Abstract
Limited data exist on whether sexual partner notification practices among HIV-infected men, particularly those who have sex with men (MSM), vary by HIV viral load. We examined factors associated with complete (all partners) vs. incomplete partner notification in 760 HIV-infected individuals across the United States, 49% of whom were MSM. Thirty-four percent reported incomplete partner notification. Incomplete partner notification was more likely among black men, MSM, and those reporting casual partners and non-condom use. Partner notification practices did not vary by HIV viral load except among those with casual partners in whom a detectable viral load was associated with incomplete partner notification. Increased sexual partner notification among HIV-infected men, especially MSM, is needed.
Keywords: HIV, sexual partners, sexually transmitted diseases/prevention and control, disease notification
Introduction
More than 170,000 individuals living in the United States(US), are unaware that they are HIV-infected(1). These individuals may not realize that they are at risk of HIV infection because their sexual partner(s) may not have disclosed their HIV status. This represents an important missed opportunity for 1) these individuals as they do not receive life-prolonging antiretroviral therapy and 2) public health as those who are not aware of their status are more likely to engage in risk behaviors than those who are aware of their HIV status(2), leading to ongoing HIV transmission. To address this, the US Centers for Disease Control and Prevention (CDC) has recommended routine HIV screening(3) as well as partner notification with active health department involvement for all individuals newly diagnosed with HIV infection and those with ongoing risk behaviors among those previously diagnosed(4). Partner notification allows partners of a patient thus diagnosed (an index patient) to be notified of their potential exposure to an infection and provided the appropriate counseling, testing and treatment. Partner notification may occur through several different approaches, including patient referral, where partners are directly notified by the index case, provider referral, where partners are notified by a provider or a public health professional such as a representative of a health department, or combinations of these two approaches. In the US, partner notification is generally considered a voluntary service with individual states holding the legal authority for notification and referral of partners of individuals with HIV(4).
Although data on sexual partner notification practices among HIV-infected patients receiving HIV care exist(5-10), little is known about whether these practices vary based on risk of HIV transmission as determined by HIV viral load. Moreover, there is a paucity of data specifically on the practices among men(11). This is concerning as men who have sex with men (MSM) are disproportionately impacted by the HIV epidemic and an estimated 44% of MSM are unaware of their HIV status(12, 13). Therefore, using data from a cohort of HIV-infected men receiving care in the Veterans Affairs Health Care System (VA) in the US, we sought to 1) describe the prevalence of partner notification practices; 2) examine whether these practices varied by HIV viral load; and 3) determine factors independently associated with incomplete partner notification.
Methods
We used data on HIV-infected men from the Veterans Aging Cohort Study (VACS), a longitudinal, multi-site study including patients receiving care through the VA Infectious Disease Clinics in Manhattan/Brooklyn, New York; Bronx, New York; Pittsburgh, Pennsylvania; Atlanta, Georgia; Houston, Texas; Baltimore, Maryland; Washington, D.C.; and Los Angeles, California VA Medical Centers(14, 15). Data sources include electronic medical records, administrative records and patient surveys. Patients are approached for potential recruitment with the permission of their providers. There are no specific exclusion criteria for the VACS. Overall, 9% of approached patients declined participation and the sample represents 58% of all HIV-infected patients seen in these clinics(15).
For this analysis, we used data collected between 2003 and 2004, the period during which items regarding partner notification were included in the VACS survey. The analytic sample was restricted to 1) men; 2) those who reported sexual activity in the prior 12 months; and 3) had available survey and HIV-1 RNA viral load data.
The dependent variable was partner notification practices. Patients were asked: Since you were diagnosed with HIV, have you told your sexual partners so that they can get tested and treated as well? Partner notification was considered complete if the patient responded I told every partner or I had the health department notify my partners for me. Partner notification was considered incomplete if the patient responded I told some partners, but not all of them; I did not tell any of them; I tried to notify my partners but could not find them; or I prefer not to answer this question.
