Abstract
Background and Aims
Brown algae are photosynthetic multicellular marine organisms evolutionarily distant from land plants, with a distinctive cell wall. They feature carbohydrates shared with plants (cellulose), animals (fucose-containing sulfated polysaccharides, FCSPs) or bacteria (alginates). How these components are organized into a three-dimensional extracellular matrix (ECM) still remains unclear. Recent molecular analysis of the corresponding biosynthetic routes points toward a complex evolutionary history that shaped the ECM structure in brown algae.
Methods
Exhaustive sequential extractions and composition analyses of cell wall material from various brown algae of the order Fucales were performed. Dedicated enzymatic degradations were used to release and identify cell wall partners. This approach was complemented by systematic chromatographic analysis to study polymer interlinks further. An additional structural assessment of the sulfated fucan extracted from Himanthalia elongata was made.
Key Results
The data indicate that FCSPs are tightly associated with proteins and cellulose within the walls. Alginates are associated with most phenolic compounds. The sulfated fucans from H. elongata were shown to have a regular α-(1→3) backbone structure, while an alternating α-(1→3), (1→4) structure has been described in some brown algae from the order Fucales.
Conclusions
The data provide a global snapshot of the cell wall architecture in brown algae, and contribute to the understanding of the structure–function relationships of the main cell wall components. Enzymatic cross-linking of alginates by phenols may regulate the strengthening of the wall, and sulfated polysaccharides may play a key role in the adaptation to osmotic stress. The emergence and evolution of ECM components is further discussed in relation to the evolution of multicellularity in brown algae.
Keywords: Brown algae, Fucales, cell wall architecture, sequential extractions, sulfated fucan, fucose-containing sulfated polysacharides, FCSP, extracellular matrix, ECM, Himanthalia elongata, plant cell wall evolution
INTRODUCTION
Land plants and macroalgae share convergent evolutionary traits, such as multicellularity and a polysaccharide-rich cell wall surrounding their cells. These two features are intrinsically interlinked as the development of an adherent extracellular matrix (ECM) probably allowed the transition from cellular autonomy to cellular co-operation, and therefore set the bases that constitute functional multicellular organisms (Popper et al., 2011). For land plants we have an extensive understanding of the structures and functions of the ECM. The cell wall contributes to cell adhesion and is therefore crucial in maintaining the physical integrity of the tissues. The ECM also exerts significant control over signalling and cell–cell communication. Positional information involves reporting to the ECM which is further integrated and used to specify growth axes and cell differentiation, thus conditioning the development of a plant. The ECM also plays a crucial role in the control of innate immunity, another common characteristic of multicellular eukaryotes. For example, pectic oligosaccharides released from the wall during wounding or pathogen attack are the best-characterized wall-derived elicitors that can lead to a defence response in land plants (Hematy et al., 2009).
Brown algae (Phaeophyceae) are a class of photoautotrophic marine macroalgae ubiquitously found on rocky intertidal shores. These organisms belong to the Stramenopiles, a phylum that comprises photosynthetic as well as non-photosynthetic members, including various protists and diatoms, but also oomycetes, a group of pseudo-fungi, many of which are notorious plant parasites such as Phytophtora (Andersen, 2004). This phylum arose about 1000 million years ago after a secondary endosymbiotic event, by which a unicellular red alga was captured by an ancestral protist (Berney and Pawlowski, 2006). Thus, brown algae evolved complex multicellularity and plant-like structures independently from the Archaeplastida phylum, which includes the red algae, green algae and land plants (Reyes-Prieto et al., 2007). The appearance of brown algae is thought to be a rather recent event dated to about 200–300 million years ago (Brown and Sorhannus, 2010; Silberfeld et al., 2010). Variations of the ECM content between land plants and brown algae are therefore evident on a phylogenetic basis, with brown algal cell walls sharing polysaccharides with both plant (cellulose) and animal (sulfated fucans) ECMs, but also with some bacteria (alginates) (Michel et al., 2010). Sulfated fucans and alginates encompass the main portion of the wall in brown algae (up to 45 % of algal dry weight), while cellulose only accounts for a small fraction (1–8 % of algal dry weight). Proteins (Quatrano and Stevens, 1976), halogenated and/or sulfated phenolic compounds known as phlorotannins (Vreeland et al., 1998; Schoenwaelder and Wiencke, 2000) and halide compounds as iodide (Verhaeghe et al., 2008) are additional components in brown algal cel walls.
