Abstract
Background
Plant cell walls form the interface between the cells and their environment. They perform different functions, such as protecting cells from biotic and abiotic stress and providing structural support during development. Maintenance of the functional integrity of cell walls during these different processes is a prerequisite that enables the walls to perform their particular functions. The available evidence suggests that an integrity maintenance mechanism exists in plants that is capable of both detecting wall integrity impairment caused by cell wall damage and initiating compensatory responses to maintain functional integrity. The responses involve 1-aminocyclopropane-1-carboxylic acid (ACC), jasmonic acid, reactive oxygen species and calcium-based signal transduction cascades as well as the production of lignin and other cell wall components. Experimental evidence implicates clearly different signalling molecules, but knowledge regarding contributions of receptor-like kinases to this process is less clear. Different receptor-like kinase families have been considered as possible sensors for perception of cell wall damage; however, strong experimental evidence that provides insights into functioning exists for very few kinases.
Scope and Conclusions
This review examines the involvement of cell wall integrity maintenance in different biological processes, defines what constitutes plant cell wall damage that impairs functional integrity, clarifies which stimulus perception and signal transduction mechanisms are required for integrity maintenance and assesses the available evidence regarding the functions of receptor-like kinases during cell wall integrity maintenance. The review concludes by discussing how the plant cell wall integrity maintenance mechanism could form an essential component of biotic stress responses and of plant development, functions that have not been fully recognized to date.
Keywords: Receptor-like kinase, RLK, plant cell wall integrity maintenance, cell wall signalling
INTRODUCTION
Two hallmark features differentiating plants from animals are their sessile lifestyle and the walls surrounding all plant cells. The sessile lifestyle implies that the ability to resist both abiotic and biotic stress is significantly more important for plants than for animals. Specialized mechanisms enabling plants to adapt to stresses, such as drought and pathogen infection, influence the survival probability of plants. Importantly, the plant cell wall is intimately involved in all these processes and influences their outcome while in parallel also being a cornerstone of developmental processes. During cell morphogenesis the wall has to be plastic to allow controlled directional expansion, whereas after termination of morphogenesis it has to be sturdy and/or waterproof to provide mechanical support and resistance to pathogen infection and to enable long-distance water transport. The integrity of the wall has to be maintained throughout these different biological processes with sometimes opposite functional requirements.
Although Saccharomyces cerevisiae represents a significantly simpler organism compared with a plant, the functional requirements for the yeast cell wall during growth and interaction with the environment, as well as the need to maintain functional integrity, are similar to those of an individual plant cell wall. Previous research has shown that a dedicated cell wall integrity (CWI) maintenance mechanism exists in yeast that monitors the functional integrity of the wall and initiates compensatory responses upon exposure to cell wall damage (Levin, 2011). In yeast, cell wall damage occurs during different processes, such as enzymatic degradation of the wall, cell cycle progression, response to hypo-/hyper-osmotic, heat or cold shock and pheromone-induced cell morphogenesis (Kopecka and Gabriel, 1992; Davenport et al., 1995; Errede et al., 1995; Kamada et al., 1995; Buehrer and Errede, 1997). Compensatory responses to maintain integrity can involve changes in cell wall composition and structure (e.g. increase in chitin), reorganization of the cytoskeleton and cell cycle arrest (Levin, 2011). These observations support the notion that the yeast CWI maintenance mechanism is active during and represents an integral element of a large number of different biological processes.
Research in yeast suggests that the primary physical consequences of cell wall impairment, in conjunction with the high turgor pressure prevalent in the cells, are changes in surface tension of the wall and plasma membrane stretch (Kamada et al., 1995; Beese et al., 2009). In yeast cells, three different sensor mechanisms (mechanoreception, turgor perception and CWI perception) have been implicated in cell wall damage detection and CWI maintenance. The available data show that, in response to cell wall damage, a stretch-activated, plasma membrane-localized channel complex (MID1 CCH1) causes an increase in intracellular Ca2+ levels (Paidhungat and Garrett, 1997). The changes in Ca2+ levels lead (via calcineurin) to activation of the transcription factor CRZ1, which regulates expression of downstream response genes such as FKS2 (the yeast β-1,3 glucan synthase) (Zhao et al., 1998). Interestingly, activity of CRZ1 is also regulated by SKN7, a transcriptional regulator exhibiting similarity to the bacterial two-component system (Maeda et al., 1994). SKN7 activity is controlled mainly by turgor pressure (SLN1 SHO1) and CWI (WSC1, 2, 3, MID2 and MTL1) sensors (Maeda et al., 1994; Alberts et al., 1998; Ketela et al., 1999; Williams and Cyert, 2001). Changes in the phosphorylation state of SLN1 enable the yeast cell to detect the occurrence of hyper- and hypo-osmolarity, which is also indicative of CWI impairment. SLN1 regulates the activity of SKN7 through signals relayed by the HOG1 signalling pathway (Levin, 2011). Recent results from the characterization of WSC1, one of the plasma membrane localized sensors of the yeast CWI pathway, using atomic force microscopy, suggest that this plasma membrane-localized protein functions as a linear nanospring (Heinisch et al., 2010). The highly O-mannosylated extracellular domain residing within the cell wall functions as a mechanical probe that undergoes a conformational change upon changes in the surface tension of the yeast cell wall, leading to activation of the small G protein RHO1 via ROM2 [a RHO1 guanine nucleotide exchange factor (GEF)]. RHO1 in turn relays the signal to PKC1, which activates a MAP kinase cascade involving MPK1 (associated with the PAF1C complex) and leads to activation of FKS2 (Kim and Levin, 2011). Interestingly, RHO1 also directly interacts with and regulates the activity of the previously mentioned SKN7, supporting the notion that the different signalling cascades are interconnected and not independent of each other (Alberts et al., 1998; Ketela et al., 1999).
While a reasonable amount of knowledge exists regarding the different sensors and their respective signal transduction cascades, information regarding the mechanisms integrating the different signals into coordinated responses is limited. Garcia et al. (2009) have shown that the HOG and CWI pathway jointly coordinate the responses to yeast cell wall degrading zymolase treatment. More recently, Baltanás et al. (2013) have provided intriguing insights into how inputs from the pheromone response and the CWI maintenance mechanism are integrated and lead to an improved ability to adapt to osmotic change. These observations highlight that, in yeast, a matrix consisting of different signalling cascades jointly regulates the processes responsible for CWI maintenance. To summarize, a sophisticated mechanism exists in yeast that is active during different biological processes, monitors the integrity of the cell wall, detects qualitatively different inputs and integrates the incoming signals to modulate cellular metabolism in an adaptive manner.
Cell wall damage in plants can be caused by changes in turgor pressure levels or physical impairment of one or more cell wall components, with effects ranging from loosening of the cell wall polysaccharide network to the generation of low-molecular weight breakage products (e.g. oligogalacturonides), which results in weakening or breakdown (i.e. integrity impairment) of the cell wall. Examples of compounds having such effects are osmotica, inhibitors of cellulose biosynthesis such as isoxaben, plant pathogen-derived enzymes such as cellulases and pectinases, and commercial enzyme preparations such as driselase (Zeiger and Hepler, 1976; Dongowski and Sembries, 2001; Scheible et al., 2001). While the use of cell wall degrading enzymes might represent a valuable approach for the characterization of the plant CWI maintenance mechanism, it is important to bear in mind that enzyme preparations from plant pathogens have the intrinsic disadvantage of also containing epitopes activating plant immune responses.
