Abstract
Background
Plant cell walls are complex matrices of carbohydrates and proteins that control cell morphology and provide protection and rigidity for the plant body. The construction and maintenance of this intricate system involves the delivery and recycling of its components through a precise balance of endomembrane trafficking, which is controlled by a plethora of cell signalling factors. Phosphoinositides (PIs) are one class of signalling molecules with diverse roles in vesicle trafficking and cytoskeleton structure across different kingdoms. Therefore, PIs may also play an important role in the assembly of plant cell walls.
Scope
The eukaryotic PI pathway is an intricate network of different lipids, which appear to be divided in different pools that can partake in vesicle trafficking or signalling. Most of our current understanding of how PIs function in cell metabolism comes from yeast and mammalian systems; however, in recent years significant progress has been made towards a better understanding of the plant PI system. This review examines the current state of knowledge of how PIs regulate vesicle trafficking and their potential influence on plant cell-wall architecture. It considers first how PIs are formed in plants and then examines their role in the control of vesicle trafficking. Interactions between PIs and the actin cytoskeleton and small GTPases are also discussed. Future challenges for research are suggested.
Keywords: Phosphoinositide, PI, plant cell wall, vesicle trafficking, endocytosis, exocytosis, cytoskeleton, actin, small GTPase
INTRODUCTION
Plant cell walls consist of a complex, largely carbohydrate-based, extracellular matrix that defines the shape of a cell and that acts as the first line of defence during environmental stress (McCann and Carpita, 2008). The cell walls also provide structural and mechanical rigidity to the plant body during growth and development (Cosgrove, 1997, 2005). Hence, plant cell walls are essential for plant growth.
Plant cell walls can broadly be classified into two major forms (primary and secondary) based on their composition (Somerville et al., 2004). The primary walls surround every plant cell and are initiated at the emerging cell plate during cell division (Matar and Catesson, 1988; Miart et al., 2014). The characteristics of the primary wall provide for directed cell growth, i.e. it must be organized to sustain anisotropic growth, which is an essential feature to generate various plant cell shapes (Ivakov and Persson, 2012). Once the cell has reached maturity, and depending on the tissue it is situated in, successive layers of secondary wall material may be deposited (Wightman and Turner, 2010). An often used example of cells that become surrounded by thick, water-resistant secondary walls that can withstand strong internal pressure changes are the water-transporting tracheary elements.
The composition of primary and secondary cell walls may vary substantially within and across different plant species. However, as a rule of thumb, primary cell walls typically contain cellulose, hemicelluloses and pectins (although pectins are regarded as only a minor component in the primary walls of many monocot species), while the secondary walls have less or almost no pectins, but contain large amounts of the polyphenolic polymer lignin (Neutelings, 2011). Most of the components of these structures, with the exception of lignins and cellulose, are synthesized in the Golgi, and are subsequently trafficked through various secretory pathways to the apoplast (Geisler et al., 2008; McFarlane et al., 2014). While these trafficking routes remain largely obscure, recent reports suggest that cellulose synthases (CesAs) might traffic through a syntaxin SYP61-related pathway (Drakakaki et al., 2012), while hemicellulose and pectins seem to be secreted via non-coated secretory vesicles regulated by an ECHIDNA (ECH)/YPT/RAB GTPase Interacting Protein (YIP) complex (Gendre et al., 2013). However, it remains unclear whether there also are overlaps/intersections between these pathways, given that the ECH/YIP complex also co-localizes with the trans-Golgi network (TGN) marker SYP61 (Gendre et al., 2013). Clearly, a fine-tuned balance of exo- and endocytosis is critical for proper cell-wall production (Johansen et al., 2006). Indeed, proteins involved in vesicle secretion have been shown to be required for correct xylem formation and for pollen tube initiation and growth (Cole et al., 2005; Li et al., 2013), and mutations in proteins involved in endocytosis lead to defects in cell plate formation (Kang et al., 2003). An often-overlooked regulatory component during these trafficking events is the composition of the lipid bilayers. Recent studies have suggested the involvement of phosphoinositides (PIs), a class of membrane lipids, as key regulatory molecules during secretion and assembly of cell-wall polymers, and even recycling processes in plants (Thole and Nielsen, 2008; Munnik and Nielsen, 2011). In addition, PIs may also affect the organization and dynamics of the actin cytoskeleton, which is crucial for the intracellular distribution of organelles, and for even deposition of cell-wall material in growing interphase cells, pollen tubes and root hairs (Ketelaar and Emons, 2001; Sampathkumar et al., 2013). In this review we mainly focus on how plant PIs affect trafficking-related aspects and how this may influence cell-wall deposition.
THE FORMATION OF PIs IN PLANTS
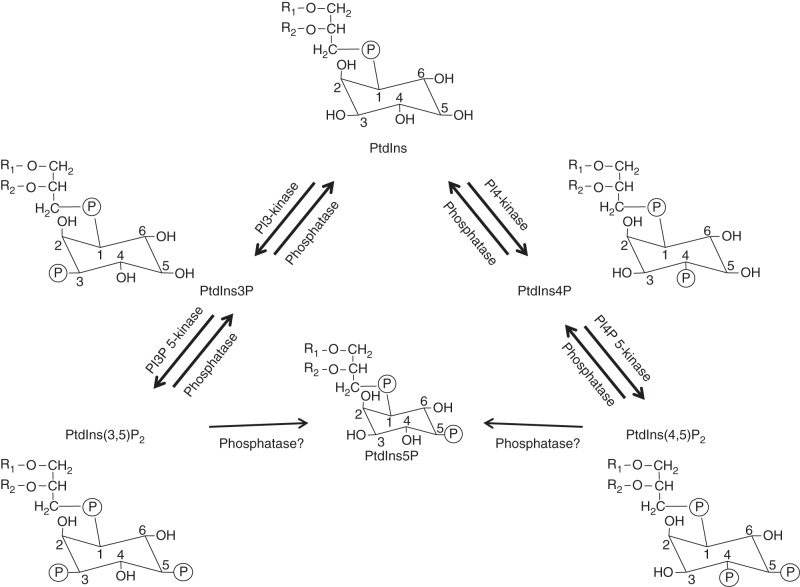
PIs constitute a group of membrane lipids that are derived from phosphatidylinositol (PtdIns) by phosphorylation of the inositol head group. PtdIns is a glycerophospholipid carrying a myo-inositol head group with five free hydroxyl groups. The hydroxyl positions 3, 4 and 5 may undergo phosphorylation in the presence of different lipid kinases, yielding PtdIns mono- and bis-phosphates (Munnik and Nielsen, 2011). The phosphorylated inositol rings can act either as precursors for soluble intracellular messengers or as binding sites for cytosolic or membrane-integral proteins that possess specific lipid recognition domains (Gillaspy, 2013). In plants, at least five forms of PIs have been identified. These include PtdIns3P, PtdIns4P, PtdIns5P, PtdIns(3,5)P2 and PtdIns(4,5)P2 (Mueller-Roeber and Pical, 2002; Fig. 1). The lipid kinases involved in the formation of PIs are largely named according to what substrate they convert and to what position of the inositol ring a phosphate group is transferred. Examples include PtdIns 3-kinase (PI3 K; phosphorylates the hydroxyl group on the 3rd hydroxyl position of the head group of PtdIns to form PtdIns3P) and PtdIns 4-kinase (PI4 K; phosphorylates the 4th hydroxyl position in the head group of PtdIns to form PtdIns4P) (Fig. 1). Certain phosphatases have also been identified in plants that may give rise to PIs such as PtdIns4P and PtdIns3P by dephosphorylation of PtdIns(4,5)P2 and PtdIns(3,5)P2, respectively (Williams et al., 2005; Vollmer et al., 2011; Novakova et al., 2014). In addition to the PIs indicated above, more recent studies have identified also lyso-PIs (derivatives of PIs in which acyl groups have been removed by hydrolysis). Due to their detergent-like properties these lysolipids are presumed to play a role in vesicle fusion and lipid re-modelling during membrane biogenesis (Boss and Im, 2012). The presence and abundance of various PIs differ across species in the animal and plant kingdoms. For example, PtdIns(3,4)P2 and PtdIns(3,4,5)P3 do not appear to be present in plants due to the absence of a Type-1 PI3 K that produces these PIs in animals (Cote and Crain, 1993). Moreover, the amount of PtdIns(4,5)P2 in plants is typically much lower than that of PtdIns4P, whereas these two PIs are found at high levels in animal membranes. Together, PtdIns4P and PtdIns(4,5)P2 contribute to less than 1 % of the total lipids found in plants.
