Abstract
Background
The epidermal cells of the seed coat of certain species accumulate polysaccharides during seed development for cell wall reinforcement or release on imbibition to form mucilage. Seed-coat epidermal cells show natural variation in their structure and mucilage production, which could explain the diverse ecophysiological roles proposed for the latter. Arabidopsis mucilage mutants have proved to be an important tool for the identification of genes involved in the production of seed-coat polysaccharides.
Scope
This review documents genes that have been characterized as playing a role in the differentiation of the epidermal cells of the arabidopsis seed coat, the natural variability in polysaccharide features of these cells and the physiological roles attributed to seed mucilage.
Conclusions
Seed-coat epidermal cells are an excellent model for the study of polysaccharide metabolism and properties. Intra- and interspecies natural variation in the differentiation of these epidermal cells is an under-exploited resource for such studies and promises to play an important part in improving our knowledge of polysaccharide production and ecophysiological function.
Keywords: Seed coat, mucilage, polysaccharides, natural variation, arabidopsis, cell wall
INTRODUCTION
The seed coat plays a vital role in ensuring the transport of an embryo through time and space with the goal of maximizing the probability that germination and development of the new seedling occur in optimal conditions. Its characteristics determine dispersal and the protection of the embryo from abiotic and biotic stress, and control dormancy and germination (Windsor et al., 2000). The seed coat is a maternal tissue formed by the differentiation of ovule integuments during seed development. During this process, the epidermal cells can undergo cell wall thickening and accumulate polysaccharides in the apoplast. These polysaccharides have high water-binding capacity so that when hydrated on imbibition they expand, fragment the outer, distal cell wall of the epidermal cells and encapsulate the seed with viscous, sticky mucilage. Although the formation of seed mucilage, termed myxospermy, is not systematic in angiosperms, this trait has been noted for seeds from a range of plant species belonging to at least 100 families (Western, 2012; Yang et al., 2012b). Furthermore, depending on the plant species, released mucilage polysaccharides can either adhere to the seed coat and/or be non-adherent and diffuse into the surrounding media. The adherence of mucilage polysaccharides to the seed is associated with cellulose (Western, 2012).
Differentiation of the epidermal cells of the seed coat has been particularly well documented for Arabidopsis thaliana (arabidopsis) (Beeckman et al., 2000; Western et al., 2000; Windsor et al., 2000) and is summarized in Fig. 1. This starts after fertilization and terminates around three-quarters of the way through seed development, when the embryo has entered maturation and is accumulating reserves. The epidermal cells are polygonal when viewed from above and rectangular in cross-section, and initially they have a large vacuole that constrains the cytoplasm to the cell edges (Fig. 1A). Differentiation starts with a large increase in the size of the cells and the central vacuole. When the embryo enters the globular stage, starch granules are produced throughout the cytoplasm, and these become localized near the distal cell wall as the embryo develops from the heart stage to the torpedo stage (Fig. 1B). This is followed by the deposition of mucilage in the apoplast at the junction between the radial and distal cell walls (these polysaccharides are clearly visible when developing seeds have torpedo-stage embryos) and continues until the embryos are at the bent cotyledon stage (Fig. 1C). The polarized secretion of polysaccharides into the apoplast pushes the cytoplasm and starch granules into a column except for the proximal cell wall, and part of the radial cell walls, where the cytoplasm remains contiguous (Fig. 1D). Finally, the starch granules are degraded and secondary cell wall material is deposited at the top of the column of cytoplasm and along the border of the cytoplasm between the mucilage and the plasma membrane (Fig. 1E). This deposition gradually reduces the cytoplasm and generates the columella. On seed desiccation, the mucilage polysaccharides dehydrate to a thin layer between the distal primary cell wall and the reinforced radial cell walls and columella (Fig. 1F). On hydration, the expanding polysaccharides exert pressure on this outer primary cell wall, which fragments at the junction with the radial cell wall, pushing residual primary cell wall material upwards above the top of the columella (Fig. 1G, H).
Fig. 1.
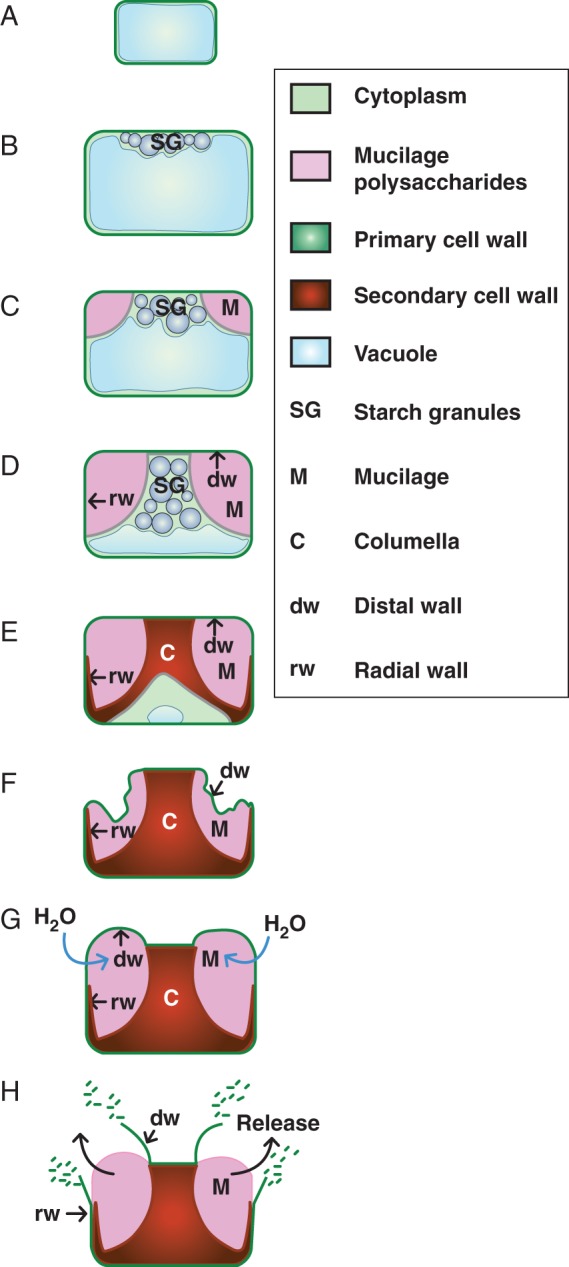
Schematic illustration of differentiation of epidermal cells of the seed coat in arabidopsis. (A) Initially, post-fertilization cells have a large single vacuole that compresses the cytoplasm to the cell edges. (B) When the embryo has passed the heart stage, cell size has increased and starch granules have formed in the cytoplasm. (C) In seeds with torpedo stage embryos, mucilage polysaccharides are accumulated in the apoplast. (D) A central column of cytoplasm, filled with starch granules, has been formed by the polar deposition of mucilage polysaccharides. (E) The cytoplasmic column is gradually filled with secondary wall material, which is also deposited along the bottom of the cell and the radial cell walls. The cytoplasm is reduced and starch granules are degraded. (F) Cells are dead at seed maturity and seed desiccation dehydrates mucilage polysaccharides to a thin layer around the central columella. (G) On imbibition, mucilage polysaccharides swell and push against the distal primary cell wall. (H) The distal cell wall breaks under the pressure exerted by the expanding polysaccharides, releasing them into the surrounding media.
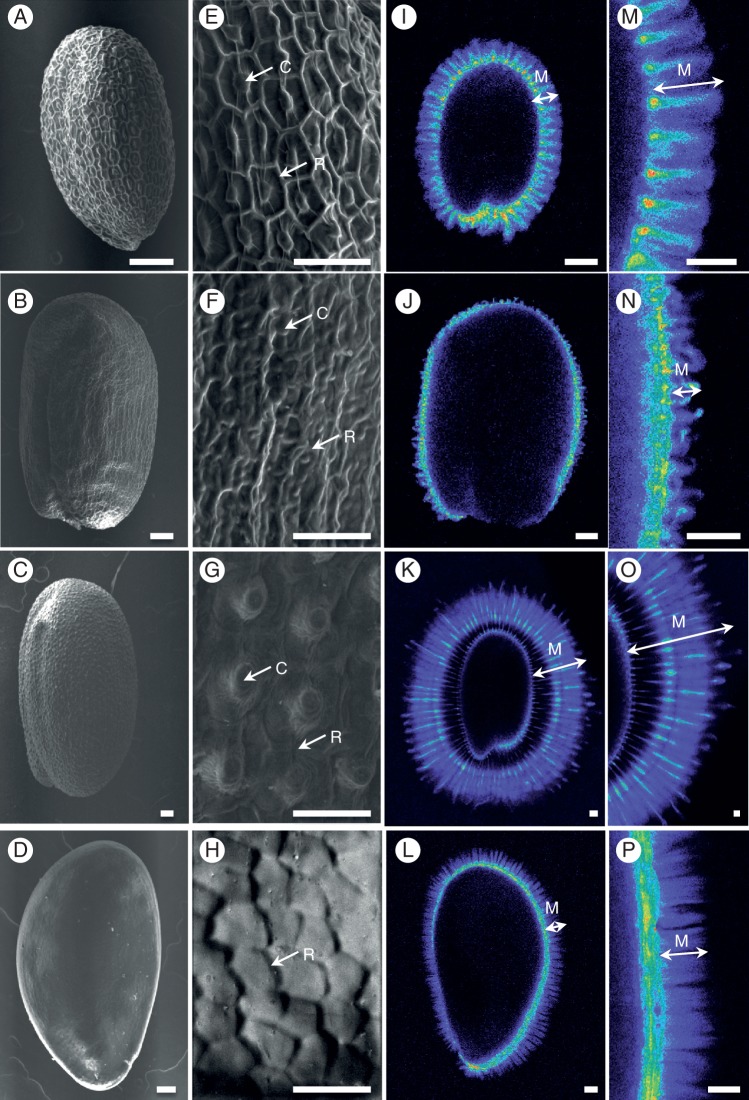
Seed coat differentiation shows natural variation that results in differences in seed coat structure and mucilage composition at both the inter- and intraspecies level. For example, only certain myxospermous species form columellae during the differentiation of their seed coat epidermal cells (Fig. 2A–P), although radial cell wall thickening is observed to some degree. Intraspecies genetic variation provides a rich resource of naturally occurring mutants for the identification and functional characterization of novel genes involved in polysaccharide metabolism, particularly now that next-generation genome sequencing techniques facilitate molecular genetic studies in species lacking genomic tools and resources. As the formation of mucilage polysaccharides and secondary wall thickening in seed coat epidermal cells is not essential for seed viability, studies of mutants affected in their production are facilitated, and in arabidopsis these have already proved to be an excellent tool for the identification of genes involved in polysaccharide metabolism (reviewed in Arsovski et al., 2010; Haughn and Western, 2012), and our current knowledge of the molecular basis of arabidopsis seed coat differentiation is detailed below. Furthermore, some of the genes have been identified thanks to natural arabidopsis variants. The polysaccharides produced during secondary cell wall thickening and secretion of mucilage polysaccharides are common cell wall components, and diverse techniques have been employed in their analysis from seed coat epidermal cells. Here we compare the information they have provided for the small number of species analysed to date and detail the advantages and drawbacks of the different methods used. Finally, we discuss the potential ecophysiological functions of seed-coat polysaccharides and how natural variation can contribute to their corroboration.