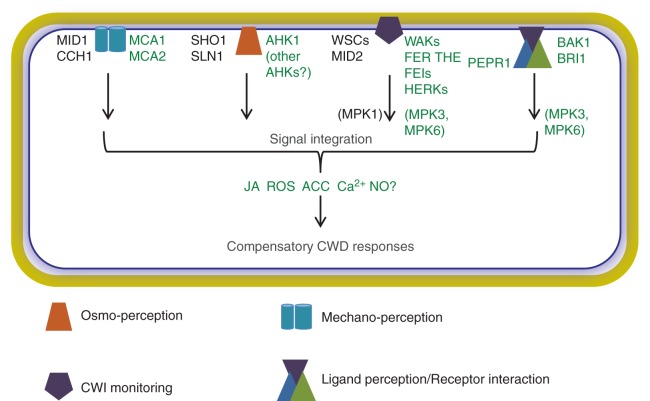
During recent years substantial evidence has accumulated supporting the existence of a CWI maintenance mechanism in plants. Several recently published articles review our current knowledge of the plant CWI maintenance mechanism competently and provide excellent global overviews (Nühse, 2012; Wolf et al., 2012). Therefore, we will focus here on particular aspects that have not been covered in detail before. Knowledge regarding the mode of action of the yeast CWI maintenance mechanism is useful when considering possible modes of action of the plant CWI maintenance mechanism. This notion is supported by the evidence available regarding the conservation of molecular activities between plants and yeast. Expression of the Arabidopsis thaliana proteins MID1 COMPLEMENTING ACTIVITY1 (MCA1) and MCA2 in MID1-deficient yeast strains leads to at least partial rescue (Nakagawa et al., 2007; Yamanaka et al., 2010). In parallel, expression of ARABIDOPSIS HISTIDINE KINASES (AHK) 1, 2, 3 and 4 complements yeast strains deficient in SLN1-dependent osmo-sensing (Urao et al., 1999; Inoue et al., 2001; Tran et al., 2007). The localization of AHK1 to the plasma membrane indicates the closest functional similarity to SLN1, while the endoplasmic reticulum-localized AHK2, 3 and 4 seem to function as organ-specific cytokinin receptors that cannot easily be integrated into a model of osmo-sensitive CWI maintenance (Inoue et al., 2001; Ueguchi et al., 2001; Yamada et al., 2001; Higuchi et al., 2004; Nishimura et al., 2004; Caesar et al., 2011; Wulfetange et al., 2011). AHK1 acts as positive regulator of stress responses, whereas the cytokinin receptor AHKs have been demonstrated to negatively regulate stress responses in a cytokinin-dependent manner, indicating opposing involvement in common pathways (Tran et al., 2007). Recently, Źd'árská et al. (2013) have shown that cytokinins regulate the abundance of proteins involved in primary metabolism, such as carbohydrate metabolism, a pathway that is controlled in an osmo-sensitive manner in the context of CWI impairment (Wormit et al., 2012). It will thus be interesting to know if, and to what extent, different AHKs contribute to this osmo-sensitive regulation. Figure 1 provides a global overview of the signalling cascades and of several key components mediating yeast CWI maintenance. It also summarizes candidate genes (and possible plant-specific signalling cascades) from arabidopsis that have been implicated in plant CWI maintenance based on currently available knowledge.
Fig. 1.
Comparative overview of CWI signalling cascades in yeast and plant cells. Black font highlights yeast genes, green indicates arabidopsis genes and grey indicates processes common to both plant and yeast cells. The cell wall is coloured yellow, while the dark blue line represents the plasma membrane and the light blue area indicates the apoplast.
However, there are also limitations of such a comparison due to differences between the model systems that have to be considered. Currently it remains to be determined how/if the single-cell situation of the yeast cell versus the multicellular plant structure affects the design and mode of action of the CWI maintenance mechanism. In parallel, it is reasonable to assume that in plants cell wall damage occurs during wounding and infection by pathogens that break down or modify cell walls, which is not a common problem in yeast. Examples of such pathogens are the necrotrophs Botrytis cinerea and Plectosphaerella cucumerina and the (hemi-)biotrophs Erysiphe cichoracearum and Pseudomonas syringae, which could be of particular interest with respect to plant CWI research, since previous work has shown that mutations affecting plant cell wall metabolism affect their infection success (Hernandez-Blanco et al., 2007; Delgado-Cerezo et al., 2011). In addition, the plant cell wall is significantly more complex than the yeast cell wall with respect to both structure and composition. The large number of different cell wall polysaccharides and proteins could give rise to a large number of ligands for potential CWI sensors. In this context it is sensible to bear in mind that although more than 600 and 1131 receptor-like kinases (RLKs) have been identified in the arabidopsis and rice genomes, respectively, corresponding ligands have been assigned only for a very small number of these RLKs (Shiu et al., 2004). Combining ligands (deriving from specific cell wall components either released or made accessible by cell wall damage) with particular RLKs would allow the generation of a large number of highly specific signals indicating plant cell wall changes.
ELICITORS OF THE SIGNAL: CELL WALL (DAMAGE)-ASSOCIATED RLK LIGANDS
In the recent past, two types of ligand have been shown to bind to cell wall (damage)-associated RLKs. The first ligand type to elicit signals upon structural changes in the cell wall resides in the wall itself and includes specific individual carbohydrate polymers and cell wall proteins that are attached to RLKs under non-stress conditions. While the scaffold structure composed of cellulose microfibrils is not readily modifiable, hemicellulosic and pectic polymers are subject to multiple and often fast modifications and turnover. For example, induction of glycosyl hydrolase expression upon carbohydrate starvation is associated with a reduction in hemicellulosic and pectic, but not cellulosic, monosaccharide content (Lee et al., 2007). Methylesterification of pectin is important for cell wall extensibility/stiffness as it regulates accessibility of pectin-degrading enzymes and controls Ca2+-dependent binding of pectic polymers (reviewed by Peaucelle et al., 2012). O-acetylation is common to pectin and hemicellulose and has been implicated in CWI, enzyme accessibility and the stress response (reviewed by Gille and Pauly, 2012). It seems likely that the availability of epitopes for binding to receptors, which function as potential sensors of CWI, is similarly affected by cell wall modification. Wall-associated kinases (WAKs) are bound to pectin in planta and bind to pectic polymers and fragments in vitro (Wagner and Kohorn, 2001; Decreux and Messiaen, 2005; Kohorn et al., 2009). In vitro binding was shown to depend on Ca2+ and de-methylesterified pectin (Decreux and Messiaen, 2005). In addition, the interaction of WAK1 with glycine-rich proteins has been observed in a yeast-two-hybrid assay, whereas an in vivo interaction has not been clearly demonstrated so far (Park et al., 2001). Another RLK that has been shown to bind pectin belongs to the proline-rich extensin-like receptor kinase (PERK) family. Bai et al. (2009) demonstrated that PERK4 can be released from root tissue by pectolyase digestion in a time-dependent manner.
The second type of ligand emerges upon damage and includes breakdown products originating in both the wall and the lumen of the cell. These molecules are commonly referred to as damage-associated molecular patterns (DAMPs), a term based on the term ‘microbe-associated molecular patterns’ (MAMPs), highly conserved molecules of microbial origin that elicit defence responses in plants. By now, DAMPs and MAMPs have been described in different species in which they enable the plant to distinguish between self and non-self and generate similar RLK-dependent signals (reviewed by Monaghan and Zipfel, 2012). Oligogalacturonides are particularly well-characterized DAMPs that can arise from pectic homogalacturonan upon digestion by wound-induced or microbial pectin-degrading enzymes in a process that is controlled by plant polygalacturonase-inhibiting proteins (Cervone et al., 1989; Bergey et al., 1999). It has been demonstrated that WAK1 binds oligogalacturonides, and that oligogalacturonide binding elicits defence responses in a chimaeric RLK consisting of an extracellular WAK1 domain and an intracellular kinase domain of the EF-Tu receptor (Brutus et al., 2010). Oligogalacturonides have been shown to antagonize auxin-dependent developmental processes in different plants and tissues, demonstrating the potential of this molecule to modulate both developmental and defence-dependent signalling (Branca et al., 1988; Bellincampi et al., 1993; Altamura et al., 1998; Savatin et al., 2011). This impact on auxin signalling is especially interesting considering the broad influence of auxin on transcription of cell wall-related genes (Lewis et al., 2013).