Fig. 1.
The plant PI pathway. The plant PI pathway consists of different PIs, and the kinases and phosphatases involved in their formation. The kinases are named depending on the carbon head group they phosphorylate. For example, the kinase PI3 K converts the hydroxyl group on the 3rd position of the head group of PtdIns to form PtdIns3P, and PtdIns(4,5)P2 is formed by phosphorylation of PtdIns4P at the 5th head group in the presence of PI4P 5-kinases. PI3P 5-kinases phosphorylate the 5th head group of PtdIns3P to give rise to PtdIns(3,5)P2. PtdIns5P is present in plants but an enzyme capable of producing it has not yet been identified. Some phosphatases have also been identified that are capable of dephosphorylation of some bi-phosphates to give PtdIns3P and PtdIns4P.
While PIs represent only a minor fraction of membrane phospholipids, they have numerous important regulatory functions in signalling pathways and membrane trafficking in plants (Table 1; Thole and Nielsen, 2008). Here, the PtdIns bis-phosphate PtdIns(4,5)P2 has been a major focus of studies due to its diverse and essential functions in plant biology. PtdIns(4,5)P2 regulates various trafficking-related processes and cytoskeletal re-arrangements, and also acts as a substrate for the production of secondary messengers (Boss and Im, 2012). PtdIns(4,5)P2 is formed by phosphorylation of PtdIns4P at the 5th position of the inositol head group by a class of kinases called PI4P 5-kinases (Fig. 1). The Arabidopsis genome encodes 11 different isoforms of PI4P 5-kinases, which impact PI metabolism in different plant tissues, partially due to differential expression and sequence divergence among the isoforms (Mueller-Roeber and Pical, 2002; Heilmann, 2009). Some of these PI4P 5-kinases have been suggested to be involved in plant abiotic stress responses, such as PIP5K1, as mRNA levels of which are rapidly induced after abscisic acid (ABA) treatment (Mikami et al., 1998). Other important functions of PI4P 5-kinases include the regulation of auxin transport (PIP5K2; Mei et al., 2012; Ischebeck et al., 2013), stomatal opening (PIP5K4; Lee et al., 2007), root hair development (PIP5K3; Kusano et al., 2008; Stenzel et al., 2008) and pollen tube growth (Ischebeck et al., 2008, 2010, 2011; Sousa et al., 2008; Gillaspy, 2013). Many of these processes also require precise control over cell-wall synthesis, deposition and remodelling.
Table 1.
Role of some PIs in vesicle trafficking and in actin cytoskeleton organization across eukaryotic kingdoms*
| Yeast | Mammals | Plants |
|---|---|---|
| Secretion | ||
| PtdIns4P involved in secretion of cargo from Golgi and the post-Golgi trafficking pathways | PtdIns4P involved in vesicle trafficking by controlling secretion of cargo from the Golgi and the TGN pathways | PI4Kβ1/PtdIns4P is involved in formation of SVs from the TGN |
| SEC3A, a subunit of the exocyst, interacts with membrane-bound PtdIns(4,5)P2 | PtdIns(4,5)P2 tethers the exocyst complex to the PM via the EXO70 subunit | PtdIns(4,5)P2 overproduction causes increased pectin secretion in pollen tubes |
| Endocytosis | ||
| PI3 K helps in endocytosis and vesicle formation at the endosomes | PtdIns3P helps in entry of fungal pathogen effectors into human cells | PtdIns3P involved in vacuolar-related vesicle trafficking |
| PIP5 K interacts with ARFs and controls membrane trafficking | PIP5Ks interact with the AP2 complex at the PM and regulate clathrin-mediated endocytosis | PI4P 5-kinases produce PtdIns(4,5)P2 important for clathrin-mediated endocytosis |
| Actin | ||
| MSS4 (a PI4P 5-kinase) involved in regulating actin cytoskeleton | PtdIns(4,5)P2 initiates actin polymerization by binding to profilin | PtdIns(4,5)P2 binds to ZmADF in maize and inhibits actin depolymerization |
| PIP5 K interacts with ARFs to regulate actin cytoskeleton | fra3 (inositol polyphosphate 5-phosphatase) Arabidopsis mutants have disturbed actin dynamics and irregular secondary cell wall in stems | |
| PtdIns(4,5)P2 binding to GDI opens the GDI/RAC complex | PtdIns(4,5)P2 mediates F-actin formation which is counteracted by GDIs | |
* PtdIns, phosphatidylinositol; TGN, trans-Golgi network; SVs, secretory vesicles; PM, plasma membrane; PI3 K, PtdIns 3-kinase; PIP5 K, PtdIns-monophosphate 5-kinase; PI4 K, PtdIns 4-kinase; ARFs, ADP-ribosylation factors; AP2, adaptin complex 2; GDIs, guanidine nucleotide dissociation inhibitors.
PIs CONTROL VESICLE TRAFFICKING IN PLANTS
Background
Vesicle trafficking represents a fundamental process that controls the transport of different cargoes, including proteins, carbohydrates and lipids, between intracellular compartments and the extracellular matrix. This cargo interchange is essential for the cell to communicate with its environment and to maintain its homeostasis (Jürgens, 2004). In addition, the endomembrane system is also crucial for cell-wall synthesis and remodelling by transporting oligosaccharides, structural proteins, enzymes of cell-wall biogenesis and cell-wall-modifying enzymes.
Endomembrane trafficking is typically categorized into two main pathways: exo- and endocytosis. Exocytosis, or the secretory route, transports cargo starting from the endoplasmic reticulum (ER), to the Golgi, TGN and subsequently to the plasma membrane (PM). The endocytotic pathway, by contrast, recruits cargoes from the PM and typically transports them to the TGN from where they are either recycled back to the PM or sent to the vacuole for degradation. In addition to the exo- and endocytosis pathways there are alternative routes, named retrograde pathways, such as those involved in Golgi to ER trafficking (Jürgens, 2004).
The best characterized trafficking pathways are mediated by vesicles and consist of several sequential steps: cargo selection and vesicle formation, vesicle trafficking through the cytoskeleton, and targeting and tethering of the vesicle to the destination membrane, followed by fusion of the vesicle and the sink membranes and cargo release (Bassham et al., 2008; Fujimoto and Ueda, 2012). With the exception of exocytosis via secretory vesicles from the TGN (Toyooka et al., 2009), the first step of plant vesicle trafficking, i.e. cargo selection and vesicle formation, relies on coat protein complexes (CPCs). Thus, COPI and COPII (coat protein complex I and II) are required for vesicle formation in ER-to-Golgi and Golgi-to-ER trafficking, respectively (Bassham et al., 2008); and clathrin-coated vesicles (CCVs) are required for sorting cargo from the TGN and from the PM (Song et al., 2006; Baisa et al., 2013; Sauer et al., 2013). PIs play a key role in mediating these membrane trafficking steps in eukaryotic cells (Wenk and De Camilli, 2004). In clathrin-mediated endocytosis in animal cells, the coat-protein docking sites at the donor membrane are defined by certain PIs for which synthesis is stimulated by small GTPases (Puertollano, 2004). The formation of certain PIs leads to the recruitment of specific coat complex adaptors and accessory proteins, which recognize the cargo and nucleate the vesicle (Robinson and Bonifacino, 2001; Jung and Haucke, 2007; Bassham et al., 2008). Once the coat proteins, or ‘cage’ subunits, stabilize the vesicle, it is detached from the membrane by the action of dynamin-related proteins (DRPs; Hong et al., 2003) that are also specific for each trafficking pathway. The vesicle identifies its target compartment and fuses to it with the help of three groups of proteins, which regulate vesicle targeting (GTPases and SM proteins), tethering (exocyst, GARP and TRAPP complexes) and fusion (SNARE proteins).