Sulfated fucans are sulfated polysaccharides containing α-l-fucosyl residues. They may be present in the form of homopolymers, called homofucans or fucans, or as heteropolymers, termed fucoidans or, more appropriately, heterofucans. The term fucose-containing sulfated polysaccharides (FCSPs) is used as a more collective term for these polysaccharides (Sakai et al., 2003; Ale et al., 2011). Sulfated fucans occur in brown algae, but also in the body wall of sea cucumbers and in the egg jelly coat of sea urchins. While echinoderm fucans have regular structures composed of linear and repetitive sequences of one, two or four residues (Pereira et al., 1999), the brown algal FCSPs encompass a continuous spectrum of highly branched polysaccharides, ranging from high-uronic acid, low-sulfate-containing polymers with significant proportions of xylose, galactose and mannose, to highly sulfated homofucan molecules (Mabeau et al., 1990). Species-dependent structural variations have also been observed: most of the sulfated fucans described from Fucales contain long stretches of alternating α-(1→3) and α-(1→4) l-fucose residues bearing one and two sulfate groups, respectively (Pereira et al., 1999; Chevolot et al., 2001; Bilan et al., 2006; Colin et al., 2006), while the sulfated fucans from Laminariales (Nishino et al., 1991; Chizhov et al., 1999; Anastyuk et al., 2010) and Ectocarpales (Nagaoka et al., 1999; Ponce et al., 2003; Sakai et al., 2003) display a basic structure resembling those of marine invertebrates and based on α-(1→3) linked l-fucose residues bearing one sulfate group in C4.
Alginates are linear polymers made of two epimers, β-(1→4)-d-mannuronate (M) and α-(1→4)-l-guluronate (G), arranged in blocks along the polysaccharide chain. While M block-rich alginate does not gel in the presence of divalent cations, G block-rich alginate will form ‘egg-box’ junctions with calcium, bridging two antiparallel chains and therefore increasing the mechanical strength of the gel (Draget et al., 2005). The epimerization of the M into G residues is mediated by mannuronan C5-epimerases acting directly on the polymer chain (Haug et al., 1974; Nyvall et al., 2003). As such, alginates and mannuronan C5-epimerases are likely to determine the wall porosity and texture, and may therefore be seen as functional analogues to pectins and pectin methylesterases in land plants, respectively.
The inherent nature of the different components of the wall points toward a complex evolutionary history that shaped the ECM structure in brown algae. Ectocarpus is a genetic and genomic model for brown algae, and the in-depth analysis of its genome sequence allowed the origin and evolution of the main cell wall components of brown algae to be resolved (Cock et al., 2010; Michel et al., 2010; Meslet-Cladière et al., 2013). Molecular evidence indicate that the biosynthetic pathways for both cellulose and sulfated fucans are ancient. Cellulose biosynthesis was initially proposed to have been gained from the red algal endosymbiont during the secondary endosymbiotic event that occurred about 1200 million years ago and that gave rise to Stramenopiles (Michel et al., 2010). However, the first genome analysis of a red macroalga (Chondrus crispus) has recently refined the phylogeny of cellulose synthases (CESAs) (Collén et al., 2013). Although the red algal origin of the CESAs from oomycetes was confirmed, the CESAs from brown algae and Eustigmatophyceae (related unicellular algae) constituted an independent clade (Collén et al., 2013), indicating that cellulose biosynthesis in brown algae has an ancient but distinct origin. The biosynthetic route for sulfated fucans in Ectocarpus involves enzymes similar to their animal counterparts for the synthesis of fucose and for the sulfation of carbohydrates, indicating an ancestral eukaryotic pathway (Michel et al., 2010). The biosynthetic routes for alginates and phlorotannins evolved more recently in brown algae. They both found their roots in a massive horizontal gene transfer (HGT) event with an ancestral actinobacterium, which occurred after the divergence of the ancestor of brown algae from diatoms and oomycetes, about 200–300 million years ago (Michel et al., 2010; Meslet-Cladière et al., 2013). The acquisition of these new biosynthetic pathways in brown algae is likely to have played a key role toward the acquisition of complex multicellularity (Michel et al., 2010).