The independent variable of interest was HIV viral load, determined based on HIV-1 RNA laboratory data collected closest to the date at which the responses regarding partner notification were obtained. Additional covariates included race/ethnicity, age and education. Clinical variables included years since HIV diagnosis (which we defined as time from the first date of CD4, HIV viral load, or enrollment, whichever came first); CD4 count closest to the survey date; Hepatitis C virus (HCV) status (based on laboratory data and ICD-9 codes); depressive symptoms (assessed using the Beck Depression Inventory [BDI]); unhealthy alcohol use (using an Alcohol Use Disorders Identification Test [AUDIT] score greater than or equal to 5); and alcohol and drug use disorders (based on ICD-9 codes). Sexual risk behaviors during the prior 12 months included: casual partners (sex with a partner who was not known ahead of time); multiple partners (defined as ≥2 partners in the prior 12 months); exchange of money or drugs for sex; non-condom use; previous diagnosis with a sexually transmitted disease; and last sex under the influence of alcohol or drugs.
We conducted descriptive statistics overall and by partner notification practices. We then used descriptive statistics to compare differences in partner notifications practices by the presence of an undetectable HIV-1 RNA viral load, defined as <500 copies/mL. Using Poisson regression, we estimated the relative risk and associated 95% confidence intervals to determine factors associated with incomplete partner notification. In the adjusted model, we included variables believed to be clinically relevant and those found to be significant (p<0.05) in bivariate analyses (unless they were collinear, Spearman coefficient >0.30). We conducted a sensitivity analysis excluding individuals who declined to answer the question about partner notification practices. We also examined whether factors associated with incomplete partner notification varied based on the presence of a casual partner during the past 12 months. We considered statistical significance to be results with a p<0.05.
Results
Patient Characteristics (Table 1)
Table 1. Factors associated with Incomplete Partner Notification, Relative Risk using Poisson Regression, n=760.
| Characteristic | Overall | Incomplete Partner Notification, N=261 (34%) | Complete Partner Notification, N=499 (66%) | p value | Unadjusted Relative Risk (95% CI) | Adjusted Relative Risk (95% CI) |
|---|---|---|---|---|---|---|
| Age (10 year increments) | 49 (9) | 49 (9) | 49 (9) | 0.55 | 0.97 (0.86, 1.08) | 1.08 (0.96, 1.22) |
| Race/Ethnicity, % | 0.45 | |||||
| White | 22 | 20 | 23 | ref | ref | |
| Black | 64 | 68 | 63 | 1.16 (0.90, 1.49) | 1.39 (1.06, 1.81) | |
| Hispanic | 9 | 8 | 10 | 0.99 (0.65, 1.49) | 1.18 (0.78, 1.77) | |
| Other | 4 | 3 | 4 | 0.85 (0.45, 1.60) | 0.91 (0.49, 1.70) | |
| Education, at least high school grad, % | 95 | 97 | 94 | 0.13 | ||
| Marriage Status | <0.001 | |||||
| Married | 15 | 9 | 18 | |||
| Divorce | 24 | 23 | 24 | |||
| Separated | 12 | 12 | 11 | |||
| Widowed | 3 | 3 | 4 | |||
| Never married | 30 | 40 | 25 | |||
| Living with a partner | 16 | 14 | 18 | |||
| MSM, % | 49 | 61 | 43 | <0.001 | 1.63 (1.33, 2.01) | 1.52 (1.21, 1.92) |
| Years Since HIV Diagnosis, median (IQR) | 6 (3, 8) | 6 (3, 8) | 6 (3, 8) | 0.68 | 1.00 (0.97, 1.03) | 1.00 (0.97, 1.03) |
| CD4 count, IQR | 422 (282, 589) | 439 (294, 588) | 411 (274, 589) | 0.39 | ||
| cART use | 93 | 93 | 93 | 0.87 | ||
| HIV-1 RNA viral load undetectable, % | 54 | 52 | 55 | 0.37 | 0.91 (0.75, 1.11) | 1.01 (0.83, 1.23) |
| HCV-infected, % | 45 | 44 | 45 | 0.83 | ||
| Depression (BDI score), median (IQR) | 3 (0, 7) | 3 (1, 7) | 3 (0, 7) | 0.74 | 1.00 (0.98, 1.02) | 1.00 (0.98, 1.02) |
| Unhealthy Alcohol Use, % | 24 | 28 | 22 | 0.07 | 1.23 (0.99, 1.52) | |
| Alcohol/Drug Use Disorder, % | 18 | 17 | 21 | 0.17 | 1.19 (0.94, 1.50) | 1.11 (0.87, 1.41) |
| Sexual Risk Behaviors, % | ||||||
| Casual partners | 19 | 33 | 12 | <0.001 | 2.10 (1.75, 2.53) | 1.80 (1.47, 2.20) |
| Multiple partners | 44 | 60 | 35 | <0.001 | 1.97 (1.63, 2.37) | |
| Exchanged money or drugs for sex | 10 | 17 | 7 | <0.001 | 1.82 (1.46, 2.27) | |
| Non-condom use | 20 | 26 | 17 | 0.003 | 1.41 (1.14, 1.74) | 1.29 (1.03, 1.62) |
| Diagnosed with a sexually transmitted infection | 13 | 17 | 11 | 0.03 | 1.34 (1.05, 1.71) | 1.21 (0.96, 1.54) |
| Last sex under the Influence of alcohol or drugs | 18 | 22 | 17 | 0.08 | 1.24 (0.98, 1.56) | 1.05 (0.84, 1.31) |
CI = Confidence Interval; MSM = men who have sex with men; STI = sexually transmitted infection; cART= combination antiretroviral therapy. IQR = interquartile range. Bold text indicates statistical significance.