Arabidopsis elicitor peptides (AtPeps) induce defence signalling after release from their wounding-/pathogen-induced PROPEP precursors (Huffaker et al., 2006). PROPEP isoforms show tissue-specific expression patterns and have been implicated in different physiological processes based on transcriptional data (Bartels et al., 2013). However, due to the subcellular localization of PROPEPs in the cytosol or the tonoplast, AtPeps are unlikely to constitute the initial signal (Huffaker and Ryan, 2007; Bartels et al., 2013). Rather, they seem to be required for amplification/modulation of defence responses after elicitation. Phosphosulphokines (PSKs) might also have a function in the RLK-dependent regulation of DAMP/MAMP signalling. PSK peptides stimulate growth and attenuate defence signalling in arabidopsis after cleavage from secreted precursor proteins and might thus function as apoplastic signals for balancing growth and defence responses (Srivastava et al., 2008; Igarashi et al., 2012).
RECEPTOR-LIKE KINASES IN CELL WALL INTEGRITY MONITORING
To date, several RLKs have been implicated in CWI monitoring, based either on the nature of their ligands or on altered responses in the respective arabidopsis mutants upon cell wall damage (summarized in Table 1). Receptor–ligand interactions are particularly well documented for WAKs (see above). The WAKs comprise a family of five members and 22 WAK-like genes that have been identified based on protein sequence homology (Verica and He, 2002). Pectin-induced activation of gene expression has been shown to depend to a great extent on WAK2 and the associated stress response was mimicked by a dominant active WAK2 allele (WAK2cTAP) (Kohorn et al., 2009, 2012). The growth phenotype of WAK2cTAP plants was suppressed by mpk6; WAK2-dependent gene expression, however, was only partially dependent on MPK6, indicating that additional pathways might be involved in signal transduction (Kohorn et al., 2012). WAKs have been shown to be required for both cell elongation and the activation of stress responses (Wagner and Kohorn, 2001; Kohorn et al., 2006, 2012). However, the activation of these different (and most likely opposing) programmes might depend on the nature of the particular ligand bound and require distinct downstream signalling events (for a model see Kohorn and Kohorn, 2012). By contrast, pectin-associated PERK4 is required for abscisic acid (ABA)-induced Ca2+ accumulation and inhibition of root cell elongation (Bai et al., 2009). While it is not known if the association with pectin influences PERK4 kinase function, this observation hints at possible interactions between CWI maintenance and the drought stress response mechanisms.
Table 1.
Receptor-like kinase (RLK) families whose members have been implicated in cell wall damage signalling
| RLK family | RLK | Ligands | Downstream signalling elements | References |
|---|---|---|---|---|
| LRR-RLK | BAK1 | Ca2+, ROS, MPK3, MPK4, MPK6 | Roux et al., 2011; Fàbregas et al., 2013; Ma et al., 2013 | |
| FEI1/2 | ACC | Xu et al., 2008 | ||
| PEPR1/2 | AtPeps | MPK3, MPK6, ethylene, Ca2+, NO, ROS | Krol et al., 2010; Yamaguchi et al., 2010; Ma et al., 2012, 2013 | |
| PSKR1/2 | PSK | Jasmonic acid | Matsubayashi et al., 2002, 2006; Igarashi et al., 2012; Mosher et al., 2013 | |
| PSY1R | PSY1 | Amano et al., 2007; Mosher et al., 2013 | ||
| CrRLK1L | ANXUR1/2 | Boisson-Dernier et al., 2009; Miyazaki et al., 2009 | ||
| FERONIA | RALF | RAC/ROP GTPases, ROS, Ca2+ | Guo et al., 2009a; Deslauriers and Larsen, 2010; Duan et al., 2010; Kessler et al., 2010; Fàbregas et al., 2013; Haruta et al., 2014 | |
| HERKULES 1/2 | Guo et al., 2009a, b | |||
| THESEUS | ROS | Hématy et al., 2007; Guo et al., 2009a; Denness et al., 2011 | ||
| PERK | PERK4 | Pectin | Ca2+ | Bai et al., 2009 |
| WAK | WAK1 | Pectin, oligogalacturonides | Decreux and Messiaen 2005; Brutus et al., 2010 | |
| WAK2 | Pectin | MPK3, MPK6, invertase | Kohorn et al., 2006, 2009, 2012 |
Known ligands, signalling molecules and pathways shown to be activated downstream of specific RLKs are listed in the table.
Leucine-rich repeat (LRR) RLKs form the largest group within the RLK superfamily (Gish and Clark, 2011). Within this family, several receptors have been shown to bind the small signalling peptides AtPeps and PSK. Recent evidence indicates that PSK receptor 1 (PSKR1) is important for jasmonic acid-dependent signalling and, together with the related RLKs PSKR2 and PSY1R, the regulation of hormone balance upon pathogen infection, suggesting a role in balancing developmental processes versus defence responses (Mosher et al., 2013). AtPeps receptor 1 (PEPR1) and its at least partially redundant homologue PEPR2 have been shown to influence defence signalling and pathogen susceptibility (Krol et al., 2010; Yamaguchi et al., 2010). Interestingly, PEPR1 interacts with BAK1, a LRR-RLK that also functions as co-receptor of the MAMP receptors FLS2 and EFR to mediate pathogen resistance, to amplify signal intensity upon elicitor binding (Schulze et al., 2010; Roux et al., 2011). Upon AtPeps or flg22 treatment, BAK1 contributes to a signalling cascade involving Ca2+, nitric oxide and reactive oxygen species (ROS), revealing functional interdependence between PEPR1- and FLS2-induced responses (Ma et al., 2013). Initially, BAK1 was identified as co-receptor of the brassinosteroid receptor BRI1 (Li et al., 2002; Nam and Li, 2002). It has been shown that co-activation of the BRI1 and FLS2/ERF pathways is differentially regulated in a phosphorylation-dependent manner (Schwessinger et al., 2011). BAK1 is thus involved in the regulation of both developmental and stress-dependent processes and might provide an interface for adjustment of different energy-consuming programmes. However, switching between these pathways is not achieved by competition for a limited BAK1 pool, but its regulation seems to be either dependent on differential binding characteristics of BAK1 with RLKs or downstream/independently of RLK interaction (Albrecht et al., 2012).
The homologous LRR-RLKs FEI1 and FEI2 have been identified based on the sucrose-dependent swollen-root phenotype of fei1 fei2 double mutant seedlings. A similar phenotype has been observed in mutants such as procuste1 (prc1; CESA6 loss-of-function allele) and isoxaben-treated wild-type seedlings, which are all impaired in the formation of load-bearing cellulose microfibrils (Fagard et al., 2000; Xu et al., 2008; Hamann et al., 2009). Interestingly, cellulose biosynthesis is also reduced in fei1 fei2 mutants, and ectopic deposition of lignin is detectable in the swollen roots. In both prc1 and fei1 fei2 mutants, isoxaben sensitivity is increased compared with wild-type, indicating impaired CWI. The fei1 fei2 root phenotype was, however, not dependent on a functional kinase domain, suggesting that interaction partners are necessary for signal transduction (Xu et al., 2008). Genetic studies suggested that the extracellular glycosylphosphatidylinositol-anchored protein SALT OVERLY SENSITIVE5 (SOS5), which was previously shown to display a similar conditional phenotype, acts in the same pathway as FEI1/2 (Shi et al., 2003; Xu et al., 2008).