Fig. 2.
Natural variation in seed coat and mucilage characteristics. (A–H) Scanning electron micrographs of the surface of seeds showing differences in cell shape and features. (I–P) Cellulose within adherent mucilage was stained with 0·01 % (w/v) pontamine S4B in 50 mm NaCl, highlighting differences in cellulose organization and mucilage width. Confocal microscope images are shown using the Rainbow 2 look-up table. (A, E, I, M) Arabidopsis thaliana; (B, F, J, N) A. lyrata; (C, G, K, O) Camelina sativa; (D, H, L, P) Linum strictum ssp. strictum. Images in (E–H) and (M–P) are magnifications of regions in (A–D) and (I–L), respectively. Scale bars (A–D, I–L) = 100 μm; (E–H, M–P) = 50 μm. C, columella; M, mucilage; R, radial cell wall.
MOLECULAR GENETICS OF DIFFERENTIATION OF THE EPIDERMAL CELLS OF THE ARABIDOPSIS SEED COAT
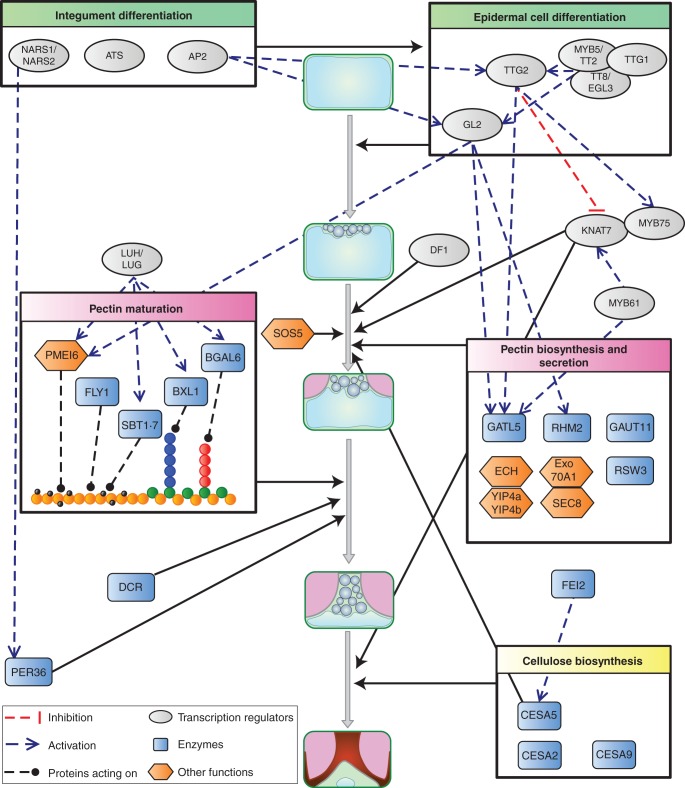
Several genes have been identified that are required for the correct differentiation of the epidermal cells of the arabidopsis seed coat and mucilage production and these are described below and summarized in Fig. 3. The gene products can be broadly grouped into two categories: transcription regulators and proteins involved in polysaccharide metabolism and secretion. Transcription factors that affect the specification of the integument during ovule formation, such as the KANADI family member ABERRANT TESTA SHAPE (ATS) (McAbee et al., 2006), will obviously affect subsequent seed coat development. Notably, APETALA2 (AP2) is necessary for the expression of the WRKY transcription factor TTG2 and the homeobox transcription factor GL2, which act during the early differentiation of seed-coat epidermal cells (Jofuku et al., 1994; Western et al., 2004). TTG2 and GL2 expression also requires the transcription complex formed from MYB (MYB5/TT2), bHLH (TT8/EGL3) and WD40 repeat (TTG1) proteins (Johnson et al., 2002; Zhang et al., 2003; Western et al., 2004; Gonzalez et al., 2009). Recently, TTG2 has also been implicated in the regulation of secondary cell wall deposition in the seed coat that occurs later in the differentiation process. The KNAT7 (Knox family) and MYB75 transcription factors appear to form a complex that modulates the production of mucilage polysaccharides, the reinforcement of radial cell walls and seed coat epidermal cell morphology, and TTG2 controls the expression of their genes (Romano et al., 2012; Bhargava et al., 2013). Interestingly, TTG2 appears to regulate KNAT7 negatively while regulating MYB75 expression positively. The regulatory network is further complicated, as KNAT7 expression requires MYB61 (Romano et al., 2012).
Fig. 3.
Schematic model of the role of, and interactions between gene products involved in the differentiation of the epidermal cells of the arabidopsis seed coat during seed development. Full black arrows indicate the developmental stage at which a protein, or group of proteins, is implicated. See main text for details of gene abbreviations, functions and interactions.
In contrast to the variety of transcription regulators mentioned above, only four enzymes have been identified to date that are directly responsible for the synthesis of the polysaccharides found in arabidopsis mucilage. The pectin component of arabidopsis mucilage is mainly rhamnogalacturonan I (RG I), which is formed from a repeating disaccharide of rhamnose (Rha) and galacturonic acid (GalA), linked →2)-α-l-Rha-(1 → 4)-α-d-GalA-(1→ (Goto, 1985; Western et al., 2000, 2004; Penfield et al., 2001; Usadel et al., 2004; Macquet et al., 2007a). Accordingly, the production of UDP-l-Rha, one of the basic subunits, is mandatory and a mutant of MUM4/RHM2 rhamnose synthase shows reduced accumulation of mucilage polysaccharides (Usadel et al., 2004; Western et al., 2004). RG I synthesis requires glycosyltransferases to add UDP-l-Rha or UDP-d-GalA to an oligo- or polysaccharide acceptor, and a mutant in the glycosyltransferase GATL5 showed a reduction in both the GalA and Rha contents of extracted mucilage (Kong et al., 2013). Interestingly, in mutants of the putative glycosyltransferase GAUT11 only a modest decrease in non-adherent mucilage GalA levels was noted (Caffall et al., 2009). This suggests that GATL5 participates in the synthesis of RG I while GAUT11 is involved in the synthesis of the more minor mucilage pectin homogalacturonan, which is uniquely (1 → 4)-α-d-GalA. GATL5 may also determine the final size of RG I polymers, as these had a higher molecular weight in gatl5 seed mucilage (Kong et al., 2013). The positive regulation of both RHM2 and GATL5 expression has been shown to require the GL2 transcription factor (Western et al., 2004; Kong et al., 2013), suggesting the integrated induction of genes involved in RG I synthesis. Nevertheless, unlike the expression of RHM2, that of GATL5 is also positively regulated by TTG2 and MYB61 (Kong et al., 2013) and it will be interesting to determine whether GATL5 is a downstream target of the network involving KNAT7. Using a whole-genome transcript correlation approach and using either GL2 or RHM2 as bait, a list of genes identified as being potentially involved in early mucilage biosynthesis has been produced (Vasilevski et al., 2012). Among these are the previously identified transcription factor genes MYB5 and TT8, as well as a putative MYB-like transcription factor named DF-1. df-1 mutants exhibit a mucilage release defect that can be partially rescued by imbibition in EDTA and is associated with reduced production of non-adherent RG I mucilage (Vasilevski et al., 2012). Further characterization is required to establish whether it is a downstream target of GL2 and regulates RHM2 expression.
Arabidopsis mucilage is cellulosic and the role of cellulose in anchoring mucilage pectin to the seed was confirmed by the phenotype of mutants with a defect in the gene for a cellulose synthase catalytic subunit (CESA), cellulose synthase5 (cesa5) (Harpaz-Saad et al., 2011; Mendu et al., 2011a; Sullivan et al., 2011). Reduced cellulose in adherent mucilage was associated with the redistribution of mucilage RG I from adherent to non-adherent layers. As some cellulose was still evident in the remaining adherent mucilage, it is likely that at least one of the other nine members of the CESA family contribute to its production. Although CESA2 and CESA9 have been excluded, as double mutants and a cesa2 cesa5 cesa9 mutant had the same phenotype as cesa5 (Harpaz-Saad et al., 2011; Mendu et al., 2011a), transcriptome data indicate that other family members, such as CESA10, are strongly expressed in the developing seed coat (http://seedgenenetwork.net/arabidopsis; Le et al., 2010) and could be involved. Similar reductions in the adhesion of mucilage pectin were observed in mutants of FEI2 and SOS5 (Harpaz-Saad et al., 2011). FEI2 is a receptor-like kinase proposed to act as a sensor of cell wall integrity and contributes to cellulose synthesis in roots (Xu et al., 2008) and the similarity of fei2 mucilage to that of cesa5 indicates that it plays the same role in seed coat epidermal cells. SOS5 is a fasciclin-like arabinogalactan protein with a glycophosphatidylinositol (GPI) anchor (Shi et al., 2003). Despite the general similarity of sos5 and cesa5 mucilage, a more detailed analysis of the single-mutant phenotypes and the more severe phenotype of the cesa5 sos5 double mutant suggest that sos5 affects mucilage RG I attachment through a mechanism different from cellulose synthesis (J. Griffiths, A. Tsai, C. Voiniciuc, B. Ellis, S.D. Mansfield and G.W. Haughn, University of British Columbia, Canada, pers. comm.).
Secretory processes play an important role in polysaccharide synthesis. Cellulose is synthesized directly at the plasma membrane, and cellulose synthase complexes appear to be transported in secretory vesicles originating from the trans-Golgi network (Crowell et al., 2009). In contrast, pectin and hemicelluloses are synthesized within the Golgi cisternae and this is followed by transport and deposition in the apoplast by Golgi-derived vesicles (reviewed in Driouich et al., 2012). Consequently, some mutants defective in components of the secretory system have been reported to show mucilage defects. Most membrane-bound and secreted proteins are N-glycosylated in the endoplasmic reticulum by glucosidase II. In the rsw3 mutant the α-subunit is affected and there are seed coat defects, including the absence of mucilage release. These could be due to the subsequent defective processing of non-glycosylated polysaccharide biosynthesis enzymes by the Golgi apparatus, or direct effects on the secretion of mucilage polysaccharides (Burn et al., 2002).
