Abstract
Background and Aims
Rhamnogalacturonan-II (RG-II) is one of the pectin motifs found in the cell wall of all land plants. It contains sugars such as 2-keto-3-deoxy-d-lyxo-heptulosaric acid (Dha) and 2-keto-3-deoxy-d-manno-octulosonic acid (Kdo), and within the wall RG-II is mostly found as a dimer via a borate diester cross-link. To date, little is known regarding the biosynthesis of this motif. Here, after a brief review of our current knowledge on RG-II structure, biosynthesis and function in plants, this study explores the implications of the presence of a Golgi-localized sialyltransferase-like 2 (SIA2) protein that is possibly involved in the transfer of Dha or Kdo in the RG-II of Arabidopsis thaliana pollen tubes, a fast-growing cell type used as a model for the study of cell elongation.
Methods
Two heterozygous mutant lines of arabidopsis (sia2-1+/– and qrt1 × sia2-2+/–) were investigated. sia2-2+/– was in a quartet1 background and the inserted T-DNA contained the reporter gene β-glucuronidase (GUS) under the pollen-specific promoter LAT52. Pollen germination and pollen tube phenotype and growth were analysed both in vitro and in vivo by microscopy.
Key Results
Self-pollination of heterozygous lines produced no homozygous plants in the progeny, which may suggest that the mutation could be lethal. Heterozygous mutants displayed a much lower germination rate overall and exhibited a substantial delay in germination (20 h of delay to reach 30 % of pollen grain germination compared with the wild type). In both lines, mutant pollen grains that were able to produce a tube had tubes that were either bursting, abnormal (swollen or dichotomous branching tip) or much shorter compared with wild-type pollen tubes. In vivo, mutant pollen tubes were restricted to the style, whereas the wild-type pollen tubes were detected at the base of the ovary.
Conclusions
This study highlights that the mutation in arabidopsis SIA2 encoding a sialyltransferase-like protein that may transfer Dha or Kdo on the RG-II motif has a dramatic effect on the stability of the pollen tube cell wall.
Keywords: Rhamnogalacturonan-II, RG-II, Dha, Kdo, pollen tube, plant cell wall, sialyltransferase-like protein, pectin motif, Arabidopsis thaliana
INTRODUCTION
The pollen tube is a fast tip-growing cell carrying the two sperm cells to the ovule, allowing the double fertilization process and seed setting (Johnson and Lord, 2006; Mollet et al., 2007; Palanivelu and Tsukamoto, 2012). It requires a massive deposition of polymers in the cell wall, including pectins, hemicelluloses and cellulose, to promote the fast pollen tube elongation, and a tight control of cell wall remodelling to modify the mechanical properties (Mollet et al., 2013). As a consequence, the most highly expressed genes in Arabidopsis thaliana (arabidopsis) pollen encode enzymes involved in cell wall remodelling (Pina et al., 2005). This research in context focuses on our current knowledge of the role of rhamnogalacturonan-II (RG-II), one of the cell wall pectic polymers with homogalacturonan (HG) and rhamnogalacturonan-I (RG-I), in pollen tube growth. Moreover, new results regarding the characterization of mutant lines impaired in a sialyltransferase-like protein that is possibly involved in RG-II synthesis are presented.
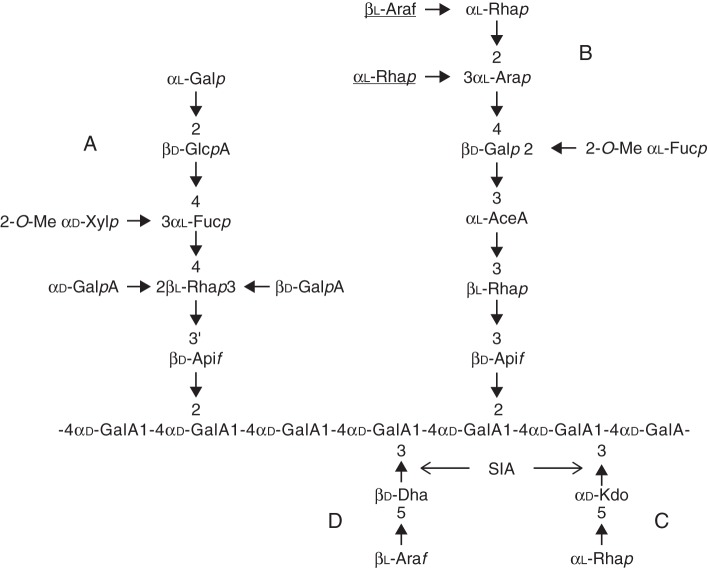
Rhamnogalacturonan-II is a highly complex polysaccharide representing between 1 and 4 % of the pectin-rich primary cell wall of eudicots (O'Neill et al., 2004). RG-II was primarily characterized from the sycamore cell wall in the late 1970s (Darvill et al., 1978). Since then, RG-II has also been isolated from the cell walls of gymnosperms (Edashige and Ishii, 1998; Shimokawa et al., 1999), lycopodiophytes and pteridophytes (Matsunaga et al., 2004). To date, RG-II has been identified in all vascular plants, with a highly conserved glycosyl sequence. This low molecular weight polysaccharide (5–10 kDa) solubilizes when subjected to an endo-polygalacturonase treatment, and contains 13 different glycosyl residues linked together by >20 different linkages, requiring 22 specific glycosyltransferases (GTs) (Bar-Peled et al., 2012). RG-II has an HG backbone made up of seven to nine α-(1,4)-d-galacturonic acid (GalA) residues that is substituted with different side chains (A–E) (Whitcombe et al., 1995; Pérez et al., 2003). Chain E is made of only one arabinosyl residue and is not always considered as a side chain. Therefore, it is usually considered that RG-II is decorated with only four oligosaccharide side chains (Fig. 1; O'Neill et al., 2004; Bar-Peled et al., 2012). The A and B side chains are composed of octa- and hepta- to nonasaccharide, respectively, whereas chains C and D are disaccharides. The glycosyl sequences of these oligosaccharide chains are conserved among vascular plant species, except for the lycophytes and several pterydophytes that show some degrees of variability on the terminal extension of the B side chain (Matsunaga et al., 2004). Nevertheless, RG-II's originality resides in the presence of sugars such as d-apiose (Api), l-aceric acid (3-C-carboxy-5-deoxy-l-xylose; AceA), 2-O-methyl l-fucose (2-O-Me-Fuc), 2-O-methyl d-xylose (2-O-Me-Xyl), l-galactose (l-Gal), 2-keto-3-deoxy-d-lyxo-heptulosaric acid (Dha) and 2-keto-3-deoxy-d-manno-octulosonic acid (Kdo). Until recently, RG-II's structure within a given plant species was considered to be unique and no modulation of the RG-II composition was thought to occur. Recently, Pabst et al. (2013) reported variations of the RG-II structure within a single individual plant concerning either the length of chain B or the monosaccharide substitution and methylation of uronic acids in chain A. However, the biological significance of structural variations of the RG-II side chains is not known.
Fig. 1.
Structure of RG-II. The sugars underlined are absent in arabidopsis RG-II. Arrows indicate the glycosyl linkages that are postulated to result from the action of sialyltransferase-like SIA1 (At1g08660) and SIA2 (At3g48820) proteins.
Even if very little is known about its biosynthesis, RG-II is believed to be synthesized in the Golgi apparatus (Mohnen, 2008). Because of its structural complexity, the synthesis requires a large number of different activated sugars, many specific GTs and also additional enzymes required for methylation and acetylation of the side chain residues (Bar-Peled et al., 2012). So far, only one GT has been fully characterized. This enzyme, named RGXT (rhamnogalacturonan-specific xylosyltransferase), is involved in the synthesis of the A side chain of RG-II by transferring an α-(1,3)-d-xylose on the internal l-fucose (Egelund et al., 2006, 2008). Recently, a bioinformatic study has listed an additional 26 putative GTs including ten sequences belonging to the GT4, 8, 29, 31, 68 and 92 CAZy families and 16 non-CAZy GTs (Voxeur et al., 2012).
Boron is an essential micronutrient, and plant boron deficiency has been known for a long time to be responsible for many anatomical, physiological and biochemical defects (Blevins and Lukaszewski, 1998). The first definitive evidence of boron requirement for the growth of higher plants was described by Warrington (1923) in legumes. To date, in agriculture, to avoid boron deficiency, calcium or sodium borate, also called borax, is commonly applied directly to the soil to promote plant growth or sprayed at the flowering stage to improve fruit and seed set or reduce fruit drop (Ganie et al., 2013). To date, the exact functions of boron in plant development have not been clearly determined (Goldbach and Wimmer, 2007), but structural studies have shown that the cell wall is a sink for boron and that it is involved in RG-II dimerization (O'Neill et al., 1996, 2004). In planta, at least 90 % of RG-II exists as a dimer that is cross-linked by a borate di-ester bond between two apiosyl residues of the A side chain. This dimer was shown to be present in angiosperms, gymnosperms, lycophytes and pteridophytes (Ishii and Matsunaga, 1996; O'Neill et al., 1996; Kaneko et al., 1997; Shimokawa et al., 1999; Vidal et al., 2001; Matsunaga et al., 2004). This dimer of RG-II is thought to play a crucial role in cell wall integrity by strengthening the pectic network, and defects in boron dimerization result in notable growth alterations (Noguchi et al., 1997; O'Neill et al., 2001; Voxeur et al., 2011).