Recently, evidence has been accumulating that members of the Catharanthus roseus RLK1-like (CrRLK1L) protein family could function as sensors of CWI during growth. Based on homology to the Xenopus laevis protein malectin, two extracellular domains of CrRLK1L proteins have been predicted to have a putative carbohydrate-binding function and might thus directly bind cell wall polymers, carbohydrate DAMPs or glycosylated proteins (Schallus et al., 2008; Boisson-Dernier et al., 2011). Cellulose deficiency in prc1 can be partially uncoupled from growth inhibition and ectopic lignification by a mutation in THESEUS1 (THE1), suggesting that THE1 mediates signals indicative of impaired CWI (Hématy et al., 2007). This notion was further supported by the finding that THE1 is involved in isoxaben-induced accumulation of ROS and ectopic root lignification (Denness et al., 2011). In addition to this role in CWI monitoring, THE1 was shown to be involved in brassinosteroid-sensitive vegetative cell elongation jointly with HERKULES1/2 (HERK1/2). Based on the growth phenotypes and results of gene expression analysis in mutant plants, FERONIA (FER) might affect the same pathway (Guo et al., 2009a, b). Deslauriers and Larsen (2010) reported that FER is required for full brassinosteroid-dependent hypocotyl elongation in etiolated seedlings, while sensitivity to exogenously applied brassinosteroid is increased in fer mutants when grown in the light. These apparently contrasting responses might reflect the absence of efficient CWI monitoring that coordinates cell elongation with cell wall expansion under particular growth conditions and/or developmental programs. Recently it has been shown that FER, BAK1, BR SIGNALING KINASE1 (BSK1) and BSK3 co-immunoprecipitate with a green fluorescent protein-tagged BRI1 homologue, BRI1-like3 (BRL3), indicating that these RLKs might form a receptor complex required for root growth (Fàbregas et al., 2013). FER and its close homologues ANXUR1 (ANX1) and ANX2 are required for functional integrity of polarly growing root hair cells and pollen tubes, respectively (Boisson-Dernier et al., 2009; Miyazaki et al., 2009; Duan et al., 2010). Recently, Haruta and colleagues (2014) demonstrated that the secreted peptide RALF (rapid alkalization factor) is bound by FER and that this interaction leads to both inhibition of H+-ATPase2 (AHA2) and a reduction in root cell elongation. The requirement of FER for rapid RALF-induced Ca2+ accumulation suggests that downstream signalling depends on Ca2+, a hypothesis that is further supported by transcriptome analysis (Haruta et al., 2014). Regulation of polar growth in root hairs also involves interaction of FER with GEFs of Rho-like RAC/ROP GTPases (ROPGEFs) to control RAC/ROP-dependent and auxin-sensitive accumulation of ROS, while some functional specificity is provided by different ROPGEFs (Duan et al., 2010, 2014; Huang et al., 2013). The same pathway seems to be responsible for suppression of ABA signalling, further supporting the comprehensive impact of FER-mediated signalling on the regulation of cell development and stress responses (Yu et al., 2012). Taking these findings together, it seems likely that FER and ANX1/2 are required for the coordination of cell elongation and cell wall assembly in fast-growing cells to permit tightly controlled cell wall breakdown. Remarkably, FER is also required for successful cell wall penetration by powdery mildew pathogens, a process that requires reorganization of the plasma membrane and the cell wall to form a novel matrix at the interface between fungal and plant cells, which also involves RAC/ROP GTPases (Kessler et al., 2010; Hückelhoven and Panstruga, 2011). Kessler et al. (2010) suggest that a similar mechanism is activated both during pollen tube reception and powdery mildew penetration, as both pathways involve FER and MILDEW RESISTANCE LOCUS O (MLO) proteins. Pollen tube recognition and rupture depend on asymmetrical accumulation of FER in synergid cells and might involve competition with ANX1/2 for the same ligand (Escobar-Restrepo et al., 2007; Kanaoka and Torii, 2010). These results suggest that CWI maintenance and pollen tube wall modification during fertilization are both regulated by CrRLK1L proteins in a cooperative manner.
SIGNAL TRANSDUCTION AND DOWNSTREAM RESPONSES
Downstream responses of impaired CWI have mainly been analysed in arabidopsis mutants either genetically or chemically impaired in cellulose biosynthesis. These analyses have shown that a wide range of signalling pathways are induced upon CWI impairment, overlapping in large part with responses to biotic and abiotic stress. Induced responses included accumulation of ROS and activation of jasmonic acid-, ABA-, salicylic acid- and ethylene-dependent signalling (Ellis et al., 2002; Manfield et al., 2004; Hernandez-Blanco et al., 2007; Hamann et al., 2009; Denness et al., 2011). Ultimately, impaired cellulose biosynthesis leads to compensatory changes in cell wall composition, which include increased uronic acid content, callose deposition and ectopic root lignification (Cano-Delgado et al., 2000, 2003; Manfield et al., 2004; Hématy et al., 2007; Hamann et al., 2009). In the presence of glucose or sucrose, impaired cellulose biosynthesis leads to a swollen root phenotype, suggesting that regulation of cell elongation and monitoring of CWI are severely impaired under favourable growth conditions (Cano-Delgado et al., 2000; Hamann et al., 2009). Ectopic lignification, however, is partially maintained in non-swollen roots in the presence of glucose or sucrose analogues, indicating that responses induced by cellulose biosynthesis inhibition are influenced by non-metabolic monitoring of sugar availability (Hamann et al., 2009).
Up to now, little has been known about the signalling cascade(s) leading to the observed changes in hormone balance and cell wall composition. Ectopic lignification depends on ROS accumulation and is inhibited by jasmonic acid, while both pathways seem to depend on Ca2+ signalling (Denness et al., 2011). Isoxaben-induced ROS accumulation depends on the NADPH oxidases RESPIRATORY BURST OXIDASE HOMOLOG D (RBOHD) and RBOHF (Denness et al., 2011), which are regulated by Ca2+ signalling and phosphorylation and contribute to both local and systemic signalling in response to a variety of stress triggers. The mutual influence of Ca2+ and ROS signalling has been studied in detail and recent reports suggest that both pathways interact directly during both signal elicitation and cell-to-cell propagation (for a recent review see Steinhorst and Kudla, 2013). Currently, however, only results from studies using different signalling inhibitors implicate Ca2+ in the response to cellulose biosynthesis inhibition (Denness et al., 2011). Treatment with oligogalacturonide elicitors induces transient Ca2+ accumulation and partially Ca2+-dependent transcriptional responses, suggesting a possible role of Ca2+ in WAK-dependent signalling (Moscatiello et al., 2006). Remarkably, isoxaben-dependent accumulation of ROS depends on THE1, indicating specific activation of this pathway upon inhibition of cellulose biosynthesis (Denness et al., 2011). The Ca2+ and ROS pathways have both been demonstrated to interact with nitric oxide signalling; nitric oxide is a signalling molecule that accumulates upon stress and oligogalacturonide-treatment and might contribute up to 50 % of oligogalacturonide-induced deregulation of gene expression according to recent analyses (Rasul et al., 2012; Scheler et al., 2013; Jeandroz et al., 2013). Accumulation of nitric oxide after treatment with flg22 or AtPeps strongly depended on the RLKs FLS2 and PEPR1, respectively, indicating tight regulation by pattern recognition pathways (Ma et al., 2013).
It is well established that root cell elongation is inhibited by both ethylene and the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC) in an auxin-dependent manner (reviewed by Muday et al., 2012). Tsang et al. (2011) have shown that this mechanism is required to mediate root growth inhibition in response to cellulose biosynthesis inhibition. Short-term responses were specifically dependent on ACC but not ethylene, suggesting that ACC represents an important signal in this process. Consistent with this observation, manipulation of ACC but not ethylene signalling resulted in rescue of the cell elongation defect observed in fei1 fei2 roots. FEI1/2 interacted with ACC synthase in a yeast two-hybrid assay, suggesting that these RLKs are required for ACC synthesis in a pathway that links cell wall biosynthesis and root cell elongation (Xu et al., 2008).