Post-Golgi vesicles are delivered to the plasma membrane in a multicomponent mechanism involving guanosine triphosphatases (GTPases) and vesicle complexes. The exocyst is an octomeric secretory vesicle complex implicated in tethering the vesicle to the plasma membrane in the final stages of exocytosis (Heider and Munson, 2012). Mutants in two of its subunits, exo70A1 and sec8, show reduced mucilage accumulation and a flattened columella, in agreement with a role for the exocyst in the polarized deposition of mucilage pectin (Kulich et al., 2010). Recently, proteins in another complex were shown to be required for post-Golgi trafficking of mucilage pectin to the plasma membrane. The YPT/RAB GTPase-interacting proteins YIP4a and YIP4b play a redundant role in this complex and interact with ECHIDNA (ECH) (Gendre et al., 2013). In yip4a yip4b and ech mutants, deposition of mucilage polysaccharides in the apoplast is reduced due to decreased export efficiency, and they accumulate in intracellular compartments (Gendre et al., 2013; McFarlane et al., 2013). Although the cytoplasmic domain where secretion of mucilage components occurs is lined with cortical microtubules, these do not appear to participate in targeting secretory vesicles to this domain, as secretion was not affected when microtubules were disrupted in the mor1 mutant (McFarlane et al., 2008). Alternative functions for these microtubules may be participation in cytoplasmic rearrangement to form a central column, or cellulose deposition in mucilage or the secondary cell wall of the columella.
After deposition, mucilage polysaccharides are matured by enzymes secreted into the apoplast. The maturation process modifies the properties of the polysaccharides, in particular their hydrophilic properties; mucilage is not released from developing seeds imbibed prior to this maturation (A. Berger and H. M. North, unpubl. res.). Accordingly, most enzymes involved in this maturation process have been identified due to mutants defective in mucilage release. BGAL6 β-d-galactosidase, which trims galactan ramifications from RG I, is affected in mucilage-modified2 (mum2) mutants (Dean et al., 2007; Macquet et al., 2007b). Mutants produce normal quantities of mucilage, but this does not swell greatly, even when the barrier imposed by cell walls is removed, demonstrating that removal of galactan side-chains from RG I modifies its hydrophilic properties. Interestingly, increased RG I branching in mum2 is also associated with increased partitioning of mucilage polysaccharides into the adherent mucilage layer (Macquet et al., 2007b), which could implicate galactan branching in mucilage adherence to the seed or the increased proximity of polysaccharides in enhancing their attachment. Trimming of arabinan side-chains from RG I appears to have similar effects on hydrophilicity, as defects in the β-xylosidase/α-arabinofuranosidase BXL1 cause delayed and patchy mucilage release (Arsovski et al., 2009a). BXL1 acts as an arabinofuranosidase in developing seed coat epidermal cells and, in addition to increased arabinan in mucilage pectin, the walls of these cells in a bxl1 mutant exhibit increased labelling with an antibody recognizing arabinan epitopes. The defect in mucilage release could therefore be due to a combination of increased cell wall stiffening and reduced mucilage hydrophilic potential (Arsovski et al., 2009a).
Other mutations that change the properties of the outer cell wall of the seed-coat epidermal cells have been shown to also alter mucilage release. Homogalacturonan is synthesized in a highly methylesterified state due to the activity of pectin methyltransferases, which add methylesters prior to deposition in the apoplast (Wolf et al., 2009). After secretion, pectin methylesterases (PMEs) remove methylesters and the resulting carboxyl groups can form Ca2+ crosslinks, which rigidify the pectin. Mutation of the PME inhibitor PMEI6, or subtilisin-like serine protease SBT1·7, results in the non-fragmentation of the outer cell wall of seed coat epidermal cells, which delays mucilage release (Rautengarten et al., 2008; Saez-Aguayo et al., 2013). When mucilage is released, the outer primary cell wall is observed as a large sheet attached to the seed hilum. Higher levels of PME activity in pmei6 or sbt1·7 mutants cause decreased homogalacturonan methylesterification, which would allow more Ca2+ crosslinks to be formed and increase cell wall strength. The SBT1·7 protease could control methylesterification of homogalacturonan through the proteolysis of a PME or removal of an inhibitory N-terminal signal sequence from a PMEI precursor (Rautengarten et al., 2008). As protein extracts of pmei6 sbt1·7 mutants showed an additive phenotype for PME activity, PMEI6 is not a target of SBT1·7, and the two proteins must modulate the activity of different PMEs (Saez-Aguayo et al., 2013). Interestingly, in seeds with normal outer cell wall rupture, most of the homogalacturonan detected in mucilage extracts is derived from wall fragments, and homogalacturonan is an extremely minor constituent of mucilage itself (Saez-Aguayo et al., 2013). Nevertheless, modification of the methylesterification of the homogalacturonan within mucilage could still have a significant effect on mucilage properties and contribute to the delay in mucilage release in mutants; arabinan and galactan side-chains are also minor mucilage constituents, but their removal dramatically modifies RG I properties. In effect, reduced homogalacturonan methylesterification in the mucilage of the flying saucer1 (fly1) mutant is associated with a novel phenotype in which released adherent mucilage appears more compact and is studded with discs corresponding to primary cell wall remnants that have detached from the columella tops (Voiniciuc et al., 2013). Mucilage release and cell wall fragmentation are normal when fly1-1 seeds are imbibed in alkaline cation chelators, indicating that Ca2+ crosslinks between homogalacturonan in the mucilage reduce mucilage swelling in the mutant. Some Ca2+ crosslinking must occur in wild-type mucilage as imbibition in EDTA results in a larger halo of adherent mucilage than imbibition in water (Rautengarten et al., 2008; Arsovski et al., 2009a; Saez-Aguayo et al., 2013), and the mutant phenotype indicates the regulation of homogalacturonan demethylesterification by FLY1 (Voiniciuc et al., 2013). The transmembrane RING E3 ubiquitin ligase encoded by FLY1 can carry out ubiquitination in concert with an E1 ubiquitin-activating enzyme and an E2 ubiquitin-conjugation enzyme, and mutants with altered expression of E1 and E2 partner enzymes in the seed coat would be expected to have the same phenotype as fly1. The intracellular localization of FLY1 in the post-Golgi endomembrane system suggests that it regulates demethylesterification indirectly, by marking proteins such as pectin methyltransferases and PMEs for retention and degradation, respectively (Voiniciuc et al., 2013).
Correct outer cell wall rupture for mucilage release was recently shown to require a class III peroxidase, PEROXIDASE36 (PER36); expression of PER36 was absent in a mutant defective for the NAM, ATAF and CUC (NAC) transcription factors NARS1 and NARS2, in which integument development is aberrant (Kunieda et al., 2008, 2013). PER36 is specifically localized to the junction between outer and radial cell walls, and on imbibition per36 mutant seeds exhibit delayed mucilage release with sheets of non-fragmented outer cell walls, similar to pmei6 and sbt1·7. The cell wall components targeted by the action of the peroxidase have yet to be identified, but one possibility is that the hydroxyl radicles generated degrade polysaccharides in a localized manner, thereby weakening the outer cell wall (Kunieda et al., 2013).
Analysis of the transcriptional regulation of proteins involved in pectin maturation has shown that BGAL6, BXL1, SBT1·7 and PMEI6 are controlled by a common regulator, LEUNIG_HOMOLOG (LUH)/MUM1 (Huang et al., 2011; Walker et al., 2011; Saez-Aguayo et al., 2013) and that this acts redundantly with LEUNIG (LUG) (Bui et al., 2011; Walker et al., 2011). Although LUH and LUG have been characterized as co-repressors using in vitro and in vivo assays (Sridhar et al., 2004), other in vitro assays indicate that LUH can activate expression, so precisely how they control the expression of seed coat epidermal cell target genes remains an open question (Huang et al., 2011). PMEI6 expression is also regulated by GL2 (Saez-Aguayo et al., 2013), but as MUM1 expression does not require GL2, AP2, TTG1 or TTG2 (Huang et al., 2011), this implies that two independent regulatory pathways participate in PMEI6 modulation.
It should be noted that factors that can affect mucilage release from seed coat epidermal cells are not necessarily related to the production of cell wall polysaccharides, but can influence the formation of cuticular waxes laid down over the outer cell wall. The defective in cuticular ridges (dcr) mutation affects an acyl transferase required for the biosynthesis of cutin polymers (Panikashvili et al., 2009). These lipid polyesters appear to influence the uptake of water by seed coat epidermal cells and it would seem that the absence of cutin polymers alters the cuticular waxes (Panikashvili et al., 2009) or introduces compensatory cell wall modifications such that water uptake is inhibited.
Polarized deposition of mucilage polysaccharides appears to be a prerequisite for the formation of the columella, as arabidopsis mutants with reduced mucilage accumulation, such as those with an impaired GLABRA2 transcription factor, have a flattened or no columella (Fig. 4). Nevertheless, the amount of mucilage polysaccharides produced in diverse mutants does not show an exact correlation with columella height (Fig. 4). This is particularly evident for the mum1 mutant and suggests that mucilage is only necessary for columella formation up to a certain threshold. Histochemical labelling has shown that the columella and radial cell walls contain both callose and cellulose (Macquet et al., 2007a; Mendu et al., 2011a). Synthesis of the cellulose in this secondary cell wall requires at least three catalytic subunits, CESA2, CESA5 and CESA9, which appear to play non-redundant roles because mutant combinations show additive phenotypes compared with single mutants (Stork et al., 2010; Mendu et al., 2011a). Defects in their genes reduce radial cell wall height and thickness, affect cell size and shape and can alter columella morphology. In contrast, knat7 and myb75 mutants have thicker radial cell walls and altered cell shape, indicating that they are negative regulators of secondary cell wall formation; columella height is also increased in myb75 mutants (Romano et al., 2012; Bhargava et al., 2013). It will be interesting to determine if CESAs are downstream targets of these transcription factors.
Fig. 4.
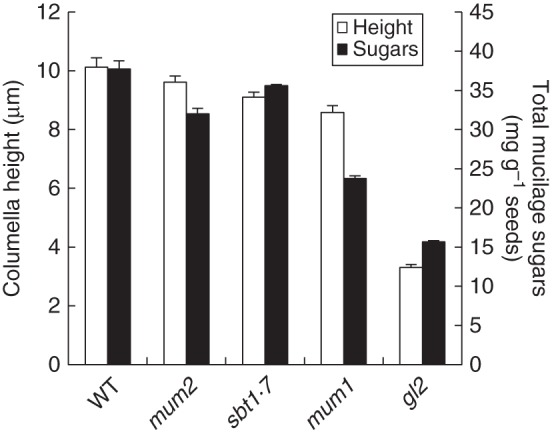
Comparison of columella height with the amount of total sugars from mucilage polysaccharides accumulated in different arabidopsis mucilage mutants. WT, wild type.