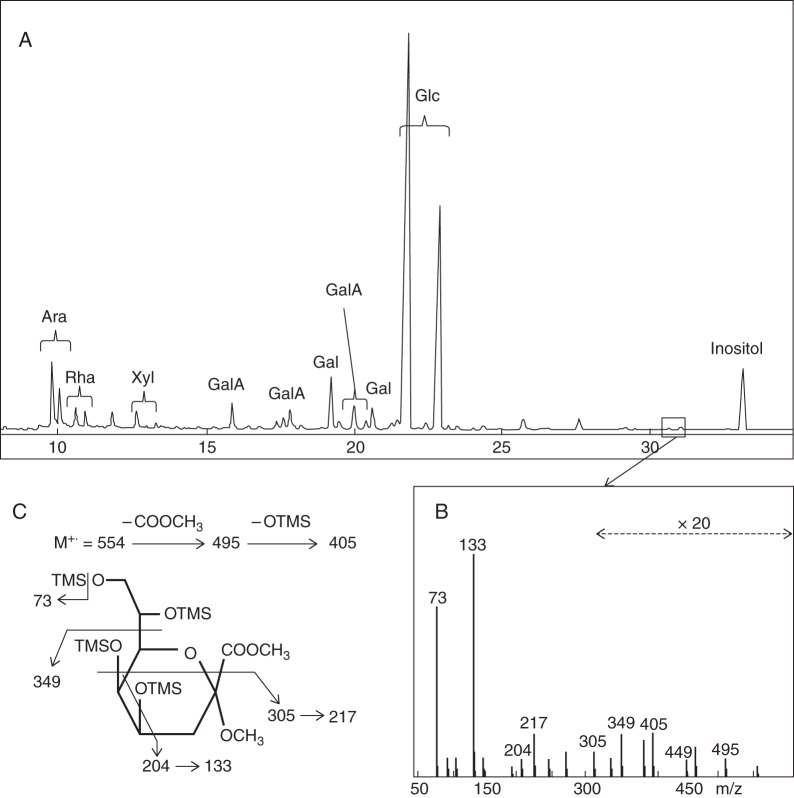
The pollen tube wall is mainly composed of cellulose, hemicellulose, callose and pectins together with HG and RG-I (Dardelle et al., 2010; Lehner et al., 2010; Chebli et al., 2012), but very little information has been reported concerning RG-II. RG-II has been localized in the cell wall of Lilium longiflorum pollen tubes using a polyclonal antibody directed against both the borate–RG-II complex and monomeric RG-II (Matoh et al., 1998). Using this antibody, we also observed staining along the cell walls of Arabidopsis thaliana, Solanum lycopersicum var. cerasiforme WVA 106 (tomato) and Nicotiana benthamiana (tobacco) pollen tubes and pollen grains (Fig. 2), suggesting that RG-II is a common structural feature of pollen tube cell walls. Moreover, this is supported by biochemical analysis. The monosaccharide composition of a hot water extract isolated from in vitro grown arabidopsis pollen tubes revealed the main sugars found in pectins (rhamnose, arabinose, galactose and GalA) as previously described by Dardelle et al. (2010). In addition, xylose, probably originating from xyloglucan, and glucose, probably arising from xyloglucan, callose and/or starch of pollen tubes (Fig. 3A), were also present. Gas chromatography coupled to electron impact mass spectrometry (GC-EIMS) has allowed the detection of specific RG-II monosaccharides. Traces of methylated sugars were detected in minor peaks eluting before the arabinose residue. Furthermore, Kdo was detected (Fig. 3A) and its structure was confirmed by analysing its electron impact (EI) mass spectrum (Fig. 3B, C) (Doco et al., 2001). The results indicate that RG-II is undoubtedly present in pollen tube cell walls of A. thaliana and other species, although in a low amount.
Fig. 2.
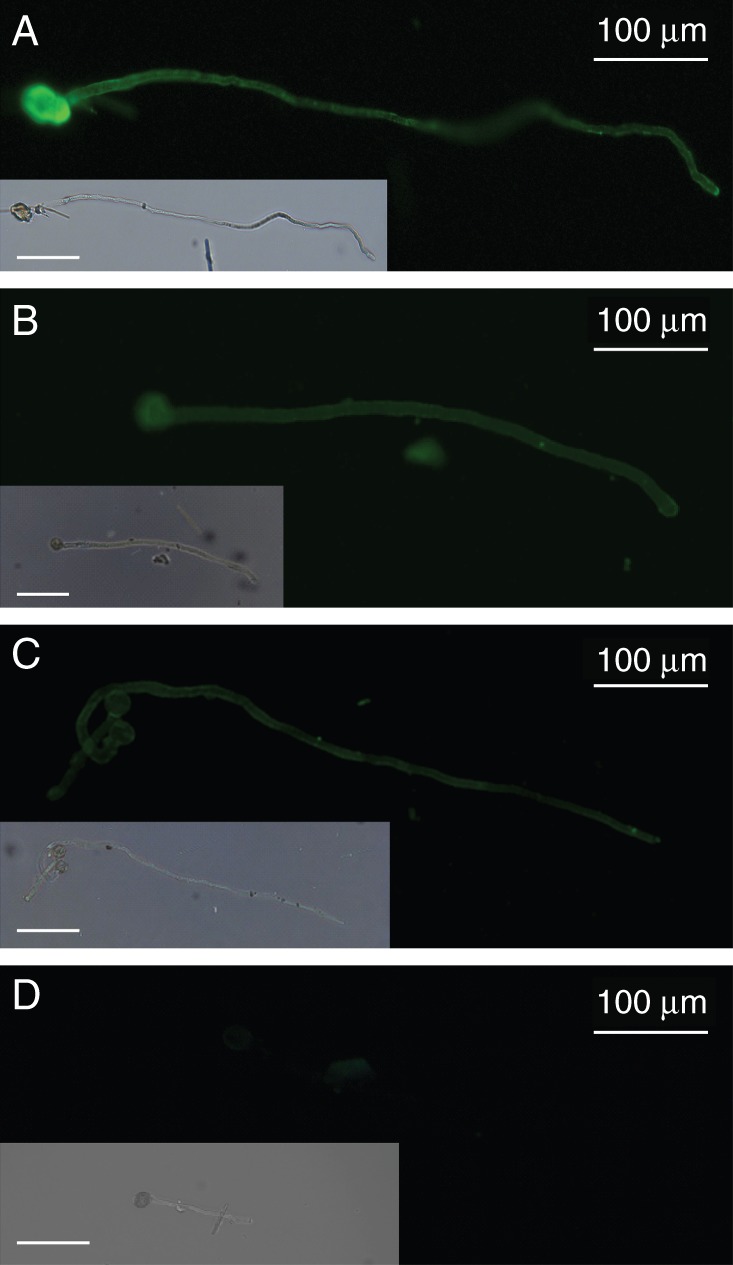
Immunolocalization of RG-II in the cell wall of pollen tubes using the anti-RG-II antibody described by Matoh et al. (1998). (A) Arabidopsis thaliana Col-0, (B) Nicotiana benthamiana and (C) Solanum lycopersicum var. cerasiforme WVA 106. (D) Negative control of A. thaliana pollen tubes. Inserts are the bright field images of the same pollen tubes. Scale bars = 100 μm.
Fig. 3.
Identification of Kdo in a hot water extract from 6-hour-old arabidopsis pollen tubes. (A) Gas chromatogram of trimethylsilyl (TMS) derivatives of the methyl glycosides, (B) electron impact mass spectrum (EIMS) of the minor peaks boxed in the chromatogram and (C) assignment of EIMS fragmentation ions showing that the minor peaks are Kdo derivatives. Ara, arabinose; Gal, galactose; GalA, galacturonic acid; Glc, glucose; Rha, rhamnose; Xyl, xylose.
Even though the RG-II structure and dimerization within the wall have not been investigated in pollen tubes, we postulate that boron-induced RG-II cross-linking is crucial for pollen tube germination and/or elongation. Pollen of most plant species requires boron to germinate both in vivo and in vitro (Table 1; García-Hernández and López, 2005) and boron is essential to control the mechanical properties of the cell wall and the oscillatory pulses during pollen tube elongation (Holdaway-Clarke et al., 2003). Wang et al. (2003) have shown that the culture of Picea meyeri pollen in a boron-deficient medium affected pollen germination and resulted in the abnormal accumulation of callose and acidic pectin in the tip region of pollen tubes compared with pollen tubes grown in optimum conditions. Moreover, Li et al. (2011) have characterized an anther-specific boric acid transporter of the aquaporin superfamily regulating the transport of this critical nutrient to the male gametophyte. However, too high concentrations of boron reduce pollen germination, slightly increase the rate of burst pollen tubes and decrease the final fruit set (Potts and Marsden-Smedley, 1989; Wang et al., 2003; Lee et al., 2009). As a consequence, the boron concentration is critical in planta and in vitro for optimal pollen germination. According to the basal Brewbaker and Kwack (1963) pollen germination medium, the boric acid concentration routinely used for in vitro germination assays of many species including arabidopsis, tobacco and tomato is 0·01 % (Table 1). Other species such as maize or rice required the supply of much less boron to promote pollen germination.
Table 1.
