Abstract
One of the most long-lived models in plant science is the belief that the long-distance transport and ratio of two plant hormones, auxin and cytokinin, at the site of action control major developmental events such as apical dominance. We have used in vivo deuterium labeling and mass spectrometry to investigate the dynamics of homeostatic cross talk between the two plant hormones. Interestingly, auxin mediates a very rapid negative control of the cytokinin pool by mainly suppressing the biosynthesis via the isopentenyladenosine-5′-monophosphate-independent pathway. In contrast, the effect of cytokinin overproduction on the entire auxin pool in the plant was slower, indicating that this most likely is mediated through altered development. In addition, we were able to confirm that the lateral root meristems are likely to be the main sites of isopentenyladenosine-5′-monophosphate-dependent cytokinin synthesis, and that the aerial tissue of the plant surprisingly also was a significant source of cytokinin biosynthesis. Our demonstration of shoot-localized synthesis, together with data demonstrating that auxin imposes a very rapid regulation of cytokinin biosynthesis, illustrates that the two hormones can interact also on the metabolic level in controlling plant development, and that the aerial part of the plant has the capacity to synthesize its own cytokinin independent of long-range transport from the root system.
Auxins and cytokinins interact in the control of many central developmental processes in plants, particularly in apical dominance and root and shoot development. The classic experiments of Skoog and Miller (1) demonstrated that the balance between auxin and cytokinin is a key regulator of in vitro organogenesis. Exposing callus cultures to a high auxin-tocytokinin ration results in root formation, whereas a low ration of these hormones promotes shoot development (1). Apical dominance is also one of the classical developmental events believed to be controlled by the ratio of auxin to cytokinin. This is supported by phenotypic observations in many Arabidopsis mutants impaired in different aspects of auxin and/or cytokinin biology (2, 3), as well as transgenic studies of plants with altered auxin or cytokinin levels (4–6). Moreover, many experiments have demonstrated the existence of synergistic, antagonistic, and additive interactions between these two plant hormones, suggesting a complex web of signal interactions (7).
It is clearly documented that auxin regulates cytokinin levels and vice versa. It has, for example, been observed that cytokinin-overproducing tobacco had lower levels of indole-3-acetic acid (IAA), and that overproduction of IAA in tobacco leads to a reduced pool size of cytokinins (8, 9). The problem with these observations, as well as many other studies on auxin–cytokinin cross talk, is the difficulty of resolving cause and effect. It is obvious there exist mechanisms used by these two hormones for interaction both through regulation of homeostasis and/or perception. It is, however, at present impossible to separate direct control of auxin and cytokinin on each other from developmental alterations in mutants and transgenic plants with constitutive overproduction/down-regulation of hormone synthesis. In many aspects of plant development, it is reasonable to believe that mechanisms of importance for the homeostatic part of the auxin–cytokinin cross talk should be relatively rapid to its nature. It is with this background that we decided to investigate the timing of the cross talk as well to elucidate the metabolic steps where such potential regulation occurs.
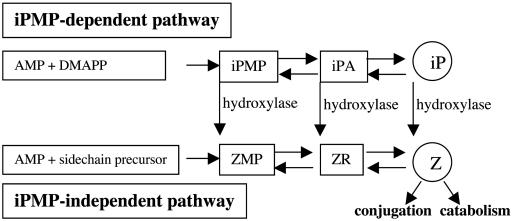
The level of active cytokinins can be diminished through reduced synthesis as well as by oxidative breakdown or conjugation. Auxin induction of cytokinin oxidase was previously demonstrated in tobacco pith explants (9) in which oxidative breakdown of radiolabeled zeatin riboside (ZR) was increased after addition of 1-naphthaleneacetic acid (NAA). This was also supported in the study by Zhang et al. (5), who observed increased in vitro conversion of zeatin (Z) type cytokinins to adenine derivatives after treatment with NAA. The action of auxins on cytokinin oxidase, however, still has to be conclusively verified in vivo, as indicated by Motyka et al. (10), who were unable to demonstrate changes in cytokinin oxidase activity after application of several synthetic auxins to the surface of tobacco callus. Cytokinin bases and ribosides can also be inactivated by conjugation (Fig. 1), and, interestingly, there are reports suggesting that auxin and auxin conjugates may regulate the conjugation of cytokinins. For example, the action of β-glucosidases catalyzing the hydrolysis of cytokinin-O-glucosides, which leads to the release of active cytokinin, has been shown to be inhibited by IAA glucose (11, 12). Unfortunately, the kinetic characteristics, which could contribute to elucidation of the physiological relevance of these enzymes, have not yet been determined.
Fig. 1.
Cytokinin biosynthesis pathways.
It has been argued that cytokinins can both up- and down-regulate auxin levels. The increase in auxin, for example, has been demonstrated either after application of exogenous cytokinin or by transformation with the bacterial ipt gene (4, 13, 14). Although the basis for these changes is not fully understood, cytokinin-induced inhibition of enzymes that conjugate free IAA into inactive IAA aspartate has been suggested as a putative mechanism (15). In contrast, studies on a whole-plant level by Eklöf et al. (8) demonstrated a decrease in auxin content of transgenic plants overproducing cytokinins via the bacterial ipt gene. These contradictory results show the complexity of the interactions between these two hormones, and the only way to get a clear answer will be to study the effects on pool size shortly after altering the content of the other hormone.
