Abstract
Background and Aims
Penium margaritaceum is a unicellular charophycean green alga with a unique bi-directional polar expansion mechanism that occurs at the central isthmus zone prior to cell division. This entails the focused deposition of cell-wall polymers coordinated by the activities of components of the endomembrane system and cytoskeletal networks. The goal of this study was to elucidate the structural organization of the cortical cytoskeletal network during the cell cycle and identify its specific functional roles during key cell-wall developmental events: pre-division expansion and cell division.
Methods
Microtubules and actin filaments were labelled during various cell cycle phases with an anti-tubulin antibody and rhodamine phalloidin, respectively. Chemically induced disruption of the cytoskeleton was used to elucidate specific functional roles of microtubules and actin during cell expansion and division. Correlation of cytoskeletal dynamics with cell-wall development included live cell labelling with wall polymer-specific antibodies and electron microscopy.
Key Results
The cortical cytoplasm of Penium is highlighted by a band of microtubules found at the cell isthmus, i.e. the site of pre-division wall expansion. This band, along with an associated, transient band of actin filaments, probably acts to direct the deposition of new wall material and to mark the plane of the future cell division. Two additional bands of microtubules, which we identify as satellite bands, arise from the isthmus microtubular band at the onset of expansion and displace toward the poles during expansion, ultimately marking the isthmus of future daughter cells. Treatment with microtubule and actin perturbation agents reversibly stops cell division.
Conclusions
The cortical cytoplasm of Penium contains distinct bands of microtubules and actin filaments that persist through the cell cycle. One of these bands, termed the isthmus microtubule band, or IMB, marks the site of both pre-division wall expansion and the zone where a cross wall will form during cytokinesis. This suggests that prior to the evolution of land plants, a dynamic, cortical cytoskeletal array similar to a pre-prophase band had evolved in the charophytes. However, an interesting variation on the cortical band theme is present in Penium, where two satellite microtubule bands are produced at the onset of cell expansion, each of which is destined to become an IMB in the two daughter cells after cytokinesis. These unique cytoskeletal components demonstrate the close temporal control and highly coordinated cytoskeletal dynamics of cellular development in Penium.
Keywords: Penium margaritaceum, microtubules, plant cell wall, actin, preprophase band, Charophycean Green Algae, cortical cytoskeletal network
INTRODUCTION
The plant cell wall consists of a complex and dynamic composite of polymers and enzymes, as well as inorganic materials and water (Keegstra, 2010; Fry, 2011). The cell wall serves as a barrier to pathogens and to breaching of the cell membrane as well as providing for cell–cell adhesion and a pathway for the movement of solutes. It also provides the tensile strength necessary to support the high internal hydrostatic pressures typical of plant cells (Kroeger et al., 2011). Developmentally, it is fundamental to the regulation of cell expansion. In an expanding plant cell, microdomains found in the wall architecture vary in resistance to turgor that results in different expansion dynamics along the cell periphery (Hepler et al., 2013). These variations in wall extensibility are reflected in variations in shape and size from cell to cell. But a plant cell must also divide, and when doing so, a new ‘partition’ wall (i.e. cross wall) must be deposited in the cytokinetic plane to complete the separation of post-telophase nuclei and protoplasts (Jurgens, 2005). Expansion and cell partitioning require precisely timed and highly coordinated interactions of multiple subcellular components responding to internal developmental signals and environmental prompts (Fendrych et al., 2013). This entails significant investment of the endomembrane system, the secretory vesicle network, the endocytic network and the cytoskeleton.
The interphase cytoskeleton of most plant cells is primarily located in the cortical cytoplasm of the protoplast and consists of two major structural elements, along with their associated sets of regulatory proteins: actin microfilaments (F-actin) and microtubules. Actin and its affiliated proteins (e.g. myosin, villin, formin) generate the motive force used for intracellular transport of cellular constituents (e.g. cytoplasmic streaming or cyclosis; Thomas et al., 2009; Verchot-Lubicz and Goldstein, 2010), including the endomembrane compartments of exocytosis and endocytosis. Likewise, actin is an important component of cell division processes (Kojo et al., 2013; Louveaux and Hamant, 2013).
Microtubules also have multiple functions in plant cells (Muller et al., 2009; Brandizzi and Wasteneys, 2013; Shaw, 2013). (1) Cortical microtubules are closely affiliated with the deposition and orientation of cellulose microfibrils in the cell wall (Paredez et al., 2006; Desprez et al., 2007; Lloyd and Chan, 2008; Crowell et al., 2009; Gutierrez et al., 2009; Shaw, 2013). (2) Microtubules also form the spindle apparatus during mitosis (Zhang and Dawe, 2011). (3) In somatic cells of land plant tissues and cells of the late divergent clades of the charophytes or ‘Charophycean Green Algae’ (CGA; Streptophyta; Leliaert et al., 2012), the production of a new cross wall during cytokinesis requires a band of two closely positioned sets of anti-parallel microtubules called the phragmoplast. Situated between the daughter nuclei, the phragmoplast coordinates Golgi vesicle aggregation, contributing to the formation of a cell plate and the deposition of a new cell wall between daughter protoplasts (Lee and Liu, 2013). (4) In land plants, cortical microtubules also form a ubiquitous but poorly understood structure known as the pre-prophase band (PPB), which consists of a distinct ring of cortical microtubules and associated actin filaments encircling the pre-prophase nucleus (Muller et al., 2009; Wright et al., 2009; Rasmussen et al., 2011, 2013). The PPB disappears before the cell enters prophase but appears to modify the cortical zone in such a way that it serves as a focal point for later division-related events such as mitosis and cytokinesis. Although the function of the PPB remains largely unresolved, it apparently leaves a positional imprint in the form of proteins such as MOR1, TON1 and FASS/TON2/DCD1, which consistently localize to the cell cortex (Van Damme, 2009; Muller, 2012). If the PPB is altered through pharmacological or genetic means, subsequent division processes are significantly altered, which in turn lead to profound changes in thallus morphogenesis (Azimzadeh et al., 2008; Wright et al., 2009; Spinner et al., 2010). The PPB represents a cytoskeletal component unique to plants that predicts the precise positioning of the division apparatus and, ultimately, the production of the new cell wall between division products. The PPB has been found in cells of most tissues of all extant land plant taxa but is absent from specialized thalli including endosperm, as well as moss protonemata and sporophytic tissue (Spinner et al., 2010; Rasmussen et al., 2013).
Modern land plants are believed to have arisen from a stock of green algae whose extant relatives are the CGA (Lewis and McCourt, 2004; Wodniok et al., 2011; Leliaert et al., 2012; Timme et al., 2012). Evidence for this has accumulated over 40 years, confirming ultrastructural, biochemical and molecular similarities between the extant CGA and land plants. Most recently, immunological and biochemical screening of the cell walls of the CGA have demonstrated that many of these algae have wall constituents that are remarkably similar to those found in land plants (Popper, 2008; Sørensen et al., 2011; Domozych et al., 2012). Many taxa of the late divergent CGA also exhibit cell and cell-wall expansion dynamics that are similar to those seen in some land plants (Domozych et al., 2013). This includes polar growth strategies whereby wall expansion is localized to precisely defined zones (e.g. desmid expansion and semi-cell morphogenesis). Likewise, previous studies have shown that the cytokinetic mechanisms of late divergent CGA include phragmoplast-directed cell plate formation that results in the production of a new cross wall (McIntosh et al., 1995; Sawitzky and Grolig, 1995; Pickett-Heaps et al., 1999; Cook, 2003). In spite of these similarities, the paucity of information on the regulation of wall development during the cell cycle has limited our understanding of the evolution of cell expansion and division mechanics in the CGA, and has hindered our ability to identify those features that may have been key to the colonization of land by green plants.
We have identified a unicellular desmid, Penium margaritaceum, itself a member of the CGA, as a potential model organism for cell-wall and expansion studies. Penium produces only a primary cell wall. Furthermore, specific wall polymers can be traced during cell development by live cell labelling with various molecular probes (Domozych et al., 2007, 2009, 2011). Prior to cell division, the alga expands in a distinct bidirectional mode in which growth is localized to the cell equator or isthmus. This entails the coordinated deposition of cellulose and pectin that are characteristic of much of the wall architecture. Here, we describe in detail the cytoskeletal dynamics that characterize this unique mode of cell morphogenesis. We report, for the first time, the presence of a microtubular band that localizes to the isthmus of the cell, and which like the PPB of the land plants, marks the future plane of cell division. Surprisingly, however, this band also marks the site of pre-division wall expansion. Additionally, satellite microtubule bands appear during the pre-division expansion mode, separating from the original band and ultimately becoming the isthmus bands of the two daughter cells.