Despite the number of transcription regulators identified that are involved in the epidermal cell differentiation of the seed coat, few have been linked to the expression of the proteins implicated in polysaccharide metabolism in these cells. Furthermore, to date no study has examined the cis elements through which the transcription factors interact with the promoters of these proteins to regulate their expression. The expression of proteins specifically in the epidermal cells of the seed coat has potential biotechnological applications, yet only one study has been aimed at defining cis elements that could direct expression in these cells. A promoter regulatory region from DIRIGENT PROTEIN 1 (DP1) was shown to be sufficient for the specific expression of downstream reporter genes in the epidermal cells of the seed coat in arabidopsis and Brassica napus (Esfandiari et al., 2013). DP1 has been linked to phenylpropanoid synthesis, and it would be of interest to determine whether there are different elements specifying the expression of polysaccharide metabolism proteins in seed coat epidermal cells. GL2 has already been shown to negatively control CESA5 expression in roots, binding to an L1 box in the CESA5 promoter (Tominaga-Wada et al., 2009), and this cis element could play a similar role in the seed coat.
ANALYSIS OF SEED MUCILAGE COMPOSITION, PROPERTIES AND STRUCTURE
The chemical composition of seed coat mucilages is highly variable over the broad range of plants in which they occur (Western, 2012). Pectin and heteroxylan are most often the predominant polymers detected. One extreme is represented by A. thaliana non-adherent mucilage, which consists almost exclusively of pectic unbranched RG I, whereas Plantago ovata (psyllium) mucilage, which is almost exclusively arabinoxylans (Guo et al., 2008), represents the other. In other species, pectin and heteroxylan mixes are found in a variety of ratios, and these are sometimes associated with heteromannans (Western, 2012). Within the Brassicaceae, arabidopsis seed mucilage has been by far the most widely studied. As mentioned above, it is organized in two distinct layers, the outer layer non-adherent and the inner layer adherent (Western et al., 2000; Macquet et al., 2007a). In wild-type arabidopsis, the outer non-adherent layer can be easily recovered by gentle extraction with water or dilute chelator (Usadel et al., 2004; Macquet et al., 2007a; Rautengarten et al., 2008; Arsovski et al., 2009a). Rha and GalA, in a molar ratio close to 1, account for 90–95 % of the sugars, and sugar linkage analysis has shown that this non-adherent mucilage is primarily 1,2-linked Rha and 1,4-linked GalA, representing unbranched RG I (Arsovski et al., 2009a; Huang et al., 2011). Branch points (1,2,3-linked Rha, 1,2,4-linked Rha and 1,3,4-linked GalA) are also present, although in extremely low proportions (Arsovski et al., 2009a; Huang et al., 2011). Trace amounts of Ara, Xyl and Gal and occasionally Man and Glc have also been detected (Usadel et al., 2004; Macquet et al., 2007a; Rautengarten et al., 2008; Arsovski et al., 2009a; Huang et al., 2011; Mendu et al., 2011b; Saez-Aguayo et al., 2013; Voiniciuc et al., 2013). Few data have been published about the macromolecular characteristics of arabidopsis mucilage. A molar mass of 5200 kg mol−1 and a gyration radius of 152 nm were first estimated on diluted chelator-extracted mucilage by multi-angle laser light scattering after field flow fractionation (Usadel et al., 2004). The use of multi-angle laser light scattering after high-performance size-exclusion chromatography allowed the separation and analysis of two populations, a major (>90 %) homogeneous one of Mn ∼650–750 kg mol−1 and a minor one of very high Mn (30 000–48 500 kg mol−1), the latter corresponding to entangled, collapsed or aggregated macromolecules (Macquet et al., 2007a; Sullivan et al., 2011). A gyration radius of 60 and 211 nm was estimated for the first and second population, respectively (Macquet et al., 2007a). The major population thus consists of large macromolecules in a slightly expanded random coil conformation. It has been hypothesized that the population with very high molar mass could represent mucilage arising from the inner adherent layer (Macquet et al., 2007a).
More aggressive extraction protocols using a strong chelator, base or acid or particularly vigorous shaking have also been employed, often to induce mucilage liberation from mutants that do not rapidly release mucilage when seeds are imbibed in water (Daviere et al., 2008; Macquet et al., 2007b; Arsovski et al., 2009a; Huang et al., 2011; Walker et al., 2011; Voiniciuc et al., 2013). A large variety of extraction protocols have been used that differ in the type of extractant, normality, temperature and time of extraction, type of shaking, and dialysis/ethanol precipitation of the extract or not, and these have been carried out either as stand alone procedures or sequentially. Additional Rha and GalA can be extracted from wild-type seeds by water with vigorous shaking (Fig. 5A; Voiniciuc et al., 2013) or by the use of 0·05 m hydrochloric acid at 85 °C followed by 0·2 m sodium hydroxide at room temperature (HCl-NaOH in Fig. 5B); the latter greatly increases the amount of Ara and Glc extracted.
Table 1.
Details of seed mucilage extraction procedures used to generate data presented in Figure 5
| Graph | Bar | Extraction method | Data source |
|---|---|---|---|
| A | White | Water with gentle shaking at RT for 1 h | Voiniciuc et al. (2013) |
| Black | Sequential extraction; water with vigorous shaking at RT for 2 h | ||
| B | White | Water with gentle shaking at RT for 3 h | M.-C. Ralet, Personal data |
| Black | Sequential extraction; gentle shaking in 0·05 m HCl at 85 °C for 30 min followed by 0·2 m NaOH at RT for 15 min | ||
| C | Black | Digestion with rhamnogalacturonan hydrolase (RGase) at 40 °C for 16 h with gentle shaking after removal of non-adherent layer (gentle shaking, 0·05 m HCl, 85 °C, 30 min; 0·2 m NaOH, RT, 15 min) | M.-C. Ralet, Personal data |
| White | 1 m NaOH with vigorous shaking at RT for 40 min after removal of non-adherent layer (vigorous shaking, 0·05 m HCl, 30 min, 85 °C) | Walker et al. (2011) | |
| Dark grey | 2 m NaOH with gentle shaking at RT for 1 h after removal of non-adherent layer (gentle shaking, water, RT, 30 min; 0·2 m NaOH, RT, 1 h) | Huang et al. (2011) | |
| D | A, white | Water with gentle shaking at RT for 3 h | M.-C. Ralet, Personal data |
| A, dark grey | Sequential extraction; gentle shaking in 0·05 m HCl at 85 °C for 30 min followed by 0·2 m NaOH at RT for 15 min | ||
| A, hatched | Sequential extraction; digestion with rhamnogalacturonan hydrolase (RGase) for 16 h at 40 °C with gentle shaking | ||
| B, white | Water with gentle shaking at RT for 1 h | Voiniciuc et al. (2013) | |
| B, black | Sequential extraction; water with vigorous shaking at RT for 2 h | ||
| C, light grey | 0·2 m NaOH with vigorous shaking at RT for 1 h | Voiniciuc et al. (2013) |
RT, room temperature.
Fig. 5.
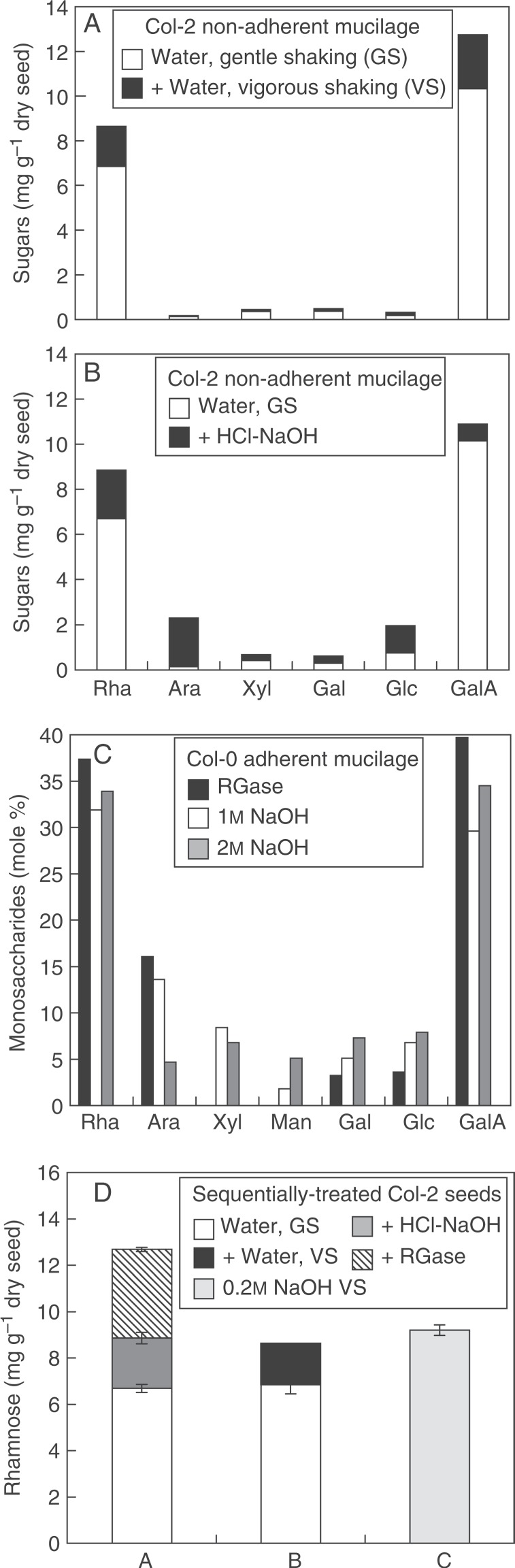
Comparison of mucilage extraction procedures. (A) Sugars present in non-adherent polysaccharides that had been extracted sequentially from intact Col-2 seeds (Voiniciuc et al., 2013). (B) Sugars present in non-adherent polysaccharides from intact Col-2 seeds that had been extracted sequentially (M.-C. Ralet, personal data). (C) Monosaccharide distribution in mole percentage of inner adherent mucilage extracted from Col-0 seeds (personal data; Huang et al., 2011; Walker et al., 2011). (D) Rhamnose solubilization from sequentially treated Col-2 seeds (M.-C. Ralet, personal data; Voiniciuc et al., 2013). See Table 1 for details of extraction procedures. GS, gentle shaking; VS, vigorous shaking; RGase, rhamnogalacturonan hydrolase.