Recently, nine ipt homologs, designated AtIPT1–AtIPT9, were described in Arabidopsis, of which seven are closely related to the bacterial ipt gene (16, 17). These genes have the capacity to produce both isopentenyladenine (iP) and Z type cytokinins when overproduced in Escherichia coli. Two of these genes, AtIPT4 and AtIPT8, caused cytokinin independence when they were overproduced in plant tissues, whereas one gene, AtIPT2, was not able to create cytokinin autonomous growth (17, 18). Tissue-specific expression patterns recently demonstrated that several of the isopentenyl transferases have distinct aerial expression patterns with AtIPT3 and AtIPT5 localized to phloem tissues (including leaves) and stems, respectively (19). The site of synthesis is a critical question for understanding the cross talk of the two hormones and how they interact in plant development. The classical view that cytokinins are mainly produced in the roots (20), whereas auxin is synthesized in the aerial parts, is now seriously questioned. For example, recent observations that substantial amount of auxin also is produced in the root system (21) show that the previous model is too simplistic, and this, in combination with the potential for tissue-specific cytokinin production governed by the large isopentenyl transferase family with their distinct expression patterns, prompted us to investigate the site of cytokinin synthesis in plants.
Taken together, it is evident that the concentration of auxins and cytokinins is under control of so far poorly understood metabolic mechanisms. Several recently discovered components of the cytokinin-signaling pathway (22, 23) and the cytokininresponse phenotype of auxin mutants (24, 25) show that these two groups of plant hormones indeed “cross talk” on the molecular level. The aim of the present study was to investigate the true nature of the interaction on the homeostatic level between these two hormones. For this purpose, we used in vivo deuterium-labeling technology to measure the rate of synthesis of both IAA and cytokinins (21, 26, 27). The advantage of the approach of probing the synthesis rate is that 2H2O penetrates all plant organs and a labeling process starts in essentially every biosynthetic pathway of the plant cell. Unlabeled molecules are replaced by deuterium-labeled molecules at a rate that depends on the turnover of the molecule of interest. We have earlier described the site of synthesis of IAA in Arabidopsis (21) and, to get a complete picture of the auxin–cytokinin interaction on a whole-plant level, we decided in this study to complement the auxin data with an analysis of the site of cytokinin biosynthesis in plants.
Materials and Methods
Plant Material and Growth Conditions. Arabidopsis thaliana Colombia and Nicotiana tabacum L. cv. Petit Havana SR1 were used in this study. A transgenic A. thaliana line (3-2) expressing the Agrobacterium tumefaciens ipt gene under the control of a glucocorticoid-inducible promoter was used for cytokinin overproduction (28). The A. thaliana axr4-2 × aux1-7 (29) double mutant was used to elucidate root-mediated cytokinin biosynthesis, and axr1 × axr4 A. thaliana mutants (29, 30) were used to investigate the effects of auxin signal transduction on cytokinin biosynthesis. Arabidopsis plants were grown in 250-ml Erlenmeyer flasks containing 50 ml of one-half Murashige and Skoog (MS) basal growth medium, 3% sucrose, pH 5.6 (25 seeds per bottle). Flasks were agitated and held at 22°C, with an 18-h photoperiod, photon density 130 μE·m–1·s–1 [E (einstein), 1 mol of photons]. Tobacco plants were grown on soil at 22°C under long day conditions, 18 h light/6 h dark.
Cytokinin and Auxin Analysis. The cytokinin pool was quantified according to ref. 31. The extraction and purification of the “auxin regulation of cytokinins, via the isopentenyladenosine-5′-monophosphate (iPMP)-independent pathway” and “sites of cytokinin biosynthesis” experiments, were also done according to ref. 31, but for separation and detection, a Micromass (Manchester, U.K.) cap liquid chromatography system coupled to Micromass Quattro Ultima triple quadrupole instrument was used. Cytokinins were separated on a Waters Symmetry C18 column (100 mm × 0.3 mm), with a flow rate of 4 μl/min and a linear elution gradient from 10% to 70% acetonitrile containing 0.7% formic acid. The in vivo deuterium-labeling experiments were performed according to ref. 27. The biosynthetic rate is presented as the tracer/tracee ratio, which is the incorporation of deuterium into the different cytokinin metabolites corrected for the natural isotope abundance obtained from unlabeled standard of the respective cytokinin metabolite. For IAA measurements, samples were extracted, purified, and analyzed according to ref. 32. The relative biosynthesis of IAA was determined as described in ref. 21. The auxin catabolite 2-oxoIAA and conjugate IAA–aspartate were measured according to ref. 33.
Auxin Regulation of Cytokinin Pool Sizes and Biosynthetic Rate. For quantification of pool sizes, 3-wk-old Arabidopsis plants were transferred to one-half of MS medium containing 5 or 25 μM NAA (Sigma) and 1.5% sucrose. In vivo deuterium-labeling experiments were performed by incubating 3-wk-old plants grown as described above with one-half of MS medium/1.5% sucrose/30% 2H2O/0, 5, or 25 μM NAA.
Cytokinin Regulation of IAA Pool Size and Biosynthetic Rate. A transgenic Arabidopsis line (3-2) carrying the A. tumefaciens ipt gene under the control of a glucocorticoid-inducible promoter (28) was used for studies of the effect of cytokinin on IAA pool size and synthesis rates. Plants (3 wk old) were incubated with one-half of MS medium containing 1.5% sucrose and 30 μM dexamethasone (Sigma) to induce cytokinin biosynthesis.