Table 1 summarizes the involvement of PIs in eukaryotic vesicle trafficking. For example, PtdIns(4,5)P2 has been suggested to mediate exocytosis in animal cells by promoting the activity of both the exocyst complex and SNARE proteins (Liu et al., 2007; Honigmann et al., 2013). It appears that a particular PI formed at the membrane of each compartment is necessary for vesicle trafficking. Accordingly, different forms of PIs are specifically present at certain endomembrane compartments and at the PM. A similar distribution can be observed in plant cells (Fig. 2); PtdIns(4,5)P2 locates at the PM, where it aids both exo- and endocytosis (Kost et al., 1999; Ischebeck et al., 2008, 2011, 2013; Sousa et al., 2008), whereas PtdIns3P localizes at all organelles implicated in vacuolar cargo sorting from the TGN (TGN, prevacuolar compartment and tonoplast; Kim et al., 2001; Vermeer et al., 2006; Novakova et al., 2014), in agreement with its putative role on this trafficking pathway. PtdIns4P, by contrast, is quite ubiquitous, being present at the TGN and PM, assisting in trafficking between these compartments (Preuss et al., 2006). Below, we comment in more detail on the role of various PIs in the two main trafficking pathways and their putative influence on plant cell-wall architecture.
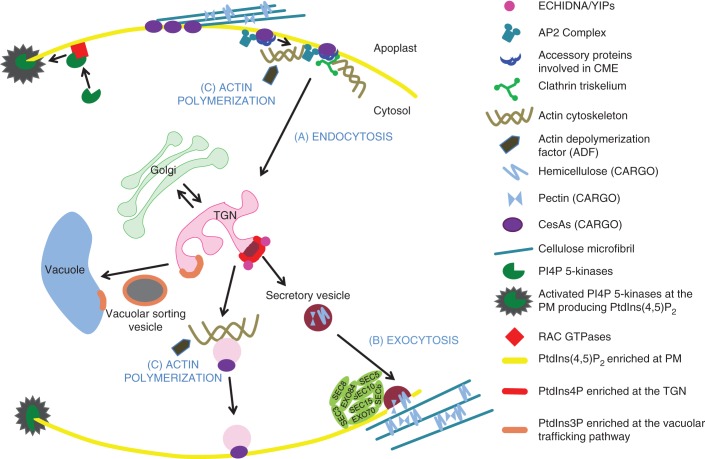
Fig. 2.
Potential functions of PIs in endomembrane trafficking and in actin organization in plant cells. (A) Endocytosis: RAC GTPases may recruit PI4P 5-kinases to the PM where they start producing PtdIns(4,5)P2 (yellow line). At the initial stages of clathrin-mediated endocytosis (CME), AP2 complex and other accessory proteins may hypothetically bind to PtdIns(4,5)P2 at the PM, as this has been shown in animal cells. This could initiate cargo recognition and vesicle nucleation with the help of clathrin triskelia. One of the cargoes presumably internalized via CME is cellulose synthase (CesA). (B) Secretion: PtdIns4P at the TGN can aid in the formation of secretory vesicles (SVs) and, along with ECHIDNIA/YIP-related vesicles, may secrete pectins/hemicelluloses to the apoplast. On the other hand, PtdIns3P appears to be involved in trafficking between the TGN and vacuole. It is plausible that one of the tethering factors, the exocyst complex, can bind to the PtdIns(4,5)P2 at the PM, as shown in other eukaryotic systems. In these studies, the binding may occur via the SEC3A and EXO70 subunits, which then facilitate secretion of different cargoes to the cell surface. (C) Actin cytoskeleton: PtdIns(4,5)P2 can bind to actin depolymerization factor (ADF) and prevent actin depolymerization, which may in turn have an effect on exocytosis of CesAs to the plasma membrane.
Secretion
PtdIns(4,5)P2 and PI4P 5-kinases appear to have crucial roles in exocytosis in animal and yeast systems (Liu et al., 2007; Funakoshi et al., 2011). In animals, PtdIns(4,5)P2 is necessary during the priming step of exocytosis of dense core vesicles (DCVs, secretory organelles in neuroendocrine cells), as the rates of DCV exocytosis could be increased or decreased by up- or down-regulating the expression of PIP5K1γ (an animal PI4P 5-kinase isoform) in these cells (Aikawa and Martin, 2003). PtdIns(4,5)P2 is, furthermore, important for the function of the exocyst complex, which tethers secretory vesicles to the PM (Liu et al., 2007). The exocyst typically consists of eight subunits: SEC3, SEC5, SEC6, SEC8, SEC10, SEC15, EXO70 and EXO84 (Munson and Novick, 2006). Some of these components have been shown to interact with PtdIns(4,5)P2 at the PM. For example, the mammalian EXO70 subunit interacts with PtdIns(4,5)P2 at the PM via its C-terminal end. Consequently, disruption of this interaction abolished the PM localization of EXO70 and subsequently resulted in defects in trafficking of exocytic cargo, such as vesicular stomatitis virus glycoproteins (VSV-Gs), to the PM (Liu et al., 2007). Overproduction of PtdIns(4,5)P2 in mammalian cells resulted in an increased pool of tethered secretory vesicles that are available for membrane fusion (Milosevic et al., 2005). In yeast, SEC3 was shown to bind to PtdIns(4,5)P2 with the help of its Pleckstrin-homology (PH) domain [a PI interacting domain], allowing the anchoring of the exocyst to the PM (Zhang et al., 2008). The exocyst complex has been shown to be essential for plant cell biology (Zárský et al., 2013), as mutations in its subunits lead to severe phenotypes in Arabidopsis, such as short hypocotyls, pollen tube growth defects and impaired pollen germination (Cole et al., 2005; Synek et al., 2006; Hála et al., 2008). The overproduction of PtdIns(4,5)P2 in tobacco pollen tubes also resulted in morphological alterations of the tube cells that were associated with massively increased secretion of the main apical cell-wall component pectin (Ischebeck et al., 2008). In another study, the degree of pectin secretion has been used as an indicator for synergies between specific coexpressed isoforms of enzymes of the PI pathway, for instance suggesting that Arabidopsis PtdIns-synthase 2 (PIS2) provides PtdIns for conversion to PtdIns4P by PI4Kβ1 and further to PtdIns(4,5)P2 by PIP5K5 (Ischebeck et al., 2010). This sequence of functionally linked enzymes is of particular interest, because there is little spatial overlap between their subcellular points of residence (reviewed by Heilmann and Heilmann, 2013). Despite these indications for a role of PtdIns(4,5)P2 and other PIs in plant secretion, a PI-dependent mechanism governing exocyst recruitment to the PM has not been established for plants. Based on the tight link between the exocyst function and PtdIns(4,5)P2 in other kingdoms, however, a similar relation may be anticipated to occur in plants.