MATERIALS AND METHODS
General
Penium margaritaceum (Skidmore College clone Skd-8) was maintained on Woods Hole Medium (Nichols, 1973) supplemented with soil extract (WHS) and grown under the conditions described by Domozych et al. (2007). Log phase cultures (i.e. 7- to 14-d-old cultures) were used for all labelling and experiments.
Rhodamine phalloidin labelling
Experimentally treated and untreated control cells (see below) were collected by centrifugation at 500g on an IEC Clinical Centrifuge (Needham, MA, USA). The supernatant was discarded and the pellets were resuspended in either WHS (untreated) or WHS containing a particular pharmacological agent, vortexed for 5 s and centrifuged again. This process was repeated twice more to ensure that the gel-like extracellular polymeric substance (EPS; Domozych et al., 2005) was removed from the cells. The final pellet was resuspended in 3 mL of WHS and to it was added MBS (3-maleimidobenzoic acid N-hydroxy succimide ester; Sigma, St Louis, MO, USA) at a final concentration of 100 nm. The cell suspension was gently shaken for 30 min at room temperature and cells were subsequently collected by centrifugation. The pellet was resuspended in a fixative consisting of 0·5 % formaldehyde (EMS, Fort Washington, PA, USA) in a microfilament stabilizing buffer (MSB) consisting of 50 mm PIPES, 5 mm MgCl2, 25 mm KCl, 5 mm CaCl2 and 5 mm EGTA (pH 6·9; Sigma) and gently shaken for 30 min at room temperature. The suspension was then centrifuged and the pellet washed three times with the MSB over 30 min. The final washed cell pellet was resuspended in a solution of 6·6 µm rhodamine phalloidin (Invitrogen, cat. no. R415, Eugene, OR, USA) in MSB containing 0·5 % Tween-20 detergent, placed in the dark and gently shaken for 90 min at room temperature. The suspension was then washed three times with MSB.
Immunofluorescence labelling with anti-tubulin antibody
Microtubules were labelled using the freeze shattering technique of Wasteneys et al. (1997). Briefly, cells were collected and washed three times with fresh WHS and then fixed in 0·5 % glutaraldehyde and 1·5 % paraformaldehyde (EMS) in a microtubule stabilizing buffer (MtbSB) containing 50 mm PIPES, 2 mm EGTA and 2 mm MgSO4 (pH 6·9) at room temperature for 30 min. The cells were then washed three times in MtbSB. A dense suspension of cells from the pellet was then placed between two glass slides to form a sandwich and plunged into liquid nitrogen (LN). The frozen sandwich was then placed on a metal block cooled with LN. The sandwich was gently tapped with the end of metal forceps for 1 min. The sandwich was then allowed to thaw to room temperature and the cells were washed into a centrifuge tube with PBST (PBS plus 1 % Triton-X, pH 6·9). The cells were then washed three times with PBST over the 30 min followed by three washes with 1 mg mL−1 NaBH4 in PBS over 10 min. The cells were then washed three times with PBS, incubated in a 1 % Driselase (Sigma)/PBS solution for 10 min, washed three times with PBS, incubated in 0·01 % trypsin (Aruga et al., 1997; Sigma) for 10 min and washed three times with PBS. The cells were subsequently washed for 20 min with 50 mm glycine/PBS, washed with PBS and labelled with anti-tubulin (T6074; Sigma, 1 : 400 dilution in PBS) overnight at 4 °C in the dark. The cells were washed with PBS three times and then labelled with anti-mouse fluorescein isothiocyanate (FITC) antibody (1 : 200 dilution; Sigma). The cells were then washed three times with PBS. For identification of the nucleus, a small aliquot of labelled cells was counterstained with 1 µg mL−1 hexidium iodide (Invitrogen) in PBS for 3 min and then washed three times in PBS before viewing.
Live cell labelling of the cell wall
Washed cells were labelled with either the following monoclonal antibodies (mAb), LM18 (sp = low esterified homogalacturonan; Plant Probes, Leeds, UK) or JIM7 (sp = high esterified homogalacturonan; Plant Probes), using the live labelling technique described by Domozych et al. (2011). For labelling of the nucleus, cells were counterstained for 1 min in 1 mg mL−1 SYTO9/WHS and then washed three times with WHS before viewing.
Light microscopy
Differential interference contrast (DIC) and fluorescence microscopy were performed using either an Olympus Fluoview 300 or an Olympus Fluoview 1200 confocal laser scanning microscope.
Field emission scanning electron microscopy (FESEM)
Harvested and washed cells were frozen in liquid nitrogen, freeze dried and placed on stubs coated with double sticky tape. Cells were sputter coated with gold/palladium and imaged using a Zeiss Neon-40 EsFIB-B scanning electron microscope.
Transmission electron microscopy (TEM)
Cells were collected by centrifugation as described above and spray frozen into liquid propane cooled to –180 °C or less using a commercial artist's airbrush (Amazon). The cells were then transferred to glass vials containing 1 % glutaraldehyde in acetone (EMS) cooled to –80 °C. The suspension was then freeze substituted for 48 h at –80 °C and then slowly warmed to room temperature (over 8 h). The cells were washed with acetone, infiltrated with Spurrs low-viscosity plastic/acetone and then embedded in 100 % plastic followed by polymerization at 65 °C for 8 h. Sections of 60–80 nm were cut using a Reichert Ultracut ultramicrotome, collected on copper grids and conventionally stained with uranyl acetate/lead citrate. Sections were viewed on a Zeiss Libra 120 transmission electron microscope at 120 kV.
Experimental treatment of cells
Centrifugation
Five hundred microlitres of washed cell suspension was placed in a 1·5-mL microcentrifuge tube and centrifuged at 10 000g for 4 min. Cells were labelled immediately with either rhodamine phalloidin or anti-tubulin, or allowed to recover for 24 h in fresh WHS. Recovered cells were labelled with LM18.
Treatment with microtubule and actin perturbation agents
Washed cells were treated with one of the following agents: 5 µg mL−1 cytochalasin E, 125 nm latrunculin B, 10 µm isopropyl phenyl carbamate (IPC) or 42 nm oryzalin, for either 4, 12, 24, 48 or 96 h. Cells were then collected by centrifugation and labelled as described above.
RESULTS
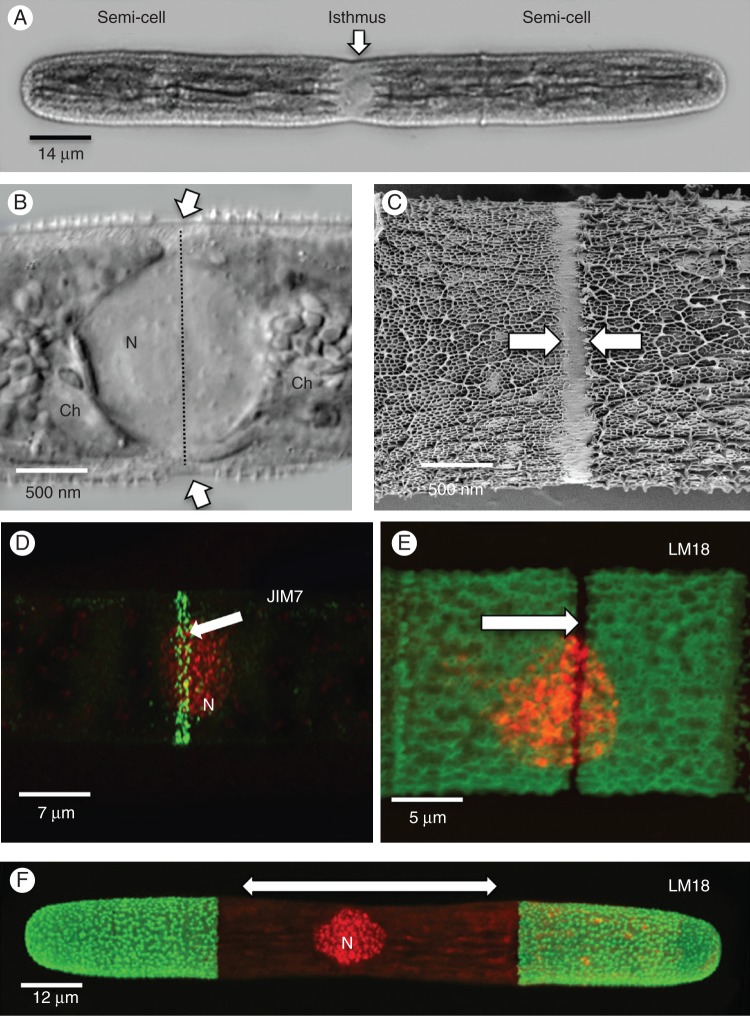
Penium margaritaceum is a unicellular desmid whose phenotype is an elongate cylinder terminating at two rounded poles (Fig. 1A). The cell is composed of two approximately equal semi-cells each containing a large multi-lobed chloroplast that surrounds a slightly narrowed cell centre or isthmus. The isthmus contains a zone of cytoplasm that includes the nucleus positioned between the two chloroplasts (Fig. 1B) for most of the cell cycle, including pre-division cell expansion. The cell wall of the isthmus contains a region of 1–2 µm that is devoid of the lattice-like projections that form the outer layer. High-resolution FESEM imaging clearly highlighted this smooth, lattice-free band (Fig. 1C). Immunocytochemical labelling of live cells with JIM7, an mAb that binds to relatively high esterified homogalacturonan (HG), revealed a labelled band that encircles the isthmus (Fig. 1D) and corresponds to the lattice-free band observed with FESEM. Labelling with LM18, an mAb that binds to relatively low esterified HG, was found throughout the cell wall except at a narrow band at the isthmus (Fig. 1E). This label-free zone corresponds to the lattice-free zone and the JIM7-labelled band found at the isthmus.