Auxin Regulation of Cytokinins via iPMP-Independent Pathway. To determine the effect of auxin on the different pathways of cytokinin biosynthesis, A. thaliana WT plants were incubated for 12 h with one-half of MS medium/1.5% sucrose/0 or 25 μM NAA/2 mM Metyrapone (Aldrich). Deuterium incorporation in iPMP and Z riboside-5′-monophosphate (ZMP) were subsequently measured as described above.
Auxin Regulation of Cytokinins via Auxin Perception. The axr1-3 and axr4-2 plants (3 wk old) were incubated in one-half of MS medium containing 1.5% sucrose, 30% 2H2O, and 0 or 25 μM NAA for 12 h, after which the biosynthetic rate of ZMP and levels of free IAA, 2-oxoIAA, and IAAaspartate were measured (33).
Sites of Cytokinin Biosynthesis. The apical part of Arabidopsis plants grown on soft agar was separated from roots at the hypocotyls junction, and the different plant tissues were incubated separately in one-half of MS medium containing 1.5% sucrose and 30% 2H2O for 12 h. The rate of cytokinin biosynthesis was measured according to ref. 27. In a second experiment, leaves of 6-wk-old tobacco plants were analyzed for pool size and synthesis rate of ZMP and ZR. The leaves analyzed represent three different developmental stages: fully expanded, ≈20-cm length; middle size, ≈13-cm length; and small developing leaves, <5-cm length. After purification, ZR and ZMP levels were determined. The leaves of 6-wk-old tobacco plants were dissected out and incubated with 30% 2H2O. After incubation of detached leaves for 24 h, samples were harvested, and ZMP and ZR biosynthesis was measured.
In a third experiment, 3-wk-old axr4-2 × aux1-7 was harvested and ZMP content determined. For the biosynthetic rate experiment, 3-wk-old axr4-2 × aux1-7 plants were incubated in one-half of MS medium/1.5% sucrose/30% 2H2O/0, 5, or 25 μM NAA. The biosynthetic rate of ZMP was subsequently measured.
Results
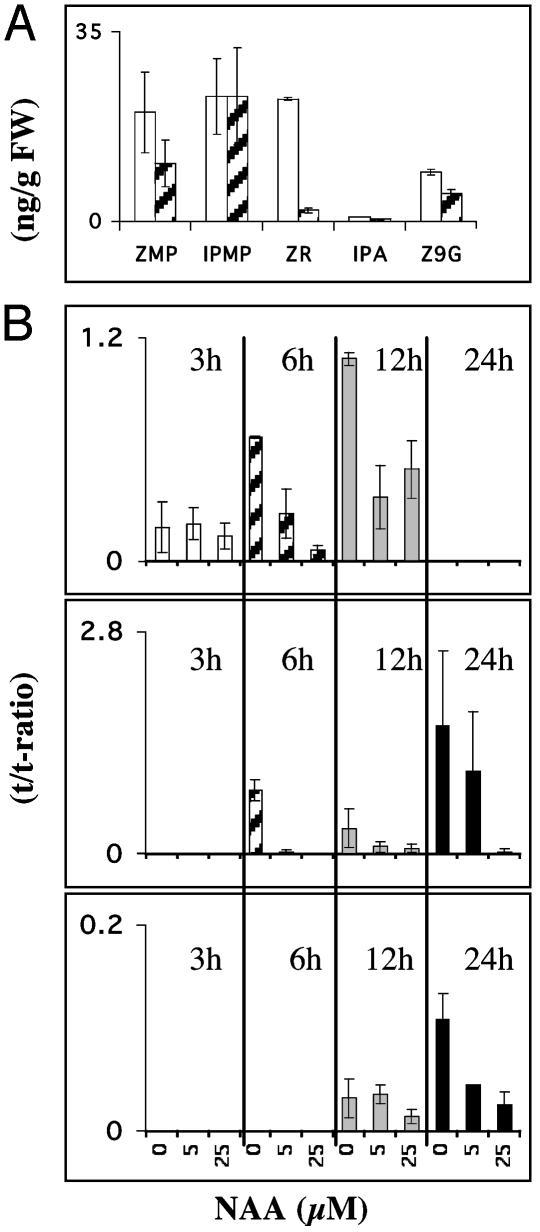
Auxin Rapidly Regulates the Rate of Biosynthesis and the Pool Size of Cytokinins. In the first experiment, Arabidopsis seedlings were treated with 25 μM NAA for 24 h, resulting in significantly reduced pool sizes of several of the major cytokinin intermediates and end products (Fig. 2 A). To measure the auxin effect on actual synthesis rate, a second experiment was performed in which seedlings were incubated in growth medium enriched with 30% 2H2O with 0 (control), 5, and 25 μM NAA, respectively. We used a range of concentrations in this study that have previously been demonstrated to be relevant for studies with exogenous auxin in plant development (34, 35). Within 3 h of incubation, labeling of the ZMP pool was observed, and a significant reduction of synthesis rate was clearly visible for both ZMP and ZR already 6 h after application of 5 μM NAA (Fig. 2B). We thus confirmed rapid repression of cytokinin biosynthesis that occurred in a clearly dose-dependent manner, as demonstrated by the differences between the 5- and 25-μM NAA treatments.
Fig. 2.