As noted above, another PI, PtdIns3P, has been found to play an important role in vesicle trafficking in the endomembrane system. This was first discovered in yeast, where VPS34, a protein involved in vesicle-mediated vacuolar protein sorting (VPS) from the TGN, was found to have PI 3-kinase activity and to play crucial roles in endocytosis (Schu et al., 1993). Interestingly, when a human endosome-binding domain (EBD; a domain from mammalian early endosome antigen-1, which specifically binds PtdIns3P with high affinity) was transiently introduced into Arabidopsis protoplasts it displayed binding to TGN, tonoplasts and endosomal membranes, indicating that PtdIns3P might be involved in similar sorting processes in plants as in yeast (Kim et al., 2001). The PtdIns3P-dependent binding of EBD makes it an interesting tool for probing intracellular PtdIns3P; however, recently a more specific PI3P biosensor (YFP-2 × FYVE) has been reported by Vermeer et al. (2006). The role of PtdIns3P in vacuolar-related vesicle trafficking in plants was further confirmed by transforming a reporter construct, sporamin-GFP, which encodes a reporter normally targeted to the large central vacuole, into protoplasts expressing EBD. EBD overexpression in Arabidopsis protoplasts resulted in a 50 % reduction in vacuolar trafficking of the sporamin-GFP, indicating a role for PtdIns3P in vescicular trafficking in plants (Kim et al., 2001; Fig. 2).
Recently, the protein ECH (Gendre et al., 2011) in cooperation with YIP proteins has been reported to be essential for the formation of secretory vesicles (SVs) at the plant TGN (Boutté et al., 2013; Gendre et al., 2013). Various cell-wall-related cargoes have been confirmed to be secreted via these SVs, including hemicellulose and pectins (Boutté et al., 2013; Gendre et al., 2013). Interestingly, the ECH/YIP complex co-localized with the TGN markers SYP41, SYP61 and VHA-a1, suggesting that they reside on a common subdomain of the TGN specific for exocytosis, given that the ECH/YIP complex did not partake in endocytosis or vacuolar targeting (Gendre et al., 2013). The formation of SVs was previously shown to be dependent on PI4Kβ1, as a mutation in the corresponding gene resulted in irregularities in the formation of SVs, affecting pectin and xyloglucan delivery to the cell wall in Arabidopsis root cells (Kang et al., 2011). Thus, it is possible that the membrane lipid environment contributes to the secretion of vesicles controlled by ECH/YIPs (Fig. 2), but direct evidence for this is lacking.
Endocytosis
The endocytotic pathways are crucial for cell growth maintenance and regulation of the PM proteome, including proteins involved in cell-wall synthesis and modification. The main endocytotic path in plants is that mediated by clathrin coats, so-called clathrin-mediated endocytosis (CME; Baisa et al., 2013). The adaptor complex that, together with various early adaptors, helps to recognize the cargo and nucleate vesicles during CME is AP2 (adaptin complex 2; Bashline et al., 2013; Di Rubbo et al., 2013; Kim et al., 2013; Yamaoka et al., 2013; Gadeyne et al., 2014). The AP2 complex has been conserved in evolution and consists of two large subunits (α, β2), a medium (μ2) and a small (σ2) subunit (Chen et al., 2011). In mouse cells, the N-terminal end of the α subunit binds to PtdIns(4,5)P2 at the PM, which can act as an anchor tethering the entire AP2 complex to the membrane (Gaidarov et al., 1996). Interestingly, this binding seems to generate a positive feedback, as the PtdIns(4,5)P2-synthesizing lipid kinase, PIP5Kγ, is recruited to the AP2 complex and undergoes dephosphorylation in the presence of calcineurin (van den Bout and Divecha, 2009). Dephosphorylated PIP5Kγ becomes activated and produces more PtdIns(4,5)P2 to further increase binding and clustering of AP2 complexes, which then recruit other accessory proteins and the clathrin coat (van den Bout and Divecha, 2009). After fission of the endocytic vesicle, PIP5Kγ is inactivated, leading to a reduction of levels of PtdIns(4,5)P2 and the dissociation of the AP2 complex and clathrin coat. In mammals, PtdIns(4,5)P2 also helps to recruit a number of accessory adaptor proteins to the PM that aid in the formation of CCVs (Jung and Haucke, 2007). Some of these accessory proteins have also been shown to have specific PtdIns(4,5)P2 binding domains, such as ENTH (found in mammalian AP180 proteins), SH3 (found in, for example, plant clathrins and other SH3-related proteins) and PH domains (found in Arabidopsis Dynamin Like 3 – DRP2B, Mikami et al., 2000). The putative binding of accessory proteins to the corresponding PI seems to occur also in other clathrin-mediated trafficking pathways, as the yeast vesicular transport system holds proteins with GLUE domains (found, for example, in Vps36 which helps to bind to PtdIns3P) (Ford et al., 2002; Teo et al., 2006).
In plants, some reports also support an important function for PtdIns(4,5)P2 in CME. For example, König et al. (2008) showed that PtdIns(4,5)P2 accumulates in CCVs formed during salt stress in Arabidopsis leaves, supporting a role for PtdIns(4,5)P2 in the formation or maturation of CCVs in plants (König et al., 2008). In tobacco pollen tubes, PtdIns(4,5)P2 has been implicated in the control of CME (Zhao et al., 2010). Moreover, an alteration in PI4P 5-kinase activity was recently shown to alter the density and size of clathrin foci at the PM in elongating root cells and to influence the endocytic recycling of PIN proteins, confirming a defect in CME (Ischebeck et al., 2013). Although direct evidence is necessary, it is possible that, as mentioned above for animal cells, PI4P 5-kinases may control CME by producing PtdIns(4,5)P2 on the cytosolic leaflet of the PM, providing anchor points for the AP2 complex at the membrane and aiding recruitment of CME accessory proteins (Gaidarov et al., 1996; Jung and Haucke, 2007; van den Bout and Divecha, 2009). Importantly, defects in CME may affect cell-wall synthesis and recycling, for example during cell plate formation where redistribution of cell-wall material from the PM of the mother cell to the growing cell plate is of great importance. Indeed, primary CesAs have recently been shown to be trafficked via CME during cell plate formation in Arabidopsis roots (Miart et al., 2014). Furthermore, CesAs producing cellulose at the PM were recently shown to be recognized by the AP2 complex (Bashline et al., 2013). Here, the μ subunit of the AP2 complex appears to recognize the CesAs at the PM and to be involved in their internalization (Fig. 2). Complete block of cellulose synthesis causes a lethal phenotype (Persson et al., 2007). Such phenotypes would also be anticipated when the internalization of the CesAs is impaired. In contrast, ap2-μ2 mutant plants were viable and surprisingly produced etiolated hypocotyls that were even longer than those of wild-type plants (Bashline et al., 2013). So while CME of CesAs is quite plausible, more clear evidence is necessary to resolve the reported phenotypic inconsistencies.
ACTIN CYTOSKELETON, SMALL GTPases AND PIs
The deposition of plant cell-wall polymers is largely dependent on the delivery and recycling of various components; this process is sustained by the actin cytoskeleton. Thus, any disturbance in the actin organization can lead to aberrant cell walls. As discussed above, cellulose is a major component of the plant cell wall, and is synthesized by CesA complexes at the PM. It has been shown that actin organization in interphase cells can affect CesA delivery patterns to, and lifetimes at, the PM (Sampathkumar et al., 2013). In addition, various studies suggest a direct role of the actin cytoskeleton in plant CME (Baluška et al., 2004; Konopka et al., 2006, 2008; Fig. 2). As PtdIns(4,5)P2 is involved in actin cytoskeleton organization and in CME, it could be argued that PtdIns(4,5)P2 and other PIs also regulate CesA dynamics at the PM. While most of the actin-related CesA data come from observations of primary wall CesAs, the actin cytoskeleton also appears to affect trafficking of secondary wall CesAs. A mutation in the FRA3 gene (Fragile Fiber 3), which encodes a type II inositol polyphosphate 5-phosphatase that hydrolyses the 5-phosphate group from PtdIns(4,5)P2, affects actin organization and causes reduced thickening of the secondary cell walls in fibre cells and xylem vessels of Arabidopsis stems (Zhong et al., 2004). A possible explanation for this observation may be that PIs are needed to maintain a uniform actin cytoskeleton, or uphold a certain actin configuration. Recently, Wang et al. (2013) showed that by inhibiting the apical F-actin assembly and PI3 K by Wortmannin in tobacco pollen tube tips, the trafficking of a tobacco-specific pectin methylesterase (NtPPME1) was affected during pollen tube cell-wall assembly and hence the pectin composition and rigidity of pollen cell walls was altered. These examples give us some initial clues that the actin cytoskeleton along with PIs may play a coordinated role during plant cell-wall assembly.