Of the 1,525 HIV-infected patients who completed the survey, 765 were excluded as they were women (n=31), did not report being sexually active (n=505), or had missing survey or HIV-1 RNA viral load data (n=229). Patients missing data were not substantially different from patients not missing data. Our final analytic sample included 760 men. Overall, participants were racially/ethnically diverse (white 22%; black 64%; Hispanic 9%; other 4%) with a mean age of 49 years and 95% with at least a high school education (Table 1). Fifteen percent of participants were married; 24% divorced; 12% separated; 3% widowed; 30% never married; and 16% living with a partner. Participants had been diagnosed with HIV for a median of 6 years (inter-quartile range [IQR]=3, 8) and had median CD4 count of 422 cells/mm3 (IQR= 282, 589). While the majority of patients were on combination antiretroviral therapy (93%), only 54% had an undetectable HIV viral load. On average, the time span between viral load tests and survey dates was approximately two weeks (median [IQR]= 17 days [5, 34]). Forty-five percent were HCV-coinfected and depressive symptoms overall were low (median [IQR] BDI score= 3 [0, 7]). Twenty-four percent of participants met criteria for unhealthy alcohol use, with 18% having an alcohol or drug use disorder.
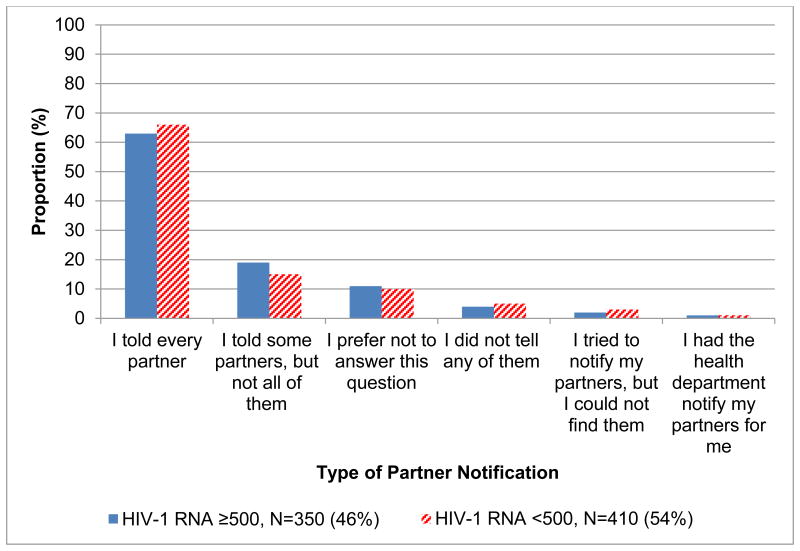
Partner Notification Practices (Figure 1)
Figure 1. Partner Notification Practices by HIV-1 RNA Viral Load, N=760.
Note: Partner notification practices did not vary based on the presence of a HIV-1 RNA ≥ 500 (p=0.65).
Two hundred and sixty one (34%) of the 760 men met criteria for incomplete partner notification. While 65% of participants reported that they told every sexual partner about their HIV status, only 1% involved the health department. A substantial proportion, therefore, did not meet criteria for complete partner notification: 17% told some partners, but not all of them; 4% did not tell any partners; 2% tried to notify partners but could not find them. Eleven percent of participants declined to answer the question. Partner notification practices did not differ based on the presence or absence of a detectable HIV viral load (p= 0.65).