(A) Pool sizes of endogenous cytokinins after incubation with 0 μM NAA (white bars) or 25 μM NAA (dashed black bars) for 24 h. (B) Relative biosynthetic rate of ZMP, ZR, and Z-9-glucoside (Z9G) after a 3- (white bars), 6- (dashed black bars), 12- (gray bars), and 24-h (black bars) incubation with 0, 5, and 25 μM, respectively. FW, fresh weight. Error bars display SD.
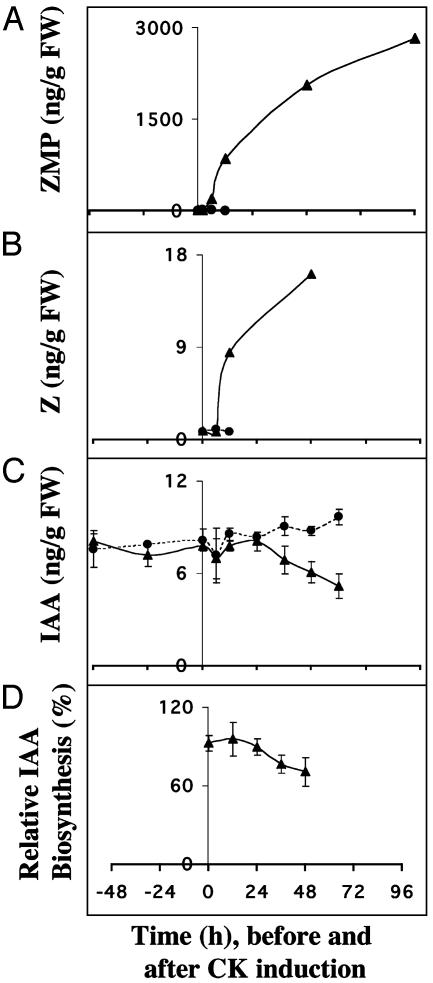
Induced Cytokinin Overproduction Does Not Lead to Rapid Changes in the Overall IAA Pool of the Plant. Arabidopsis plants with glucocorticoid-inducible ipt expression (28) were used to study the effect and timing of cytokinin overproduction on the plant pool size and synthesis rate of IAA. This system has the advantage that it overcomes the problem of application of exogenous cytokinins in a controlled manner, because it delivers the produced cytokinins directly into the cell, thereby avoiding problems related to a controlled uptake. The system gives 100-fold increase in the endogenous ZMP level and 7-fold increase in the active cytokinin, Z, 12 h after induction. Using this system to study the effect of increased endogenous cytokinin levels of auxin biosynthesis, we first observed that both the pool size and synthesis rate of IAA were unaffected during the first 24 h. Between 24 and 36 h, the rate of IAA biosynthesis started to drop, and after 48 h, both the pool size and synthesis rate of IAA were considerably diminished (Fig. 3 C and D). Levels of Z, the active cytokinin, were drastically increased 12 h after induction, and still the reduction of IAA synthesis occurred first after 36–48 h. Thus it is unlikely that cytokinin control of IAA biosynthesis is a direct effect; rather, that it may be mediated via changes in plant development.
Fig. 3.
(A) Endogenous ZMP after induction of cytokinin biosynthesis (t = 0) in the inducible cytokinin overproducing line 3-2-1 (▴) and control (•). (B) Pool size of Z in WT plants (•) and the 3-2-1 line (▴) before and after induction of cytokinin synthesis. (C) Pool size of IAA in WT plants (•) and the 3-2-1 line (▴) before and after induction of cytokinin synthesis. (D) The relative biosynthetic rate of IAA after induction of cytokinin biosynthesis in line 3-2-1. FW, fresh weight. Error bars display SD.
Auxin Regulates Cytokinin Biosynthesis via the iPMP-Independent Pathway. Arabidopsis has two major biosynthesis pathways for synthesis of cytokinins, the iPMP-dependent pathway and the alternative iPMP-independent pathway (36) (Fig. 1). By addition of metyrapone, an inhibitor of isopentenylhydroxylase, the two pathways were uncoupled, and biosynthesis in both pathways was measured in an independent manner. We incubated Arabidopsis seedlings for 12 h with and without 25 μM NAA together with 2 mM metyrapone and 30% 2H2O. Analysis of isotopomer clusters as tracer/tracee ratios revealed that the biosynthetic rate of ZMP, in contrast to iPMP, was significantly reduced after auxin treatment (one-tailed t test; P > 0.05) (Fig. 4). These data thereby demonstrate that NAA represses the synthesis of ZMP independent of the iPMP pathway. This finding was supported by the data in Fig. 2A, showing reduced levels of Z type cytokinins, whereas the levels of iP-type cytokinins are less affected.
Fig. 4.
The biosynthetic rate of iPMP and ZMP in A. thaliana incubated with 0 μM NAA plus 2 mM metyrapone (white bars) and 25 μM NAA plus 2 mM metyrapone (black bars). Error bars display SD.