Fig. 1.
General expansion mechanics during pre-division. (A) DIC image of cell prior to cell division. The isthmus zone is positioned at the cell centre between the two semi-cells. (B) The nucleus (N) is situated in the cytoplasm of the isthmus between two chloroplasts (Ch). External to this zone is a 1-μm-wide band where the cell wall is incompletely formed (arrows and line). This zone is the expansion band where new wall material is deposited during the ‘bi-directional’ expansion mechanism. (C) FESEM image showing the absence of the outer layer at the isthmus (arrows). (D) LM20 labels a narrow band (arrow) where high esterified HG is secreted at the isthmus. (E) LM18, an mAb with specificity for low esterified HG, labels all of the cell wall except for the narrow band (arrow) found at the isthmus. This unlabelled band corresponds with the LM20-labelled band seen in (D). (F) When LM18-labelled cells are placed back in culture for 24 h, new deposited wall is unlabelled (arrow) and illustrates bi-directional expansion occurring at the central isthmus (N, nucleus).
Prior to cell division, Penium expands in a ‘bipolar’ or bidirectional fashion (Fig. 1E). New cell-wall materials are deposited and incorporated into the wall at the isthmus band, which, in turn, displaces older cell wall axially toward both poles. Detailed information about this expansion process is also given by Domozych et al. (2009).
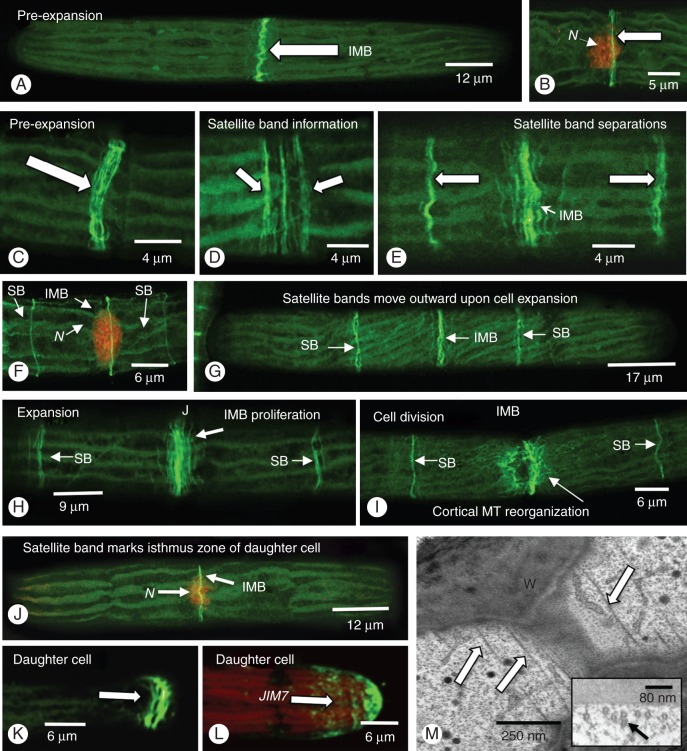
Immunocytochemical labelling of the microtubule cytoskeleton was performed using the freeze shattering technique of Wasteneys et al. (1997) as described above. During assessment of the labelling, we chose only those cells that were intact and not injured during the fixation process. This was done by DIC imaging as well as confocal laser scanning microscopy (CLSM) imaging of chlorophyll/chloroplast autofluorescence (e.g. use of argon laser and FITC filter set). By measuring the lengths of individual cells, we were also able to correlate band number/position with general cell cycle phase. Finally, at least 100 cells each with either single or tri-labelled bands were examined. In all interphase cells prior to pre-division expansion, a band of microtubules was found at the isthmus zone (Fig. 2A). This isthmus microtubule band (IMB) was located at the cell cortex surrounding the nucleus (Fig. 2B) and contained 10–20 microtubules (Fig. 2C). However, immediately prior to or during early pre-division expansion, three bands appeared at the cell isthmus (Fig. 2D). Based on subsequent observations, we interpret this array as two satellite microtubule bands which separate from the original IMB. As cell expansion proceeded, the two outer satellite bands migrated further from the isthmus as a result of cell expansion (Fig. 2E–G). As expansion continued, the IMB expanded to 20–30 microtubules (Fig. 2H). As the cell entered into cell division, the IMB disassembled and was replaced by the mitotic spindle (Fig. 2I) in a manner similar to the PPB. The satellite bands persisted, however, and continued to migrate away from the isthmus. After cytokinetic separation of daughter cells, each satellite band became the IMB of a daughter cell (Fig. 2J). In many post-cytokinetic cells, the polar tip of the expanding semi-cell of a daughter cell also contained an assemblage of microtubules (Fig. 2K). This site was also highlighted by JIM7 labelling (Fig. 2L). The presence of the microtubule bands was corroborated by TEM analysis (Fig. 2M). Microtubules were found in parallel arrays located just under the plasma membrane at the isthmus zone.
Fig. 2.
Cortical microtubular bands. (A) Throughout most of the cell cycle, including the pre-division expansion phase, a band of microtubules (IMB or isthmus microtubule band) that are aligned perpendicular to the long axis of the cell is found at the isthmus (arrows). (B) This band (arrow) surrounds the central nucleus (N) as labelled with SYTO9. (C) The IMB consists of 10–15 microtubules and starts to expand as the cell enters pre-division expansion. (D) At this time, two satellite bands (arrows) emerge from the periphery of the IMB. (E) As expansion begins, the satellite bands (arrows) move away from the IMB. (F) The IMB remains at the isthmus as marked by the nucleus (N). The two satellite bands displace outward toward both poles (SB, arrows). (G) As expansion continues, the satellite bands (SB) move further away from the IMB. (H) During the expansion process, the IMB grows (arrow) and the satellite bands move further towards the poles. (I) As the cell enters into cell division, the IMB disassembles (arrow), probably contributing to the mitotic spindle and phragmoplast. The satellite bands (SB) remain intact. (J) After cytokinesis, a satellite band is found at the isthmus of each daughter cell (N, nucleus). This band now constitutes the new IMB. (K) Immediately after cytokinesis, remnants of the cell division apparatus remain for a short period at the pole of the expanding semi-cell of a daughter cell (arrow). (L) This zone also is a site of additional wall deposition, as noted by LM 20 labelling (arrow). (M) TEM analysis reveals the parallel arrays of microtubules at the isthmus (arrows) just inside the cell wall (W). Inset: cross-section of this zone.