As for land plants, cell walls of brown algae are involved in a number of essential biological functions. The zygotes of Fucales species have long served as practical models to study cell polarization and asymmetrical cell division, pointing towards the role of the cell wall and targeted secretions in early morphogenesis in plant systems (Berger et al., 1994; Belanger and Quatrano, 2000; Paciorek and Bergmann, 2010). The cell wall was shown to be a source of position-dependent information required for polarization in the Fucus zygote (Kropf et al., 1988) and in cell fate determination in the two-celled embryo (Berger et al., 1994; Bouget et al., 1998). In the brown algal model Ectocarpus siliculosus, the cell wall is involved in maintaining the life cycle generation stage when the developmental programme has been engaged (Arun et al., 2013). Finally, cell wall components are thought to be involved in controlling cell differentiation in the sporophytic phase of E. siliculosus (Le Bail et al., 2011).
The cell wall was also shown to mediate innate immunity in brown algae. In Laminaria digitata, oligoalginates released from the wall elicit an oxidative burst and activation of defence responses (Kupper et al., 2001). The oxidative stress is often associated with a massive production of volatile halogenated organic compounds. Their release is catalysed by haloperoxidases, which oxidize the halide compounds present in the wall (i.e. iodide, bromide and chloride), leading to the halogenation of various organic substrates (Leblanc et al., 2006).
Polyanionic wall components in brown algae might also be major players in ionic and hydric regulation. Differences in the cation selectivity of alginate and sulfated fucans, combined with their distinct in situ localization, are likely to result in gradients of cation distribution throughout the wall (Kloareg et al., 1986). Although the molecular mechanisms involved are unknown, such gradients might confer an adaptive advantage to resist desiccation or osmotic shocks at low tides. The sulfate content of the cell wall polysaccharides in brown algae was also seen as a driving force for species zonation (Mabeau and Kloareg, 1987). In the genus Ectocarpus, one strain isolated from a genuine freshwater environment was shown to have retained its ability to grow in normal seawater (Dittami et al., 2012). The transition between high and low salinities is reversible, yet it is accompanied by profound morphological, transcriptomic and metabolic changes. Interestingly, drastic changes in gene expression were observed regarding metabolism of cell wall polysaccharides and phlorotannins (Dittami et al., 2012; Meslet-Cladière et al., 2013).
It is noteworthy also that fucans or FSCPs display a variety of biological activities in different mammalian systems, such as antithrombic and anticoagulant (Nishino and Nagumo, 1991; Albuquerque et al., 2004; Zhu et al., 2010; Camara et al., 2011), antitumour (Yamasaki-Miyamoto et al., 2009; Ale et al., 2011), antivirus (Baba et al., 1988; Ponce et al., 2003), contraceptive (Mahony et al., 1993) and antioxidant (Camara et al., 2011) properties, among others. These potent activities probably reside in the ability of the FCSPs to mimic the structure of the carbohydrate moieties of mammalian glycosaminoglycans (Nishino et al., 1991; Albuquerque et al., 2004; Anastyuk et al., 2010).
Here we describe the comprehensive analysis of the cell wall composition of five species of Fucales. Our data provide a global snapshot of the cell wall architecture in brown algae. We suggest that the FCSPs are interlocking the cellulosic scaffold while the alginate–phenol linkages are key players in regulating the rigidity of the wall. The core fucan backbone in Himanthalia elongata differs in its structure from that of other Fucales. We speculate that this variation might be related to osmotic regulation and species zonation. The emergence of ECM components is discussed in relation to the evolution of multicellularity in brown algae.