The inner adherent layer of arabidopsis mucilage is difficult to analyse since it is partly resistant to extraction by standard chemical protocols (Macquet et al., 2007a). It has, however, been claimed to be largely solubilized by 0·2 m sodium hydroxide with vigorous shaking (Voiniciuc et al., 2013) or by 1 or 2 m sodium hydroxide (Huang et al., 2011; Walker et al., 2011). Digestion of adherent mucilage can be carried out on intact seeds after removal of the non-adherent mucilage using rhamnogalacturonan hydrolase, a pectolytic enzyme that cleaves unbranched RG I to oligomers, to solubilize the inner adherent mucilage layer (Macquet et al., 2007a; Sullivan et al., 2011; Saez-Aguayo et al., 2013; Kong et al., 2013). It should be noted that this enzyme is not commercially available. Monosaccharide distribution in mole percentages shows that the majority of sugars are Rha and GalA with the presence of Ara, Gal and Glc (Fig. 5C). Similar results were observed for 1 or 2 m sodium hydroxide fractions obtained after removal of non-adherent mucilage with HCl or NaOH (Fig. 5C; Huang et al., 2011; Walker et al., 2011). Expression of the results of quantitative sugar analysis as mass units of sugar per mass unit of seed is preferable as it provides a better understanding of the distribution of mucilage constituents between the two mucilage layers. The implementation of a sequential extraction with water, soft chemical and RG I hydrolase indicates that in Col-2, RG I is distributed in three layers: (1) a non-adherent one (6·7 mg Rha/g seed); (2) a loosely attached one (2·2 mg Rha/g seed); and (3) a strongly adherent one (3·8 mg Rha/g seed) (Fig. 5D). Interestingly, very similar trends were observed after sequential extraction by gentle shaking in water (6·9 mg Rha/g seed) followed by vigorous shaking in water (1·8 mg Rha/g seed) (Fig. 5D; Voiniciuc et al., 2013), the fraction recovered after vigorous shaking corresponding to the loosely attached RG I. Finally, vigorous shaking in 0·2 m sodium hydroxide would not seem to solubilize the strongly adherent layer (Fig. 5D; Voiniciuc et al., 2013).
Whatever the extraction process chosen for the release of non-adherent, loosely attached and strongly attached mucilage, it is noteworthy that unbranched RG I constitutes the predominant polymer in all arabidopsis extracts. Other polymers are, however, solubilized, and their amount increases with the severity of the extraction treatment. Homogalacturonan, type I and type II arabinogalactans, arabinoxylans and galacto(gluco)mannans have been identified (Walker et al., 2011). It is, however, particularly challenging to determine whether these polymers are part of the mucilage itself or if they originate from primary cell walls of the outer seed coat layer. As mentioned above, the majority of the methylesterified homogalacturonan present in non-adherent and adherent mucilage is derived from fragments of the outer cell wall (Saez-Aguayo et al., 2013).
Within the Brassicaceae, RG I-rich pectic components are generally predominant but large variability in mucilage chemical composition has been observed, with mainly unbranched RG I in arabidopsis, as detailed above, Gal-branched RG I in camelina (Camelina sativa) and 4-O-Me-GlcA-Gal-branched RG I in peppergrass (Lepidium sativum) (Deng et al., 2009; Pattathil et al., 2010). Mucilages extracted from these three species and mustard exhibit different monoclonal antibody recognition patterns (Pattathil et al., 2010), indicating major structural differences. All four mucilage extracts bound strongly to pectic backbone-directed monoclonal antibodies. Arabidopsis mucilage bound to pectic backbone monoclonal antibodies only, while camelina mucilage also bound to a specific RG I-directed monoclonal antibody set, peppergrass mucilage to a specific arabinan–galactan–arabinogalactan-related monoclonal antibody set and mustard mucilage to four different arabinan–galactan–arabinogalactan-related monoclonal antibody sets.
Due to its commercial use, such as a texturing agent in cosmetics, the chemical composition of flax (Linum usitatissimum) seed mucilage has also been much studied and has been characterized as a mixture of RG I, heteroxylans and presumably homogalacturonan (Paynel et al., 2013). The RG I polymer is unusual, with Rha residues that are highly branched at O-3 by single non-reducing l-Gal or l-Fuc residues (Naran et al., 2008). The heteroxylan polymer presents the distinctive characteristic of being highly (75 %) doubly substituted at O-2 and O-3 by single non-reducing Ara, Xyl, GlcA, Gal or Fuc residues and short 1,3 or 1,5-linked Ara chains (Warrand et al., 2005; Naran et al., 2008). Comprehensive physico-chemical analysis of flax mucilage is particularly difficult. Heteroxylan was claimed to exhibit shear thinning behaviour and RG I typical Newtonian-like behaviour (Cui et al., 1996). However, more recently Naran et al. (2008) observed Newtonian behaviour for both polymers. It is also unclear whether heteroxylan only or heteroxylan, homogalacturonan and RG I contribute to viscosity (Warrand et al., 2005; Naran et al., 2008; Paynel et al., 2013). Furthermore, some of the RG I molecules were found to associate with heteroxylan polymers, this composite exhibiting a viscosity substantially higher than those of the individual RG I and heteroxylan polysaccharides (Naran et al., 2008). In the latter study, however, viscosity measurements were conducted in distilled water and the high viscosity observed for RG I and RG I–heteroxylan composite is likely to reflect intra- and intermolecular electrostatic repulsions due to the acidic nature of RG I. Major variations in the chemical composition of flax seed mucilage have been associated with differences in the genotype, mother plant culture environment and extraction process used (cited by Paynel et al., 2013). In particular, variations in the distribution of different polymers and in their size occurred over the course of seed imbibition. These variations have been attributed, at least partly, to endogenous enzymatic activities (Paynel et al., 2013).
As mentioned above, the major constituent of psyllium mucilage is arabinoxylan (Guo et al., 2008). Psyllium mucilage can be totally recovered by sequential extraction of husks with water and 0·5 m sodium hydroxide. After neutralization the sodium hydroxide extract separates into two sub-fractions; one soluble and one gel-like. All the fractions are particularly rich in Xyl and Ara. Interestingly, the water-soluble fraction contains significant amounts of uronic acid and Rha residues, while the sodium hydroxide-neutralized, soluble one consists of uronic acid residues only (Guo et al., 2008). From sugar composition and yields, one can calculate that RG I and homogalacturonan are likely to constitute 4 and 1·5 % of psyllium mucilage, respectively, and arabinoxylan >90 %. Sugar linkage analysis showed that the mucilage is mainly composed of mixed linked arabinoxylan, with both 1,4- and 1,3-linked β-d-Xylp residues in the main chain branched at O-2 and/or O-3 by single non-reducing Ara and Xyl residues and short 1,3-linked arabinan chains. Classical arabinoxylans (1,4-linked β-d-Xylp main chain substituted at O-3 by single non-reducing Ara residues), typical of many dicotyledonous species, were also present. All fractions exhibited high apparent hydrodynamic diameters (95–184 nm, depending on the solubilization conditions) (Guo et al., 2008).
In addition to variability in the pectic and hemicellulosic components of mucilage, dispersed cellulose microfibrils can be present or absent, depending on plant families (Western, 2012). In arabidopsis this is required for the formation of an adherent mucilage layer (Harpaz-Saad et al., 2011; Mendu et al., 2011a; Sullivan et al., 2011). Cellulose in the adherent mucilage can be observed by cytochemical staining, immunolabelling or the use of polarized light. Diffuse cellulose is observed within the mucilage, interspersed with cellulose rays that extend from the top of the columella across the inner adherent mucilage layer (Fig. 2I, M; Windsor et al., 2000; Willats et al., 2001; Blake et al., 2006; Macquet et al., 2007a; Harpaz-Saad et al., 2011; Sullivan et al., 2011). Digestion of the inner adherent mucilage layer by pectolytic and cellulolytic enzymes in admixture allows degradation of both cellulose and RG I and has allowed cellulose content to be estimated at approximately 3 mg of cellulose per g of dry seed (Macquet et al., 2007a). Although cellulose has been shown to be involved in anchoring RG I to the seed, precisely how this is achieved is not yet understood (Harpaz-Saad et al., 2011; Sullivan et al., 2011).
Immunolabelling using antibodies against glycan epitopes has highlighted other spatial distinctions within the adherent mucilage layer of arabidopsis (Macquet et al., 2007a; Young et al., 2008). The LM5 and LM6 monoclonal antibodies recognize (1 → 4)-β-galactan and (1 → 5)-arabinan, respectively (Jones et al., 1997; Willats et al., 1998), and although galactan epitopes are detected throughout the adherent mucilage, arabinans appear mainly in a peripheral region (Macquet et al., 2007a). The localization and presence of hemicellulose in arabidopsis requires clarification, as although arabinoxylan has been detected in mucilage extracted with sodium hydroxide (Walker et al., 2011), the LM10 monoclonal antibody (McCartney et al., 2005) shows no labelling of adherent mucilage while a polyclonal antibody to xyloglucan (Moore et al., 1986) cross-reacts with epitopes in the adherent mucilage (Young et al., 2008).
NATURAL VARIATION IN SEED COAT EPIDERMAL CELL DIFFERENTIATION
Variation in the differentiation of seed-coat epidermal cells is observed as differences in final cell structure as well as mucilage composition and organization. Obvious cell structure differences include final cell shape or size, the presence of a columella and the width of the reinforced radial cell walls. These occur not just between different families, but also among taxa. For example, Arabidopsis lyrata has a small columella and thick radial cell walls, whereas camelina has a large, prominent columella and thin radial cell walls compared with A. thaliana (Fig. 2A–C, E–G). In the Brassicaceae, the final cell shape when viewed from above approximates to a hexagon, whereas the epidermal cell shape of flax seeds is less uniform and the seed surface appears smooth as cells do not have a columella and radial cell well reinforcement is limited (Fig. 2D, H).
Disparities in mucilage composition have been documented, as mentioned in the previous section, ranging from divergence in RG I ramifications within the Brassicaceae to more major differences in polysaccharide type between families (Guo et al., 2008; Deng et al., 2009; Pattathil et al., 2010; Paynel et al., 2013). Furthermore, analysis of natural variability within A. thaliana has found that the amount of non-adherent mucilage RG I produced can vary widely, as can its physico-chemical properties (J. Tran, H. M. North, INRA, Versailles, France, and D. Poulain, M-C Ralet, INRA, Nantes, France, unpubl. res.). Differences in the amount of water-extractable mucilage produced within a species have also been reported for flax seed and oilseed rape (Eskin, 1992; Diederichsen et al., 2006; Soto-Cerda et al., 2012). Moreover, mucilage production can vary between seeds produced by the same plant. The production of such heteromorphic seeds has been reported for Diptychocarpus strictus and shepherd's purse (Capsella bursa-pastoris) and might also be found in arabidopsis, in which thicker seed coats have been observed for seeds produced by secondary inflorescences (Kulich et al., 2010; Lu et al., 2010; Toorop et al., 2012).