So far, no direct targets for phosphorylation by candidate CWI-monitoring RLKs have been identified. However, MAPK cascades are involved in WAK2-dependent signalling upon perception of pectin and oligogalacturonides (Kohorn et al., 2009, 2012). In the future, it will be pivotal to discover phosphorylation targets of the discussed RLKs and additional players in downstream kinase cascades. Furthermore, it will be interesting to study the interactions of RLK-dependent pathways with signalling cascades involved in turgor pressure and mechano-perception that are activated by cell wall damage.
CONCLUSION
To summarize, recently a significant amount of evidence supporting the existence of a dedicated plant CWI maintenance mechanism has accumulated. The available data suggest some degree of similarity between the plant and yeast mechanisms with respect to the signalling cascades and proteins involved. They also highlight differences. For example, the yeast genome does not encode a large number of RLKs (like plant genomes tend to), hinting at possible fundamental differences between the modes of action of the plant and yeast CWI maintenance mechanisms. In addition, the possible consequences of the multicellular organization of plants with respect to the processes maintaining CWI integrity have not been discussed here simply because there is still pretty much a black hole in our knowledge.
Probably the most interesting questions that need to be addressed in the near future regard whether cell wall derived DAMPs may actually represent the signals indicating CWI impairment, and which specific cell wall components are affected by cell wall damage. This type of qualitative information, in conjunction with quantitative information about physical stimuli generated by mechanosensors and turgor sensors (such as MCA1 and 2, as well as AHKs), would provide the plant cell with a detailed overview of events taking place at its interface with the environment and enable it to produce adaptive responses that increase its probability of survival.
ACKNOWLEDGEMENTS
T.E. is supported by a DFG postdoctoral fellowship and research in the laboratory of T.H. is supported by the Peder Sather Center, the Marie Curie Initiative of the Seventh EU Framework Programme and the Gatsby Charitable Foundation.
LITERATURE CITED
- Alberts AS, Bouquin N, Johnston LH, Treisman R. Analysis of RhoA-binding proteins reveals an interaction domain conserved in heterotrimeric G protein beta subunits and the yeast response regulator protein Skn7. Journal of Biological Chemistry. 1998;273:8616–8622. doi: 10.1074/jbc.273.15.8616. [DOI] [PubMed] [Google Scholar]
- Albrecht C, Boutrot F, Segonzac C, et al. Brassinosteroids inhibit pathogen-associated molecular pattern-triggered immune signaling independent of the receptor kinase BAK1. Proceedings of the National Academy of Sciences of the USA. 2012;109:303–308. doi: 10.1073/pnas.1109921108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altamura MM, Zaghi D, Salvi G, De Lorenzo G, Bellincampi D. Oligogalacturonides stimulate pericycle cell wall thickening and cell divisions leading to stoma formation in tobacco leaf explants. Planta. 1998;204:429–436. [Google Scholar]
- Amano Y, Tsubouchi H, Shinohara H, Ogawa M, Matsubayashi Y. Tyrosine-sulfated glycopeptide involved in cellular proliferation and expansion in Arabidopsis. Proceedings of the National Academy of Sciences of the USA. 2007;104:18333–18338. doi: 10.1073/pnas.0706403104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L, Zhang G, Zhou Y, et al. Plasma membrane-associated proline-rich extensin-like receptor kinase 4, a novel regulator of Ca signalling, is required for abscisic acid responses in Arabidopsis thaliana. Plant Journal. 2009;60:314–327. doi: 10.1111/j.1365-313X.2009.03956.x. [DOI] [PubMed] [Google Scholar]
- Baltanás R, Bush A, Couto A, Durrieu L, Hohmann S, Colman-Lerner A. Pheromone-induced morphogenesis improves osmoadaptation capacity by activating the HOG MAPK pathway. Science Signaling. 2013;6 doi: 10.1126/scisignal.2003312. pra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels S, Lori M, Mbengue M, et al. The family of AtPeps and their precursors in Arabidopsis: differential expression and localization but similar induction of pattern-triggered immune responses. Journal of Experimental Botany. 2013;64:5309–5321. doi: 10.1093/jxb/ert330. [DOI] [PubMed] [Google Scholar]
- Beese SE, Negishi T, Levin DE. Identification of positive regulators of the yeast fps1 glycerol channel. PLoS Genetics. 2009;5 doi: 10.1371/journal.pgen.1000738. e1000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellincampi D, Salvi G, Lorenzo G, et al. Oligogalacturonides inhibit the formation of roots on tobacco explants. Plant Journal. 1993;4:207–213. [Google Scholar]
- Bergey DR, Orozco-Cardenas M, de Moura DS, Ryan CA. A wound- and systemin-inducible polygalacturonase in tomato leaves. Proceedings of the National Academy of Sciences of the USA. 1999;96:1756–1760. doi: 10.1073/pnas.96.4.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson-Dernier A, Roy S, Kritsas K, et al. Disruption of the pollen-expressed FERONIA homologs ANXUR1 and ANXUR2 triggers pollen tube discharge. Development. 2009;136:3279–3288. doi: 10.1242/dev.040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson-Dernier A, Kessler SA, Grossniklaus U. The walls have ears: the role of plant CrRLK1Ls in sensing and transducing extracellular signals. Journal of Experimental Botany. 2011;62:1581–1591. doi: 10.1093/jxb/erq445. [DOI] [PubMed] [Google Scholar]
- Branca C, Lorenzo G De, Cervone F. Competitive inhibition of the auxin-induced elongation by α-D-oligogalacturonides in pea stem segments. Physiologia Plantarum. 1988;72:499–504. [Google Scholar]
- Brutus A, Sicilia F, Macone A, Cervone F, De Lorenzo G. A domain swap approach reveals a role of the plant wall-associated kinase 1 (WAK1) as a receptor of oligogalacturonides. Proceedings of the National Academy of Sciences of the USA. 2010;107:9452–9457. doi: 10.1073/pnas.1000675107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehrer BM, Errede B. Coordination of the mating and cell integrity mitogen-activated protein kinase pathways in Saccharomyces cerevisiae. Molecular and Cellular Biology. 1997;17:6517–6525. doi: 10.1128/mcb.17.11.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caesar K, Thamm AM, Witthöft J, et al. Evidence for the localization of the Arabidopsis cytokinin receptors AHK3 and AHK4 in the endoplasmic reticulum. Journal of Experimental Botany. 2011;62:5571–5580. doi: 10.1093/jxb/err238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano-Delgado AI, Metzlaff K, Bevan MW. The eli1 mutation reveals a link between cell expansion and secondary cell wall formation in Arabidopsis thaliana. Development. 2000;127:3395–3405. doi: 10.1242/dev.127.15.3395. [DOI] [PubMed] [Google Scholar]
- Cano-Delgado A, Penfield S, Smith C, Catley M, Bevan M. Reduced cellulose synthesis invokes lignification and defense responses in Arabidopsis thaliana. Plant Journal. 2003;34:351–362. doi: 10.1046/j.1365-313x.2003.01729.x. [DOI] [PubMed] [Google Scholar]
- Cervone F, Hahn MG, De Lorenzo G, Darvill A, Albersheim P. Host-pathogen interactions: XXXIII. A plant protein converts a fungal pathogenesis factor into an elicitor of plant defense responses. Plant Physiology. 1989;90:542–548. doi: 10.1104/pp.90.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport KR, Sohaskey M, Kamada Y, Levin DE, Gustin MC. A second osmosensing signal transduction pathway in yeast. Hypotonic shock activates the PKC1 protein kinase-regulated cell integrity pathway. Journal of Biological Chemistry. 1995;270:30157–30161. doi: 10.1074/jbc.270.50.30157. [DOI] [PubMed] [Google Scholar]