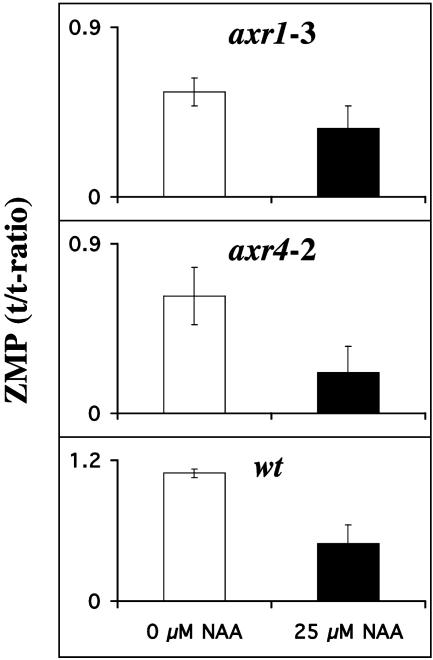
Auxin Regulation of Cytokinin Biosynthesis Is Regulated via Auxin Perception. Our observation that addition of auxin rapidly represses cytokinin biosynthesis was the basis for experiments to elucidate whether the auxin effect was mediated via perception and/or signal transduction of this hormone. For this purpose, we analyzed the effects of added NAA on cytokinin biosynthesis in two mutants that were originally described as auxin resistant, axr1 (30) and axr4 (29). axr1 is a truly auxin-insensitive mutant that contains increased amounts of free IAA [axr1 21.96 ± 4.6 ng/g fresh weight (FW) compared to WT 17.9 ± 0.8 ng/g FW], indicating that the plant cannot sense the increased pool size and thus does not respond to it. The advantage of using these mutants is that both axr1 and axr4 show only minor changes in phenotype compared to WT at an early developmental stage, as used in this study, thereby avoiding effects caused by detrimental growth changes. axr1 shows only a minor reduction in cytokinin biosynthesis upon addition of NAA (Fig. 5). The second mutant in this study, axr4, was originally described as auxin resistant but, in reality in many aspects is auxin sensitive, and the phenotype is rather a reflection of auxin depletion as demonstrated by the levels of free IAA and the metabolites 2-oxoIAA (oxIAA) and IAA aspartate (there is a 60% reduction of free IAA and a 40% increase of oxIAA). In agreement with the auxin effect on cytokinin biosynthesis being mediated via perception, this auxinsensitive mutant shows the same rate of reduction of biosynthesis upon addition of auxin as WT (Fig. 5). We can thus conclude that normal perception and signal transduction are needed for auxin to be able to fully repress the cytokinin biosynthesis.
Fig. 5.
The biosynthetic rate of ZMP in axr1-3, axr4-2, and WT measured after incubation for 12 h with 0 μM NAA (white bars) and 25 μM NAA (black bars). Error bars display SD.
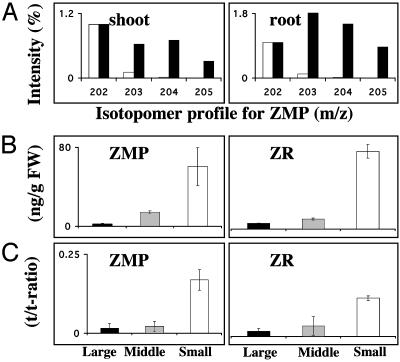
Cytokinins Are Synthesized also in the Aerial Part of the Plant. In an attempt to elucidate the site of cytokinin biosynthesis, Arabidopsis roots and shoots were separated and incubated in liquid growth medium enriched with 30% 2H2O. The in vivo deuterium labeling resulted in an isotopomeric cluster for root and shoot clearly demonstrating the capacity to synthesize ZMP in both types of tissues (Fig. 6A). This observation, that the aerial part of the plant is a significant source of cytokinin synthesis, triggered a second experiment where we used tobacco to elucidate the localization of this source. We analyzed the pool sizes and synthesis rates of ZR and ZMP in detached leaves of different sizes: large leaves where expansion has ended, leaves in expansion phase, and small leaves with active cell division and only limited expansion growth. The results in Fig. 6 B and C clearly show that young developing leaves with active cell division have the highest capacity to synthesize ZMP and ZR, which also is reflected in the endogenous pool sizes. Both data from experiments with Arabidopsis and tobacco show the importance of aerial tissues for cytokinin biosynthesis and clearly establish developing leaves with active cell division as major sites for biosynthesis of Z type cytokinins.
Fig. 6.
(A) In vivo deuterium labeling of the ZMP pool in shoot and roots after separate incubation on 2H2O. Biosynthesis capacity is presented as amount of deuterium labeling into the ZMP fragment m/z 202 and its isotopomers, +1 to +3 (isotopomer profile). White bars represents isotope distribution in standard ZMP with only minor signal in m/z + 1 (203) and m/z + 2 (204) from natural isotope abundance. Black bars show isotope distribution after in vivo deuterium labeling, demonstrating incorporation into m/z + 1 (203) to m/z + 3 (205), thereby verifying massive biosynthesis. (B) Pool size of endogenous ZMP and ZR in tobacco leaves of different size, large (black bars, 20-cm length), middle (gray bars, 13-cm length), and small (white bars, <5-cm length). (C) Biosynthetic rate of ZMP and ZR in tobacco leaves with same legend as B. FW, fresh weight. Error bars display SD.