Adherent mucilage formation is also subject to variability, with some natural variants of arabidopsis producing almost uniquely non-adherent mucilage (Saez-Aguayo et al., 2014). The cellulose present within the adherent mucilage layer also displays structural and quantitative differences that can be observed using the cellulose-specific cytochemical stain pontamine S4B. Although rays of cellulose are observed within A. lyrata adherent mucilage, there is less of the diffuse staining that is visible in A. thaliana adherent mucilage (Fig. 2I, J, M, N). In contrast, camelina has a large and dense halo of cellulose staining at the periphery of the adherent mucilage layer that is much wider than that of A. thaliana (Fig. 2K, O). Curiously, the domain closest to the camelina seed is only weakly labelled, despite clear labelling of cellulose on the seed surface, suggesting that the cellulose is masked from interacting with the stain and that the adherent mucilage shows spatial differences in composition. Flax seed has previously been shown to lack long cellulose microfibrils when examined by scanning electron microscopy or stained with methylene blue (Mühlethaler, 1950; Western, 2012), but another member of the Linaceae, Linum strictum, shows cellulose staining (Fig. 2L, P). Interestingly, this staining is diffuse without rays, and as the cellulose rays observed in Brassiaceae mucilage appear to originate from the top of the columella it is tempting to speculate that their presence is associated with columella formation.
The wealth of diversity observed in the polysaccharide components of the seed coat epidermal cells indicates that a large variety of genetic elements and mechanisms are involved in their production. To date, naturally occurring seed coat variants have only been exploited for the identification of two genes involved in polysaccharide production, both from A. thaliana (Macquet et al., 2007b; Saez-Aguayo et al., 2013). The advent of next-generation sequencing will facilitate the development of this approach in other species. One likely handicap for such studies is the size of the available seed germplasm. Most myxospermous species are wild plants, and although seed collection through the Kew Millennium Seed Bank Partnership has incited the collection and storage of wild plant seeds, this is focused on the plants and regions most threatened by climate change. Commercially exploited myxospermous species, such as flax seed and psyllium, are the most likely to be taken advantage of initially due to the range of genotypes available.
An alternative strategy to the forward genetic approach using variants within a species has exploited the facility of dissecting the cells producing mucilage from pysllium seeds during seed development. Expressed sequence tag profiling was used to find abundant transcripts with homologues that are also present in arabidopsis tissues undergoing secondary wall formation. Two arabidopsis genes were identified, IRREGULAR XYLEM (IRX) 15 and IRX15-LIKE, that are involved in xylan biosynthesis (Jensen et al., 2011). This technique could easily be extended to other species in which the separation of the epidermal cells of the seed coat is less straightforward, using the technique of laser capture microdissection.
ADAPTIVE FUNCTION OF MUCILAGE
Mucilage production during seed development is a significant metabolic drain on the mother plant; mucilage can represent at least 2 % of flax seed mass (Naran et al., 2008) and 3 % of arabidopsis seed mass (Macquet et al., 2007a). The adaptive value of mucilage has long interested scientists, including Darwin (1859), and diverse functions have been proposed for seed mucilage, yet its precise ecophysiological role remains unclear. The production of mucilage by the seed coat occurs mainly in phylogenetically advanced plant families, suggesting that it is an evolutionarily advanced trait (Yang et al., 2012b). The production of two different mucilage layers, non-adherent and adherent, appears to occur in other species as well as arabidopsis, such as flax seed (Fig. 2I–P; Naran et al., 2008; Western, 2012). The apparent differences in the structure and properties of these layers could be related to differing ecophysiological functions (Macquet et al., 2007a), which might explain in part the diversity of the proposed roles of mucilage. Predicting mucilage function is further complicated as mucilage properties may also be modified over time, by glycosyl hydrolases released from the seed itself or from soil microorganisms. Flax seed mucilage composition and physico-chemical properties were found to vary with time after release due to the action of enzymes released from the seeds (Paynel et al., 2013), and biodegradation of Artemisia sphaerocephala mucilage was observed in soil microbiomes derived from natural habitats (Yang et al., 2012a). Differences in mucilage composition and properties when it is released on imbibition may not, therefore, directly reflect those required for its ecophysiological function. Derivatives of mucilage degradation, in addition to increasing the biomass of soil microbes in the surrounding rhizosphere, were taken up by A. sphaerocephala seedlings and promoted their establishment (Yang et al., 2012a). Mucilage could thus play multiple and non-exclusive roles before and after degradation.
Frequently mucilage has been proposed to play a role in dispersion mechanisms aimed either at long-distance seed transport or maintaining the seed close to the mother plant (Yang et al., 2012b). A number of arabidopsis accessions have been identified that do not release mucilage on imbibition (Macquet et al., 2007b; Saez-Aguayo et al., 2013, 2014). When water is added to seeds of these variants they float, instead of sinking due to the increased seed specific gravity from released and hydrated mucilage (Saez-Aguayo et al., 2014). Floating could enable them to be dispersed by water, as has previously been observed for seeds of the myxospermic species Plantago coronopus (Gutterman and Shem-Tov, 1996).
Mutations that generate intra- and interspecies natural variation could produce ecophysiological advantages; genetic polymorphisms will only be retained if they provide an advantage or are neutral. The identification of similar mucilage characteristics in sites with a common factor is indicative of natural selection for a physiological function. Thus, in addition to being tools for the identification of novel genes, natural variants can yield clues as to the adaptive function of seed mucilage by comparison of the habitats from which they have been collected. A study of mucilage production in Artemisia found that most myxospermic taxa occurred in dry habitats (Kreitschitz, 2012). Due to its hydrogel properties, mucilage has frequently been implicated in the enhancement of water uptake by seeds during imbibition, with a consequent improvement of germination (reviewed by Western, 2012; Yang et al., 2012b). Low-field nuclear magnetic resonance analysis of water mobility during the imbibition of seeds showed that although water is taken up rapidly by released mucilage in wild-type seeds, this does not improve water transfer to internal seed tissues, compared with arabidopsis mucilage release mutants (Saez-Aguayo et al., 2014), indicating that its hydrogel properties serve uniquely in the retention of water around the seed.
Mucilage may influence other aspects of seed physiology, such as seed bank maintenance and germination. Seeds of A. sphaerocephala with mucilage have improved DNA repair compared with those lacking mucilage, when subjected to cycles of hydration by desert dew (Huang et al., 2008; Yang et al., 2011). Maintenance of DNA integrity is critical for preserving seed viability, and water retention by mucilage in dry environments would prolong the imbibed state, thereby contributing to seed longevity. A number of arabidopsis mucilage mutants exhibit delayed germination (reviewed by Western, 2012; Yang et al., 2012b). Nevertheless, many of the mutants studied have pleiotropic phenotypes, such as reduced tannins, that also affect germination and complicate the interpretation of results. Comparison of heteromorphic seeds from shepherd's purse indicated that seeds with mucilage had stronger secondary dormancy than those without mucilage (Toorop et al., 2012), but again other traits were variable between seed types, such as nitrogen content, which can also affect dormancy. Comparison of different aspects of seed physiology in mutants uniquely affected in mucilage characteristics should permit clarification of the adaptive function of mucilage.
FUTURE PERSPECTIVES
The mucilage-producing epidermal cells of the seed coat are an excellent model for studying different types of polysaccharide found in plant cell walls. Since the first detailed descriptions of their differentiation in arabidopsis in 2000, their potential has gradually been recognized; for example, there was a boom in the number of presentations concerning mucilage production in several species at the Plant Cell Wall meeting held in Nantes in 2013. Nonetheless, the identification of mucilage mutants through screens has not yet reached saturation, and there are clear gaps in the schematic model of seed coat epidermal cell differentiation (Fig. 3), such as the identification of the PME targets of PMEI6 and SBT1·7, and additional genes involved in the synthesis of mucilage polysaccharides. Furthermore, the molecular identity of genes defective in a number of mutants remains to be identified: mum enhancer (men)1, men2, men4, men5, men6, mum5 and prairie (Western et al., 2001; Western, 2006; Arsovski et al., 2009b). The use of natural variation in the differentiation of the seed coat is currently an under-exploited resource for the identification of polysaccharide metabolism genes. Screens of natural mucilage variants have proved fruitful as a screen of 488 genotypes identified 71 natural mucilage release mutants, in which three different genes were mutated (Saez-Aguayo et al., 2014). The interspecies variation in mucilage composition also remains to be fully exploited for the identification of genes involved in heteroxylan synthesis. With increased use of seed coat epidermal cells for the study of polysaccharide metabolism, the coming years promise to be rich with new discoveries.
ACKNOWLEDGEMENTS
This work was supported in part by the ANR programme (grant number ANR-08-BLAN-0061), including a doctoral fellowship to S.S.-A. S.S.-A. also received financial support from the Fondecyt project 3140415. We thank Yves Pauthier (Muséum National d'Histoire Naturelle, Département des Jardins Botaniques et Zoologiques, Graineterie, Paris, France) for providing seeds. The Zeiss 710 confocal microscope used for the acquisition of images is located at the IJPB Observatoire du Végétal and was cofinanced by a grant from the Conseil Général des Yvelines.