- Decreux A, Messiaen J. Wall-associated kinase WAK1 interacts with cell wall pectins in a calcium-induced conformation. Plant & Cell Physiology. 2005;46:268–278. doi: 10.1093/pcp/pci026. [DOI] [PubMed] [Google Scholar]
- Delgado-Cerezo M, Sanchez-Rodriguez C, Escudero V, et al. Arabidopsis heterotrimeric g-protein regulates cell wall defense and resistance to necrotrophic fungi. Molecular Plant. 2011;5:98–114. doi: 10.1093/mp/ssr082. [DOI] [PubMed] [Google Scholar]
- Denness L, McKenna JF, Segonzac C, et al. Cell wall damage-induced lignin biosynthesis is regulated by a ROS- and jasmonic acid-dependent process in Arabidopsis thaliana. Plant Physiology. 2011;156:1364–1374. doi: 10.1104/pp.111.175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslauriers SD, Larsen PB. FERONIA is a key modulator of brassinosteroid and ethylene responsiveness in Arabidopsis hypocotyls. Molecular Plant. 2010;3:626–640. doi: 10.1093/mp/ssq015. [DOI] [PubMed] [Google Scholar]
- Dongowski G, Sembries S. Effects of commercial pectolytic and cellulolytic enzyme preparations on the apple cell wall. Journal of Agricultural and Food Chemistry. 2001;49:4236–4242. doi: 10.1021/jf001410+. [DOI] [PubMed] [Google Scholar]
- Duan Q, Kita D, Li C, Cheung AY, Wu HM. FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proceedings of the National Academy of Sciences of the USA. 2010;107:17821–17826. doi: 10.1073/pnas.1005366107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Q, Kita D, Johnson EA, et al. Reactive oxygen species mediate pollen tube rupture to release sperm for fertilization in Arabidopsis. Nature Communications. 2014;5(3129) doi: 10.1038/ncomms4129. doi:10.1038/ncomms4129. [DOI] [PubMed] [Google Scholar]
- Ellis C, Karafyllidis I, Wasternack C, Turner JG. The Arabidopsis mutant cev1 links cell wall signaling to jasmonate and ethylene responses. Plant Cell. 2002;14:1557–1566. doi: 10.1105/tpc.002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errede B, Cade RM, Yashar BM, et al. Dynamics and organization of MAP kinase signal pathways. Molecular Reproduction and Development. 1995;42:477–485. doi: 10.1002/mrd.1080420416. [DOI] [PubMed] [Google Scholar]
- Escobar-Restrepo JM, Huck N, Kessler S, et al. The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science. 2007;317:656–660. doi: 10.1126/science.1143562. [DOI] [PubMed] [Google Scholar]
- Fàbregas N, Li N, Boeren S, Nash TE, et al. The BRASSINOSTEROID INSENSITIVE1-LIKE3 signalosome complex regulates Arabidopsis root development. Plant Cell. 2013;25:3377–3388. doi: 10.1105/tpc.113.114462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagard M, Desnos T, Desprez T, et al. PROCUSTE1 encodes a cellulose synthase required for normal cell elongation specifically in roots and dark-grown hypocotyls of Arabidopsis. Plant Cell. 2000;12:2409–2424. doi: 10.1105/tpc.12.12.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia R, Rodriguez-Pena JM, Bermejo C, Nombela C, Arroyo J. The high osmotic response and cell wall integrity pathways cooperate to regulate transcriptional responses to zymolyase-induced cell wall stress in Saccharomyces cerevisiae. Journal of Biological Chemistry. 2009;284:10901–10911. doi: 10.1074/jbc.M808693200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille S, Pauly M. O-acetylation of plant cell wall polysaccharides. Frontiers in Plant Science. 2012;3 doi: 10.3389/fpls.2012.00012. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gish LA, Clark SE. The RLK/Pelle family of kinases. Plant Journal. 2011;66:117–127. doi: 10.1111/j.1365-313X.2011.04518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Li L, Ye H, Yu X, Algreen A, Yin Y. Three related receptor-like kinases are required for optimal cell elongation in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the USA. 2009a;106:7846–7653. doi: 10.1073/pnas.0812346106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Ye H, Li L, Yin Y. A family of receptor-like kinases are regulated by BES1 and involved in plant growth in Arabidopsis thaliana. Plant Signaling & Behavior. 2009b;4:784–786. doi: 10.4161/psb.4.8.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann T, Bennett M, Mansfield J, Somerville C. Identification of cell-wall stress as a hexose-dependent and osmosensitive regulator of plant responses. Plant Journal. 2009;57:1015–1026. doi: 10.1111/j.1365-313X.2008.03744.x. [DOI] [PubMed] [Google Scholar]
- Haruta M, Sabat G, Stecker K, Minkoff BB, Sussman MR. A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science. 2014;343:408–411. doi: 10.1126/science.1244454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinisch JJ, Dupres V, Alsteens D, Dufrene YF. Measurement of the mechanical behavior of yeast membrane sensors using single-molecule atomic force microscopy. Nature Protocols. 2010;5:670–677. doi: 10.1038/nprot.2010.19. [DOI] [PubMed] [Google Scholar]
- Hématy K, Sado PE, Van Tuinen A, et al. A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Current Biology. 2007;17:922–931. doi: 10.1016/j.cub.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Hernandez-Blanco C, Feng DX, Hu J, et al. Impairment of cellulose synthases required for Arabidopsis secondary cell wall formation enhances disease resistance. Plant Cell. 2007;19:890–903. doi: 10.1105/tpc.106.048058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M, Pischke MS, Mähönen AP, et al. In planta functions of the Arabidopsis cytokinin receptor family. Proceedings of the National Academy of Sciences of the USA. 2004;101:8821–8826. doi: 10.1073/pnas.0402887101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G-Q, Li E, Ge F-R, et al. Arabidopsis RopGEF4 and RopGEF10 are important for FERONIA-mediated developmental but not environmental regulation of root hair growth. New Phytologist. 2013;200:1089–1101. doi: 10.1111/nph.12432. [DOI] [PubMed] [Google Scholar]
- Hückelhoven R, Panstruga R. Cell biology of the plant-powdery mildew interaction. Current Opinion in Plant Biology. 2011;14:738–746. doi: 10.1016/j.pbi.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Huffaker A, Ryan CA. Endogenous peptide defense signals in Arabidopsis differentially amplify signaling for the innate immune response. Proceedings of the National Academy of Sciences of the USA. 2007;104:10732–10736. doi: 10.1073/pnas.0703343104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffaker A, Pearce G, Ryan CA. An endogenous peptide signal in Arabidopsis activates components of the innate immune response. Proceedings of the National Academy of Sciences of the USA. 2006;103:10098–10103. doi: 10.1073/pnas.0603727103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi D, Tsuda K, Katagiri F. The peptide growth factor, phytosulfokine, attenuates pattern-triggered immunity. Plant Journal. 2012;71:194–204. doi: 10.1111/j.1365-313X.2012.04950.x. [DOI] [PubMed] [Google Scholar]
- Inoue T, Higuchi M, Hashimoto Y, et al. Identification of CRE1 as a cytokinin receptor from Arabidopsis. Nature. 2001;409:1060–1063. doi: 10.1038/35059117. [DOI] [PubMed] [Google Scholar]
- Jeandroz S, Lamotte O, Astier J, et al. There's more to the picture than meets the eye: nitric oxide cross talk with Ca2+ signaling. Plant Physiology. 2013;163:459–470. doi: 10.1104/pp.113.220624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada Y, Jung US, Piotrowski J, Levin DE. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes & Development. 1995;9:1559–1571. doi: 10.1101/gad.9.13.1559. [DOI] [PubMed] [Google Scholar]
- Kanaoka MM, Torii KU. FERONIA as an upstream receptor kinase for polar cell growth in plants. Proceedings of the National Academy of Sciences of the USA. 2010;107:17461–17462. doi: 10.1073/pnas.1013090107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler SA, Shimosato-Asano H, Keinath NF, et al. Conserved molecular components for pollen tube reception and fungal invasion. Science. 2010;330:968–971. doi: 10.1126/science.1195211. [DOI] [PubMed] [Google Scholar]