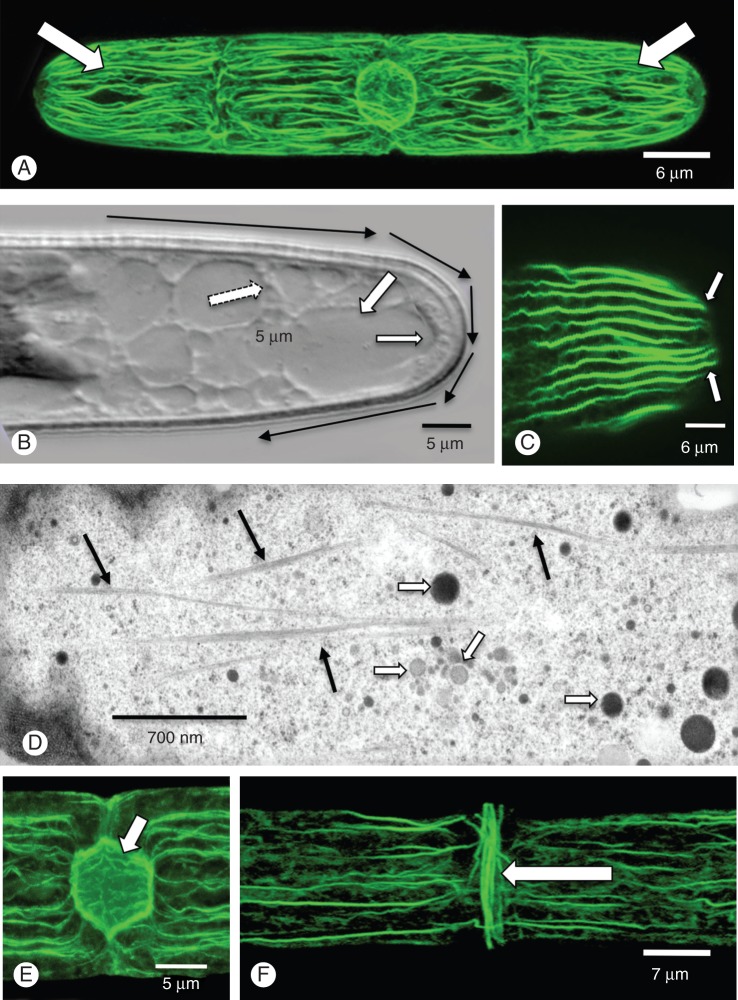
Rhodamine phalloidin labelling was used to visualize the actin cytoskeleton. During interphase, and prior to pre-division expansion, the cortical cytoplasm contained parallel arrays of actin filament bundles aligned parallel to the long axis of the cell (Fig. 3A). The cortical cytoplasm was also the site of active cytoplasmic streaming (Supplementary Data Video) that occurred in cortical cytoplasmic channels that were lined internally by an extensive vacuolar meshwork (Fig. 3B). Cytoplasmic streaming ran along the longitudinal axis of the cell and curved around the rounded polar zones to continue back through the centre of the cell. The actin filament bundles also curved around the rounded poles (Fig. 3C). TEM analysis of the cortical cytoplasm revealed numerous actin filament bundles interspersed with various vesicles (Fig. 3D). During most of interphase, actin filament bundles converged and covered the outer surface of the nucleus (Fig. 3E). During pre-division expansion, a band of actin filament bundles formed at the isthmus (Fig. 3F) at the same location as the IMB. Once the cell entered into cell division, both bands disappeared.
Fig. 3.
The cortical actin network. (A) The cortical cytoplasm is highlighted by numerous actin filament bundles that run parallel to the long axis of the cell (arrows; rhodamine phalloidin labelling). (B) These bundles are located in the cortical cytoplasm (small arrow) that most likely fuel cytoplasmic streaming around the cell (black arrows). The cortical cytoplasmic channels are interspersed with vacuoles (large arrows). (C) At the polar zones, the bundles curve around back into the cell (arrows). (D) TEM analysis shows the numerous bundles in the cortical cytoplasm (dark arrows). Multiple vesicles are found in this zone. (E) At the nucleus, the actin filament bundles line the nuclear envelope (arrow). (F) When the cell enters into pre-division expansion, a band of actin filament bundles appears aligned perpendicular to the long axis (arrow) at the same position of the IMB.
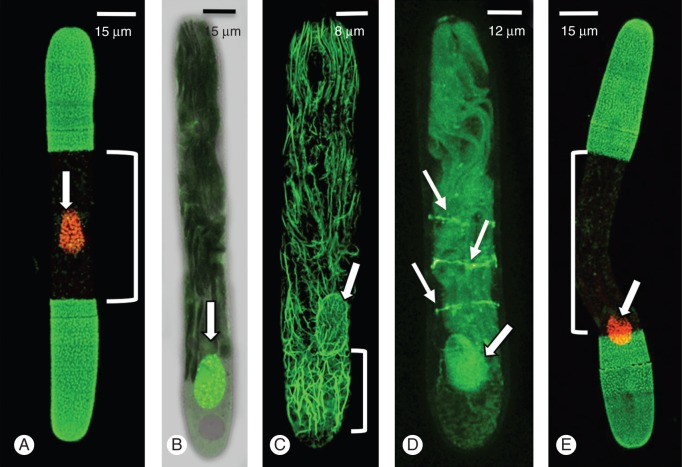
Our initial observations showed that the bands of microtubules and actin were closely associated with the isthmus zone of a cell; but because the nucleus is also normally located at the isthmus the question arises as to whether it is the IMB or the nucleus that defines the region or point of polar expansion and the site of cell division. To determine whether cell separation is nucleus-controlled or IMB-controlled we centrifuged cells to displace the nucleus but not the microtubule bands from the isthmus region. After a short recovery period, cells were labelled and imaged to determine if there were changes to where polar expansion occurred (e.g. at the original isthmus zone or near the displaced nucleus). In control cells, expansion occurred exclusively at the central isthmus, the site where the nucleus is normally located (Fig. 4A). After centrifugation, the nucleus may displace a considerable distance from the isthmus (Fig. 4B). When these cells were labelled with rhodamine phalloidin (Fig. 4C), actin filament bundles were observed throughout the cell but with higher density at the ‘centrifugal force-directed pole’ (i.e. the pole at the point of maximum centrifugal force). This was probably due to the displacement of cortical cytoplasm to this pole. However, during recovery, the actin filament network returned to its normal architecture within a few hours. In cells where the nucleus displaced from the isthmus but not the IMB (Fig. 4D), cell-wall expansion occurred at the site of the IMB and not at the site of the displaced nucleus (Fig. 4E).
Fig. 4.
Effects of centrifugation on expansion. (A) A non-centrifuged cell labelled with LM18 and SYTO9. Wall expansion is roughly equally on either side of the nucleus (N). (B) DIC image of a cell centrifuged for 4 min at 12 000g. The nucleus (arrow) is displaced to one end of the cell. (C) Rhodamine phalloidin labelling of a centrifuged cell. Note that the nucleus (arrow) of actin filaments accumulates at the ‘centrifugal’ end. (D) The microtubule bands remain at the isthmus while the nucleus is at the ‘centrifugal’ end (arrow). (E) When placed back into culture for 24 h, expansion still occurs at the isthmus. The position of the displaced nucleus does not affect where expansion occurs.
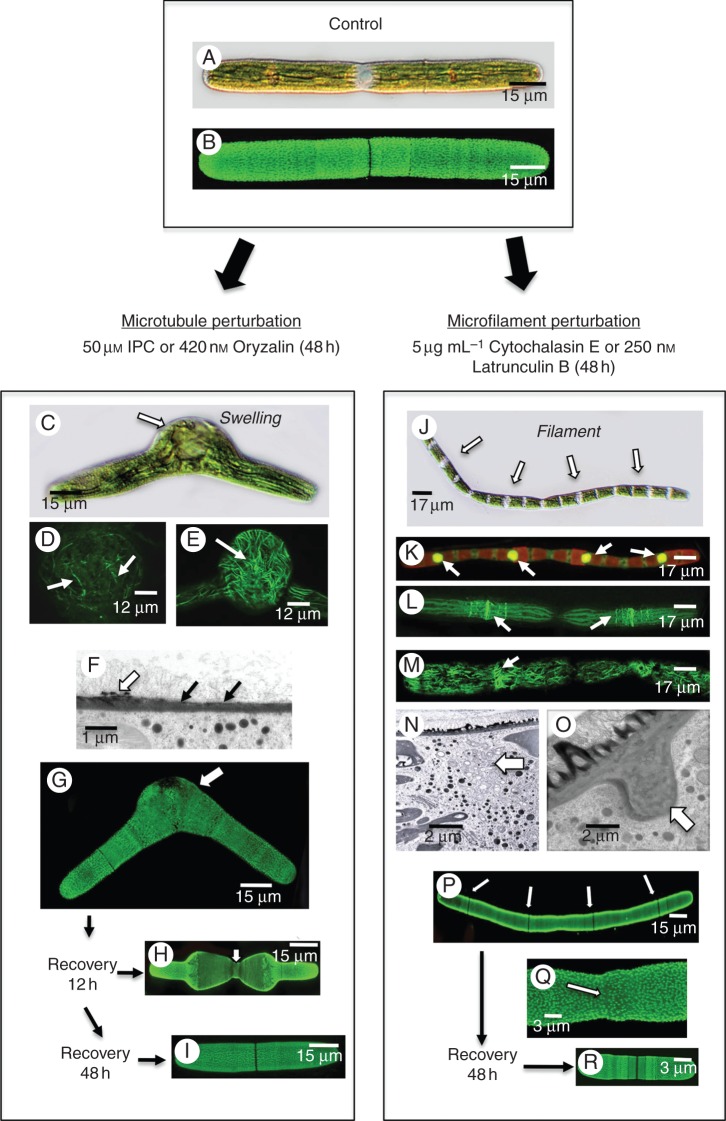
To determine the function of the cortical microtubule bands or cortical actin filament network, cells were treated either with the microtubule perturbation agents IPC and oryzalin or the actin perturbation agents (Fig. 5C–I) cytochalasin E and latrunculin B (Fig. 5J–R). The results presented here for IPC and cytochalasin E were virtually the same as those for the two actin perturbation agents. When cells were treated with IPC for 24 h, significant swelling occurred in the isthmus (Fig. 5C; compare with Fig. 5A and B). The microtubules were in a random array and no bands were observable in the swollen isthmus (Fig. 5D). Microfilaments were likewise found randomly distributed (Fig. 5E). TEM analysis of the swollen isthmus revealed that the inner cellulosic layer was thinner than that of untreated cells (Fig. 5F) and that the outer lattice was either missing or irregular (Fig. 5G). This result was similar to that reported for oryzalin treatment (Domozych et al., 2014).