MATERIALS AND METHODS
Materials
Pelvetia canaliculata, Ascophyllum nodosum, Fucus serratus, Fucus vesiculosus and Himanthalia elongata were collected in Roscoff (Brittany, France; corresponding co-ordinates 48°43′N, 3°59′W) and used for the sequential extraction procedures. Before extractions, algae were cleaned from epiphytes and rinsed with seawater.
Chemical sequential extractions
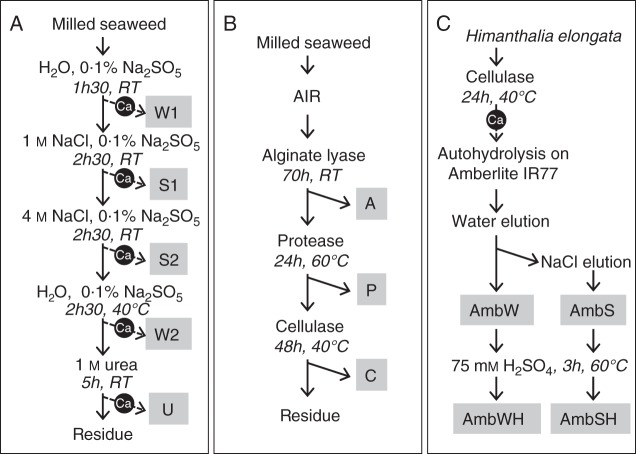
Five hundred grams of fresh algal tissues were milled and homogenized using a tissue lyser. Five extraction conditions were used (Fig. 1A): (1) two serial incubations of 45 min at room temperature in deionized water containing 0·1 % Na2SO5 as an antioxidant agent; (2) incubation of 2 h 30 min at room temperature in 1 m NaCl containing 0·1 % Na2SO5; (3) incubation of 2 h 30 min at room temperature in 4 m NaCl containing 0·1 % Na2SO5; (4) incubation of 2 h 30 min at 40 °C in deionized water containing 0·1 % Na2SO5; and (5) a final incubation of 5 h at room temperature in 1 m urea. The rationale for this was to sequentially extract polysaccharides bound to other cell wall polymers through linkages of increasing force, namely unbound compounds (1), ionically interacting compounds (2 and 3) and hydrogen-bound compounds (4, 5). Each extraction was performed in approx. 1 L of solvent and with magnetic stirring. Soluble materials were isolated by filtration, and alginates were precipitated in all extracts by the addition of calcium chloride (4 % as a final concentration). The respective supernatants (W1, S1, S2, W2 and U) were dialysed against deionized water and freeze-dried (Fig. 1A). The names given refer to the extractants used: water (W), salts (S) and urea (U), respectively.
Fig. 1.
Extraction and fractionation of the cell wall content from algal biomass. (A) Chemical sequential extraction procedure. The final extracts were obtained after elimination of the alginate content from the solubilized materials by a calcium chloride precipitation. (B) Enzymatic sequential extraction procedure. An alcohol-insoluble residue is used as a starting material. (C) Partial hydrolytic extraction procedure used to study fucan structures from Himanthalia elongata. The alginate content is removed by a calcium chloride precipitation.