Depression and Substance Use by Partner Notification Practices
BDI scores among participants with incomplete partner notification were similar to those with complete partner notification (median [IQR]= 3[1, 7], vs. 3[0, 7], p=0.74). The prevalence of unhealthy alcohol use was 28% among those with incomplete and 22% among those with complete partner notification (p=0.07). Seventeen percent of those with incomplete partner notification had an alcohol or drug use disorder compared to 21% of those with complete partner notification (p=0.17).
Sexual Practices and Risk Behaviors by Partner Notification Practices
Forty-nine percent of participants were MSM; this was more common among those who met criteria for incomplete partner notification compared to those with complete partner notification (61% vs. 43%, p<0.001). Fifty-eight percent of participants reported engaging in sexual risk behaviors in the past 12 months. Those with incomplete partner notification were more likely to report engaging in sexual risk behaviors, including having casual partners (33 vs. 12%, p<0.001), multiple partners (60% vs. 35%, p<0.001), exchanging money or drugs for sex (17% vs. 7%, p<0.001), non-condom use (26% vs. 17%, p=0.003), and being diagnosed with an sexually transmitted infection (17% vs. 11%, p=0.03). Those with incomplete partner notification were more likely to report that their last sexual encounter occurred under the influence of alcohol or drugs than those with complete partner notification (22% vs. 17%, p=0.08).
Factors Associated with Incomplete Partner Notification
In unadjusted analyses, MSM, compared to men who reported only having sex with women, and those who endorsed sexual risk behaviors were more likely to have incomplete partner notification. Having an undetectable viral load was not associated with partner notification practices.
In the adjusted analysis, black men (RR [95% CI]= 1.39 [1.06, 1.81]), MSM (RR [95% CI]= 1.52 [1.21, 1.92]) and those reporting sexual risk behaviors, including casual partners (RR [95% CI]= 1.80 [1.47, 2.20]) and non-condom use (RR [95% CI]= 1.29 [1.03, 1.62]) were more likely to have incomplete partner notification. Having an undetectable viral load remained unassociated with partner notification practices (RR [95%CI]= 1.01 [0.83, 1.23]).
In the sensitivity analysis that excluded those who declined to answer the question regarding partner notification, neither race/ethnicity nor non-condom use was associated with incomplete partner notification, though the trends remained similar. A previous diagnosis with a sexually transmitted infection was associated with incomplete partner notification. Our findings were otherwise unchanged. When we stratified by those who reported having casual partners versus not, the presence of an undetectable HIV-1 RNA viral load was not associated with partner notification practices among those without casual partners (RR [95%CI]= 1.20[0.93, 1.56]), but was negatively associated with incomplete partner notification (RR [95%CI]= 0.61 [0.44, 085]) among those with casual partners. In other words, those with a detectable viral load were less likely to notify partners. Non-condom use was no longer significantly associated with partner notification practices (RR [95%CI]= 0.95 [0.69, 1.30]). Findings were otherwise consistent with the main analysis.
Discussion
These data extend the existing literature by examining partner notification practices in a sample reflecting men from various sites in the US and while considering differences by HIV viral load. Importantly, as nearly half of our sample are MSM, our findings are highly relevant given the nature of the current HIV epidemic in the US(13). We found that more than one third of HIV-infected men failed to meet criteria for complete partner notification. Partner notification through the health department was exceptionally uncommon. The relative risk of incomplete partner notification was greater among black men, compared to white men, and MSM, compared to those who only have sex with women. Individuals with sexual risk behaviors, specifically casual partners and non-condom use, were less likely to have complete partner notification. Finally, partner notification practices did not vary based on the presence of a detectable HIV viral load overall, though among those with casual partners, those with a detectable HIV-1 RNA viral load were less likely to report complete partner notification.