Fig. 5.
Effects of microtubule and actin perturbation agents. (A) DIC image of an untreated cell. (B) LM18 labelling of an untreated cell highlighting the pectin lattice. (C) DIC image of a cell treated with 50 µm IPC for 24 h. Note the distinct swelling at the isthmus. (D) Anti-tubulin labelling of the swollen isthmus region of an IPC-treated cell. No banding is noted and the microtubules are in disarray. (E) Rhodamine phalloidin labelling of the swollen isthmus of an IPC-treated cell. The actin network is disorganized. (F) TEM image of the cell wall at the interface of the regular wall and the isthmus at the swollen isthmus. The cell wall in the isthmus is thinner (arrows) and has no pectin lattice (white arrow). (G) LM18 labelling of an IPC-treated cell. Note the irregular labelling at the swollen isthmus zone. (H) LM18 labelling of a cell washed free of IPC and allowed to recover for 2 h. Note that the cell is thinner at the isthmus. (I) Forty-eight hours after recovery, cells return to normal shape. (J) Pseudofilament formed after cells are treated with cytochalasin E for 48 h. Note the individual cell units (arrows). (K) SYTO9 labelling of pseudofilament demonstrates that each cell unit contains a nucleus. (L) Anti-microtubule labelling of a psuedofilament. Note that the microtubule bands are still present. (M) Rhodamine phalloidin labelling of a pseudofilament showing the disorganization of the cortical actin filaments. No actin filament band is seen near the nucleus. (N) TEM image of the zone between cellular units in a cytochalasin E-treated cell. Note that the cytoplasm consists of a collection of vesicles (arrow). (O) TEM image of the isthmus region of a cytochalasin E-treated cell. Note the large wall plug found at the cell periphery (arrow). (P) LM18 labelling of the pseudofilament showing that little alteration to the pectin lattice (arrows). (Q) LM18 labelling of the isthmus zone between units. Note that the lattice remains intact. (R) Upon 48 h of recovery, cells return to a unicellular phenotype.
When cells were washed free of IPC and placed back in culture, they ultimately returned to normal shape. This included a return to cylindrical shape for the isthmus-based expansion zone of the cell and, ultimately, the production of a complete cylindrical shape after the second cell division (Fig. 5H, I). When treated with the actin perturbation agent, cytochalasin E, a very different result was noted. After 48 h, ‘pseudofilaments’ formed and consisted of cell-like units that attach in a regular, filament-like chain (Fig. 5J). When labelled with SYTO9 to reveal the position of nuclei (Fig. 5K), each cell unit possessed a nucleus which suggested that post-mitotic nuclear separation still occurred. When labelled with anti-tubulin, the microtubular bands were still found near the isthmus of the each cell unit (Fig. 5L). However, the cortical actin network rearranged into a random network and no actin filament band was ever noted at the isthmus (Fig. 5M). TEM analysis revealed that in the zone between cell units, i.e. zones where cytokinesis should have occurred, a region of cytoplasm containing many vesicles and partially fused vesicle elements was present (Fig. 5N). At the outer edge of these zones, a distinct peg-like wall protrusion was found consisting of fibrillar aggregates (Fig. 5O). Examination of the pectin of the cell wall using LM18 showed that the outer lattice was still intact, including the isthmus regions (Fig. 5P, Q). Finally, when cells were washed free of cytochalasin E and allowed to recover, normal cell division resumed and complete cells were produced within two division cycles (Fig. 5R).
DISCUSSION
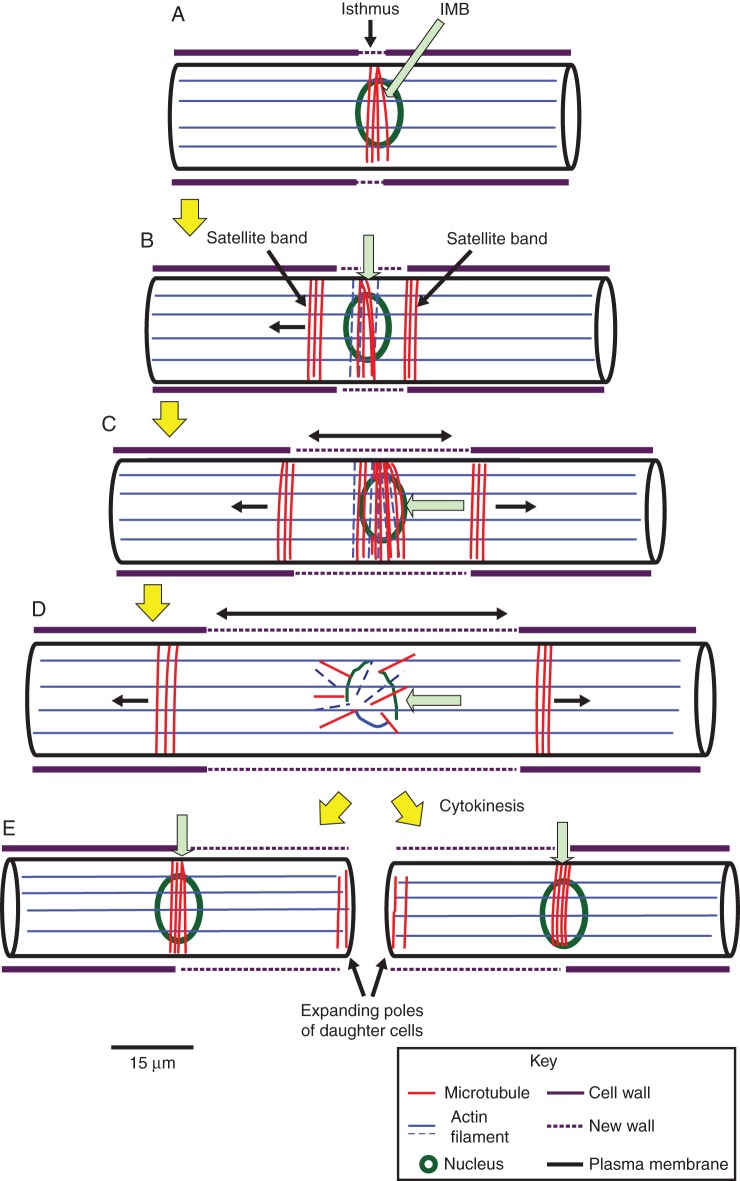
This study has demonstrated that organized and dynamic arrays of cortical cytoskeletal elements are present during cell-wall deposition events and cell development in Penium margaritaceum, a member of the CGA. A model that illustrates their positioning during cell-wall/cell expansion and cell division is provided in Fig. 6. The most conspicuous band of microtubules that we identified, the IMB, is situated at the isthmus throughout most of the cell cycle. This zone is the site of bi-directional cell-wall/cell expansion that occurs just prior to cell division and phragmoplast/cell plate formation during cell division. At the same cortical (axial) location on the cell as the IMB, a more transient band of actin filaments also appears during pre-division expansion. Both disassemble as the cell enters into cell division and may be recycled into the mitotic spindle and cytokinetic apparatus. However, before this happens, two satellite microtubule bands form near, and translocate from, the IMB and toward the poles of the cell during pre-division expansion. After cytokinesis is completed, each of the satellite bands becomes an IMB of a daughter cell. The satellite microtubule bands thus represent ‘predictive’ structures in that they will move to regions that will ultimately become expansion and division sites of future daughter cells (i.e. they are ‘pre-IMBs’). All of these observations demonstrate a previously unknown and complex set of cytoskeleton-based events during cell morphogenesis in Penium.