Examples of species that required boron for optimum in vitro pollen germination and pollen tube growth
| Plant species | H3BO3 (%) | Reference |
|---|---|---|
| Areca catechu | 0·04–0·06 | Liu et al. (2013) |
| Cajanus cajan | 0·025 | Jayaprakash and Sarla (2001) |
| Cucumis sativus | 0·025 | Vižintin and Bohanec (2004) |
| Arabidopsis thaliana | 0·01 | Boavida and McCormick (2007) |
| Solanum lycopersicum | 0·01 | Covey et al. (2010) |
| Solanum chacoense | 0·01 | Parre and Geitmann (2005b) |
| Nicotiana tabacum | 0·01 | Persia et al. (2008) |
| Gossypium hirsutum | 0·01 | Kakani et al. (2005) |
| Triticum aestivum | 0·01 | Cheng et al. (1992) |
| Lilium longiflorum | 0·01 | Rounds et al. (2011) |
| Pinus sylvestris | 0·01 | Fang et al. (2008) |
| Pinus bungeana | 0·01 | Wang et al. (2003) |
| Picea meyeri | 0·01 | McKenna et al. (2009) |
| Luffa aegyptica | 0·005 | Prajapati and Jain (2010) |
| Zea mays | 0·005 | Schreiber and Dresselhaus (2003) |
| Oryza sativa | 0·004 | Dai et al. (2006) |
| Pisum sativum | 0·002 | McGee and Baggett (1992) |
| Brassica oleracea | 0·001 | Roberts et al. (1983) |
Further evidence of the importance of the borate–RG-II complex in pollen tube development was deduced from the analysis of arabidopsis mutants. Alteration of the expression of genes involved in RG-II biosynthesis was reported to impair male fertility (Delmas et al., 2008; Deng et al., 2010; Kobayashi et al., 2011; Liu et al., 2011) and consequently no homozygous lines can be obtained. Two mutants affected in genes encoding the Kdo-8-P synthase (AtkdsA1 and AtkdsA2) have been characterized (Delmas et al., 2008). These mutants affected in the synthesis of this cytosolic monosaccharide are probably impaired in RG-II because Kdo is exclusively present in this pectic polymer (Fig. 1). A single mutation in the KDSA1 or KDSA2 gene did not display any phenotype, but the generation of a double knock-out mutant AtkdsA1/AtkdsA2 failed. In order to test whether this was due to a gametophytic or sporophytic defect, the authors generated AtkdsA mutants in the quartet (qrt) mutant background. Analysis of pollen tetrads revealed that a maximum of two out of the four pollen grains from the quartet 1-2 × AtkdsA1–/– AtkdsA2+/– were able to germinate and form a proper pollen tube. These results showed that the absence of Kdo biosynthesis in arabidopsis pollen impairs pollen tube growth.
To date, the α-(1,3)-xylosyltransferase RGXT is the only reported GT that has been demonstrated to be involved in RG-II biosynthesis. This type II enzyme belongs to the CAZy GT77 family and is involved in the synthesis of chain A by transferring an α-d-xylose residue on the internal α-l-fucose (Egelund et al., 2006). Four isoforms, RGXT1–RGXT4, are expressed in arabidopsis. The biological function of the last one (RGXT4) was investigated in arabidopsis. The mutant line was called mgp4 for male gametophyte defective4 because of the severe defect observed in pollen tube growth (Liu et al., 2011). Genetic analyses indicated that mgp4 completely suppressed the genetic transmission of the male gametophyte without affecting the female function. Pollen grain formation was not affected in the mutant, but in vitro germination revealed short or burst pollen tubes by comparison with the wild type. Homozygous mgp4–/– plants were generated by introducing into the heterozygous line the full-length cDNA of MGP4 under the control of a pollen-specific promoter. The seedlings harvested from the pollen-rescued mgp4 homozygous plants exhibited severe defects in root growth and swollen root cells. These growth defects were partially suppressed by supplying the plants with exogenous boric acid. Restoration of wild-type phenotypes in RG-II mutants by supplementation with borate was previously reported and supports the crucial role of borate-induced RG-II dimerization in plant cell wall elongation (O'Neill et al., 2001; Voxeur et al., 2011). Together with the study of Kdo mutants, this work on RGXT transferase demonstrated that RG-II biosynthesis is crucial for proper pollen tube growth.
Two sialyltransferase-like sequences that belong to the GT29 family were selected in a bioinformatic screening recently reported by Voxeur and co-workers (2012). These two proteins encoded by At1g08660 and At3g48820 contain the four conserved sialyl motifs (Audry et al., 2011) of mammalian sialyltransferases and are located in the Golgi apparatus (Dunkley et al., 2006; Daskalova et al., 2009). While At1g08660 is found in the Affimetrix ATH1 microarray chips, At3g48820 is not, but is present in the Complete Arabidopsis Transcriptome Microarray (CATMA, http://www.catma.org/). Moreover, RNA-seq analyses have detected RNA fragments from At3g48820 in arabidopsis pollen, although not with an extensive coverage (Loraine et al., 2013). Sialic acid is a nine-carbon acidic sugar that is involved in a large variety of structural and biological roles in mammalian cells. This monosaccharide has not been detected in plants (Séveno et al., 2004). However, sialic acid, Kdo and Dha share common features; for example, they result from the condensation in the cytosol of phosphoenol pyruvate to a monosaccharide phosphate, and GTs of the GT29 family use CMP-activated nucleotide sugars as substrates. As a consequence, we have previously proposed that these two plant sialyltransferase-like GTs may be involved in the transfer of Kdo and/or Dha on the HG backbone of RG-II (Fig. 1) (Voxeur et al., 2012). Deng et al. (2010) have investigated one of these two putative sialyltransferase-like proteins encoded by At1g08660 in pollen tube growth. The isolated heterozygous mgp2 (male gametophyte defective2) mutant had a loss of male gametophytic function without affecting the female gametophyte. While mgp2 pollen grains did not show any morphological abnormality, in vivo analysis revealed that mgp2 pollen tubes were restricted to the stigmatic tissues and were not able to reach and fertilize the ovules in comparison with the wild-type pollen tubes. Moreover, introduction of the MGP2 genomic fragment into mgp2+/– plants could restore the genetic transmission of the mgp2 mutation through the male gametophyte.
Herein, we report on the effect of inactivation of the At3g48820 gene, predicted to encode the second sialyltransferase-like protein, on pollen tube development. We hypothesized that, as postulated for its homologue At1g08660, At3g48820 may be involved in the transfer of Kdo and/or Dha on the HG backbone of RG-II and that this protein is important for efficient pollen grain germination and pollen tube elongation in arabidopsis. For a better understanding, the two sialyltransferase-like proteins were named SIA1 (sialyltransferase-like 1, MGP2, At1g08660) and SIA2 (sialyltransferase-like 2, At3g48820).
MATERIALS AND METHODS
Plant material and growth conditions
The Arabidopsis thaliana plant lines were from the Columbia (Col) ecotype. The T-DNA At3g48820 insertion lines SALK_059690 and SAIL_259_H07, named sia2-1 and qrt1 × sia2-2 respectively, were obtained from the Nottingham Arabidopsis Stock Centre. The sia2-2 (SAIL_259_H07) mutant line has a quartet1 (qrt1, At5g55590) background and the T-DNA vector pCSA110 encodes a β-glucuronidase (GUS) reporter gene under the control of the post-meiotic pollen-specific LAT52 promoter. The qrt1 line expressing LAT52::GUS was from Mark Johnson's lab (Brown University, USA). Wild-type and mutant seeds were spread on the surface of sterile soil and cultured in a growth chamber with a photoperiod of 16 h light/8 h dark cycle at 20 °C during the light phase and 16 °C in the dark phase, with 60 % relative humidity.
For comparison of RG-II distribution in pollen tubes, Nicotiana benthamiana and Solanum lycopersicum var. cerasiforme WVA 106 were grown in soil with a photoperiod of 16 h light/8 h dark cycle at 25 °C and 22 °C during the light and dark phase, respectively. Relative humidity was maintained at 60 % and plants were watered every 2 d.
Genetic analysis of sia2-1+/– and qrt1 × sia2-2+/– mutants
Genotypes of the sia2-1+/– arabidopsis plants were confirmed by PCR using the combination of the gene-specific primers (P1, 5′-GCAAATGGTTTGGGACTACAA-3′; and P2, 5′-TGTTTCAGGAAGCACCAATG-3′) and a T-DNA-pecific primer (LBb1·3: 5′-ATTTTGCCGATTTCGGAAC-3′). Similarly, the genotypes of the qrt1 × sia2-2+/– plants were identified with gene-specific primers (P3, 5′-CGCAGCGTTTTATAAAGTGAAA-3′; and P4, 5′-ACAAGCATGGGACAATGATG-3′) and a T-DNA-specific primer (LB2: 5′-GCTTCCTATTATATCTTCCCAAATTACCAATACA-3′).
Pollen tube growth conditions
Arabidopsis pollen germination was performed in liquid medium [5 mm CaCl2, 0·01 % H3BO3, 1 mm MgSO4, 5 mm KCl, 10 % (w/v) sucrose, pH 7·5] as described by Boavida and McCormick (2007). Nicotiana benthamiana and S. lycopersicum var. cerasiforme WVA 106 pollen grains were grown in BK medium [1·62 mm H3BO3, 1·25 mm Ca(NO3)2·4H2O, 2·97 mm KNO3 and 1·65 mm MgSO4·7H2O, pH 7] (Brewbaker and Kwack, 1963) containing 10 % (w/v) sucrose at 22 °C in the dark for 6 h under agitation.