- Ketela T, Green R, Bussey H. Saccharomyces cerevisiae mid2p is a potential cell wall stress sensor and upstream activator of the PKC1-MPK1 cell integrity pathway. Journal of Bacteriology. 1999;181:3330–3340. doi: 10.1128/jb.181.11.3330-3340.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K-Y, Levin DE. Mpk1 MAPK association with the Paf1 complex blocks Sen1-mediated premature transcription termination. Cell. 2011;144:745–756. doi: 10.1016/j.cell.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohorn BD, Kohorn SL. The cell wall-associated kinases, WAKs, as pectin receptors. Frontiers in Plant Science. 2012;3 doi: 10.3389/fpls.2012.00088. 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohorn BD, Kobayashi M, Johansen S, et al. An Arabidopsis cell wall-associated kinase required for invertase activity and cell growth. Plant Journal. 2006;46:307–316. doi: 10.1111/j.1365-313X.2006.02695.x. [DOI] [PubMed] [Google Scholar]
- Kohorn BD, Johansen S, Shishido A, et al. Pectin activation of MAP kinase and gene expression is WAK2 dependent. Plant Journal. 2009;60:974–982. doi: 10.1111/j.1365-313X.2009.04016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohorn BD, Kohorn SL, Todorova T, Baptiste G, Stansky K, McCullough M. A dominant allele of Arabidopsis pectin-binding wall-associated kinase induces a stress response suppressed by MPK6 but not MPK3 mutations. Molecular Plant. 2012;5:841–851. doi: 10.1093/mp/ssr096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecka M, Gabriel M. The influence of congo red on the cell wall and (1–3)-beta-D-glucan microfibril biogenesis in Saccharomyces cerevisiae. Archives of Microbiology. 1992;158:115–126. doi: 10.1007/BF00245214. [DOI] [PubMed] [Google Scholar]
- Krol E, Mentzel T, Chinchilla D, et al. Perception of the Arabidopsis danger signal peptide 1 involves the pattern recognition receptor AtPEPR1 and its close homologue AtPEPR2. Journal of Biological Chemistry. 2010;285:13471–13479. doi: 10.1074/jbc.M109.097394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Matsumura Y, Soga K, Hoson T, Koizumi N. Glycosyl hydrolases of cell wall are induced by sugar starvation in Arabidopsis. Plant Cell Physiol. 2007;48:405–413. doi: 10.1093/pcp/pcm009. [DOI] [PubMed] [Google Scholar]
- Levin DE. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: the cell wall integrity signaling pathway. Genetics. 2011;189:1145–1175. doi: 10.1534/genetics.111.128264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DR, Olex AL, Lundy SR, Turkett WH, Fetrow JS, Muday GK. A kinetic analysis of the auxin transcriptome reveals cell wall remodeling proteins that modulate lateral root development in Arabidopsis. Plant Cell. 2013;25:3329–3346. doi: 10.1105/tpc.113.114868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wen J, Lease KA, Doke JT, Tax FE, Walker JC. BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell. 2002;110:213–222. doi: 10.1016/s0092-8674(02)00812-7. [DOI] [PubMed] [Google Scholar]
- Ma Y, Walker RK, Zhao Y, Berkowitz GA. Linking ligand perception by PEPR pattern recognition receptors to cytosolic Ca2+ elevation and downstream immune signaling in plants. Proceedings of the National Academy of Sciences of the USA. 2012;109:19852–19857. doi: 10.1073/pnas.1205448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Zhao Y, Walker RK, Berkowitz GA. Molecular steps in the immune signaling pathway evoked by plant elicitor peptides (Peps): CPKs, NO and ROS are downstream from the early Ca2+ signal. Plant Physiology. 2013;163:1459–1471. doi: 10.1104/pp.113.226068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Wurgler-Murphy SM, Saito H. A two-component system that regulates an osmosensing MAP kinase cascade in yeast. Nature. 1994;369:242–245. doi: 10.1038/369242a0. [DOI] [PubMed] [Google Scholar]
- Manfield IW, Orfila C, McCartney L, et al. Novel cell wall architecture of isoxaben-habituated Arabidopsis suspension-cultured cells: global transcript profiling and cellular analysis. Plant Journal. 2004;40:260–275. doi: 10.1111/j.1365-313X.2004.02208.x. [DOI] [PubMed] [Google Scholar]
- Matsubayashi Y, Ogawa M, Morita A, Sakagami Y. An LRR receptor kinase involved in perception of a peptide plant hormone, phytosulfokine. Science. 2002;296:1470–1472. doi: 10.1126/science.1069607. [DOI] [PubMed] [Google Scholar]
- Matsubayashi Y, Shinohara H, Ogawa M. Identification and functional characterization of phytosulfokine receptor using a ligand-based approach. Chemical Records. 2006;6:356–364. doi: 10.1002/tcr.20090. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Murata T, Sakurai-Ozato N, et al. ANXUR1 and 2, sister genes to FERONIA/SIRENE, are male factors for coordinated fertilization. Current Biology. 2009;19:1327–1331. doi: 10.1016/j.cub.2009.06.064. [DOI] [PubMed] [Google Scholar]
- Monaghan J, Zipfel C. Plant pattern recognition receptor complexes at the plasma membrane. Current Opinion in Plant Biology. 2012;15:349–357. doi: 10.1016/j.pbi.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Moscatiello R, Mariani P, Sanders D, Maathuis FJ. Transcriptional analysis of calcium-dependent and calcium-independent signalling pathways induced by oligogalacturonides. Journal of Experimental Botany. 2006;57:2847–2865. doi: 10.1093/jxb/erl043. [DOI] [PubMed] [Google Scholar]
- Mosher S, Seybold H, Rodriguez P, et al. The tyrosine-sulfated peptide receptors PSKR1 and PSY1R modify the immunity of Arabidopsis to biotrophic and necrotrophic pathogens in an antagonistic manner. Plant Journal. 2013;73:469–482. doi: 10.1111/tpj.12050. [DOI] [PubMed] [Google Scholar]
- Muday GK, Rahman A, Binder BM. Auxin and ethylene: collaborators or competitors? Trends in Plant Science. 2012;17:181–195. doi: 10.1016/j.tplants.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Katagiri T, Shinozaki K, et al. Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proceedings of the National Academy of Sciences of the USA. 2007;104:3639–3644. doi: 10.1073/pnas.0607703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam KH, Li J. BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell. 2002;110:203–212. doi: 10.1016/s0092-8674(02)00814-0. [DOI] [PubMed] [Google Scholar]
- Nishimura C, Ohashi Y, Sato S, Kato T, Tabata S, Ueguchi C. Histidine kinase homologs that act as cytokinin receptors possess overlapping functions in the regulation of shoot and root growth in Arabidopsis. Plant Cell. 2004;16:1365–1377. doi: 10.1105/tpc.021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nühse TS. Cell wall integrity signaling and innate immunity in plants. Frontiers in Plant Science. 2012;3:280. doi: 10.3389/fpls.2012.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paidhungat M, Garrett S. A homolog of mammalian, voltage-gated calcium channels mediates yeast pheromone-stimulated Ca2+ uptake and exacerbates the cdc1(Ts) growth defect. Molecular and Cellular Biology. 1997;17:6339–6347. doi: 10.1128/mcb.17.11.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park AR, Cho SK, Yun UJ, et al. Interaction of the Arabidopsis receptor protein kinase Wak1 with a glycine-rich protein, AtGRP-3. Journal of Biological Chemistry. 2001;276:26688–26693. doi: 10.1074/jbc.M101283200. [DOI] [PubMed] [Google Scholar]
- Peaucelle A, Braybrook S, Höfte H. Cell wall mechanics and growth control in plants: the role of pectins revisited. Frontiers in Plant Science. 2012;3:121. doi: 10.3389/fpls.2012.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasul S, Dubreuil-Maurizi C, Lamotte O, et al. Nitric oxide production mediates oligogalacturonide-triggered immunity and resistance to Botrytis cinerea in Arabidopsis thaliana. Plant, Cell & Environment. 2012;35:1483–1499. doi: 10.1111/j.1365-3040.2012.02505.x. [DOI] [PubMed] [Google Scholar]