Although approximately three in five respondents reported notifying all partners, this still leaves a substantial proportion of HIV-infected men in the VACS cohort who do not report notifying all their partners of their exposure to HIV. Importantly, the demographics of the groups more likely to have incomplete partner notification are similar to those who are disproportionately impacted by the HIV epidemic (blacks, MSM, sexual risk behaviors). Further, despite evidence supporting the role of the health department in facilitating partner notification(5, 16), these resources appear to be underused among patients receiving care in the VA setting. For example, based on their systematic review of the literature between 1988 and 2004, Passin et. al found that the majority of individuals (55-97%) would be willing to notify their partners directly if they tested positive for HIV and that there was high acceptability for partner services, including direct involvement of the health department(5). While these data are consistent with our results among a sample of men known to be HIV-infected, our findings indicate a lack of health department interaction. Moreover, the low rate of complete partner notification among individuals with a detectable HIV viral load, especially in the setting of casual partners, is particularly concerning and supports the need for ongoing prevention efforts for all individuals engaging in risk behaviors.
These findings should be interpreted in the context of several limitations. First, this study relies on data collected from 2003 to 2004, which may not reflect changes that have occurred in response to subsequent CDC recommendations(4). The data are valuable as estimates against which to compare current practices (especially veteran partner notification). Second, these participants were all receiving care through the VA; referral patterns to the health department for partner notification may differ in other healthcare settings. However, the VA has no independent system for conducting partner notification. Third, participants in the study had been diagnosed with HIV for an average of 6 years, leading to the potential for recall bias.
Fourth, the partner notification items were asked at a single point in time and focused on notifications since the time the men were diagnosed with HIV. Accordingly, whether a patient had a detectable HIV viral load when partners were exposed is unknown. Future research, with more frequent assessment of partner notification practices and viral load determination is warranted. Fifth, previous analyses have found that individuals are most likely to inform main partners of their status(5). We found that partner notification practices were less likely to be complete in the setting of a detectable viral load among those with recent casual partners. Our survey asked about relationships during the past 12 months, which may not accurately classify all the partners the participant has had since the time of their HIV diagnosis. Sixth, we are unable to account for the fact that some participants may have known the status of their partners. However, 45% of participants reported that since they were diagnosed with HIV, they have had sex with a person with HIV or a sexually transmitted disease. Therefore, we can assume that for many participants, partner notification would be highly relevant. Moreover, bidirectional partner notification remains important given the risk of transmission of different HIV viral strains. Seventh, as these data are based on self-report, they are subject to social desirability bias. Lastly, due to the sample size, we may have been underpowered to detect existing differences.
These limitations notwithstanding, these data support the need for interventions to optimize partner notification. Strategies, such as integrating public health authorities into clinical settings, have demonstrated favorable outcomes(9, 17). Novel approaches tailored to the specific needs of vulnerable populations, such as black MSM who engage in risk behaviors, are needed(18). Future studies examining system and provider-level factors contributing to low rates of partner notification and trends across different HIV treatment eras are warranted.
Conclusions
In summary, HIV-infected patients commonly report low rates of notification of their sexual partners. Interventions to optimize partner notification, especially among MSM, are desperately needed as an essential step towards curbing the HIV epidemic.
Acknowledgments
Sources of Funding: This work was generously supported by funding from the Robert Wood Johnson Foundation Clinical Scholars Program, Department of Veteran Affairs, National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism (U10-AA13566). Dr. Edelman was funded as Yale-Drug Abuse, Addiction, and HIV Research Scholar (K12DA033312-01A1) during the conduct of this work.
Footnotes
Conflicts of Interest: None declared.
This work was presented as a poster presentation at the Society of General Internal Medicine Annual Meeting. April 25th, 2013.
Disclosures: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the National Institute on Alcohol Abuse and Alcoholism, the Department of Veterans Affairs or the Centers for Disease Control and Prevention.