In vitro phenotypic characterization of pollen tubes
The sia2-1+/– germinating pollen grains were scored after 4, 6, 8 and 24 h of incubation at 22 °C. A pollen grain was considered germinated if the pollen tube length was greater than the pollen grain diameter. To discriminate the sia2-2+/– pollen grains from wild-type pollen grains within the tetrads by GUS staining, the germinated pollen grains from qrt1 × sia2-2+/– plants were fixed for 15 min in 80 % acetone and washed twice with GUS buffer (2 mm potassium ferrocyanide, 2 mm potassium ferricyanide, 50 mm NaPO4 pH 7, 0·2 % Triton X-100). Pollen grains were incubated at 37 °C overnight in GUS staining solution [1 mm 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid (X-Gluc)] and observed.
In vivo phenotypic characterization of pollen tubes
To investigate the growth of pollen tubes in vivo, a double staining was performed on self-pollinated flowers. Flowers were fixed in 80 % acetone for 30 min, washed twice with GUS buffer and incubated overnight at 37 °C in GUS staining solution. Samples were rinsed several times with 70 % ethanol (EtOH) at room temperature and successively treated with 50 and 30 % EtOH and distilled water. The flowers were deposited on a glass slide and treated with 8 m NaOH overnight at room temperature in a wet chamber. After careful washing in distilled water, the flowers were incubated in decolorized aniline blue solution (Johnson-Brousseau and McCormick, 2004) for at least 2 h in the dark.
Immunolabelling of pollen tubes
Pollen tubes were fixed and immunolabelled as described by Dardelle et al. (2010) Briefly, pollen tubes were mixed (v/v) with a fixation solution containing 100 mm PIPES buffer pH 6·9, 4 mm MgSO4·7H2O, 4 mm EGTA, 10 % (w/v) sucrose and 5 % (v/v) formaldehyde, and incubated for 1 h at room temperature. Pollen tubes were rinsed three times by centrifugation (1 min, 3000 g) with CMF-DPBS (calcium- and magnesium-free Dulbecco's phosphate-buffered saline: 137 mm NaCl, 2·7 mm KCl, 7 mm Na2HPO4·7H2O, 1·5 mm KH2PO4). A saturation step was carried out for 30 min in CMF-DPBS supplemented with 3 % fat-free milk. After three washes, pollen tubes were incubated overnight at 4 °C in the dark in the anti-RG-II antibody (Matoh et al., 1998) diluted 1:20 with CMF-DPBS. Pollen tubes were rinsed with the buffer and incubated for 2 h at 30 °C with a goat secondary antibody anti-rabbit IgG (whole molecule) combined with fluorescein isothiocyanate (FITC; Sigma) diluted 1:50. Controls were carried out by incubation of the pollen tubes without the primary antibody.
Microscope observation and image acquisition
Pollen grains, pollen tubes or pistils were observed under bright field using an inverted Leica DMI6000B microscope or an upright Leica DLMB microscope equipped with a Leica DFC300FX camera. For immunolocalization of RG-II epitopes, pollen tubes were observed under fluorescence with an FITC filter (absorption, 485–520 nm; emission, 520–560 nm). The UV illumination was used to detect the aniline blue-stained pollen tubes. Pollen grain germination and pollen tube length were measured from the images using the ImageJ program (Abramoff et al., 2004). Images were assembled using the program GIMP (GNU Image Manipulation Program; http://www.gimp.org/).
Sugar composition of a hot water extract from arabidopsis pollen tubes
Six-hour-old pollen tubes from 3800 arabidopsis flowers were pooled after addition of 3 vols of 95 % EtOH to the germination medium. The EtOH-insoluble residue was then prepared as previously reported (Dardelle et al., 2010) and pectins were then extracted in boiling water. The monosaccharide composition of the pectin-enriched extract was determined by gas chromatography coupled to an electron impact mass spectrometer (GC-EIMS). The extract was first hydrolysed with 2 m trifluoroacetic acid (TFA) for 2 h at 110 °C and then submitted to a methanolysis for 16 h at 80 °C with 500 μL of dried 1 m methanolic-HCl (Supelco). After evaporation of the methanol, the methyl glycosides were converted into their trimethylsilyl (TMS) derivatives at 110 °C for 20 min with 200 μL of the silylation reagent (HMDS:TMCS:pyridine, 3:1:9, Supelco). Monosaccharides were then separated by GC (HP6890 series) on a Zebron Z5-MSi capillary column (length 30 m, i.d. 0·25 mm) (Macherey-Nagel) and analysed by EIMS using an Autospec GC-MS (Micromass, Manchester, UK) equipped with an Opus 3·1 data system.
RT-qPCR analysis
Total RNA was extracted from inflorescences of 6-week-old wild-type, quartet1, sia2-1+/– and qrt1 × sia2-2+/– plants using the NucleoSpin® RNA Plant kit (Macherey-Nagel) as described by the supplier. After RNA quantification using NanoDrop spectrophotometry, and a DNase treatment, 400 ng of RNA were converted into single cDNAs with a QuantiTect Reverse Transcription Kit (Qiagen, Valencia, CA, USA) following the instructions of the supplier. Real-time quantitative PCR (RT-qPCR) analyses were performed on 1/2 diluted cDNA. For RT-qPCR, the LightCycler® 480 SYBR Green I Master (Roche, Cat. No. 04887352001) was used in 384-well plates in the LightCycler® 480 Real-Time PCR System (Roche). The CT values for each sample (crossing threshold values are the number of PCR cycles required for the accumulated fluorescence signal to cross a threshold above the background) were acquired with the LightCycler 480 software (Roche) using the second derivative maximum method. Primers used are shown in Supplementary Data Table S1. Stably expressed reference genes (At3g28750, At3g57690 and At5g59370), selected using GeNorm software (Vandesompele et al., 2002), were used as internal controls to calculate the relative expression of target genes, according to the method described in Gutierrez et al. (2009). Each amplicon was first sequenced to ensure the specificity of the amplified sequence.
Statistical analysis
The data were analysed statistically by Student's t-test (GraphPad Software, La Jolla, CA, USA; www.graphpad.com).
RESULTS
Phenotypic characterization of the sia2-1 mutant
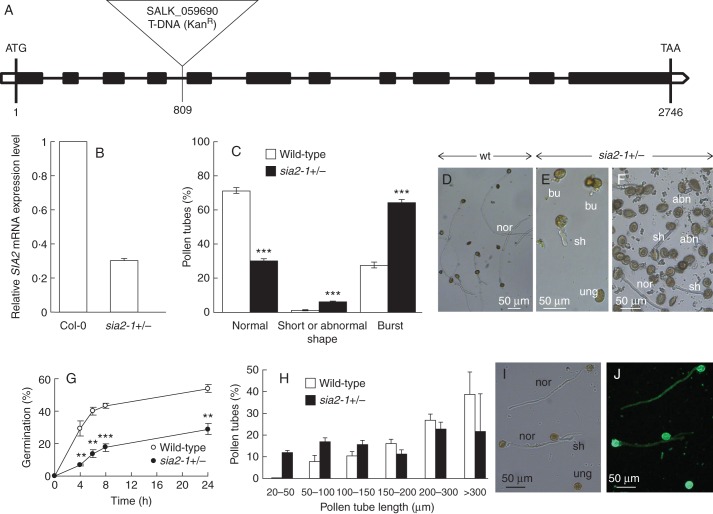
sia2-1 (SALK_059690) is a T-DNA insertion mutant in the At3g48820 gene. The T-DNA insertion is located in the fourth intron, 809 bp downstream of the start codon (Fig. 4A). PCR analyses of the progeny from the self-pollinated heterozygous sia2-1+/– plants showed a 1:1 segregation ratio between the heterozygous sia2-1+/– (308/609 plants, 50·6 %) and the wild-type (301/609, 49·4 %) plants. Of the 609 plants analysed in the progeny, no homozygous sia2-1–/– plant was identified, which may suggest that the mutation is lethal. However, we cannot rule out that the number of screened plants may not be enough to obtain homozygous lines. On the other hand, the heterozygous plants did not show any obvious visible phenotype compared with wild-type plants. The RT-qPCR analysis revealed that the expression level of SIA2 in the inflorescences of sia2-1+/– plants was reduced by about 70 % in comparison with Col-0 plants (Fig. 4B). Similar results were reported for SIA1 in the mgp2–1 mutant (Deng et al., 2010).
Fig. 4.
Characterization of the sia2-1 mutant. (A) Genomic organization of the SIA2–1 gene and location of the T-DNA insertion site. The black boxes indicate the exons. (B) Relative expression levels of SIA2 in Col-0 and sia2-1+/– quantified in inflorescences using three references genes (At3g28750, At3g57690 and At5g59370). Similar variations were observed with the three references genes and the three biological replicates. Only the results obtained with At3g28750 are shown using the SIA2–1-flanking primer pair. (C) In vitro germination of pollen grains from sia2-1+/– and wild-type plants (see key) showing the percentage of normal and abnormal pollen tubes (n >2000) after 6 h of culture. (D) In vitro culture of wild-type pollen tubes. Pollen grains were cultured for 6 h at 22 °C. (E, F) In vitro culture of 6-hour-old sia2-1+/– pollen tubes showing ungerminated pollen grains (ung), normal (nor), short (sh), burst (bu) and abnormal (abn) pollen tubes. (G) Time course of pollen germination from sia2-1+/– and wild-type plants (n >3000). (H) Comparison of sia2-1+/– and wild-type pollen tube length after 6 h of culture (n >300). (I, J) Bright field (I) and epifluorescent (J) images of sia2-1+/– pollen tubes labelled with the anti-RG-II antibody. **P <0·01; ***P <0·001.