Enzymatic sequential extractions
The fresh algal tissues were milled and homogenized using a tissue lyser, in a volume of boiling ethanol solution brought up to a final ethanol concentration of 70 %, taking into account the water content of the tissues. The insoluble material was repeatedly washed until colourless with 70 % ethanol, then 96 % ethanol, then acetone, and finally dried in a ventilated oven. The final residue was referred to as the alcohol-insoluble residue (AIR). Approximately 1 g of AIR was sequentially extracted by enzymes using their corresponding buffers as the resuspending solutions (Fig. 1B): (1) 2·5 U of alginate lyase (A) purified from Haliotis tuberculata digestive glands (Heyraud et al., 1996), in 100 mL of 10 mm Tris–MES pH 7, 100 mm NaCl, 20 mm MgCl2, for 70 h at room temperature; (2) 230 U of papain (P) (Sigma), in 100 mL of 100 mm sodium acetate pH 6, 5 mm EDTA, 5 mm cysteine, for 24 h at 60°C; and (3) cellulase (C; dilution 1/13, Gist Brocades) added twice at 0 h and 24 h of incubation, respectively, in 50 mL of 0·2 % sodium azide solution, for 48 h at 40 °C. The reactions were stopped by boiling and the soluble materials were recovered by filtration. The respective extracts (A, P and C) were dialysed against deionized water and freeze-dried (Fig. 1B).
Extraction and acid hydrolysis of sulfated fucans from Himanthalia elongata
Approximately 40 g of fresh algal tissue were milled and homogenized using a tissue lyser in 50 mm sodium acetate buffer pH 5·0 containing 0·2 % sodium azide. The suspension was incubated at 40 °C and cellulase (800 mg, Gist Brocades) was added successively at 0 and 24 h (Fig. 1C). The reactions were stopped by boiling and the soluble materials were recovered by filtration. Alginates were precipitated by the addition of calcium chloride (2 % final concentration). The CaCl2-soluble fraction was recovered by filtration and applied on an Amberlite IR-77 resin using H+ as a counterion (Sigma). The AmbW and AmbS extracts were recovered by water and 2 m NaCl, respectively. Extracts were neutralized with NaOH and dialysed against deionized water, and then freeze-dried. They were further hydrolysed (0·5 % final concentration) in 75 mm H2SO4 for 3 h at 60 °C. The respective extracts (AmbWH and AmbSH) were dialysed against deionized water and freeze-dried (Fig. 1C). The names given refer to the extracts hydrolysed onto the Amberlite resin (Amb) and eluted by water (AmbW) or salts (AmbS) and further hydrolysed by strong acid (AmbWH and AmbSH).
Chemical characterization
Uronic acid and fucose contents were assayed using the m-hydroxydiphenyl method (Knutson and Jeanes, 1968) and the cysteine–H2SO4 method (Dische and Shettles, 1948), respectively. Sulfate content was assayed using the Azure A method (Jaques et al., 1968). The fucan content was determined by summation of the fucose and sulfate contents. The protein and polyphenol contents were determined by the Bradford method (Bradford, 1976) and the Folin–Ciocalteu method (Singleton et al., 1999), respectively.
Size exclusion chromatography
Extracts were resuspended in water (0·5–1 %, w/v) and subjected to chromatography with 50 mm ammonium carbonate using a Superdex 200 column (Amersham Biosciences) equipped with a refractometric detector. A similar procedure was used for the AmbW, AmbS, AmbWH and AmbSH extracts using a Superdex 30 column (Amersham Biosciences).
Anion-exchange chromatography
Extracts were further fractionated on a DEAE–Sepharose CL 6B column (Amersham Biosciences, total volume 100 mL, flow rate 1·3 mL min–1, 20 °C) equilibrated with a 50 mm sodium acetate buffer pH 5. They were resuspended in the same buffer (0·5–1 %, w/v) and applied on the column. Unbound materials were first collected and anionic polymers were eluted with buffers of increasing NaCl concentrations. Hydrogen-bound compounds were released during regeneration of the column by 1 m NaOH. All eluted fractions were dialysed against deionized water before freeze-drying and chemical characterization.
Carbohydrate-polyacrylamide gel electrophoresis (C-PAGE) analyses
The oligosaccharidic patterns obtained after acidic hydrolyses of the sulfated polysaccharides from H. elongata were monitored by a C-PAGE assay (Zablackis and Perez, 1990). Briefly the corresponding fractions were electrophoresed through 6 % (w/v) stacking and 27 % running, 1 mm thick polyacrylamide gels in 50 mm Tris–HCl pH 8·7, 2 mm EDTA, and stained with Alcian Blue followed by silver nitrate (Min and Cowman, 1986). Anionic oligosaccharide bands were detected in the bottom part of the running gel.