Fig. 6.
Putative model of cytoskeletal dynamics. (A) The interphase cell is highlighted by a network of actin filament bundles that run parallel to the long axis of the cell. In addition, the isthmus microtubule band (IMB) marks the cortical cytoplasm at the isthmus. (B) At the onset of pre-division expansion, two satellite microtubule bands appear on either side of the IMB (green arrow). (C) As the cell undergoes its bidirectional expansion (double arrow), the satellite bands move toward the poles (single arrows). The IMB (green arrow) increases in size at the isthmus and an actin band forms in the same location as the IMB. The satellite bands displace further toward the poles (single arrow). (D) During cell division, the satellite bands continue to move toward the poles and the IMB and actin band disassembles (green arrow). (E) After cytokinesis, each satellite band becomes the IMB of a daughter cell (green arrows). At the poles of the expanding semi-cell of each daughter cell, an assemblage of microtubules is found. Most likely it is derived from microtubule remnants of the cytokinetic machinery and marks the site of residual expansion at the poles.
The IMB functions like a PPB
The IMB of Penium appears to behave in a manner similar to the PPB of land plants. Typically, a PPB consists of a band of microtubules that co-localizes with actin filaments and marks the site of future cell division (Dhonukshe and Gadella, 2003; Wright et al., 2009; Muller et al., 2009; Janski et al., 2012; Shaw, 2013; Smolarkiewicz and Dhonukshe, 2013). PPB formation is a product of microtubule-associated proteins (MAPs) that control rates of microtubule polymerization/depolymerization, stabilization/destabilization and de novo nucleation (Rasmussen et al., 2013). It is believed that the PPB organizes proteins and lipids in the cortical cytoplasm as spatial markers for cell division, which are subsequently maintained after the PPB disappears (Rasmussen et al., 2011). When the PPB is altered by pharmacological or genetic means, cell division is altered and significant morphogenetic changes occur (Wright et al., 2009; Spinner et al., 2010). The IMB of Penium is also a cortical cytoskeletal microtubular band that co-localizes with an actin filament band and marks the future division plane. However, unlike the land plant PPB, the IMB is more persistent (i.e. it appears throughout virtually all of the cell cycle) and only disassembles at cell division. When the constituent cytoskeletal components of the IMB are disrupted by pharmacological agents (e.g. microtubule- or actin-perturbation compounds), cell division fails to occur. When these agents are removed, cell division resumes. At present, the cell division mechanism has not been described in detail for Penium. However, preliminary observations suggest that like other Zygnematalean taxa (Marchant and Pickett-Heaps, 1973; Goto and Ueda, 1988; Sawitzky and Grolig, 1995; Pickett-Heaps et al., 1999; Cook, 2003), cytokinesis entails a small centripetal furrow and a centrifugal phragmoplast-cell plate that eventually meet to form the cross wall. We postulate that the IMB and associated actin band probably create cortical sites that mark where both the furrow and the cell plate will form, like that of the PPB. The exact biochemical constituents of the IMB and surrounding cortical cytoplasm in Penium along with more detailed comparisons with the PPB await future study.
The satellite microtubule bands predict the site of wall expansion and cell division in daughter cells
The satellite bands formed prior to pre-division expansion are distinct pre-IMBs that dynamically change after cytokinetic separation of the daughter cells. Previous analyses of cell-wall expansion in Penium (Domozych et al., 2007, 2009) have shown that deposition of the two dominant cell-wall layers, the cellulosic inner layer and HG-rich outer layer, occur almost exclusively at the isthmus. During expansion, new wall polymer deposition at the isthmus pushes older wall axially toward the poles of the cell. Except for some residual wall expansion at the growing pole of recently divided daughter cells (Fig. 2K, L), no evidence has yet been found to show that wall deposition occurs outside the isthmus during the cell cycle. This means that wall polymer deposition does not track the migration of the satellite bands and implies that the presence of the IMB is necessary but not sufficient to enable new wall synthesis. The satellite bands are not associated with wall polymer deposition until after cell division is completed. The molecular mechanics that underlie these behaviours await further detailed examination but our data strongly suggest a temporal and geographical regulation of wall deposition associated with the microtubule bands. For example, a cortical microtubule band in Penium may only activate (i.e. to direct wall deposition) when associated or interacting with a nucleus or with the actin network near a nucleus. This would explain why satellite bands before cytokinesis are not associated with wall deposition. However, satellite bands clearly do become focal points of wall deposition after cytokinetic partitioning of the daughter cells and positioning of a daughter nucleus at the isthmus zone of each daughter protoplast, i.e. the exact location of each satellite band. Interestingly, once the satellite band transforms into an IMB, the association with a nucleus is no longer necessary for correct wall deposition and cell division plane positioning, as demonstrated by our nuclear displacement experiments with centrifugation. Perhaps most remarkably, the formation of the future morphogenetic centres represented by the satellite bands occurs in a cell that is one division cycle previous to the time when they become functional in the control of expansion and cell division. One might interpret this as an anticipatory phase shift in the assembly of the future IMB. The significance of this is not readily apparent, but this unique sequence of developmental events may be characteristic of desmids such as Penium with distinct and highly symmetrical cell morphogenesis. Future surveys of other desmid taxa will be needed to confirm this.
Extant CGA also possess cytoskeletal structures like a PPB
Previous studies have suggested that the presence of cortical microtubule bands that anticipate critical morphogenetic events in the cell cycle are characteristic of multicellular land plants (Pickett-Heaps et al., 1999; Spinner et al., 2010). We show that cortical microtubular bands linked to morphogenetic events are also found in the extant CGA.
In the higher plant taxa, cell-wall extension growth tends to be evenly spread over the side walls of extending cells, effectively producing the ‘diffuse growth’ habit we associate with axiation, and contrasts with the PPB, which is confined to a region that anticipates the future division plane; but in Penium, an alga with simple morphology, the zones of cell-wall deposition/expansion and cell division-based cross wall formation occupy the same space and their cytoskeletal support structures are superimposed on each other at the same point of the cell, the isthmus. Therefore, because the cytoskeleton appears to be critical for both wall deposition and cell division dynamics, a more permanent cortical cytoplasmic site marked by the IMB and co-localizing actin band may be necessary to sustain this highly focused region of wall development. In land plants, where wall deposition and expansion typically occur more diffusely around the cell periphery, a diffuse microtubule distribution in the cortex may only be needed to support interphase wall deposition and extension. However, the transient PPB may be an evolutionary derivative of an IMB that is required to support the brief ‘spurt’ of highly focused wall deposition that will occur during phragmoplast-cell plate formation and cross wall deposition
The IMB directs wall deposition at the isthmus, the site of a bipolar expansion mechanism
Plant cell expansion entails the alteration of the microarchitecture of the cell wall through secretion of specific polymers and/or post-secretory modulations at particular zones of the cell wall. These events may increase the extensibility of the wall, softening the wall so that outward-directed turgor pressure causes the wall and cell to expand at these zones (Hepler et al., 2013). In plant cells that grow by unipolar extension, wall modification and expansion occur at a specific point or front. For example, in pollen tubes, polar expansion occurs at the tip of the tube, resulting in the formation of a highly elongate tube (i.e. unipolar). At this expanding pole, the secretion and subsequent remodelling of pectin, specifically high-esterified HG, results in a ‘softened’ wall that can expand in a controlled manner under turgor pressure (Palin and Geitmann, 2012). The wall of the sides or shanks of the pollen tube have polymers that resist turgor. For example, the HG in these zones complexes calcium (Ca2+), to form a rigid gel. Likewise, other polymers (e.g. callose, cellulose) may be deposited, further enhancing the rigidity of the wall. In turn, the secretion of wall polymers requires the coordinated activity of the cell's secretion apparatus that interfaces with the cytoskeleton, specifically the actin microarchitecture.
Penium also has a tubular construction but differs in that cell extension and cell division are both constrained to share the same space. Prior to cell division, the alga undergoes an extended period of localized expansion that is focused at the isthmus. New wall material, HG and cellulose, is deposited at a narrow band or midzone at the central isthmus. Continued deposition displaces pre-existing cell wall toward the poles. This type of polar expansion is unusual in that it appears to yield new growth on two fronts (i.e. a type of bi-directional or ‘bipolar’ expansion). This expansion also corresponds with the time when the HG, now positioned in the outer wall layer, binds to Ca2+ and forms a rigid lattice. The exact mechanisms responsible for wall polymer deposition in the isthmus wall have yet to be fully resolved. However, the results of this study demonstrate that narrow bands of cytoskeletal components located in the cytoplasmic cortex are prominently and reliably associated with the isthmus-based expansion site, and that by manipulating their behaviour the growth behaviour of the cell can also be altered.