LITERATURE CITED
- Arsovski AA, Popma TM, Haughn GW, Carpita NC, McCann MC, Western TL. AtBXL1 encodes a bifunctional beta-D-xylosidase/alpha-L-arabinofuranosidase required for pectic arabinan modification in Arabidopsis mucilage secretory cells. Plant Physiology. 2009a;150:1219–1234. doi: 10.1104/pp.109.138388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsovski AA, Villota MM, Rowland O, Subramaniam R, Western TL. MUM ENHANCERS are important for seed coat mucilage production and mucilage secretory cell differentiation in Arabidopsis thaliana. Journal of Experimental Botany. 2009b;60:2601–2612. doi: 10.1093/jxb/erp102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsovski AA, Haughn GW, Western TL. Seed coat mucilage cells of Arabidopsis thaliana as a model for plant cell wall research. Plant Signaling & Behavior. 2010;5:796–801. doi: 10.4161/psb.5.7.11773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeckman T, De Rycke R, Viane R, Inzé D. Histological study of seed coat development in Arabidopsis thaliana. Journal of Plant Research. 2000;113:139–148. [Google Scholar]
- Bhargava A, Ahad A, Wang S, et al. The interacting MYB75 and KNAT7 transcription factors modulate secondary cell wall deposition both in stems and seed coat in Arabidopsis. Planta. 2013;237(1199–1211) doi: 10.1007/s00425-012-1821-9. [DOI] [PubMed] [Google Scholar]
- Blake AW, McCartney L, Flint JE, et al. Understanding the biological rationale for the diversity of cellulose-directed carbohydrate-binding modules in prokaryotic enzymes. Journal of Biological Chemistry. 2006;281:29321–29329. doi: 10.1074/jbc.M605903200. [DOI] [PubMed] [Google Scholar]
- Bui M, Lim N, Sijacic P, Liu Z. LEUNIG_HOMOLOG and LEUNIG regulate seed mucilage extrusion in Arabidopsis. Journal of Integrative Plant Biology. 2011;53:399–408. doi: 10.1111/j.1744-7909.2011.01036.x. [DOI] [PubMed] [Google Scholar]
- Burn JE, Hurley UA, Birch RJ, Arioli T, Cork A, Williamson RE. The cellulose-deficient Arabidopsis mutant rsw3 is defective in a gene encoding a putative glucosidase II, an enzyme processing N-glycans during ER quality control. Plant Journal. 2002;32:949–960. doi: 10.1046/j.1365-313x.2002.01483.x. [DOI] [PubMed] [Google Scholar]
- Caffall KH, Pattathil S, Phillips SE, Hahn MG, Mohnen D. Arabidopsis thaliana T-DNA mutants implicate GAUT genes in the biosynthesis of pectin and xylan in cell walls and seed testa. Molecular Plant. 2009;2:1000–1014. doi: 10.1093/mp/ssp062. [DOI] [PubMed] [Google Scholar]
- Crowell EF, Bischoff V, Desprez T, et al. Pausing of Golgi bodies on microtubules regulates secretion of cellulose synthase complexes in Arabidopsis. Plant Cell. 2009;21:1141–1154. doi: 10.1105/tpc.108.065334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W, Kenaschuk E, Mazza G. Influence of genotype on chemical composition and rheological properties of flaxseed gums. Food Hydrocolloids. 1996;10:221–227. [Google Scholar]
- Darwin C. On the origin of species by means of natural selection. London: Murray; 1859. [Google Scholar]
- Daviere JM, de Lucas M, Prat S. Transcriptional factor interaction: a central step in DELLA function. Current Opinion in Genetics & Development. 2008;18:295–303. doi: 10.1016/j.gde.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Dean GH, Zheng H, Tewari J, et al. The Arabidopsis MUM2 gene encodes a β-galactosidase required for the production of seed coat mucilage with correct hydration properties. Plant Cell. 2007;19:4007–4021. doi: 10.1105/tpc.107.050609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C, O'Neill MA, Hahn MG, York WS. Improved procedures for the selective chemical fragmentation of rhamnogalacturonans. Carbohydrate Research. 2009;344:1852–1857. doi: 10.1016/j.carres.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Diederichsen A, Raney JP, Duguid SD. Variation of mucilage in flax seed and its relationship with other seed characteristics. Crop Science. 2006;46:365–371. [Google Scholar]
- Driouich A, Follet-Gueye ML, Bernard S, et al. Golgi-mediated synthesis and secretion of matrix polysaccharides of the primary cell wall of higher plants. Frontiers in Plant Science. 2012;3 doi: 10.3389/fpls.2012.00079. 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esfandiari E, Jin Z, Abdeen A, Griffiths JS, Western TL, Haughn GW. Identification and analysis of an outer-seed-coat-specific promoter from Arabidopsis thaliana. Plant Molecular Biology. 2013;81:93–104. doi: 10.1007/s11103-012-9984-0. [DOI] [PubMed] [Google Scholar]
- Eskin NAM. Effect of variety and geographical location on the incidence of mucilage in canola seeds. Canadian Journal of Plant Science. 1992;72:1223–1225. [Google Scholar]
- Gendre D, McFarlane HE, Johnson E, et al. Trans-Golgi network localized ECHIDNA/Ypt interacting protein complex is required for the secretion of cell wall polysaccharides in Arabidopsis. Plant Cell. 2013;25:2633–2646. doi: 10.1105/tpc.113.112482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A, Mendenhall J, Huo Y, Lloyd A. TTG1 complex MYBs, MYB5 and TT2, control outer seed coat differentiation. Developmental Biology. 2009;325:412–421. doi: 10.1016/j.ydbio.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Goto N. A mucilage polysaccharide secreted from testa of Arabidopsis thaliana. Arabidopsis Information Service. 1985;22:143–145. [Google Scholar]
- Guo Q, Cui SW, Wang Q, Young JC. Fractionation and physicochemical characterization of psyllium gum. Carbohydrate Polymers. 2008;73:35–43. [Google Scholar]
- Gutterman Y, Shem-Tov S. Structure and function of the mucilaginous seed coats of Plantago coronopus inhabiting the Negev desert of Israel. Israel Journal of Plant Sciences. 1996;44:125–133. [Google Scholar]
- Harpaz-Saad S, McFarlane HE, Xu S, et al. Cellulose synthesis via the FEI2 RLK/SOS5 pathway and CELLULOSE SYNTHASE 5 is required for the structure of seed coat mucilage in Arabidopsis. Plant Journal. 2011;68:941–953. doi: 10.1111/j.1365-313X.2011.04760.x. [DOI] [PubMed] [Google Scholar]
- Haughn GW, Western TL. Arabidopsis seed coat mucilage is a specialized cell wall that can be used as a model for genetic analysis of plant cell wall structure and function. Frontiers in Plant Science. 2012;3:64. doi: 10.3389/fpls.2012.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heider MR, Munson M. Exorcising the exocyst complex. Traffic. 2012;13:898–907. doi: 10.1111/j.1600-0854.2012.01353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, DeBowles D, Esfandiari E, Dean G, Carpita NC, Haughn GW. The Arabidopsis transcription factor LUH/MUM1 is required for extrusion of seed coat mucilage. Plant Physiology. 2011;156:491–502. doi: 10.1104/pp.111.172023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Boubriak I, Osborne DJ, Dong M, Gutterman Y. Possible role of pectin-containing mucilage and dew in repairing embryo DNA of seeds adapted to desert conditions. Annals of Botany. 2008;101:277–283. doi: 10.1093/aob/mcm089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JK, Kim H, Cocuron JC, Orler R, Ralph J, Wilkerson CG. The DUF579 domain containing proteins IRX15 and IRX15-L affect xylan synthesis in Arabidopsis. Plant Journal. 2011;66:387–400. doi: 10.1111/j.1365-313X.2010.04475.x. [DOI] [PubMed] [Google Scholar]
- Jofuku KD, den Boer BG, Van Montagu M, Okamuro JK. Control of Arabidopsis flower and seed development by the homeotic gene APETALA2. Plant Cell. 1994;6:1211–1225. doi: 10.1105/tpc.6.9.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CS, Kolevski B, Smyth DR. TRANSPARENT TESTA GLABRA2, a trichome and seed coat development gene of Arabidopsis, encodes a WRKY transcription factor. Plant Cell. 2002;14:1359–1375. doi: 10.1105/tpc.001404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L, Seymour GB, Knox JP. Localization of pectic galactan in tomato cell walls using a monoclonal antibody specific to (1→4)-β-D-galactan. Plant Physiology. 1997;113:1405–1412. doi: 10.1104/pp.113.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y, Zhou G, Abdeen AA, et al. GALACTURONOSYLTRANSFERASE-LIKE5 is involved in the production of Arabidopsis seed coat mucilage. Plant Physiology. 2013;163:1203–1217. doi: 10.1104/pp.113.227041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitschitz A. Mucilage formation in selected taxa of the genus Artemisia L. (Asteraceae, Anthemideae) Seed Science Research. 2012;22:177–189. [Google Scholar]
- Kulich I, Cole R, Drdova E, et al. Arabidopsis exocyst subunits SEC8 and EXO70A1 and exocyst interactor ROH1 are involved in the localized deposition of seed coat pectin. New Phytologist. 2010;188:615–625. doi: 10.1111/j.1469-8137.2010.03372.x. [DOI] [PubMed] [Google Scholar]
- Kunieda T, Mitsuda N, Ohme-Takagi M, et al. NAC family proteins NARS1/NAC2 and NARS2/NAM in the outer integument regulate embryogenesis in Arabidopsis. Plant Cell. 2008;20:2631–2642. doi: 10.1105/tpc.108.060160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunieda T, Shimada T, Kondo M, Nishimura M, Nishitani K, Hara-Nishimura I. Spatiotemporal secretion of PEROXIDASE36 is required for seed coat mucilage extrusion in Arabidopsis. Plant Cell. 2013;25:1355–1367. doi: 10.1105/tpc.113.110072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le BH, Cheng C, Bui AQ, et al. Global analysis of gene activity during Arabidopsis seed development and identification of seed-specific transcription factors. Proceedings of the National Academy of Sciences of the USA. 2010;107:8063–8070. doi: 10.1073/pnas.1003530107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Tan D, Baskin JM, Baskin CC. Fruit and seed heteromorphism in the cold desert annual ephemeral Diptychocarpus strictus (Brassicaceae) and possible adaptive significance. Annals of Botany. 2010;105:999–1014. doi: 10.1093/aob/mcq041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macquet A, Ralet MC, Kronenberger J, Marion-Poll A, North HM. In situ, chemical and macromolecular study of the composition of Arabidopsis thaliana seed coat mucilage. Plant Cell Physiology. 2007a;48:984–999. doi: 10.1093/pcp/pcm068. [DOI] [PubMed] [Google Scholar]
- Macquet A, Ralet MC, Loudet O, et al. A naturally occurring mutation in an Arabidopsis accession affects a β-D-galactosidase that increases the hydrophilic potential of rhamnogalacturonan I in seed mucilage. Plant Cell. 2007b;19:3990–4006. doi: 10.1105/tpc.107.050179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAbee JM, Hill TA, Skinner DJ, et al. ABERRANT TESTA SHAPE encodes a KANADI family member, linking polarity determination to separation and growth of Arabidopsis ovule integuments. Plant Journal. 2006;46:522–531. doi: 10.1111/j.1365-313X.2006.02717.x. [DOI] [PubMed] [Google Scholar]
- McCartney L, Marcus SE, Knox JP. Monoclonal antibodies to plant cell wall xylans and arabinoxylans. Journal of Histochemistry and Cytochemistry. 2005;53:543–546. doi: 10.1369/jhc.4B6578.2005. [DOI] [PubMed] [Google Scholar]