Nuclear magnetic resonance (NMR) spectroscopy
All samples were dissolved in D2O and exchanged twice. 1H-NMR spectra were recorded at 25 °C on a Bruker Avance 500 spectrometer equipped with an indirect 5 mm triple resonance probehead TBI 1H/{BB}/13C using standard pulse sequences available in the Bruker software (NMR facility, University Bretagne Occidentale, Brest, France). Two-dimensional 1H-COSY spectra were recorded at 60 °C. Double-quantum filtered 1H–1H correlated spectroscopy (DQF COSY) was performed according to standard pulse sequences to assign 1H and 13C resonances. Chemical shifts are expressed in ppm by reference to an external standard based on the methyl signal of TSP (trimethylsilylpropionic acid).
RESULTS
Enzymatic treatments significantly increase cell wall extraction yields
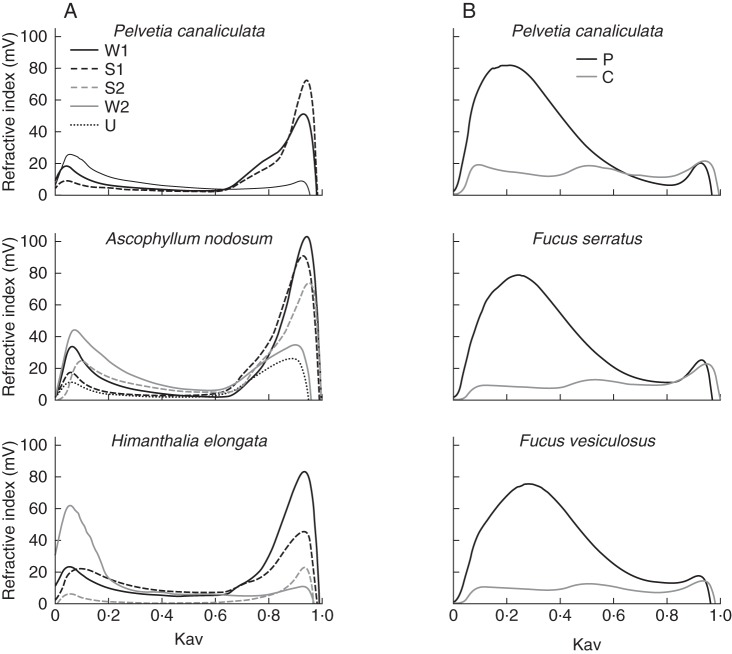
In order to study interactions between cell wall polymers, FCSPs were extracted sequentially from five Fucales brown algae by two independent procedures summarized in Fig. 1A and B. In brief, the first procedure used treatments based on ionic solutions and urea on fresh milled algae, while the second procedure used enzymatic incubations of alcohol-insoluble residues. Yields of extracted material are given in Table 1. The overall yields showed that the enzymatic sequential extraction procedure recovered about five times more cell wall material compared with the chemical sequential extraction procedure, with yields of 35–54 % and 6–9 % of the total algal dry weight, respectively. Except for H. elongata, the protease and the cellulase treatments both led to the release of large amounts of compounds (16–19 % and 16–32 % of the total algal dry weight, respectively), probably indicating that they cleaved important interlinks within the walls. In comparison, the alginate lyase digestion, while degrading a main compartment of the wall, produced the release of only a small amount of additional compounds (between 1 and 3 % of the total algal dry weight). The situation is slightly different with H. elongata where the alginate lyase treatment extracted twice as much material compared with the protease treatment, albeit in a limited range (10 and 5 % of the total algal dry weight, respectively). In the case of the chemical sequential extraction procedure, despite the low extraction yields, the profiles show that most compounds were extracted in the W1 and S1 samples, so hence were released by solutions of low ionic strengths. These results support the idea that FCSPs are tightly associated with proteins and cellulose in brown algal cell walls but not with alginates. Those interlinks limit the extractability of FCSPs, and the polymers extracted by the chemical sequential extraction procedure represent only a limited fraction of the wall.