The plant actin cytoskeleton plays a major role in plant growth and development, largely because of its central role in actinomyosin-based cytoplasmic streaming (Franklin-Tong, 1999). The regulation of formation and dissociation of actin filaments is controlled by various cytosolic factors, including PIs. Among them, PtdIns(4,5)P2 has, again, been suggested to play an important role, especially in the organization of the actin cytoskeleton during polar tip growth in plants. Actin depolymerization factors (ADFs) sever actin filaments (Pei et al., 2012) and in maize pollen tubes the function of ZmADF3 has been shown to be inhibited in vitro by PtdIns(4,5)P2 (Gungabissoon et al., 1998; Fig. 2). Similarly, PtdIns(4,5)P2 has also been shown to promote actin polymerization in animals by binding to profilin (an actin binding protein) and detaching it from the profilin–actin complex to release G-actin (Pei et al., 2012). G-actin is then available for actin polymerization at a later stage. In yeast, the mss4 mutant (which lacks the sole PI4P 5-kinase) exhibited defects in exocytosis and actin organization, suggesting a connection between PtdIns(4,5)P2 and cytoskeleton structure (Desrivières et al., 1998).
Another important link between the PI pathway and the actin cytoskeleton arises from the effects of PtdIns(4,5)P2 on ROP/RAC proteins (Kost, 2008). ROP/RAC proteins are a subfamily of small GTPases (Vernoud et al., 2003) that potentially interact with cell surface-associated signal receptors that control diverse cellular pathways to mediate growth, differentiation, development and defence responses (Nibau et al., 2006). Using extracts from tobacco pollen tubes, AtRAC2 was shown to co-precipitate with a PI4P 5-kinase activity, and to co-localize with PtdIns(4,5)P2 in pollen tube tips (Kost et al., 1999). Interestingly, a mutation in AtRAC2 showed pollen growth phenotypes rationalized by changes in the actin cytoskeleton (Kost et al., 1999). It has been proposed that PtdIns(4,5)P2 influences the plant actin cytoskeleton by controlling the membrane-associated pool of inactive Rac-GDP, which is maintained by Rac-GDP binding to guanidine nucleotide dissociation inhibitors (GDIs) holding them in the cytosol (Klahre et al., 2006; Kost, 2008). In tobacco pollen tubes (based on structural studies on mammalian GDIs; Fauré et al., 1999), it was proposed that binding of PtdIns(4,5)P2 to the GDI would cause the GDI/Rac-GDP complex to open and release Rac-GDP at the PM where it can be activated to Rac-GTP by membrane-associated guanidine nucleotide exchange factors (GEFs). Rac-GTP could then mediate the controlled polymerization of F-actin (Kost, 2008), which is required, for example, for directional movement of internalized CCVs to the recycling endosome. In support of this hypothesis, apical tip swelling of tobacco pollen tubes arising from overexpression of PI4P 5-kinases and overproduction of PtdIns(4,5)P2 was counterbalanced by co-overexpression of a tobacco GDI (Ischebeck et al., 2011). Previous data also indicate that the PI4P 5-kinases may be recruited to the PM by ROP/RAC to determine the sites of PtdIns(4,5)P2 production (Kost et al., 1999). A similar relationship among PI4P 5-kinases and small GTPases has been identified in yeast and animal systems (van den Bout and Divecha, 2009). In addition, the small GTPase subfamily of ADP-ribosylation factors (ARFs) has been shown to control actin dynamics and vesicle trafficking in animal cells (Schafer et al., 2000). Interestingly, overexpression of ARF6 caused an increase in PtdIns(4,5)P2 levels at the PM. This overexpression resulted in the formation of large internal vesicles rich in PtdIns(4,5)P2, even though this lipid is rarely found in internal vesicles. The abnormal PtdIns(4,5)P2 accumulation in cytosolic compartments might be a consequence of a defect in PtdIns(4,5)P2 recycling back to the PM after endocytic vesicle formation (Aikawa and Martin, 2003). Thus, RAC/ROPS and ARFs control PtdIns(4,5)P2 levels, which can in turn affect actin dynamics, and various trafficking processes, which indirectly control various factors that affect the deposition of plant cell walls.
FUTURE CHALLENGES
The eukaryotic PI pathway is an intricate network of different lipids, which appear to be divided in different pools that can partake in vesicle trafficking or signalling. Most of our current understanding of how PIs function in cell metabolism comes from yeast and mammalian systems. However, in recent years significant progress has been made towards a better understanding of the plant PI system; that said, many important questions remain unanswered. In context of this review, an obvious point is the role of PIs in plant cell-wall synthesis and remodelling. It is clear that putting the enzymes and products related to PIs in the context of the exo- and endocytic machineries will be of importance. More specifically, narrowing the specific activity and location of the enzymes of the PI pathway, their influence on the levels of different pools of PIs in different cellular compartments and the effects on cargo trafficking will become important tasks. Many of these questions/tasks are currently being tackled by taking advantage of new tools and techniques, for example the recently identified fluorescent imaging probes to track various PIs, proteomic techniques to identify potential PI enzyme interactors and confocal microscopy for live-cell imaging of cargo trafficking in cells with altered PIs. We therefore expect many interesting new studies in the coming years, which hopefully will shed light on the molecular targets of PIs in vesicle trafficking and their influence on plant cell-wall design, which will ultimately dictate plant shape and possibly the accumulation of biomass.
ACKNOWLEDGEMENTS
The research leading to this article received funding from the European Union Seventh Framework Programme (FP7 2007–2013) under the WallTraC project (Grant Agreement 263916). This article reflects the author's views only. The European Community is not liable for any use that may be made of the information contained herein.