References
- 1.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data - United States and 6 U.S. dependent areas - 2011. 2013 Contract No.: 5. [Google Scholar]
- 2.Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005 Aug 1;39(4):446–53. doi: 10.1097/01.qai.0000151079.33935.79. [DOI] [PubMed] [Google Scholar]
- 3.Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006 Sep 22;55(RR-14):1–17. quiz CE1-4. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Recommendations for partner services programs for HIV infection, syphilis, gonorrhea, and chlamydial infection. MMWR Recomm Rep. 2008 Nov 7;57(RR-9):1–83. quiz CE1-4. [PubMed] [Google Scholar]
- 5.Passin WF, Kim AS, Hutchinson AB, Crepaz N, Herbst JH, Lyles CM. A systematic review of HIV partner counseling and referral services: client and provider attitudes, preferences, practices, and experiences. Sex Transm Dis. 2006 May;33(5):320–8. doi: 10.1097/01.olq.0000194597.16236.48. [DOI] [PubMed] [Google Scholar]
- 6.Mimiaga MJ, Reisner SL, Tetu AM, Cranston K, Bertrand T, Novak DS, et al. Psychosocial and behavioral predictors of partner notification after HIV and STI exposure and infection among MSM. AIDS Behav. 2009 Aug;13(4):738–45. doi: 10.1007/s10461-008-9424-y. [DOI] [PubMed] [Google Scholar]
- 7.Golden MR, Dombrowski JC, Wood RW, Fleming M, Harrington RD. A controlled study of the effectiveness of public health HIV partner notification services. Aids. 2009 Jan 2;23(1):133–5. doi: 10.1097/QAD.0b013e32831fb52f. [DOI] [PubMed] [Google Scholar]
- 8.Chen MJ, Pipkin S, Marcus JL, Bernstein KT, Scheer S. Using HIV testing history to measure the success of HIV partner services. Sex Transm Dis. 2013 May;40(5):419–21. doi: 10.1097/OLQ.0b013e318283bfcb. [DOI] [PubMed] [Google Scholar]
- 9.Udeagu CC, Shah D, Shepard CW, Bocour A, Guiterrez R, Begier EM. Impact of a New York City Health Department Initiative to Expand HIV Partner Services Outside STD Clinics. Public Health Rep. 2012 Jan;127(1):107–14. [PMC free article] [PubMed] [Google Scholar]
- 10.Song B, Begley EB, Lesondak L, Voorhees K, Esquivel M, Merrick RL, et al. Partner Referral by HIV-Infected Persons to Partner Counseling and Referral Services (PCRS) - Results from a Demonstration Project. Open AIDS J. 2012;6:8–15. doi: 10.2174/1874613601206010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mimiaga MJ, Reisner SL, Tetu AM, Bonafide KE, Cranston K, Bertrand T, et al. Partner notification after STD and HIV exposures and infections: knowledge, attitudes, and experiences of Massachusetts men who have sex with men. Public Health Rep. 2009 Jan-Feb;124(1):111–9. doi: 10.1177/003335490912400114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prejean J, Song R, Hernandez A, Ziebell R, Green T, Walker F, et al. Estimated HIV incidence in the United States, 2006-2009. PLoS One. 2011;6(8):e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Prevalence and awareness of HIV infection among men who have sex with men --- 21 cities, United States, 2008. MMWR Morb Mortal Wkly Rep. 2010 Sep 24;59(37):1201–7. [PubMed] [Google Scholar]
- 14.Justice AC, Erdos J, Brandt C, Conigliaro J, Tierney W, Bryant K. The Veterans Affairs Healthcare System: A unique laboratory for observational and interventional research. Med Care. 2006 Aug;44(8 Suppl 2):S7–12. doi: 10.1097/01.mlr.0000228027.80012.c5. [DOI] [PubMed] [Google Scholar]
- 15.Justice AC, Dombrowski E, Conigliaro J, Fultz SL, Gibson D, Madenwald T, et al. Veterans Aging Cohort Study (VACS): Overview and description. Med Care. 2006 Aug;44(8 Suppl 2):S13–24. doi: 10.1097/01.mlr.0000223741.02074.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hogben M, McNally T, McPheeters M, Hutchinson AB. The effectiveness of HIV partner counseling and referral services in increasing identification of HIV-positive individuals a systematic review. Am J Prev Med. 2007 Aug;33(2 Suppl):S89–100. doi: 10.1016/j.amepre.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 17.Taylor MM, Mickey T, Winscott M, James H, Kenney K, England B. Improving partner services by embedding disease intervention specialists in HIV-clinics. Sex Transm Dis. 2010 Dec;37(12):767–70. doi: 10.1097/OLQ.0b013e3181e65e8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edelman EJ, Cole CA, Richardson W, Boshnack N, Jenkins H, Rosenthal MS. Opportunities for Improving Partner Notification for HIV: Results from a Community-Based Participatory Research Study. AIDS Behav. 2014 Jan 28; doi: 10.1007/s10461-013-0692-9. [DOI] [PubMed] [Google Scholar]