Specifically, we show that a distinct and persistent band of microtubules, the IMB, and a transient actin band appear together in the cortical cytoplasm at the isthmus during pre-division expansion. It is highly likely that both are involved in the delivery and incorporation of wall precursors (Akkerman et al., 2011; Brandizzi and Wasteneys, 2013). The IMB and its associated actin filament band may be spatially linked to ensure that cellulose deposition and HG secretion at the isthmus are strongly coupled. It has been previously shown that formation of the cellulosic inner layer forms the scaffold necessary for the incorporation of pectic polymers into the wall and for the subsequent formation of the outer lattice layer (Domozych et al., 2009).
Based on results of this study, we suggest that the actin filament band accomplishes the transport and directs cellulose synthase (CESA) and precursor-containing vesicles to a restricted region of the plasma membrane at the midzone of the isthmus where CESA would then process the synthesis of microfibrils. This aspect of wall assembly follows the classical model in that the orientation of microfibril deposition at the inner wall surface follows the orientation of IMB microtubules in the cortical cytoplasm. It is in topographic restriction of wall synthesis and the delivery of pectic polymer precursors to the cell surface that our model differs from the classical model because pectin secretion does not occur at the midzone of the isthmus. We propose that this results from the fact that the nascent cellulose layer at the midline is either incomplete or insufficiently consolidated to allow proper pectin incorporation (Domozych et al., 2009). As deposition of the cellulose microfibrils continues, the cellulosic layer thickens while at the same time being continuously transported away from the isthmus midzone. Once the cellulosic infrastructure reaches the proper thickness pectin secretion and incorporation into the wall can occur. It is also possible that the pectin-carrying vesicles might be physically blocked by the dense aggregation of IMB microtubules or exocytic vesicles carrying β-glucan wall precurors/CESA from fusing with the plasma membrane at the centre of the isthmus but are capable of fusing on either side of this zone.
The IMB and its associated actin band may function in other ways, however. For example, they may alter the physical characteristics of the cell wall at the isthmus that ultimately control wall extensibility and expansion. This might be through connections of the cytoskeletal elements with plasma membrane proteins that are connected to cell-wall constituents (Baluska et al., 2003).
Elucidation of IMB function should benefit from the use of transformed cell lines expressing fluorescent protein–cytoskeletal components (e.g. microtubules, microfilaments) and wall-synthesizing enzymes such as CESA. In conjunction with live cell labelling of specific wall polymers with specific mAbs, these transgenic lines would provide unique and valuable systems for acquiring novel insight into the coordinated interactions of secretory, cytoskeletal and wall processing mechanisms.
CONCLUSIONS
The study of unicellular CGA such as Penium shows that the cellular mechanisms normally associated with higher plant morphogenesis have antecedents in simpler life-forms that can inform us about the evolution of plant structure in general. Furthermore, the mechanistic congruence between these two developmental systems can reveal the presence of critical control points that might not otherwise be apparent. This study has revealed novel information about the cortical cytoskeleton in the expansion and division processes in Penium. A distinct cytoskeletal band like the PPB has now been shown to have its origins in CGA and therefore pre-dates the emergence of land plants or complex tissues. Furthermore, the IMB of Penium illustrates the close association of the cortical cytoskeleton with highly focused cell-wall deposition events during the cell cycle. A next step will be deciphering the specific interactions of the membrane trafficking apparatus with the cytoskeletal assemblages identified in this study, including proteins that link membrane compartments to microtubules and actin filaments. Likewise, the simple unicellular phenotype and growth characteristics of Penium will allow for a convenient means to elucidate specific wall polymer deposition strategies as they relate to cytoskeletal dynamics in highly synchronized cells. Penium represents a powerful model organism for understanding the cell-wall biology of green plant cells.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank Alicia Britton and Amanda Andreas for technical assistance and Dr Philip Lintilhac (University of Vermont, Burlington, VT, USA) for valuable discussions. This work was supported by grants from the National Science Foundation to D.S.D. (NSF-MCB 0919925 and NSF-DBI 0922805).
LITERATURE CITED
- Akkerman M, Overdijk EJR, Schel JHN, et al. Golgi body motility in the plant cortex correlates with actin cytoskeleton organization. Plant Cell Physiology. 2011;52:1844–1855. doi: 10.1093/pcp/pcr122. [DOI] [PubMed] [Google Scholar]
- Aruga H, Motomura T, Ichimura T. A proteolytic enzyme, trypsin, for immunofluorescence observation of microtubules in algal cells. Phycological Research. 1997;45:141–144. [Google Scholar]
- Azimzadeh J, Nacry P, Christodoulidou A, et al. Arabidopsis TONNEAU1 proteins are essential for preprohase band formation and interact with centrin. Plant Cell. 2008;20:2146–2159. doi: 10.1105/tpc.107.056812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluska F, Samaj J, Wojtaszek P, et al. Cytoskeleton–plasma membrane–cell wall continuum in plants. Emerging links revisited. Plant Physiology. 2003;133:482–491. doi: 10.1104/pp.103.027250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandizzi F, Wasteneys GO. Cytoskeleton-dependent endomembrane organization in plant cells: an emerging role for microtubules. Plant Journal. 2013;75:339–349. doi: 10.1111/tpj.12227. [DOI] [PubMed] [Google Scholar]
- Cook ME. Cytokinesis in Coleochaete orbicularis (Charophyceae): an ancestral mechanism inherited by plants. American Journal of Botany. 2003;91:313–320. doi: 10.3732/ajb.91.3.313. [DOI] [PubMed] [Google Scholar]
- Crowell EF, Bischoff V, Desprez T, et al. Pausing of Golgi bodies on microtubules regulates secretion of cellulose synthase complexes in Arabidopsis. Plant Cell. 2009;21:1141–1154. doi: 10.1105/tpc.108.065334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desprez T, Juraniec M, Crowell EF, et al. Organization of cellulose synthase complexes involved in primary cell wall synthesis in Arabidopsis thaliana. Proceedings of the National Academy of Sciences USA. 2007;104:15572–15577. doi: 10.1073/pnas.0706569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhonukshe P, Gadella TW. Alteration of microtubule dynamic instability during pre-prophase band formation revealed by yellow fluorescent protein-CLIP170 microtubule plus-end labeling. Plant Cell. 2003;15:597–611. doi: 10.1105/tpc.008961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domozych DS, Kort S, Benton S, Yu T. The extracellular polymeric substance of the green alga Penium margaritaceum and its role in biofilm formation. Biofilms. 2005;2:1–16. [Google Scholar]
- Domozych DS, Serfis A, Keimle S, et al. The structure and biochemistry of the homogalacturonans of the cell wall of the desmid, Penium margaritaceum. Protoplasma. 2007;230:99–115. doi: 10.1007/s00709-006-0197-8. [DOI] [PubMed] [Google Scholar]
- Domozych DS, Lambiasse L, Kiemle SN, et al. Cell wall development and bipolar growth in the desmid Penium margaritaceum. Asymmetry in a symmetric world. Journal of Phycology. 2009;45:879–893. doi: 10.1111/j.1529-8817.2009.00713.x. [DOI] [PubMed] [Google Scholar]
- Domozych DS, Brechka H, Britton A, et al. Cell wall growth and modulation dynamics in a model unicellular green alga – Penium margaritaceum: live cell labeling with monoclonal antibodies. Journal of Botany. 2011;2011:2–8. [Google Scholar]