LITERATURE CITED
- Aikawa Y, Martin TFJ. ARF6 regulates a plasma membrane pool of phosphatidylinositol (4,5) bisphosphate required for regulated exocytosis. Journal of Cell Biology. 2003;162:647–659. doi: 10.1083/jcb.200212142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baisa GA, Mayers JR, Bednarek SY. Budding and braking news about clathrin-mediated endocytosis. Current Opinion in Plant Biology. 2013;16:718–725. doi: 10.1016/j.pbi.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Baluška F, Šamaj J, Hlavacka A, Kendrick-Jones J, Volkmann D. Actin-dependent fluid-phase endocytosis in inner cortex cells of maize root apices. Journal of Experimental Botany. 2004;55:463–473. doi: 10.1093/jxb/erh042. [DOI] [PubMed] [Google Scholar]
- Bashline L, Li S, Anderson CT, Lei L, Gu Y. The endocytosis of cellulose synthase in arabidopsis is dependent on μ2, a clathrin-mediated endocytosis adaptin. Plant Physiology. 2013;163:150–160. doi: 10.1104/pp.113.221234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassham DC, Brandizzi F, Otegui MS, Sanderfoot AA. The secretory system of Arabidopsis. Arabidopsis Book. 2008;6:e0116. doi: 10.1199/tab.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss WF, Im YJ. Phosphoinositide signaling. Annual Review of Plant Biology. 2012;63:409–429. doi: 10.1146/annurev-arplant-042110-103840. [DOI] [PubMed] [Google Scholar]
- Boutté Y, Jonsson K, McFarlane HE, et al. ECHIDNA-mediated post-Golgi trafficking of auxin carriers for differential cell elongation. Proceedings of the National Academy of Sciences of the USA. 2013;110:16259–16264. doi: 10.1073/pnas.1309057110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Irani NG, Friml J. Clathrin-mediated endocytosis: the gateway into plant cells. Current Opinion in Plant Biology. 2011;14:674–682. doi: 10.1016/j.pbi.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Cole RA, Synek L, Zarsky V, Fowler JE. SEC8, a subunit of the putative Arabidopsis exocyst complex, facilitates pollen germination and competitive pollen tube growth. Plant Physiology. 2005;138:2005–2018. doi: 10.1104/pp.105.062273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ. Creeping walls, softening fruit, and penetrating pollen tubes: the growing roles of expansions. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:5504–5555. doi: 10.1073/pnas.94.11.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ. Growth of the plant cell wall. Nature Reviews. Molecular Cell Biology. 2005;6:850–861. doi: 10.1038/nrm1746. [DOI] [PubMed] [Google Scholar]
- Cote GG, Crain RC. Biochemistry of phosphoinositides. Annual Review of Plant Physiology and Plant Molecular Biology. 1993;44:333–356. [Google Scholar]
- Desrivières S, Cooke FT, Parker PJ, Hall MN. MSS4, a phosphatidylinositol-4-phosphate 5-kinase required for organization of the actin cytoskeleton in Saccharomyces cerevisiae. Journal of Biological Chemistry. 1998;273:15787–15793. doi: 10.1074/jbc.273.25.15787. [DOI] [PubMed] [Google Scholar]
- Di Rubbo S, Irani NG, Kim SY, et al. The clathrin adaptor complex AP-2 mediates endocytosis of brassinosteroid INSENSITIVE1 in Arabidopsis. Plant Cell. 2013;25:2986–2997. doi: 10.1105/tpc.113.114058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakakaki G, van de Ven W, Pan S, et al. Isolation and proteomic analysis of the SYP61 compartment reveal its role in exocytic trafficking in Arabidopsis. Cell Research. 2012;22:413–424. doi: 10.1038/cr.2011.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauré J, Vignais PV, Dagher M-C. Phosphoinositide-dependent activation of Rho A involves partial opening of the RhoA/Rho-GDI complex. European Journal of Biochemistry. 1999;262:879–889. doi: 10.1046/j.1432-1327.1999.00458.x. [DOI] [PubMed] [Google Scholar]
- Ford MGJ, Mills IG, Peter BJ, et al. Curvature of clathrin-coated pits driven by epsin. Nature. 2002;419:361–366. doi: 10.1038/nature01020. [DOI] [PubMed] [Google Scholar]
- Franklin-Tong VE. Signaling and the modulation of pollen tube growth. Plant Cell. 1999;11:727–738. doi: 10.1105/tpc.11.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M, Ueda T. Conserved and plant-unique mechanisms regulating plant post-Golgi traffic. Frontiers in Plant Science. 2012;3:197. doi: 10.3389/fpls.2012.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi Y, Hasegawa H, Kanaho Y. Regulation of PIP5 K activity by Arf6 and its physiological significance. Journal of Cellular Physiology. 2011;226:888–895. doi: 10.1002/jcp.22482. [DOI] [PubMed] [Google Scholar]
- Gadeyne A, Sánchez-Rodríguez C, Vanneste S, et al. The TPLATE adaptor complex drives clathrin-mediated endocytosis in plants. Cell. 2014;156:691–704. doi: 10.1016/j.cell.2014.01.039. [DOI] [PubMed] [Google Scholar]
- Gaidarov I, Chen Q, Falck JR, Reddy KK, Keen JH. A functional phosphatidylinositol 3,4,5-trisphosphate/phosphoinositide binding domain in the clathrin adaptor AP-2 alpha subunit. Implications for the endocytic pathway. Journal of Biological Chemistry. 1996;271:20922–20929. doi: 10.1074/jbc.271.34.20922. [DOI] [PubMed] [Google Scholar]
- Geisler DA, Sampathkumar A, Mutwil M, Persson S. Laying down the bricks: logistic aspects of cell wall biosynthesis. Current Opinion in Plant Biology. 2008;11:647–652. doi: 10.1016/j.pbi.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Gendre D, Oh J, Boutté Y, et al. Conserved Arabidopsis ECHIDNA protein mediates trans-Golgi-network trafficking and cell elongation. Proceedings of the National Academy of Sciences of the USA. 2011;108:8048–8053. doi: 10.1073/pnas.1018371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendre D, McFarlane HE, Johnson E, et al. Trans-golgi network localized ECHIDNA/Ypt interacting protein complex is required for the secretion of cell wall polysaccharides in Arabidopsis. Plant Cell. 2013;25:2633–2646. doi: 10.1105/tpc.113.112482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillaspy GE. The role of phosphoinositides and inositol phosphates in plant cell signaling. Advances in Experimental Medicine and Biology. 2013;991:141–157. doi: 10.1007/978-94-007-6331-9_8. [DOI] [PubMed] [Google Scholar]
- Gungabissoon RA, Jiang C, Drøbak BK, Maciver SK, Hussey PJ. Interaction of maize actin-depolymerising factor with actin and phosphoinositides and its inhibition of plant phospholipase C. Plant Journal. 1998;16:689–696. [Google Scholar]
- Hála M, Cole R, Synek L, et al. An exocyst complex functions in plant cell growth in Arabidopsis and tobacco. Plant Cell. 2008;20:1330–1345. doi: 10.1105/tpc.108.059105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilmann I. Using genetic tools to understand plant phosphoinositide signalling. Trends in Plant Science. 2009;14:171–179. doi: 10.1016/j.tplants.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Heilmann M, Heilmann I. Arranged marriage in lipid signalling? The limited choices of PtdIns(4,5)P2 in finding the right partner. Plant Biology. 2013;15:789–797. doi: 10.1111/plb.12025. [DOI] [PubMed] [Google Scholar]
- Hong Z, Bednarek SY, Blumwald E, et al. A unified nomenclature for Arabidopsis dynamin-related large GTPases based on homology and possible functions. Plant Molecular Biology. 2003;53:261–265. doi: 10.1023/b:plan.0000007000.29697.81. [DOI] [PubMed] [Google Scholar]
- Honigmann A, van den Bogaart G, Iraheta E, et al. Phosphatidylinositol 4,5-bisphosphate clusters act as molecular beacons for vesicle recruitment. Nature Structural and Molecular Biology. 2013;20:679–686. doi: 10.1038/nsmb.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ischebeck T, Stenzel I, Heilmann I. Type B phosphatidylinositol-4-phosphate 5-kinases mediate pollen tube growth in Nicotiana tabacum and Arabidopsis by regulating apical pectin secretion. Plant Cell. 2008;20:3312–3330. doi: 10.1105/tpc.108.059568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ischebeck T, Vu LH, Jin X, Stenzel I, Löfke C, Heilmann I. Functional cooperativity of enzymes of phosphoinositide conversion according to synergistic effects on pectin secretion in tobacco pollen tubes. Molecular Plant. 2010;3:870–881. doi: 10.1093/mp/ssq031. [DOI] [PubMed] [Google Scholar]
