [DOI] [PubMed] [Google Scholar]
- 25.Krisinger, J., Jeung, E. B., Simmen, R. C. & Leung, P. C. (1995) Biol. Reprod. 52, 115–123. [DOI] [PubMed] [Google Scholar]
- 26.Walters, J. R. (1989) Am. J. Physiol. 256, G124–G128. [DOI] [PubMed] [Google Scholar]
- 27.Belkacemi, L., Simoneau, L. & Lafond, J. (2002) Endocrine J. 19, 57–64. [DOI] [PubMed] [Google Scholar]
- 28.Wang, J., Mayernik, L. & Armant, D. R. (2002) Dev. Biol. 245, 270–279. [DOI] [PubMed] [Google Scholar]
- 29.Wang, J., Mayernik, L., Schultz, J. F. & Armant, D. R. (2000) Development (Cambridge, U.K.) 127, 33–44. [DOI] [PubMed] [Google Scholar]
- 30.Tombal, B., Denmeade, S. R., Gillis, J. M. & Isaacs, J. T. (2002) Cell Death Differ. 9, 561–573. [DOI] [PubMed] [Google Scholar]
- 31.Bellido, T., Huening, M., Raval-Pandya, M., Manolagas, S. C. & Christakos, S. (2000) J. Biol. Chem. 275, 26328–26332. [DOI] [PubMed] [Google Scholar]