- Domozych DS, Ciancia M, Fangel JU, et al. The cell walls of green algae: a journey through evolution and diversity. Frontiers in Plant Science. 2012;3 doi: 10.3389/fpls.2012.00082. doi:10.3389/fpls.2012.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domozych DS, Fujimoto C, LaRue T. Polar expansion dynamics in the plant kingdom: a diverse and multifunctional journey on the path to pollen tubes. Plants. 2013;2:148–173. doi: 10.3390/plants2010148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domozych DS, Sørensen I, Sacks C, et al. Disruption of the microtubule network alters cellulose deposition and causes major changes in pectin distribution in the cell wall of the green alga. Penium margaritaceum. Journal of Experimental Botany. 2014;65:465–479. doi: 10.1093/jxb/ert390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendrych M, Synek L, Pecenkova T, et al. Visualization of the exocyst complex dynamics at the plasma membrane of Arabidopsis thaliana. Molecular Biology of the Cell. 2013;24:510–520. doi: 10.1091/mbc.E12-06-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry SC. Cell wall polysaccharide composition and covalent cross-linking. In: Ulvskov P, editor. Annual plant reviews: plant polysaccharides, biosynthesis and bioengineering. Vol. 41. Oxford: Blackwell Publishing; 2011. pp. 1–42. [Google Scholar]
- Goto Y, Ueda K. Microfilament bundle of F-actin in Spirogyra observed by fluorescence microscopy. Planta. 1988;173:442–446. doi: 10.1007/BF00958955. [DOI] [PubMed] [Google Scholar]
- Gutierrez R, Lindeboom JJ, Paredez AR, et al. Arabidopsis cortical microtubules position cellulose synthase delivery to the plasma membrane and interact with cellulose synthase trafficking compartments. Nature Cell Biology. 2009;11:797–806. doi: 10.1038/ncb1886. [DOI] [PubMed] [Google Scholar]
- Hepler PK, Rounds CM, Winship LJ. Control of cell wall extensibility during pollen tube growth. Molecular Plant. 2013;6:998–1017. doi: 10.1093/mp/sst103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janski N, Masoud K, Batzenschlager M, et al. The GCP3-interacting proteins GIP1 and GIP2 are required for y-tubulin complex protein localization, spindle integrity, and chromosomal stability. Plant Cell. 2012;24:1171–1187. doi: 10.1105/tpc.111.094904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens G. Plant cytokinesis: fission by fusion. Trends in Cell Biology. 2005;15:277–283. doi: 10.1016/j.tcb.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Keegstra K. Plant cell walls. Plant Physiology. 2010;154:483–486. doi: 10.1104/pp.110.161240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojo KH, Higaki T, Kutsuna N, et al. Roles of cortical actin microfilament patterning in division plane orientation in plants. Plant and Cell Physiology. 2013;54:1491–1503. doi: 10.1093/pcp/pct093. [DOI] [PubMed] [Google Scholar]
- Kroeger JH, Zerzour R, Geitmann A. Regulator or driving force? The role of turgor pressure in oscillatory plant cell growth. PLoS One. 2011;6 doi: 10.1371/journal.pone.0018549. e18549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y-RJ, Liu B. The rise and fall of the phragmoplast microtubule array. Current Opinion in Plant Biology. 2013;16:1–7. doi: 10.1016/j.pbi.2013.10.008. [DOI] [PubMed] [Google Scholar]
- Leliaert F, Smith DR, Moreau H, et al. Phylogeny and molecular evolution of the green algae. Critical Reviews in Plant Sciences. 2012;31:1–46. [Google Scholar]
- Lewis LA, McCourt RM. Green algae and the origin of land plants. American Journal of Botany. 2004;91:1535–1556. doi: 10.3732/ajb.91.10.1535. [DOI] [PubMed] [Google Scholar]
- Lloyd C, Chan J. The parallel lives of microtubules and cellulose microfibrils. Current Opinion in Plant Biology. 2008;11:641–646. doi: 10.1016/j.pbi.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Louveaux M, Hamant O. The mechanics behind cell division. Current Opinion in Plant Biology. 2013;16:774–779. doi: 10.1016/j.pbi.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Marchant HJ, Pickett-Heaps JD. Mitosis and cytokinesis in Coleochaete scutata. Journal of Phycology. 1973;9:461–471. [Google Scholar]
- McIntosh K, Pickett-Heaps JD, Gunning BES. Cytokinesis in Spirogyra: integration of cleavage and cell-plate formation. International Journal of Plant Science. 1995;156:1–8. [Google Scholar]
- Muller S. Universal rules for division plane selection in plants. Protoplasma. 2012;249:239–253. doi: 10.1007/s00709-011-0289-y. [DOI] [PubMed] [Google Scholar]
- Muller S, Wright AJ, Smith LG. Division plane control in plants: new players in the band. Trends in Cell Biology. 2009;19:180–188. doi: 10.1016/j.tcb.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Nichols HW. Growth media-freshwater. In: Stein JR, editor. Handbook of phycological methods: culture methods and growth measurements. New York: Cambridge University Press; 1973. pp. 39–78. [Google Scholar]
- Palin R, Geitmann A. The role of pectin in plant morphogenesis. Biosystems. 2012;109:397–402. doi: 10.1016/j.biosystems.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Paredez AR, Somerville CR, Ehrhardt DW. Visualization of cellulose synthase demonstrates functional association with microtubules. Science. 2006;312:1491–1495. doi: 10.1126/science.1126551. [DOI] [PubMed] [Google Scholar]
- Pickett-Heaps JD, Gunning BES, Brown RC, et al. The cytoplast concept in dwindling plant cells: cytoplasmic domains and the evolution of spatially organized cell division. American Journal of Botany. 1999;86:153–172. [PubMed] [Google Scholar]
- Popper ZA. Evolution and diversity of green plant cell walls. Current Opinion in Plant Biology. 2008;11:286–292. doi: 10.1016/j.pbi.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Rasmussen CG, Sun B, Smith LG. Tangled localization at the cortical division site of plant cells occurs by several mechanisms. Journal of Cell Science. 2011;124:270–279. doi: 10.1242/jcs.073676. [DOI] [PubMed] [Google Scholar]
- Rasmussen CG, Wright AJ, Muller S. The role of the cytoskeleton and associated proteins in determination of the plant cell division plane. Plant Journal. 2013;75:258–269. doi: 10.1111/tpj.12177. [DOI] [PubMed] [Google Scholar]
- Sawitzky H, Grolig F. Phragmoplast of the green alga Spirogyra is functionally distinct from the higher plant phragmoplast. Journal of Cell Biology. 1995;130:1359–1371. doi: 10.1083/jcb.130.6.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw SL. Reorganization of the plant cortical microtubule array. Current Opinion in Plant Biology. 2013;16:1–5. doi: 10.1016/j.pbi.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Smolarkiewicz M, Dhonukshe P. Formative cell divisions: principal determinants of plant morphogenesis. Plant Cell Physiology. 2013;54:333–342. doi: 10.1093/pcp/pcs175. [DOI] [PubMed] [Google Scholar]
- Sørensen I, Pettolino FA, Bacic A, et al. The Charophycean green algae provide insights into the early origins of plant cell walls. Plant Journal. 2011;68:201–211. doi: 10.1111/j.1365-313X.2011.04686.x. [DOI] [PubMed] [Google Scholar]
- Spinner L, Pastuglia M, Belcram K, et al. The function of TONNEAU1 in moss reveals ancient mechanisms of division plane specification and cell elongation in land plants. Development. 2010;137:2733–2742. doi: 10.1242/dev.043810. [DOI] [PubMed] [Google Scholar]
- Thomas C, Tholl S, Moes D, et al. Actin bundling in plants. Cell Motility and Cytoskeleton. 2009;66:940–957. doi: 10.1002/cm.20389. [DOI] [PubMed] [Google Scholar]
- Timme RE, Bachvaroff TR, Delwiche CF. Broad phylogenomic sampling and the sister lineage of land plants. Plos One. 2012;7 doi: 10.1371/journal.pone.0029696. e29696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme D. Division plane determination duing plant somatic cytokinesis. Current Opinion in Plant Biology. 2009;13:745–751. doi: 10.1016/j.pbi.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Verchot-Lubicz J, Goldstein RE. Cytoplasmic streaming enables the distribution of molecules and vesicles in large plant cells. Protoplasma. 2010;240:99–107. doi: 10.1007/s00709-009-0088-x. [DOI] [PubMed] [Google Scholar]
- Wasteneys G, Willingale-Theune J, Menzel D. Freeze shattering: a simple and effective method for permeabilizing higher plant cell walls. Journal of Microscopy. 1997;188:51–61. doi: 10.1046/j.1365-2818.1977.2390796.x. [DOI] [PubMed] [Google Scholar]
- Wodniok S, Brinkmann H, Glockner G, et al. Origin of land plants: do conjugating green algae hold the key? BMC Evolutionary Biology. 2011;11:104. doi: 10.1186/1471-2148-11-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AJ, Gallagher K, Smith LG. discordia1 and alternative discordia1 function redundantly at the cortical division site to promote preprophase band formation and orient division planes in maize. Plant Cell. 2009;21:234–247. doi: 10.1105/tpc.108.062810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Dawe RK. Mechanisms of plant spindle formation. Chromosome Research. 2011;19:335–344. doi: 10.1007/s10577-011-9190-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.