- McFarlane HE, Young RE, Wasteneys GO, Samuels AL. Cortical microtubules mark the mucilage secretion domain of the plasma membrane in Arabidopsis seed coat cells. Planta. 2008;227:1363–1375. doi: 10.1007/s00425-008-0708-2. [DOI] [PubMed] [Google Scholar]
- McFarlane HE, Watanabe Y, Gendre D, et al. Cell wall polysaccharides are mislocalized to the vacuole in echidna mutants. Plant & Cell Physiology. 2013 doi: 10.1093/pcp/pct129. in press. [DOI] [PubMed] [Google Scholar]
- Mendu V, Griffiths JS, Persson S, et al. Subfunctionalization of cellulose synthases in seed coat epidermal cells mediates secondary radial wall synthesis and mucilage attachment. Plant Physiology. 2011a;157:441–453. doi: 10.1104/pp.111.179069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendu V, Stork J, Harris D, DeBolt S. Cellulose synthesis in two secondary cell wall processes in a single cell type. Plant Signaling & Behavior. 2011b;6:1638–1643. doi: 10.4161/psb.6.11.17709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PJ, Darvill AG, Albersheim P, Staehelin LA. Immunogold localization of xyloglucan and rhamnogalacturonan I in the cell walls of suspension-cultured sycamore cells. Plant Physiology. 1986;82:787–794. doi: 10.1104/pp.82.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlethaler K. The structure of plant slimes. Experimental Cell Research. 1950;1:341–350. [Google Scholar]
- Naran R, Chen G, Carpita NC. Novel rhamnogalacturonan I and arabinoxylan polysaccharides of flax seed mucilage. Plant Physiology. 2008;148:132–141. doi: 10.1104/pp.108.123513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panikashvili D, Shi JX, Schreiber L, Aharoni A. The Arabidopsis DCR encoding a soluble BAHD acyltransferase is required for cutin polyester formation and seed hydration properties. Plant Physiology. 2009;151:1773–1789. doi: 10.1104/pp.109.143388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattathil S, Avci U, Baldwin D, et al. A comprehensive toolkit of plant cell wall glycan-directed monoclonal antibodies. Plant Physiology. 2010;153:514–525. doi: 10.1104/pp.109.151985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paynel F, Pavlov A, Ancelin G, et al. Polysaccharide hydrolases are released with mucilages after water hydration of flax seeds. Plant Physiology and Biochemistry. 2013;62:54–62. doi: 10.1016/j.plaphy.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Penfield S, Meissner RC, Shoue DA, Carpita NC, Bevan MW. MYB61 is required for mucilage deposition and extrusion in the Arabidopsis seed coat. Plant Cell. 2001;13:2777–2791. doi: 10.1105/tpc.010265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautengarten C, Usadel B, Neurnetzler L, Hartmann J, Buessis D, Altmann T. A subtilisin-like serine protease essential for mucilage release from Arabidopsis seed coats. Plant Journal. 2008;54:466–480. doi: 10.1111/j.1365-313X.2008.03437.x. [DOI] [PubMed] [Google Scholar]
- Romano JM, Dubos C, Prouse MB, et al. AtMYB61, an R2R3-MYB transcription factor, functions as a pleiotropic regulator via a small gene network. New Phytologist. 2012;195:774–786. doi: 10.1111/j.1469-8137.2012.04201.x. [DOI] [PubMed] [Google Scholar]
- Saez-Aguayo S, Ralet MC, Berger A, et al. PECTIN METHYLESTERASE INHIBITOR6 promotes Arabidopsis mucilage release by limiting methylesterification of homogalacturonan in seed coat epidermal cells. Plant Cell. 2013;25:308–323. doi: 10.1105/tpc.112.106575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez-Aguayo S, Rondeau-Mouro C, Macquet A, et al. Local evolution of seed flotation in Arabidopsis. PLoS Genetics. 2014 doi: 10.1371/journal.pgen.1004221. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Kim Y, Guo Y, Stevenson B, Zhu JK. The Arabidopsis SOS5 locus encodes a putative cell surface adhesion protein and is required for normal cell expansion. Plant Cell. 2003;15:19–32. doi: 10.1105/tpc.007872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Cerda BJ, Maureira-Butler I, Muñoz G, Rupayan A, Cloutier S. SSR-based population structure, molecular diversity and linkage disequilibrium analysis of a collection of flax (Linum usitatissiumum L.) varying for mucilage seed-coat content. Molecular Breeding. 2012;30(875–888) [Google Scholar]
- Sridhar VV, Surendrarao A, Gonzalez D, Conlan RS, Liu Z. Transcriptional repression of target genes by LEUNIG and SEUSS, two interacting regulatory proteins for Arabidopsis flower development. Proceedings of the National Academy of Sciences of the USA, 2004;101:11494–11499. doi: 10.1073/pnas.0403055101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork J, Harris D, Griffiths J, et al. CELLULOSE SYNTHASE9 serves a nonredundant role in secondary cell wall synthesis in Arabidopsis epidermal testa cells. Plant Physiology. 2010;153:580–589. doi: 10.1104/pp.110.154062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan S, Ralet MC, Berger A, et al. CESA5 is required for the synthesis of cellulose with a role in structuring the adherent mucilage of Arabidopsis seeds. Plant Physiology. 2011;156:1725–1739. doi: 10.1104/pp.111.179077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga-Wada R, Iwata M, Sugiyama J, et al. The GLABRA2 homeodomain protein directly regulates CESA5 and XTH17 gene expression in Arabidopsis roots. Plant Journal. 2009;60:564–574. doi: 10.1111/j.1365-313X.2009.03976.x. [DOI] [PubMed] [Google Scholar]
- Toorop PE, Cuerva RC, Begg GS, Locardi B, Squire GR, Iannetta PP. Co-adaptation of seed dormancy and flowering time in the arable weed Capsella bursa-pastoris (shepherd's purse) Annals of Botany. 2012;109:481–449. doi: 10.1093/aob/mcr301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usadel B, Kuschinsky AM, Rosso MG, Eckermann N, Pauly M. RHM2 is involved in mucilage pectin synthesis and is required for the development of the seed coat in Arabidopsis. Plant Physiology. 2004;134:286–295. doi: 10.1104/pp.103.034314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilevski A, Giorgi FM, Bertinetti L, Usadel B. LASSO modeling of the Arabidopsis thaliana seed/seedling transcriptome: a model case for detection of novel mucilage and pectin metabolism genes. Molecular BioSystems. 2012;8:2566–2574. doi: 10.1039/c2mb25096a. [DOI] [PubMed] [Google Scholar]
- Voiniciuc C, Dean GH, Griffiths JS, et al. FLYING SAUCER1 is a transmembrane RING E3 ubiquitin ligase that regulates the degree of pectin methylesterification in Arabidopsis seed mucilage. Plant Cell. 2013;25:944–959. doi: 10.1105/tpc.112.107888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M, Tehseen M, Doblin MS, et al. The transcriptional regulator LEUNIG_HOMOLOG regulates mucilage release from the Arabidopsis testa. Plant Physiology. 2011;156:46–60. doi: 10.1104/pp.111.172692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrand J, Michaud P, Picton L, et al. Structural investigations of the neutral polysaccharide of Linum usitatissimum L. seeds mucilage. International Journal of Biological Macromolecules. 2005;35:121–125. doi: 10.1016/j.ijbiomac.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Western TL. Changing spaces: the Arabidopsis mucilage secretory cells as a novel system to dissect cell wall production in differentiating cells. Canadian Journal of Botany. 2006;84:622–630. [Google Scholar]
- Western TL. The sticky tale of seed coat mucilages: production, genetics, and role in seed germination and dispersal. Seed Science Research. 2012;22:1–25. [Google Scholar]
- Western TL, Skinner DJ, Haughn GW. Differentiation of mucilage secretory cells of the Arabidopsis seed coat. Plant Physiology. 2000;122:345–356. doi: 10.1104/pp.122.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Western TL, Burn J, Tan WL, et al. Isolation and characterization of mutants defective in seed coat mucilage secretory cell development in Arabidopsis. Plant Physiology. 2001;127:998–1011. [PMC free article] [PubMed] [Google Scholar]
- Western TL, Young DS, Dean GH, Tan WL, Samuels AL, Haughn GW. MUCILAGE-MODIFIED4 encodes a putative pectin biosynthetic enzyme developmentally regulated by APETALA2, TRANSPARENT TESTA GLABRA1, and GLABRA2 in the Arabidopsis seed coat. Plant Physiology. 2004;134:296–306. doi: 10.1104/pp.103.035519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willats WG, Marcus SE, Knox JP. Generation of a monoclonal antibody specific to (1→5)-α-L-arabinan. Carbohydrate Research. 1998;308:149–152. doi: 10.1016/s0008-6215(98)00070-6. [DOI] [PubMed] [Google Scholar]
- Willats WG, McCartney L, Knox JP. In-situ analysis of pectic polysaccharides in seed mucilage and at the root surface of Arabidopsis thaliana. Planta. 2001;213:37–44. doi: 10.1007/s004250000481. [DOI] [PubMed] [Google Scholar]
- Windsor JB, Symonds VV, Mendenhall J, Lloyd AM. Arabidopsis seed coat development: morphological differentiation of the outer integument. Plant Journal. 2000;22:483–493. doi: 10.1046/j.1365-313x.2000.00756.x. [DOI] [PubMed] [Google Scholar]
- Wolf S, Mouille G, Pelloux J. Homogalacturonan methyl-esterification and plant development. Molecular Plant. 2009;2:851–860. doi: 10.1093/mp/ssp066. [DOI] [PubMed] [Google Scholar]
- Xu SL, Rahman A, Baskin TI, Kieber JJ. Two leucine-rich repeat receptor kinases mediate signaling, linking cell wall biosynthesis and ACC synthase in Arabidopsis. Plant Cell. 2008;20:3065–3079. doi: 10.1105/tpc.108.063354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Zhang W, Dong M, Boubriak I, Huang Z. The achene mucilage hydrated in desert dew assists seed cells in maintaining DNA integrity: adaptive strategy of desert plant Artemisia sphaerocephala. PloS ONE. 2011;6 doi: 10.1371/journal.pone.0024346. e24346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Baskin CC, Baskin JM, Zhang W, Huang Z. Degradation of seed mucilage by soil microflora promotes early seedling growth of a desert sand dune plant. Plant Cell & Environment. 2012a;35:872–883. doi: 10.1111/j.1365-3040.2011.02459.x. [DOI] [PubMed] [Google Scholar]
- Yang X, Baskin J, Baskin C, Huang Z. More than just a coating: ecological importance, taxonomic occurrence and phylogenetic relationships of seed coat mucilage. Perspectives in Plant Ecology, Evolution and Systematics. 2012b;14:434–442. [Google Scholar]
- Young RE, McFarlane HE, Hahn MG, Western TL, Haughn GW, Samuels AL. Analysis of the Golgi apparatus in Arabidopsis seed coat cells during polarized secretion of pectin-rich mucilage. Plant Cell. 2008;20:1623–1638. doi: 10.1105/tpc.108.058842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Gonzalez A, Zhao M, Payne CT, Lloyd A. A network of redundant bHLH proteins functions in all TTG1-dependent pathways of Arabidopsis. Development. 2003;130:4859–4869. doi: 10.1242/dev.00681. [DOI] [PubMed] [Google Scholar]