In order to investigate how the mutation in At3g48820 was affecting male gametophyte function, we compared the pollen tube phenotypes of the heterozygous sia2-1+/– plants (which contain a population of 50 % mutant and 50 % wild-type pollen grains) with those of wild-type plants. In in vitro conditions, 64 % of sia2-1+/– pollen tubes had burst (Fig. 4C, E) and 6 % were short or had an abnormal shape (swollen or dichotomous branching tip) (Fig. 4C, E, F). In contrast, 70 % of pollen tubes from wild-type plants were normal (Fig. 4C, D), 27 % had burst and 1 % displayed short or abnormal tubes. Time course analyses over a 24 h period revealed an important delay in germination of pollen grains from sia2-1+/– plants (Fig. 4G). After 6 h of growth, 12 % of the sia2-1+/– pollen grains were germinated compared with 43 % of the wild-type pollen grains. With the same culture period, only 20 % of the sia2-1+/– pollen tubes reached 300 μm compared with 38 % for the wild-type pollen tubes (Fig. 4H), and 3 0 % of the sia2-1+/– pollen tubes were <100 μm in length. For wild-type pollen tubes, only 8 % were <100 μm in length. These data suggest that the differences observed between wild-type and sia2-1+/– pollen may be due to the disruption of SIA2 expression in the mutant pollen grains. Immunolabelling of pollen tubes from the sia2-1+/– plants with the RG-II-specific antibody showed no visible difference between the short and normal pollen tubes (Fig. 4I, J), which may suggest that the epitopes recognized by the antibody are still synthesized and incorporated in the cell wall. However, we cannot rule out that the short pollen tubes were wild type. To assess the function of SIA2 more precisely, another mutant, sia2-2+/– in the qrt1 background with a GUS reporter gene, was studied which allowed the discrimination between wild-type and mutant pollen grains within a tetrad.
Phenotypic characterization of in vitro grown qrt1 × sia2-2 pollen
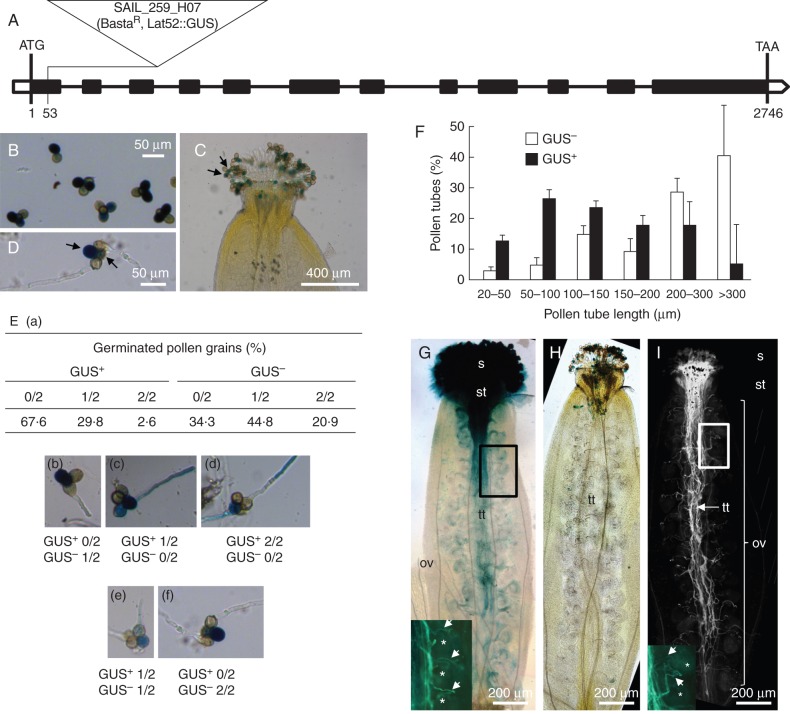
The sia2-2 mutant (SAIL_259_H07) has a T-DNA insertion in the first intron (53 bp downstream of the start codon) in the arabidopis qrt background (Fig. 5A). Surprisingly, in contrast to data obtained in sia2-1+/–, overexpression of SIA2 in the inflorescences of qrt1 × sia2-2+/– plants compared with the qrt1 plants was observed by RT-qPCR. This could be due to the pCSA110 vector used to generate the SAIL lines, which contains a 1′2′ bidirectional promoter at the left border of the T-DNA leading to overexpression or antisense RNA production (Ülker et al., 2008). Moreover, insertion near the transcription start site, as in qrt1 × sia2-2+/–, can create alternative transcripts (Missihoun et al., 2012) which have a lower stability and undergo a post-transcriptional degradation that prevents their translation into protein. It is noteworthy that, as described by Wang (2008), the transcript level may not be correlated with the protein level.
Fig. 5.
Characterization of the qrt1 × sia2-2 mutant. (A) Genomic organization of the SIA2–2 gene and location of the T-DNA insertion site. The black boxes indicate the exons. (B) GUS staining of the qrt1 × sia2-2+/– tetrads showing two GUS+ and two GUS– pollen grains. (C) GUS staining of the qrt1 × sia2-2+/– pollen grains on the stigma. Arrows show the two GUS+ pollen grains in the tetrad. (D) GUS staining of the qrt1 × sia2-2+/– pollen grains and pollen tubes grown for 6 h. Arrows show the two GUS+ pollen grains. (E) Percentages of GUS+ and GUS– germinated pollen grains in qrt1 × sia2-2+/– tetrads (n >800 grains). (a) Table summarizing the percentages of pollen tubes growing from the two GUS+ and the two GUS– pollen grains of the tetrads. (b–f) Images representing the different cases summarized in the table (a). (F) Distribution of the pollen tube length between the GUS+ and GUS– in qrt1 × sia2-2+/– tetrads after 6 h of culture (n >200). (G) GUS staining of a self-pollinated qrt1 plant showing pollen grains on the stigma and the in vivo growth of pollen tubes in the transmitting tract. Pollen tubes have reached the base of the ovary. The close-up picture at the bottom left shows the aniline blue staining of the same pistil. Arrows indicate pollen tubes, and asterisks indicate ovules. (H, I) GUS (H) and aniline blue (I) staining of a self-pollinated qrt1 × sia2-2+/– plant showing GUS+ and GUS– pollen grains on the stigma (H). No GUS+ pollen tubes were visible in the transmitting tract (H) but GUS– pollen tubes were stained with aniline blue and able to produce pollen tubes that reached the base of the ovary (I). The close-up image at the bottom left in (I) shows pollen tubes (arrows) and ovules (asterisks). ov, ovary; s, stigma; st, style; tt, transmitting tract.
At the mature stage, the qrt1 mutant releases tetrads as the microspores fail to separate during pollen development (Rhee and Somerville, 1998) without affecting pollen tube growth considerably (Boavida and McCormick, 2007). As the T-DNA contains a GUS reporter gene under the pollen LAT-52 promoter, mutant pollen can be discriminated from the wild-type pollen within a single tetrad after GUS staining (Fig. 5B, C). This allows the unambiguous in vitro and in vivo phenotypic study of the qrt1 × sia2-2+/– mutant pollen tubes (Fig. 5D). About 67 % of the GUS+ pollen failed to germinate (Fig. 5Ea, b, f) whereas only 2·6 % of the tetrads produced two GUS+ pollen tubes (Fig. 5Ea, d). Of the two GUS– pollen grains (wild type) in the tetrads, 65·7 % had produced one or two tubes (Fig. 5Ea, b, e, f). When the GUS+ pollen grains had produced a pollen tube, the tubes were much shorter than the GUS– pollen tubes (Fig. 5F). Approximately 50 % of the GUS+ pollen tubes were 50–150 μm long after 6 h of growth whereas 40 % of the wild-type pollen tubes (GUS–) were >300 μm long.
In vivo study of pollen tube elongation in qrt1 × sia2-2
The GUS staining of the self-pollinated qrt1 plants revealed that the pollen tubes were able to grow normally inside the transmitting tract, to reach the base of the ovary and allow double fertilization (Fig. 5G). On the other hand, the GUS+ qrt1 × sia2-2+/– pollen grains and pollen tubes were restricted to the stigma and style (Fig. 5H) and no pollen tubes were detected in the transmitting tract of the ovary. However, aniline blue staining of the same pistil revealed that pollen tubes were growing in the transmitting tract and have reached the base of the ovary (Fig. 5I), indicating that the GUS– pollen tubes (wild type) were able to perform the fertilization but the GUS+ qrt1 × sia2-2 pollen tubes were not.
DISCUSSION
Sialyltransferase-like sequences belonging to the GT29 family are predicted to occur in plant genomes. These proteins contain the four conserved sialyl motifs of mammalian sialyltransferases (Audry et al., 2011). Sialic acids are acidic sugars involved in multiple functions in mammals. Since endogenous sialyltransferase activity has not been detected in plants (Séveno et al., 2004), it was postulated that these transferases could be involved in the transfer of Kdo and/or Dha on the HG backbone of RG-II, considering that sialic acid and Kdo transferases share common features such as the use of CMP-activated nucleotide sugars as substrates (Voxeur et al., 2012). Studying the biological function of Kdo transferases is not achievable through an enzymatic assay since the nucleotide sugar CMP-Kdo, required for the bioassay, is an unstable compound (Belunis et al., 1995). The activated form of Dha is as yet unidentified, although it is likely to be CMP-Dha as for other phosphoenolpyruvate-derived monosaccharides.