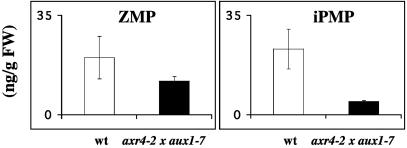
Root-Mediated Cytokinin Synthesis Depends on Developed Lateral Root Primordia. To further elucidate the localization of the root-mediated synthesis, we used the axr4-2 × aux1-7 double mutant that has almost no lateral root formation (29). Quantification of endogenous pool sizes of the ribotides revealed that the mutant contained drastically reduced levels of iPMP, indicating that the reduced number of root meristems resulted in a reduced amount of iPMP produced (one-tailed t test; P > 0.05), whereas only a minor, not significant, reduction of the ZMP pool was observed. These data suggest that the iPMP pool, to a larger extent than the ZMP pool, would originate from root-localized biosynthesis (Fig. 7). We were also able to demonstrate that the ZMP biosynthesis in axr4-2 × aux1-7 was as sensitive to added NAA as in WT (data not shown), showing that auxin control of ZMP synthesis is independent of lateral root primordia.
Fig. 7.
Pool size of endogenous ZMP and iPMP in WT (white bars) and in the double mutant axr4-2 × aux1-7 (black bars). FW, fresh weight. Error bars display SD.
Discussion
The concept of auxin to cytokinin concentration ratios controlling plant development has been around for many years, despite investigations demonstrating that auxins are known to regulate cytokinin pool sizes and vice versa (6, 7). Most of these studies are based on transgenic systems with constitutive overproduction in which normal regulatory mechanisms of the hormones are often overruled. We therefore decided to study the direct effect of auxin and cytokinin on each other's biosynthetic rate to investigate the timing of potential cross talk. For our studies, we made use of NAA with experimental conditions that is standard in auxin studies related to plant development (34, 35). NAA is the only auxin that easily penetrates the plasma membrane without the need for active uptake, thereby ensuring a rapid increase of NAA level inside the cells. This is not as straightforward for treatment with cytokinins, due to problems related to control of exact uptake. Thus for this hormone, we used an Arabidopsis line carrying the bacterial ipt gene under an inducible promoter. We were able to demonstrate that auxin rapidly suppresses both the pool size and synthesis rate of cytokinins. During incubation with NAA for 24 h, ZMP, ZR, and Z-9-glucoside levels were reduced, and in vivo deuterium incorporation experiments showed that application of the auxin rapidly reduced deuterium incorporation into ZMP and ZR already after 6 h (Fig. 2B).
Tobacco plants with constitutive overproduction of cytokinin have earlier been shown to have reduced auxin biosynthesis (6). To investigate whether this result is a direct effect of an increased amount of endogenous cytokinins or an effect of prolongated changes of development, a time-resolved study was performed by using transgenic Arabidopsis plants carrying the bacterial ipt gene under the control of an inducible promoter (28). A 7-fold increase in the active cytokinin Z was detected 12 h after induction of ipt expression. The elevated cytokinin levels resulted in long-term reduction of the pool size and biosynthesis rate of IAA (Fig. 3 C and D). However, we observed a 36-h lag phase, until significant changes in both the pool size and synthesis rate of IAA were detected, indicating an indirect mechanism of regulation, probably mediated through a change in development. We thus clearly demonstrated that the auxin effect on cytokinin biosynthesis is rapid and therefore a potential mechanism for the plant to control the pool size of cytokinins, whereas the reverse, cytokinin effect on auxin synthesis, is long-term and therefore on a whole-plant level not a useful mechanism for rapid control development.
Two de novo cytokinin biosynthesis pathways (Fig. 1) exist in Arabidopsis (36). The iPMP-dependent pathway produces isopentenyladenine nucleotides as the first cytokinin intermediates, and the alternative iPMP-independent pathway produces Z nucleotides without involving iP-type cytokinins as intermediates. By use of metyrapone, an inhibitor of isopentenylhydroxylase, the two pathways are uncoupled because the conversion of iP-type cytokinins to their Z type analogs is blocked. When metyrapone was added simultaneously with NAA and the cytokinin biosynthesis measured, it was demonstrated that NAA acts as a regulator of the alternative pathway, directly producing Z nucleotides (Fig. 4). Thus, the pathways form a biosynthesis network with differentiated regulation. A basal level of cytokinins is probably formed via the iPMP-dependent pathway, and the alternative pathway might contribute to an additional cytokinin supply. The decrease of ZMP synthesis upon NAA addition was well correlated with a marked reduction of the ZMP pool size (data not shown).
Current models for auxin–cytokinin interactions are built on concentration ratios. Our finding that auxin is a rapid and potent regulator of cytokinin biosynthesis will impact this model, and it is therefore necessary to determine where auxin and cytokinin are synthesized in the plant to clarify whether there exist alternatives to the previously proposed root-localized synthesis of cytokinins (20). To investigate whether any other part of plant has the ability to synthesize cytokinins, biosynthetic capacity of this hormone was analyzed in dissected Arabidopsis root and shoot tissues separately. The isotopomer pattern showed that both tissues have the ability to synthesize cytokinins de novo (Fig. 6A), and because root and shoot were incubated separately, no transport could account for the de novo synthesized hormone in the aerial tissue. Due to technical difficulties in dissecting large amounts of well defined organs from a small plant like Arabidopsis, tobacco was chosen for experiments aiming to pinpoint where the synthesis occurs in the aerial parts. When ZMP and ZR were quantified and their biosynthesis probed in tobacco leaves of three developmental stages, an inverse correlation was found among age of leaves, content, and biosynthesis of Z type cytokinins (Fig. 6 B and C). Thus, cytokinin synthesis is very active in small developing leaves in which a substantial part of the cells still divides. Our observation that the highest synthesis of an important cell cycle regulator like cytokinin occurs in tobacco leaves that still have the capacity for cell division, manifested by expression of the cyclin D promoter (data not shown), contradicts the hypothesis of cytokinins as long-distance mediators in root–shoot communication. It has also been shown that very young developing leaves possess the highest capacity for de novo synthesis of IAA (21), which, in combination with our study showing that dividing leaf tissue has a high capacity to synthesize both cytokinins and auxins, is a previously undescribed observation. This was a surprise, considering our recently discovered rapid auxin suppression of cytokinin biosynthesis. One possible explanation that will be a subject for future studies is based on the fact that auxin control of cytokinin synthesis is mediated via auxin signal transduction (Fig. 5) or that there exist cell-specific differences in timing and strength of specific regulatory mechanisms. It is possible that dividing tissues synthesizing large amounts of both hormones may have uncoupled the auxin effect on cytokinin biosynthesis.