- Ischebeck T, Stenzel I, Hempel F, Jin X, Mosblech A, Heilmann I. Phosphatidylinositol-4,5-bisphosphate influences Nt-Rac5-mediated cell expansion in pollen tubes of Nicotiana tabacum. Plant Journal. 2011;65:453–468. doi: 10.1111/j.1365-313X.2010.04435.x. [DOI] [PubMed] [Google Scholar]
- Ischebeck T, Werner S, Krishnamoorthy P, et al. Phosphatidylinositol 4,5-bisphosphate influences PIN polarization by controlling clathrin mediated membrane trafficking. Plant Cell. 2013;25:4894–4911. doi: 10.1105/tpc.113.116582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivakov A, Persson S. Plant cell walls. In: eLS. Chichester: John Wiley & Sons. 2012 http://www.els.net . doi:10.1002/9780470015902.a0001682.pub2. [Google Scholar]
- Johansen JN, Vernhettes S, Höfte H. The ins and outs of plant cell walls. Current Opinion in Plant Biology. 2006;9:616–620. doi: 10.1016/j.pbi.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Jung N, Haucke V. Clathrin-mediated endocytosis at synapses. Traffic. 2007;8:1129–1136. doi: 10.1111/j.1600-0854.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- Jürgens G. Membrane trafficking in plants. Annual Review of Cell and Developmental Biology. 2004;20:481–504. doi: 10.1146/annurev.cellbio.20.082503.103057. [DOI] [PubMed] [Google Scholar]
- Kang B-H, Busse JS, Bednarek SY. Members of the Arabidopsis dynamin-like gene family, ADL1, are essential for plant cytokinesis and polarized cell growth. Plant Cell. 2003;15:899–913. doi: 10.1105/tpc.009670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B-H, Nielsen E, Preuss ML, Mastronarde D, Staehelin LA. Electron tomography of RabA4b- and PI-4Kβ1-labeled trans Golgi network compartments in Arabidopsis. Traffic. 2011;12:313–329. doi: 10.1111/j.1600-0854.2010.01146.x. [DOI] [PubMed] [Google Scholar]
- Ketelaar T, Emons AMC. The cytoskeleton in plant cell growth: lessons from root hairs. New Phytologist. 2001;152:409–418. doi: 10.1046/j.0028-646X.2001.00278.x. [DOI] [PubMed] [Google Scholar]
- Kim DH, Eu Y-J, Yoo CM, et al. Trafficking of phosphatidylinositol 3-phosphate from the trans-Golgi network to the lumen of the central vacuole in plant cells. Plant Cell. 2001;13:287–301. doi: 10.1105/tpc.13.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Xu Z-Y, Song K, et al. Adaptor protein complex 2-mediated endocytosis is crucial for male reproductive organ development in Arabidopsis. Plant Cell. 2013;25:2970–2985. doi: 10.1105/tpc.113.114264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klahre U, Becker C, Schmitt AC, Kost B. Nt-RhoGDI2 regulates Rac/Rop signaling and polar cell growth in tobacco pollen tubes. Plant Journal. 2006;46:1018–1031. doi: 10.1111/j.1365-313X.2006.02757.x. [DOI] [PubMed] [Google Scholar]
- König S, Ischebeck T, Lerche J, Stenzel I, Heilmann I. Salt-stress-induced association of phosphatidylinositol 4,5-bisphosphate with clathrin-coated vesicles in plants. Biochemical Journal. 2008;415:387–399. doi: 10.1042/BJ20081306. [DOI] [PubMed] [Google Scholar]
- Konopka CA, Schleede JB, Skop AR, Bednarek SY. Dynamin and cytokinesis. Traffic. 2006;7:239–247. doi: 10.1111/j.1600-0854.2006.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka CA, Backues SK, Bednarek SY. Dynamics of Arabidopsis dynamin-related protein 1C and a clathrin light chain at the plasma membrane. Plant Cell. 2008;20:1363–1380. doi: 10.1105/tpc.108.059428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost B. Spatial control of Rho (Rac-Rop) signaling in tip-growing plant cells. Trends in Cell Biology. 2008;18:119–127. doi: 10.1016/j.tcb.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Kost B, Lemichez E, Spielhofer P, et al. Rac homologues and compartmentalized phosphatidylinositol 4, 5-bisphosphate act in a common pathway to regulate polar pollen tube growth. Journal of Cell Biology. 1999;145:317–330. doi: 10.1083/jcb.145.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano H, Testerink C, Vermeer JEM, et al. The Arabidopsis phosphatidylinositol phosphate 5-kinase PIP5K3 is a key regulator of root hair tip growth. Plant Cell. 2008;20:367–380. doi: 10.1105/tpc.107.056119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Kim YW, Jeon BW, et al. Phosphatidylinositol 4,5-bisphosphate is important for stomatal opening. Plant Journal. 2007;52:803–816. doi: 10.1111/j.1365-313X.2007.03277.x. [DOI] [PubMed] [Google Scholar]
- Li S, Chen M, Yu D, et al. EXO70A1-mediated vesicle trafficking is critical for tracheary element development in Arabidopsis. Plant Cell. 2013;25:1774–1786. doi: 10.1105/tpc.113.112144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zuo X, Yue P, Guo W. Phosphatidylinositol 4,5-bisphosphate mediates the targeting of the exocyst to the plasma membrane for exocytosis in mammalian cells. Molecular Biology of the Cell. 2007;18:4483–4492. doi: 10.1091/mbc.E07-05-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matar D, Catesson AM. Cell plate development and delayed formation of the pectic middle lamella in root meristems. Protoplasma. 1988;146:10–17. [Google Scholar]
- McCann MC, Carpita NC. Designing the deconstruction of plant cell walls. Current Opinion in Plant Biology. 2008;11:314–320. doi: 10.1016/j.pbi.2008.04.001. [DOI] [PubMed] [Google Scholar]
- McFarlane HE, Doering A, Persson S. The cell biology of cellulose synthesis. Annual Review of Plant Biology. 2014;65 doi: 10.1146/annurev-arplant-050213-040240. in press. [DOI] [PubMed] [Google Scholar]
- Mei Y, Jia WJ, Chu YJ, Xue HW. Arabidopsis phosphatidylinositol monophosphate 5-kinase 2 is involved in root gravitropism through regulation of polar auxin transport by affecting the cycling of PIN proteins. Cell Research. 2012;22:581–597. doi: 10.1038/cr.2011.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miart F, Desprez T, Biot E, et al. Spatio-temporal analysis of cellulose synthesis during cell plate formation in Arabidopsis. Plant Journal. 2014;77:71–84. doi: 10.1111/tpj.12362. [DOI] [PubMed] [Google Scholar]
- Mikami K, Katagiri T, Iuchi S, Yamaguchi-Shinozaki K, Shinozaki K. A gene encoding phosphatidylinositol-4-phosphate 5-kinase is induced by water stress and abscisic acid in Arabidopsis thaliana. Plant Journal. 1998;15:563–568. doi: 10.1046/j.1365-313x.1998.00227.x. [DOI] [PubMed] [Google Scholar]
- Mikami K, Iuchi S, Yamaguchi-Shinozaki K, Shinozaki K. A novel Arabidopsis thaliana dynamin-like protein containing the pleckstrin homology domain. Journal of Experimental Botany. 2000;51:317–318. doi: 10.1093/jexbot/51.343.317. [DOI] [PubMed] [Google Scholar]
- Milosevic I, Sørensen JB, Lang T, et al. Plasmalemmal phosphatidylinositol-4,5-bisphosphate level regulates the releasable vesicle pool size in chromaffin cells. Journal of Neuroscience. 2005;25:2557–2565. doi: 10.1523/JNEUROSCI.3761-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Roeber B, Pical C. Inositol phospholipid metabolism in Arabidopsis. Characterized and putative isoforms of inositol phospholipid kinase and phosphoinositide-specific phospholipase C. Plant Physiology. 2002;130:22–46. doi: 10.1104/pp.004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnik T, Nielsen E. Green light for polyphosphoinositide signals in plants. Current Opinion in Plant Biology. 2011;14:489–497. doi: 10.1016/j.pbi.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Munson M, Novick P. The exocyst defrocked, a framework of rods revealed. Nature Structural and Molecular Biology. 2006;13:577–581. doi: 10.1038/nsmb1097. [DOI] [PubMed] [Google Scholar]