In arabidopsis, two sialyltransferase-like sequences, encoded by At1g08660 and At3g48820, are predicted. These two transferases were found in the Golgi apparatus, as expected for GTs involved in the biosynthesis of non-cellulosic cell wall polysaccharides (Dunkley et al., 2006; Daskalova et al., 2009). Furthermore, these two candidate genes were selected from a bioinformatic study based on the selection of candidate GT genes that are tightly co-expressed in rice and arabidopsis with previously characterized genes encoding enzymes involved in the synthesis of RG-II (Voxeur et al., 2012). Deng et al. (2010) have investigated the pollen growth features in one of these two putative sialyltransferase-like proteins encoded by At1g08660. The isolated heterozygous mgp2 mutant (named sia1+/– in this study) had a loss of male gametophytic function without any effect on the female gametophyte. In vitro, the mutant pollen grains failed to germinate or the pollen tubes either burst, were short or had an abnormal shape. In vivo, the mutant pollen tubes were restricted to the stigma 24 h after pollination and could not reach the ovules, whereas wild-type pollen tubes had already grown to the base of the ovary (Deng et al., 2010). In our study, the same conclusions are drawn for the two sia2 mutant lines. Mutation in the SIA2 gene has a dramatic effect in vitro on the stability of the pollen tube cell wall. This results in a large number of pollen tubes that burst in the tip region or showed a significantly reduced length. This effect was correlated in vivo with the inability of the pollen tubes to grow further down than the style, possibly explaining the lack of homozygous line.
From the conclusions on both the sia1+/– and qrt1 × sia2+/– mutants, it is worth noting that no functional compensation was observed between the two SIA1 and SIA2 sequences, which may suggest that the two enzymes are responsible for either Kdo or Dha transfers. The strong homology between the two transferases does not allow the discrimination between the two biosynthetic pathways. Furthermore, two CMP-sialic acid transporter-like proteins are predicted in plant genomes (Bakker et al., 2008; Daskalova et al., 2009) one of which was shown to complement the transport of CMP-sialic acid in CHO Lec2 mutant cells which were unable to transport CMP-sialic acid to the Golgi lumen (Bakker et al., 2008). As a consequence, plant CMP-sialic acid transporter-like proteins are probably able to transport other CMP-nucleotide sugars such as CMP-Kdo. As observed for SIA1 and SIA2, T-DNA insertion lines of A. thaliana targeting these genes exhibited a lethal phenotype (Takashima et al., 2009). Based on these observations, we postulate that Kdo and Dha are synthesized, transported and integrated into the RG-II side chains through two independent pathways.
Many studies have shown that the integrity of the cell wall is important for pollen tube growth and for the tube to resist the turgor pressure. Studies on single or double pollen mutants affected in the biosynthesis of more abundant polymers than RG-II such as cellulose (Wang et al., 2011) and HG (Wang et al., 2013) resulted in abnormal pollen tube shape with a swollen tip and/or bursting of the pollen tubes in the tip region. The same phenotypes were observed on mutant pollen tubes impaired in cell wall remodelling enzymes such as pectin methylesterases (PMEs) (Jiang et al., 2005; Tian et al., 2006) or by exogenous application to pollen grains or pollen tubes of moderate concentrations of pectinase, cellulase, lyticase, PME (Parre and Geitmann, 2005a, b), a PME inhibitor (Woriedh et al., 2013; Paynel et al., 2014) or drugs such as the cellulose inhibitor isoxaben (Lazzaro et al., 2003). All these data indicate that the proper biosynthesis, assembly and remodelling of the polymers in the pollen tube cell wall need to be tightly controlled and are required to create a network sufficiently rigid to support internal pressure but with adequate plasticity at the tip to promote fast growth.
Conclusions
Our study and three others (Delmas et al., 2008; Deng et al., 2010; Liu et al., 2011) have indicated that mutations in genes coding for proteins possibly implicated in the machinery of building RG-II have a dramatic effect on the integrity of the cell wall and correct pollen tube elongation. However, more studies are required to verify if the RG-II structure is impaired in the mutant pollen tubes, but the low abundance of this motif in the cell wall of pollen tubes, the high levels of burst tubes and short pollen tubes, and the mixture of mutant and wild-type pollen tubes will make this task very difficult. Finally, we lack conclusive evidence for Dha or Kdo transferase activity of SIA. It will require the setting up of an appropriate bioassay by incubating the catalytic domains of SIA1 and SIA2 proteins with short HG chains harbouring or not the A, the B or both side chains, and CMP-Kdo synthesized in vitro by incubating Kdo, CTP and a bacterial CMP-Kdo synthase as reported in the study of the biosynthesis of bacterial lipopolysaccharides (White et al., 1997).
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
This work was supported by the University of Rouen and the ‘Trans Channel Wallnet’ project that was selected by the INTERREG IVA program France (Channel)–England European cross-border co-operation programme, which is co-financed by the ERDF. The authors are grateful to the Grand Réseau de Recherche VASI de Haute Normandie for the use of equipment, to Mark Johnson (Brown University, USA) for the seeds of the pLAT-52::GUS quartet mutant line, to Corinne Loutelier-Bourhis for the GC-EIMS analysis, to Hélène Dauchel for her advice on bioinformatics, and to Gaëtan Vannier for preliminary results. We also thank the Molecular Biology Platform (CRRBM) of the UPJV for scientific and technical support with the RT-qPCR.
LITERATURE CITED
- Abramoff MD, Magalhães PJ, Ram SJ. Image processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- Audry M, Jeanneau C, Imberty A, Harduin-Lepers A, Delannoy P, Breton C. Current trends in the structure–activity relationships of sialyltransferases. Glycobiology. 2011;21:716–726. doi: 10.1093/glycob/cwq189. [DOI] [PubMed] [Google Scholar]
- Bakker H, Routier F, Ashikov A, Neumann D, Bosch D, Gerardy-Schahn R. A CMP-sialic acid transporter cloned from Arabidopsis thaliana. Carbohydrate Research. 2008;343:2148–2152. doi: 10.1016/j.carres.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Bar-Peled M, Urbanowicz BR, O'Neill MA. The synthesis and origin of the pectic polysaccharide rhamnogalacturonan II – Insights from nucleotide sugar formation and diversity. Frontiers in Plant Science. 2012;3:1–12. doi: 10.3389/fpls.2012.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belunis CJ, Clementz T, Carty SM, Raetz CRH. Inhibition of lipopolysaccharide biosynthesis and cell growth following inactivation of the kdtA gene in Escherichia coli. Journal of Biological Chemistry. 1995;270:27646–27652. doi: 10.1074/jbc.270.46.27646. [DOI] [PubMed] [Google Scholar]
- Blevins DG, Lukaszewski KM. Boron in plant structure and function. Annual Review of Plant Physiology and Plant Molecular Biology. 1998;49:481–500. doi: 10.1146/annurev.arplant.49.1.481. [DOI] [PubMed] [Google Scholar]
- Boavida LC, McCormick S. Temperature as a determinant factor for increased and reproducible in vitro pollen germination in Arabidopsis thaliana. The Plant Journal. 2007;52:570–582. doi: 10.1111/j.1365-313X.2007.03248.x. [DOI] [PubMed] [Google Scholar]
- Brewbaker JL, Kwack BH. The essential role of calcium ion in pollen germination and pollen tube growth. American Journal of Botany. 1963;50:859. [Google Scholar]
- Chebli Y, Kaneda M, Zerzour R, Geitmann A. The cell wall of the Arabidopsis pollen tube – spatial distribution, recycling, and network formation of polysaccharides. Plant Physiology. 2012;160:1940–1955. doi: 10.1104/pp.112.199729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, McComb JA, Rerkasem B. Techniques to study the anther in wheat. In: Mann CE, Rerkasem B, editors. Wheat Special Report No. 11. Boron deficiency in wheat. Mexico: CIMMYT; 1992. pp. 32–33. [Google Scholar]
- Covey PA, Subbaiah CC, Parsons RL, et al. A pollen-specific RALF from tomato that regulates pollen tube elongation. Plant Physiology. 2010;153:703–715. doi: 10.1104/pp.110.155457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S, Chen T, Chong K, Xue Y, Liu S, Wang T. Proteomics identification of differentially expressed proteins associated with pollen germination and tube growth reveals characteristics of germinated Oryza sativa pollen. Molecular and Cellular Proteomics. 2006;6:207–230. doi: 10.1074/mcp.M600146-MCP200. [DOI] [PubMed] [Google Scholar]