To confirm the importance of root meristems for cytokinin biosynthesis, the Arabidopsis double mutant axr4-2 × aux1-7 was examined, which is impaired in the lateral root formation. The plant's total ZMP biosynthesis was not significantly altered, and the reduction caused by NAA was not significantly different from the reduction observed in WT during the same experimental conditions (data not shown). A major part of the plant's ZMP must therefore be formed in apical parts and in this mutant synthesized via the NAA-sensitive iPMP-independent pathway. The mutant's total iPMP pool, however, was reduced by 80% compared to WT. Thus a reduced root-based cytokinin biosynthesis had an impact on the plant's total iPMP content, indicating that the isopentenyl-dependent pathway dominates in the root meristems.
In tobacco roots, isopentenyladenine nucleotides are the major cytokinins detected (data not shown). The very low levels of ZMP and ZR in this tissue (data not shown) support the finding from Arabidopsis that mainly the iPMP-dependent pathway of cytokinin biosynthesis is present in roots. The shoot localization of the iPMP-independent pathway may be explained in terms of exclusive access of the side-chain donor in green plant tissue. The putative side-chain precursor, (2E)-4-hydroxy-3-methylbut-2-enyl diphosphate, has been identified as a key intermediate in the 2-C-methyl-d-erythritol 4-phosphate pathway of terpenoid biosynthesis (37). This pathway, initially detected in prokaryotes, is expressed in eukaryotes that harbor plastids within their cells (38). Chloroplasts might thus be a prerequisite for cytokinin biosynthesis via the alternative pathway, restricting this route of synthesis to green plant tissue.
Based on our global studies, we propose a model where auxin exercises a rapid negative control over the iPMP-independent cytokinin biosynthesis pathway that also is expressed in apical tissues. Thus, cytokinins are synthesized in both shoots and roots and preferentially in tissues rich in dividing cells, such as very young leaves and root tips. Our model also rules out cytokinin root–shoot transport as the only important mechanism maintaining shoot cytokinin pool sizes. This is in agreement with earlier studies where a paracrine mode of action for cytokinin synthesis has been suggested (39).
Acknowledgments
We thank Roger Granbom for skillful technical assistance and Prof. Nam Hai Chua for access to the A. thaliana line expressing the A. tumefaciens ipt gene under the control of a glucocorticoid-inducible promoter. This work was supported by grants from the Swedish Foundation for Strategic Research and the Swedish Research Council.
Abbreviations: iP, isopentenyladenine; iPMP, isopentenyladenosine-5′-monophosphate; Z, zeatin; ZR, Z riboside; ZMP, ZR-5′-monophosphate; MS, Murashige and Skoog; IAA, indole-3-acetic acid; NAA, 1-naphthaleneacetic acid.
References
- 1.Skoog, F. & Miller, C. O. (1957) Symp. Soc. Exp. Biol. 11, 118–131. [PubMed] [Google Scholar]
- 2.Hobbie, L. & Estelle, M. (1994) Plant Cell Environ. 17, 525–540. [DOI] [PubMed] [Google Scholar]
- 3.Catterou, M., Dubois, F., Smets, R., Vaniet, S., Kichey, T., Van Onckelen, H., Sangwan-Norreel, B. S. & Sangwan, R. S. (2002) Plant J. 30, 273–287. [DOI] [PubMed] [Google Scholar]
- 4.Binns, A. N., Labriola, J. & Black, R. C. (1987) Planta 171, 539–548. [DOI] [PubMed] [Google Scholar]
- 5.Zhang, R., Zhang, X., Wang, J., Letham, D. S., Mckinney, S. A. & Higgins, T. J. V. (1995) Planta 196, 84–94. [Google Scholar]
- 6.Eklöf, S., Åstot, C., Blackwell, J., Moritz, T., Olsson, O. & Sandberg, G. (1997) Plant Cell Physiol. 38, 225–235. [Google Scholar]
- 7.Coenen, C. & Lomax, T. L. (1997) Trends Plant Sci. 2, 351–356. [DOI] [PubMed] [Google Scholar]
- 8.Eklöf, S., Åstot, C., Sitbon, F., Moritz, T., Olsson, O. & Sandberg, G. (2000) Plant J. 23, 279–284. [DOI] [PubMed] [Google Scholar]
- 9.Palni, L. M. S., Burch, L. & Horgan, R. (1988) Planta 174, 231–234. [DOI] [PubMed] [Google Scholar]
- 10.Motyka, V. & Kaminek, M. (1992) in Physiology and Biochemistry of Cytokinins in Plants, eds. Kaminek, M., Mok, D. W. S. & Zazimalova, E. (Academic, The Hague, The Netherlands), pp. 33–39.