- Roux M, Schwessinger B, Albrecht C, et al. The Arabidopsis leucine-rich repeat receptor-like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell. 2011;23:2440–2455. doi: 10.1105/tpc.111.084301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savatin DV, Ferrari S, Sicilia F, De Lorenzo G. Oligogalacturonide-auxin antagonism does not require posttranscriptional gene silencing or stabilization of auxin response repressors in Arabidopsis. Plant Physiology. 2011;157:1163–74. doi: 10.1104/pp.111.184663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallus T, Jaeckh C, Fehér K, et al. Malectin: a novel carbohydrate-binding protein of the endoplasmic reticulum and a candidate player in the early steps of protein N-glycosylation. Molecular and Cellular Biology. 2008;19:3404–3414. doi: 10.1091/mbc.E08-04-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheible WR, Eshed R, Richmond T, Delmer D, Somerville C. Modifications of cellulose synthase confer resistance to isoxaben and thiazolidinone herbicides in Arabidopsis Ixr1 mutants. Proceedings of the National Academy of Sciences of the USA. 2001;98:10079–10084. doi: 10.1073/pnas.191361598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheler C, Durner J, Astier J. Nitric oxide and reactive oxygen species in plant biotic interactions. Current Opinion in Plant Biology. 2013;16:534–539. doi: 10.1016/j.pbi.2013.06.020. [DOI] [PubMed] [Google Scholar]
- Schulze B, Mentzel T, Jehle AK, et al. Rapid heteromerization and phosphorylation of ligand-activated plant transmembrane receptors and their associated kinase BAK1. Journal of Biological Chemistry. 2010;285:9444–9451. doi: 10.1074/jbc.M109.096842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwessinger B, Roux M, Kadota Y, et al. Phosphorylation-dependent differential regulation of plant growth, cell death, and innate immunity by the regulatory receptor-like kinase BAK1. PLoS Genetics. 2011;7 doi: 10.1371/journal.pgen.1002046. e1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Kim Y, Guo Y, Stevenson B, Zhu JK. The Arabidopsis SOS5 locus encodes a putative cell surface adhesion protein and is required for normal cell expansion. Plant Cell. 2003;15:19–32. doi: 10.1105/tpc.007872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu S, Karlowski WM, Pan R, Tzeng Y, Mayer KFX, Li W. Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant Cell. 2004;16:1220–1234. doi: 10.1105/tpc.020834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava R, Liu J-X, Howell SH. Proteolytic processing of a precursor protein for a growth-promoting peptide by a subtilisin serine protease in Arabidopsis. Plant Journal. 2008;56:219–227. doi: 10.1111/j.1365-313X.2008.03598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhorst L, Kudla J. Calcium and reactive oxygen species rule the waves of signaling. Plant Physiology. 2013;163:471–485. doi: 10.1104/pp.113.222950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran LS, Urao T, Qin F, et al. Functional analysis of AHK1/ATHK1 and cytokinin receptor histidine kinases in response to abscisic acid, drought, and salt stress in Arabidopsis. Proceedings of the National Academy of Sciences of the USA. 2007;104:20623–20628. doi: 10.1073/pnas.0706547105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang DL, Edmond C, Harrington JL, Nuhse TS. Cell wall integrity controls root elongation via a general 1-aminocyclopropane-1-carboxylic acid-dependent, ethylene-independent pathway. Plant Physiology. 2011;156:596–604. doi: 10.1104/pp.111.175372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urao T, Yakubov B, Satoh R, et al. A transmembrane hybrid-type histidine kinase in Arabidopsis functions as an osmosensor. Plant Cell. 1999;11:1743–1754. doi: 10.1105/tpc.11.9.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueguchi C, Sato S, Kato T, Tabata S. The AHK4 gene involved in the cytokinin-signaling pathway as a direct receptor molecule in Arabidopsis thaliana. Plant Cell Physiology. 2001;42:751–755. doi: 10.1093/pcp/pce094. [DOI] [PubMed] [Google Scholar]
- Verica JA, He ZH. The cell wall-associated kinase (WAK) and WAK-like kinase gene family. Plant Physiology. 2002;129:455–459. doi: 10.1104/pp.011028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner TA, Kohorn BD. Wall-associated kinases are expressed throughout plant development and are required for cell expansion. Plant Cell. 2001;13:303–318. doi: 10.1105/tpc.13.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KE, Cyert MS. The eukaryotic response regulator Skn7p regulates calcineurin signaling through stabilization of Crz1p. The EMBO Journal. 2001;20:3473–3483. doi: 10.1093/emboj/20.13.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S, Hématy K, Höfte H. Growth control and cell wall signaling in plants. Annual Review of Plant Biology. 2012;63:381–407. doi: 10.1146/annurev-arplant-042811-105449. [DOI] [PubMed] [Google Scholar]
- Wormit A, Butt SM, Chairam I, et al. Osmosensitive changes of carbohydrate metabolism in response to cellulose biosynthesis inhibition. Plant Physiology. 2012;159:105–117. doi: 10.1104/pp.112.195198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulfetange K, Lomin SN, Romanov GA, Stolz A, Heyl A, Schmülling T. The cytokinin receptors of Arabidopsis are located mainly to the endoplasmic reticulum. Plant Physiology. 2011;156:1808–1818. doi: 10.1104/pp.111.180539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SL, Rahman A, Baskin TI, Kieber JJ. Two leucine-rich repeat receptor kinases mediate signaling, linking cell wall biosynthesis and ACC synthase in Arabidopsis. Plant Cell. 2008;20:3065–3079. doi: 10.1105/tpc.108.063354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Suzuki T, Terada K, et al. The Arabidopsis AHK4 histidine kinase is a cytokinin-binding receptor that transduces cytokinin signals across the membrane. Plant Cell Physiology. 2001;42:1017–1023. doi: 10.1093/pcp/pce127. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Huffaker A, Bryan AC, Tax FE, Ryan CA. PEPR2 is a second receptor for the Pep1 and Pep2 peptides and contributes to defense responses in Arabidopsis. Plant Cell. 2010;22:508–522. doi: 10.1105/tpc.109.068874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka T, Nakagawa Y, Mori K, et al. MCA1 and MCA2 that mediate Ca2+ uptake have distinct and overlapping roles in Arabidopsis. Plant Physiology. 2010;152:1284–1296. doi: 10.1104/pp.109.147371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Qian L, Nibau C, et al. FERONIA receptor kinase pathway suppresses abscisic acid signaling in Arabidopsis by activating ABI2 phosphatase. Proceedings of the National Academy of Sciences of the USA. 2012;109:14693–14938. doi: 10.1073/pnas.1212547109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Źd'árská M, Zatloukalová P, Benítez M, et al. Proteome analysis in Arabidopsis reveals shoot- and root-specific targets of cytokinin action and differential regulation of hormonal homeostasis. Plant Physiology. 2013;161:918–930. doi: 10.1104/pp.112.202853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger E, Hepler PK. Production of guard cell protoplasts from onion and tobacco. Plant Physiology. 1976;58:492–498. doi: 10.1104/pp.58.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Jung US, Garrett-Engele P, Roe T, Cyert MS, Levin DE. Temperature-induced expression of yeast FKS2 is under the dual control of protein kinase C and calcineurin. Molecular and Cellular Biology. 1998;18:1013–1022. doi: 10.1128/mcb.18.2.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]