Table 1.
Yields of sequential extraction procedures
| Extract | Extraction yields (% algal d. wt) |
||||
|---|---|---|---|---|---|
| P. caniculata | H. elongata | A. nodosum | F. vesiculosus | F. serratus | |
| Chemical sequential extraction procedure | |||||
| W1 | 2·2 | 4·4 | 2·8 | ||
| S1 | 0·9 | 2·5 | 1·5 | – | – |
| S2 | 0·3 | 0·5 | 0·7 | – | – |
| W2 | 0·3 | 0·8 | 0·8 | – | – |
| U | 2·0 | 0·4 | 0·5 | – | – |
| Total | 5·7 | 8·6 | 6·3 | – | – |
| Enzymatic sequential extraction procedure | |||||
| A | 1·6 | 9·9 | 1·4 | 2·9 | 3·2 |
| P | 17·4 | 4·9 | 18·5 | 15·6 | 19·4 |
| C | 15·6 | 21·9 | 19·2 | 21·4 | 31·7 |
| Total | 34·6 | 36·7 | 39·1 | 39·9 | 54·3 |
Associations of cell wall components
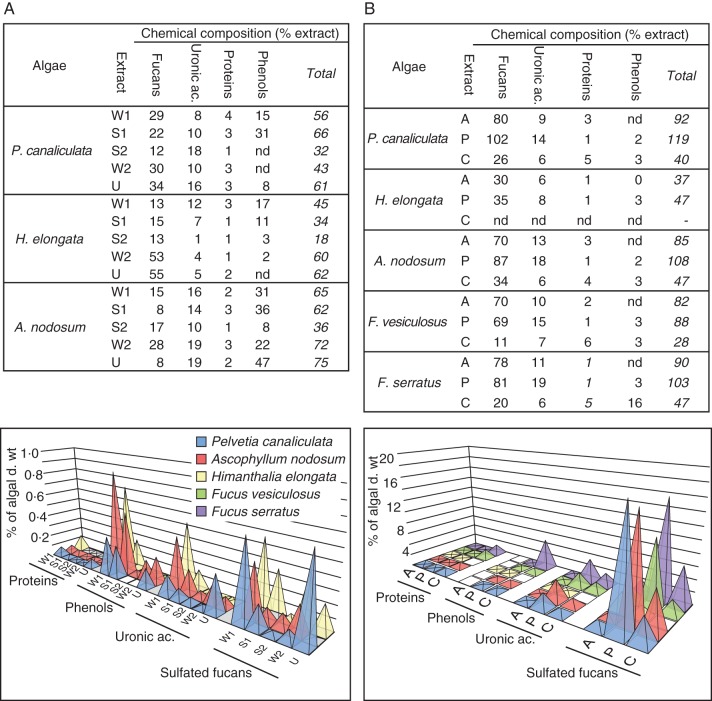
The chemical characterization revealed that most extracts can be referred to as FCSP-containing fractions, since they are mostly composed of sulfated fucans. This was confirmed by the 1H-NMR spectra which were systematically determined on all extracts discussed herein (data not shown), and which displayed the characteristic chemical shifts of fucans from Fucales observed in earlier works (Chevolot et al., 2001; Bilan et al., 2006; Colin et al., 2006). The 1H-NMR spectra discussed in more detail in Figure 6 were those obtained for the specific case of H. elongata. The resolution of the spectra was directly related to the degree of contamination of the samples by additional components. Indeed, a pure fucan fraction was never observed since as well as fucose and sulfate residues, substantial amounts of uronic acids, phenols and proteins were always observed (Fig. 2). These co-extractions were particularly pronounced in the chemical extraction sequential procedure, while the enzymatic treatments led to better purified fucan fractions. Uronic acids represented regular contaminants, and similar observations were previously reported in other studies (Kloareg and Quatrano, 1988; Ponce et al., 2003; Camara et al., 2011). Whether these originated as constitutive residues of the FSCPs or contaminating alginate blocks has not yet been determined. Phenols were mostly co-extracted during the chemical procedure where they can represent a significant portion of the extract (e.g. the W1 extract in A. nodosum contains 15 % of fucans and 31 % of phenols). Their associations with other compounds were further investigated in representative W1 and U extracts by an additional alginate lyase treatment which released most phenols (Table 2). These results support the idea of some CaCl2-soluble alginates being present in the samples and which are associated with most of the phenols from these fractions. In all extracts, proteins were observed at lower levels. This is virtually masked in the enzymatic procedure by the use of a protease treatment, which cleaved most proteins and helped the release of abundant fucan fractions.