- Neutelings G. Lignin variability in plant cell walls: contribution of new models. Plant Sciences. 2011;181:379–386. doi: 10.1016/j.plantsci.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Nibau C, Wu H, Cheung AY. RAC/ROP GTPases: hubs for signal integration and diversification in plants. Trends in Plant Science. 2006;11:309–315. doi: 10.1016/j.tplants.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Novakova P, Hirsch S, Feraru E, et al. SAC domain phosphoinositide phosphatases at the tonoplast mediate vacuolar function in Arabidopsis. Proceedings of the National Academy of Sciences of the USA. 2014;111:2818–2823. doi: 10.1073/pnas.1324264111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei W, Du F, Zhang Y, He T, Ren H. Control of the actin cytoskeleton in root hair development. Plant Sciences. 2012;187:10–18. doi: 10.1016/j.plantsci.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Persson S, Paredez A, Carroll A, et al. Genetic evidence for three unique components in primary cell-wall cellulose synthase complexes in Arabidopsis. Proceedings of the National Academy of Sciences of the USA. 2007;104:15566–15571. doi: 10.1073/pnas.0706592104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss ML, Schmitz AJ, Thole JM, Bonner HKS, Otegui MS, Nielsen E. A role for the RabA4b effector protein PI-4Kβ1 in polarized expansion of root hair cells in Arabidopsis thaliana. Journal of Cell Biology. 2006;172:991–998. doi: 10.1083/jcb.200508116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puertollano R. Clathrin-mediated transport: assembly required. EMBO Reports. 2004;5:942–946. doi: 10.1038/sj.embor.7400249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MS, Bonifacino JS. Adaptor-related proteins. Current Opinion in Cell Biology. 2001;13:444–453. doi: 10.1016/s0955-0674(00)00235-0. [DOI] [PubMed] [Google Scholar]
- Sampathkumar A, Gutierrez R, McFarlane HE, et al. Patterning and lifetime of plasma membrane-localized cellulose synthase is dependent on actin organization in Arabidopsis interphase cells. Plant Physiology. 2013;162:675–688. doi: 10.1104/pp.113.215277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer M, Delgadillo MO, Zouhar J, et al. MTV1 and MTV4 encode plant-specific ENTH and arf GAP proteins that mediate clathrin-dependent trafficking of vacuolar cargo from the trans-Golgi network. Plant Cell. 2013;25:2217–2235. doi: 10.1105/tpc.113.111724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DA, D'souza-Schorey C, Cooper JA. Actin assembly at membranes controlled by ARF6. Traffic. 2000;1:896–907. doi: 10.1034/j.1600-0854.2000.011108.x. [DOI] [PubMed] [Google Scholar]
- Schu PV, Takegawa K, Fry MJ, Stack JH, Waterfield MD, Emr SD. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science. 1993;260:88–91. doi: 10.1126/science.8385367. [DOI] [PubMed] [Google Scholar]
- Somerville C, Bauer S, Brininstool G, et al. Toward a systems approach to understanding plant cell walls. Science. 2004;306:2206–2211. doi: 10.1126/science.1102765. [DOI] [PubMed] [Google Scholar]
- Song J, Lee MH, Lee G-J, Yoo CM, Hwang I. Arabidopsis EPSIN1 plays an important role in vacuolar trafficking of soluble cargo proteins in plant cells via interactions with clathrin, AP-1, VTI11, and VSR1. Plant Cell. 2006;18:2258–2274. doi: 10.1105/tpc.105.039123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa E, Kost B, Malhó R. Arabidopsis phosphatidylinositol-4-monophosphate 5-kinase 4 regulates pollen tube growth and polarity by modulating membrane recycling. Plant Cell. 2008;20:3050–3064. doi: 10.1105/tpc.108.058826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenzel I, Ischebeck T, Konig S, et al. The type B phosphatidylinositol-4-phosphate 5-kinase 3 is essential for root hair formation in Arabidopsis thaliana. Plant Cell. 2008;20:124–141. doi: 10.1105/tpc.107.052852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synek L, Schlager N, Eliáš M, Quentin M, Hauser M-T, Žárský V. AtEXO70A1, a member of a family of putative exocyst subunits specifically expanded in land plants, is important for polar growth and plant development. Plant Journal. 2006;48:54–72. doi: 10.1111/j.1365-313X.2006.02854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo H, Gill DJ, Sun J, et al. ESCRT-I core and ESCRT-II GLUE domain structures reveal role for GLUE in linking to ESCRT-I and membranes. Cell. 2006;125:99–111. doi: 10.1016/j.cell.2006.01.047. [DOI] [PubMed] [Google Scholar]
- Thole JM, Nielsen E. Phosphoinositides in plants: novel functions in membrane trafficking. Current Opinion in Plant Biology. 2008;11:620–631. doi: 10.1016/j.pbi.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Toyooka K, Goto Y, Asatsuma S, Koizumi M, Mitsui T, Matsuoka K. A mobile secretory vesicle cluster involved in mass transport from the Golgi to the plant cell exterior. Plant Cell. 2009;21:1212–1229. doi: 10.1105/tpc.108.058933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bout I, Divecha N. PIP5K-driven PtdIns(4,5)P2 synthesis: regulation and cellular functions. Journal of Cell Science. 2009;122:3837–3850. doi: 10.1242/jcs.056127. [DOI] [PubMed] [Google Scholar]
- Vermeer JE, van Leeuwen W, Tobeña-Santamaria R, et al. Visualization of PtdIns3P dynamics in living plant cells. Plant Journal. 2006;47:687–700. doi: 10.1111/j.1365-313X.2006.02830.x. [DOI] [PubMed] [Google Scholar]
- Vernoud V, Horton AC, Yang Z, Nielsen E. Analysis of the small GTPase gene superfamily of Arabidopsis. Plant Physiology. 2003;131:1191–1208. doi: 10.1104/pp.013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer A, Youssef N, DeWald D. Unique cell wall abnormalities in the putative phosphoinositide phosphatase mutant AtSAC9. Planta. 2011;234:993–1005. doi: 10.1007/s00425-011-1454-4. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhuang X, Cai Y, Cheung AY, Jiang L. Apical F-actin-regulated exocytic targeting of NtPPME1 is essential for construction and rigidity of the pollen tube cell wall. Plant Journal. 2013;76:367–379. doi: 10.1111/tpj.12300. [DOI] [PubMed] [Google Scholar]
- Wenk MR, De Camilli P. Protein-lipid interactions and phosphoinositide metabolism in membrane traffic: insights from vesicle recycling in nerve terminals. Proceedings of the National Academy of Sciences of the USA. 2004;101:8262–8269. doi: 10.1073/pnas.0401874101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman R, Turner S. Trafficking of the cellulose synthase complex in developing xylem vessels. Biochemical Society Transactions. 2010;38:755–760. doi: 10.1042/BST0380755. [DOI] [PubMed] [Google Scholar]
- Williams ME, Torabinejad J, Cohick E, et al. Mutations in the Arabidopsis phosphoinositide phosphatase gene SAC9 lead to overaccumulation of PtdIns(4,5)P2 and constitutive expression of the stress-response pathway. Plant Physiology. 2005;138:686–700. doi: 10.1104/pp.105.061317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka S, Shimono Y, Shirakawa M, et al. Identification and dynamics of Arabidopsis adaptor protein-2 complex and its involvement in floral organ development. Plant Cell. 2013;25:2958–2969. doi: 10.1105/tpc.113.114082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zárský V, Kulich I, Fendrych M, Pečenková T. Exocyst complexes multiple functions in plant cells secretory pathways. Current Opinion in Plant Biology. 2013;16:726–733. doi: 10.1016/j.pbi.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Zhang X, Orlando K, He B, et al. Membrane association and functional regulation of Sec3 by phospholipids and Cdc42. Journal of Cell Biology. 2008;180:145–158. doi: 10.1083/jcb.200704128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Yan A, Feijó JA, et al. Phosphoinositides regulate clathrin-dependent endocytosis at the tip of pollen tubes in Arabidopsis and tobacco. Plant Cell. 2010;22:4031–4044. doi: 10.1105/tpc.110.076760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong R, Burk DH, Morrison WH, Ye Z-H. FRAGILE FIBER3, an Arabidopsis gene encoding a type II inositol polyphosphate 5-phosphatase, is required for secondary wall synthesis and actin organization in fiber cells. Plant Cell. 2004;16:3242–3259. doi: 10.1105/tpc.104.027466. [DOI] [PMC free article] [PubMed] [Google Scholar]