- 11.Brzobohaty, B., Moore, I., Kristoffersen, P., Bako, L., Campos, N., Schell, J. & Palme, K. (1993) Science 262, 1051–1054. [DOI] [PubMed] [Google Scholar]
- 12.Brzobohaty, B., Moore, I. & Palme, K. (1994) Plant Mol. Biol. 26, 1483–1497. [DOI] [PubMed] [Google Scholar]
- 13.Bourquin, M. & Pilet, P. E. (1990) Physiol. Plant. 80, 342–349. [Google Scholar]
- 14.Bertell, G. & Eliasson, L. (1992) Physiol. Plant. 84, 255–261. [Google Scholar]
- 15.Yip, W. & Yang, S. F. B. (1986) Plant Physiol. 80, 515–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takei, K., Sakakibara, H. & Sugiyama, T. (2001) J. Biol. Chem. 276, 26405–26410. [DOI] [PubMed] [Google Scholar]
- 17.Kakimoto, T. (2001) Plant Cell Physiol. 42, 677–685. [DOI] [PubMed] [Google Scholar]
- 18.Sun, J., Niu, Q.-W., Tarkowski, P., Zheng, B., Tarkowska, D., Sandberg, G., Chua, N.-H. & Zuo, J. (2003) Plant Physiol. 131, 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyawaki, K., Matsumoto-kitano, M. & Kakimoto, T. (2004) Plant J. 37, 128–138. [DOI] [PubMed] [Google Scholar]
- 20.Hopkins, W. G. (1995) in Introduction to Plant Physiology (Wiley, New York), pp. 326–327.
- 21.Ljung, K., Bhalerao, R. P. & Sandberg, G. (2001) Plant J. 28, 465–474. [DOI] [PubMed] [Google Scholar]
- 22.Inoue, T., Higuchi, M., Hashimoto, Y., Seki, M., Kobayashi, M., Kato, T., Tabata, S., Shinozaki, K. & Kakimoto, T. (2001) Nature 409, 1060–1063. [DOI] [PubMed] [Google Scholar]
- 23.Schmuelling, T. (2001) Trends Plant Sci. 6, 281–284. [DOI] [PubMed] [Google Scholar]
- 24.Hobbie, L., Timpte, C. & Estelle, M. (1994) Plant Mol. Biol. 26, 1499–1519. [DOI] [PubMed] [Google Scholar]
- 25.Wilson, A. K., Picket, F. B., Turner, J. C. & Estelle, M. (1990) Mol. Gen. Genet. 222, 377–383. [DOI] [PubMed] [Google Scholar]
- 26.Pengelly, W. L. & Bandurski, R. S. (1983) Plant Physiol. 73, 445–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Åstot, C., Dolezal, K., Moritz, T. & Sandberg, G. (2000) J. Mass Spectrom. 35, 13–22. [DOI] [PubMed] [Google Scholar]
- 28.Kunkel, T., Niu, Q.-W., Chan, Y.-S. & Chua, N.-H. (1999) Nat. Biotechnol. 17, 916–919. [DOI] [PubMed] [Google Scholar]
- 29.Hobbie, L. & Estelle, M. (1995) Plant J. 7, 211–220. [DOI] [PubMed] [Google Scholar]
- 30.Estelle, M. A. & Somerville, C. (1987) Mol. Gen. Genet. 206, 200–206. [Google Scholar]
- 31.Åstot, C., Dolezal, K., Moritz, T. & Sandberg, G. (1998) J. Mass Spectrom. 33, 892–902. [DOI] [PubMed] [Google Scholar]
- 32.Edlund, A., Eklöf, S., Sundberg, B., Moritz, T. & Sandberg, G. (1995) Plant Physiol. 108, 1043–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kowalczyk, M. & Sandberg, G. (2001) Plant Physiol. 127, 1845–1853. [PMC free article] [PubMed] [Google Scholar]
- 34.Friml, J., Vieten, A., Sauer, M., Weijers, D., Schwarz, H., Hamann, T., Offringa, R. & Jurgens, G. (2003) Nature 426, 147–153. [DOI] [PubMed] [Google Scholar]
- 35.Benkova, E., Michniewicz, M., Sauer, M., Teichmann, T., Seifertova, D., Jurgens, G. & Friml, J. (2003) Cell 115, 591–602. [DOI] [PubMed] [Google Scholar]
- 36.Åstot, C., Dolezal, K., Nordström, A., Wang, Q., Kunkel, T., Moritz, T., Chua, N.-H. & Sandberg, G. (2000) Proc. Natl. Acad. Sci. USA 97, 14778–14783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hintz, M., Reichenberg, A., Altincicek, B., Bahr, U., Gschwind, R. M., Kollas, A.-K., Beck, E., Wiesner, J., Eberl, M. & Jomaa, H. (2001) FEBS Lett. 509, 317–322. [DOI] [PubMed] [Google Scholar]
- 38.Lange, B. M., Rujan, T., Martin, W. & Croteau, R. (2000) Proc. Natl. Acad. Sci. USA 97, 13172–13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faiss, M., Zalubilova, J., Strnad, M. & Schmuelling, T. (1997) Plant J. 12, 401–415. [DOI] [PubMed] [Google Scholar]