- Dardelle F, Lehner A, Ramdani Y, et al. Biochemical and immunocytological characterizations of Arabidopsis pollen tube cell wall. Plant Physiology. 2010;153:1563–1576. doi: 10.1104/pp.110.158881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvill AG, McNeil M, Albersheim P. Structure of plant cell walls. Plant Physiology. 1978;62:418–422. doi: 10.1104/pp.62.3.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalova SM, Pah AR, Baluch DP, Lopez LC. The Arabidopsis thaliana putative sialyltransferase resides in the Golgi apparatus but lacks the ability to transfer sialic acid. Plant Biology. 2009;11:284–299. doi: 10.1111/j.1438-8677.2008.00138.x. [DOI] [PubMed] [Google Scholar]
- Delmas F, Séveno M, Northey JGB, et al. The synthesis of the rhamnogalacturonan II component 3-deoxy-d-manno-2-octulosonic acid (Kdo) is required for pollen tube growth and elongation. Journal of Experimental Botany. 2008;59:2639–2647. doi: 10.1093/jxb/ern118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Wang W, Li W-Q, et al. MALE GAMETOPHYTE DEFECTIVE 2, encoding a sialyltransferase-like protein, is required for normal pollen germination and pollen tube growth in Arabidopsis. Journal of Integrative Plant Biology. 2010;52:829–843. doi: 10.1111/j.1744-7909.2010.00963.x. [DOI] [PubMed] [Google Scholar]
- Doco T, O'Neill MA, Pellerin P. Determination of the neutral and acidic glycosyl-residue compositions of plant polysaccharides by GC-EI-MS analysis of the trimethylsilyl methyl glycoside derivatives. Carbohydrate Polymers. 2001;46:249–259. [Google Scholar]
- Dunkley TPJ, Hester S, Shadforth IP, et al. Mapping the Arabidopsis organelle proteome. Proceedings of the National Academy of Sciences, USA; 2006. pp. 6518–6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edashige Y, Ishii T. Rhamnogalacturonan II from cell walls of Cryptomeria japonica. Phytochemistry. 1998;49:681–690. doi: 10.1016/s0031-9422(98)00238-6. [DOI] [PubMed] [Google Scholar]
- Egelund J, Petersen BL, Motawia MS, et al. Arabidopsis thaliana RGXT1 and RGXT2 encode Golgi-localized (1,3)-alpha-d-xylosyltransferases involved in the synthesis of pectic rhamnogalacturonan-II. The Plant Cell. 2006;18:2593–2607. doi: 10.1105/tpc.105.036566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egelund J, Damager I, Faber K, Olsen C-E, Ulvskov P, Petersen BL. Functional characterisation of a putative rhamnogalacturonan II specific xylosyltransferase. FEBS Letters. 2008;582:3217–3222. doi: 10.1016/j.febslet.2008.08.015. [DOI] [PubMed] [Google Scholar]
- Fang K, Wang Y, Yu T, et al. Isolation of de-exined pollen and cytological studies of the pollen intines of Pinus bungeana Zucc. Ex Endl. and Picea wilsonii Mast. Flora. 2008;203:332–340. [Google Scholar]
- Ganie MA, Akhter F, Bhat MA, et al. Boron – a critical nutrient element for plant growth and productivity with reference to temperate fruits. Current Science. 2013;104:76–85. [Google Scholar]
- García-Hernández ER, Cassab Lόpez GI. Structural cell wall proteins from five pollen species and their relationship with boron. Brazilian Journal of Plant Physiology. 2005;17:375–381. [Google Scholar]
- Goldbach HE, Wimmer MA. Boron in plants and animals: is there a role beyond cell wall structure? Journal of Plant Nutrition and Soil Science. 2007;170:39–48. [Google Scholar]
- Gutierrez L, Bussell JD, Pacurar DI, Schwambach J, Pacurar M, Bellini C. Phenotypic plasticity of adventitious rooting in Arabidopsis is controlled by complex regulation of AUXIN RESPONSE FACTOR transcripts and microRNA abundance. The Plant Cell. 2009;21:3119–3132. doi: 10.1105/tpc.108.064758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdaway-Clarke TL, Weddle NM, Kim S, et al. Effect of extracellular calcium, pH and borate on growth oscillations in Lilium formosanum pollen tubes. Journal of Experimental Botany. 2003;54:65–72. doi: 10.1093/jxb/erg004. [DOI] [PubMed] [Google Scholar]
- Ishii T, Matsunaga T. Isolation and characterization of a boron–rhamnogalacturonan-II complex from cell walls of sugar beet pulp. Carbohydrate Research. 1996;284:1–9. [Google Scholar]
- Jayaprakash P, Sarla N. Development of an improved medium for germination of Cajanus cajan (L.) Millsp. pollen in vitro. Journal of Experimental Botany. 2001;52:851–855. doi: 10.1093/jexbot/52.357.851. [DOI] [PubMed] [Google Scholar]
- Jiang L, Yang S-L, Xie L-F, et al. VANGUARD1 encodes a pectin methylesterase that enhances pollen tube growth in the Arabidopsis style and transmitting tract. The Plant Cell. 2005;17:584–596. doi: 10.1105/tpc.104.027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MA, Lord E. Extracellular guidance cues and intracellular signalling pathways that direct pollen tube growth. In: Malhó R, editor. Plant Cell Monographs. The pollen tube. Berlin: Springer; 2006. pp. 223–242. [Google Scholar]
- Johnson-Brousseau SA, McCormick S. A compendium of methods useful for characterizing Arabidopsis pollen mutants and gametophytically-expressed genes. The Plant Journal. 2004;39:761–775. doi: 10.1111/j.1365-313X.2004.02147.x. [DOI] [PubMed] [Google Scholar]
- Kakani VG, Reddy KR, Koti S, et al. Differences in in vitro pollen germination and pollen tube growth of cotton cultivars in response to high temperature. Annals of Botany. 2005;96:59–67. doi: 10.1093/aob/mci149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S, Ishii T, Matsunaga T. A boron–rhamnogalacturonan-II complex from bamboo shoot cell walls. Phytochemistry. 1997;44:243–248. [Google Scholar]
- Kobayashi M, Kouzu N, Inami A, et al. Characterization of Arabidopsis CTP:3-deoxy-d-manno-2-octulosonate cytidylyltransferase (CMP-KDO synthetase), the enzyme that activates KDO during rhamnogalacturonan II biosynthesis. Plant and Cell Physiology. 2011;52:1832–1843. doi: 10.1093/pcp/pcr120. [DOI] [PubMed] [Google Scholar]
- Lazzaro MD, Donohue JM, Soodavar FM. Disruption of cellulose synthesis by isoxaben causes tip swelling and disorganizes cortical microtubules in elongating conifer pollen tubes. Protoplasma. 2003;220:201–207. doi: 10.1007/s00709-002-0042-7. [DOI] [PubMed] [Google Scholar]
- Lee S-H, Kim W-S, Han T-H. Effects of post-harvest foliar boron and calcium applications on subsequent season's pollen germination and pollen tube growth of pear (Pyrus pyrifolia) Scientia Horticulturae. 2009;122:77–82. [Google Scholar]
- Lehner A, Dardelle F, Soret-Morvan O, Lerouge P, Driouich A, Mollet J-C. Pectins in the cell wall of Arabidopsis thaliana pollen tube and pistil. Plant Signaling and Behavior. 2010;5:1282–1285. doi: 10.4161/psb.5.10.13040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Choi W-G, Wallace IS, Baudry J, Roberts DM. Arabidopsis thaliana NIP7.1: an anther-specific boric acid transporter of the aquaporin superfamily regulated by an unusual tyrosine in helix 2 of the transport pore. Biochemistry. 2011;50:6633–6641. doi: 10.1021/bi2004476. [DOI] [PubMed] [Google Scholar]
- Liu L, Huang L, Li Y. Influence of boric acid and sucrose on the germination and growth of Areca pollen. American Journal of Plant Sciences. 2013;4:1669–1674. [Google Scholar]
- Liu X-L, Liu L, Niu Q-K, et al. MALE GAMETOPHYTE DEFECTIVE 4 encodes a rhamnogalacturonan II xylosyltransferase and is important for growth of pollen tubes and roots in Arabidopsis. The Plant Journal. 2011;65:647–660. doi: 10.1111/j.1365-313X.2010.04452.x. [DOI] [PubMed] [Google Scholar]
- Loraine AE, McCormick S, Estrada A, Patel K, Qin P. RNA-Seq of Arabidopsis pollen uncovers novel transcription and alternative splicing. Plant Physiology. 2013;162:1092–1109. doi: 10.1104/pp.112.211441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoh T, Takasaki M, Takabe K, Kobayashi M. Immunocytochemistry of rhamnogalacturonan II in cell walls of higher plants. Plant and Cell Physiology. 1998;39:483–491. [Google Scholar]
- Matsunaga T, Ishii T, Matsumoto S, et al. Occurrence of the primary cell wall polysaccharide rhamnogalacturonan II in pteridophytes, lycophytes, and bryophytes. Implications for the evolution of vascular plants. Plant Physiology. 2004;134:339–351. doi: 10.1104/pp.103.030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee RJ, Baggett JR. Unequal growth rate of pollen tubes from normal and stringless pea genotypes. HortScience. 1992;27:833–834. [Google Scholar]
- McKenna ST, Kunkel JG, Bosch M, et al. Exocytosis precedes and predicts the increase in growth in oscillating pollen tubes. The Plant Cell. 2009;21:3026–3040. doi: 10.1105/tpc.109.069260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missihoun TD, Kirch H-H, Bartels D. T-DNA insertion mutants reveal complex expression patterns of the aldehyde dehydrogenase 3H1 locus in Arabidopsis thaliana. Journal of Experimental Botany. 2012;63:3887–3898. doi: 10.1093/jxb/ers081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohnen D. Pectin structure and biosynthesis. Current Opinion in Plant Biology. 2008;11:266–277. doi: 10.1016/j.pbi.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Mollet J-C, Faugeron C, Morvan H. Cell adhesion, separation and guidance in compatible plant reproduction. Annual Plant Reviews. 2007;25:69–90. [Google Scholar]
- Mollet J-C, Leroux C, Dardelle F, Lehner A. Cell wall composition, biosynthesis and remodelling during pollen tube growth. Plants. 2013;2:107–147. doi: 10.3390/plants2010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi K, Yasumori M, Imai T, et al. bor1–1, an Arabidopsis thaliana mutant that requires a high level of boron. Plant Physiology. 1997;115:901–906. doi: 10.1104/pp.115.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill MA, Warrenfeltz D, Kates K, et al. Rhamnogalacturonan-II, a pectic polysaccharide in the walls of growing plant cell, forms a dimer that is covalently cross-linked by a borate ester. In vitro conditions for the formation and hydrolysis of the dimer. Journal of Biological Chemistry. 1996;271:22923–22930. doi: 10.1074/jbc.271.37.22923. [DOI] [PubMed] [Google Scholar]
- O'Neill MA, Eberhard S, Albersheim P, Darvill AG. Requirement of borate cross-linking of cell wall rhamnogalacturonan II for Arabidopsis growth. Science. 2001;294:846–849. doi: 10.1126/science.1062319. [DOI] [PubMed] [Google Scholar]
- O'Neill MA, Ishii T, Albersheim P, Darvill AG. Rhamnogalacturonan II: structure and function of a borate cross-linked cell wall pectic polysaccharide. Annual Review of Plant Biology. 2004;55:109–139. doi: 10.1146/annurev.arplant.55.031903.141750. [DOI] [PubMed] [Google Scholar]
- Pabst M, Fischl RM, et al. Rhamnogalacturonan II structure shows variation in the side chains monosaccharide composition and methylation status within and across different plant species. The Plant Journal. 2013;76:61–72. doi: 10.1111/tpj.12271. [DOI] [PubMed] [Google Scholar]
- Palanivelu R, Tsukamoto T. Pathfinding in angiosperm reproduction: pollen tube guidance by pistils ensures successful double fertilization. Developmental Biology. 2012;1:96–113. doi: 10.1002/wdev.6. [DOI] [PubMed] [Google Scholar]
- Parre E, Geitmann A. More than a leak sealant. The mechanical properties of callose in pollen tubes. Plant Physiology. 2005a;137:274–286. doi: 10.1104/pp.104.050773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parre E, Geitmann A. Pectin and the role of the physical properties of the cell wall in pollen tube growth of Solanum chacoense. Planta. 2005b;220:582–592. doi: 10.1007/s00425-004-1368-5. [DOI] [PubMed] [Google Scholar]
- Paynel F, Leroux C, Surcouf O, Schaumann A, Pelloux J, Driouich A, Mollet JC, Lerouge P, Lehner A, Mareck A. Kiwi fruit PMEI inhibits PME activity, modulates root elongation and induces pollen tube burst in Arabidopsis thaliana. Plant Growth Regulation. 2014 doi:10.1007/s10725-014-9919-7. [Google Scholar]
- Pérez S, Rodríguez-Carvajal MA, Doco T. A complex plant cell wall polysaccharide: rhamnogalacturonan II. A structure in quest of a function. Biochimie. 2003;85:109–121. doi: 10.1016/s0300-9084(03)00053-1. [DOI] [PubMed] [Google Scholar]
- Persia D, Cai G, Casino CD, Faleri C, Willemse MTM, Cresti M. Sucrose synthase is associated with the cell wall of tobacco pollen tubes. Plant Physiology. 2008;147:1603–1618. doi: 10.1104/pp.108.115956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pina C. Gene family analysis of the Arabidopsis pollen transcriptome reveals biological implications for cell growth, division control, and gene expression regulation. Plant Physiology. 2005;138:744–756. doi: 10.1104/pp.104.057935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts B, Marsden-Smedley J. In vitro germination of Eucalyptus pollen: response to variation in boric acid and sucrose. Australian Journal of Botany. 1989;37:429–441. [Google Scholar]
- Prajapati PP, Jain BK. Effect of sucrose, boron, calcium, magnesium and nitrate during in vitro pollen germination in Luffa aegyptica Mill. Prajna. 2010;18:5–8. [Google Scholar]
- Rhee SY, Somerville CR. Tetrad pollen formation in quartet mutants of Arabidopsis thaliana is associated with persistence of pectic polysaccharides of the pollen mother cell wall. The Plant Journal. 1998;15:79–88. doi: 10.1046/j.1365-313x.1998.00183.x. [DOI] [PubMed] [Google Scholar]
- Roberts IN, Gaude TC, Harrod G, Dickinson HG. Pollen–stigma interactions in Brassica oleracea; a new pollen germination medium and its use in elucidating the mechanism of self incompatibility. Theoretical and Applied Genetics. 1983;65:231–238. doi: 10.1007/BF00308074. [DOI] [PubMed] [Google Scholar]
- Rounds CM, Winship LJ, Hepler PK. Pollen tube energetics: respiration, fermentation and the race to the ovule. AoB Plants. 2011;2011:plr019. doi: 10.1093/aobpla/plr019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber DN, Dresselhaus T. In vitro pollen germination and transient transformation of Zea mays and other plant species. Plant Molecular Biology Reporter. 2003;21:31–41. [Google Scholar]
- Séveno M, Bardor M, Paccalet T, Gomord V, Lerouge P, Faye L. Glycoprotein sialylation in plants? Nature Biotechnology. 2004;22:1351–1352. doi: 10.1038/nbt1104-1351. [DOI] [PubMed] [Google Scholar]
- Shimokawa T, Ishii T, Matsunaga T. Isolation and structural characterization of rhamnogalacturonan II–borate complex from Pinus densiflora. Journal of Wood Science. 1999;45:435–439. [Google Scholar]
- Takashima S, Seino J, Nakano T, et al. Analysis of CMP-sialic acid transporter-like proteins in plants. Phytochemistry. 2009;70:1973–1981. doi: 10.1016/j.phytochem.2009.09.017. [DOI] [PubMed] [Google Scholar]
- Tian G-W, Chen M-H, Zaltsman A, Citovsky V. Pollen-specific pectin methylesterase involved in pollen tube growth. Developmental Biology. 2006;294:83–91. doi: 10.1016/j.ydbio.2006.02.026. [DOI] [PubMed] [Google Scholar]
- Ülker B, Peiter E, Dixon DP, et al. Getting the most out of publicly available T-DNA insertion lines. The Plant Journal. 2008;56:665–677. doi: 10.1111/j.1365-313X.2008.03608.x. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology. 2002;3:research0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal S, Williams P, O'Neill MA, Pellerin P. Polysaccharides from grape berry cell walls. Part I : tissue distribution and structural characterization of the pectic polysaccharides . Carbohydrate Polymers. 2001;45:315–323. [Google Scholar]
- Vižintin L, Bohanec B. In vitro manipulation of cucumber (Cucumis sativus L.) pollen and microspores: isolation procedures, viability tests, germination, maturation. Acta Biologica Cracoviensia Series Botanica. 2004;46:177–183. [Google Scholar]
- Voxeur A, Gilbert L, Rihouey C, et al. Silencing of the GDP-d-mannose 3,5-epimerase affects the structure and cross-linking of the pectic polysaccharide rhamnogalacturonan II and plant growth in tomato. Journal of Biological Chemistry. 2011;286:8014–8020. doi: 10.1074/jbc.M110.198614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voxeur A, André A, Breton C, Lerouge P. Identification of putative rhamnogalacturonan-II specific glycosyltransferases in Arabidopsis using a combination of bioinformatics approaches. PLoS One. 2012;7:e51129. doi: 10.1371/journal.pone.0051129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wang W, Wang Y-Q, et al. Arabidopsis galacturonosyltransferase (GAUT) 13 and GAUT14 have redundant functions in pollen tube growth. Molecular Plant. 2013;6:1131–1148. doi: 10.1093/mp/sst084. [DOI] [PubMed] [Google Scholar]
- Wang Q, Lu L, Wu X, Li Y, Lin J. Boron influences pollen germination and pollen tube growth in Picea meyeri. Tree Physiology. 2003;23:345–351. doi: 10.1093/treephys/23.5.345. [DOI] [PubMed] [Google Scholar]
- Wang W, Wang L, Chen C, et al. Arabidopsis CSLD1 and CSLD4 are required for cellulose deposition and normal growth of pollen tubes. Journal of Experimental Botany. 2011;62:5161–5177. doi: 10.1093/jxb/err221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH. How effective is T-DNA insertional mutagenesis in Arabidopsis? Journal of Biochemical Technology. 2008;1:11–20. [Google Scholar]
- Warrington K. The effect of boric acid and borax on the broad bean and certain other plants. Annals of Botany. 1923;37:629–672. [Google Scholar]
- Whitcombe AJ, O'Neill MA, Steffan W, Albersheim P, Darvill AG. Structural characterization of the pectic polysaccharide, rhamnogalacturonan-II. Carbohydrate Research. 1995;271:15–29. doi: 10.1016/0008-6215(94)00002-w. [DOI] [PubMed] [Google Scholar]
- White KA, Kaltashov IA, Cotter RJ, Raetz CR. A mono-functional 3-deoxy-d-manno-octulosonic acid (Kdo) transferase and a Kdo kinase in extracts of Haemophilus influenzae. Journal of Biological Chemistry. 1997;272:16555–16563. doi: 10.1074/jbc.272.26.16555. [DOI] [PubMed] [Google Scholar]
- Woriedh M, Wolf S, Márton ML, et al. External application of gametophyte-specific ZmPMEI1 induces pollen tube burst in maize. Plant Reproduction. 2013;26:255–266. doi: 10.1007/s00497-013-0221-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.