Fig. 6.
1H-NMR spectra of the fucan fractions from H. elongata after autohydrolysis and acid hydrolysis.
Fig. 2.
Composition of sequential extracts. (A) Chemical sequential extraction procedure. (B) Enzymatic sequential extraction procedure. The tables refer to the composition of the extracts (nd, not determined). The graphs refer to the composition of the extracts with respect to the respective extraction yields as shown in Table 1. The empty squares in the graphs indicate that values were not determined.
Table 2.
Phenol composition (%) and loss observed after an alginate lyase treatment
| Extract | Alginate lyase treatment |
Corresponding loss (%) | |
|---|---|---|---|
| – | + | ||
| P. canaliculata (W1) | 15·0 | 5·7 | 62·0 |
| A. nodosum (U) | 46·7 | 14·1 | 69·8 |
| H. elongata (W1) | 16·6 | 1·7 | 89·8 |
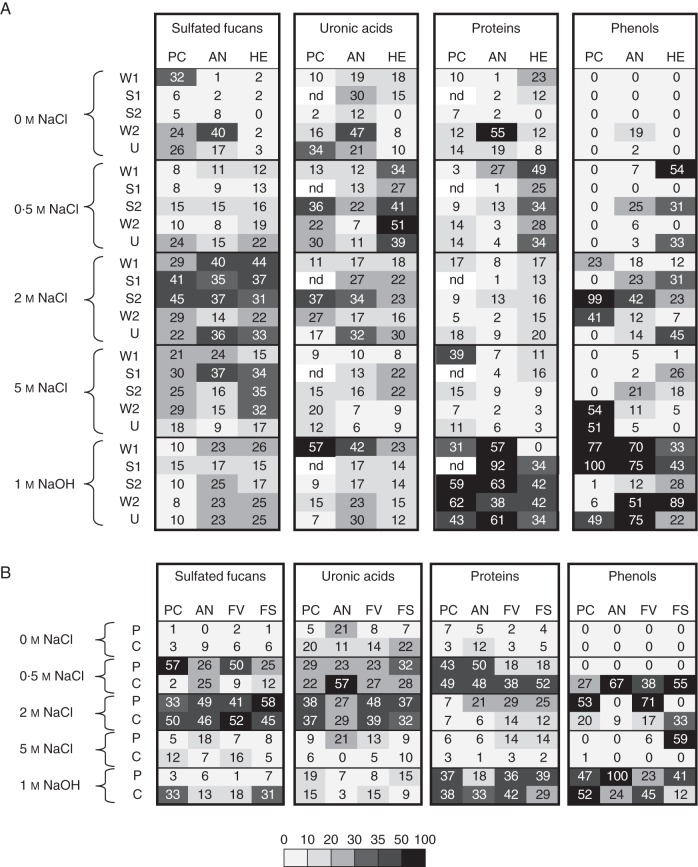
The different extracts were further fractionated by anion-exchange chromatography (AEC). All of the 23 extracts were individually loaded on the column and eluted in five AEC fractions by an NaCl gradient followed by a regenerating NaOH treatment. The resulting 115 fractions were further analysed for their chemical composition in terms of sulfated fucans, uronic acids, phenols and proteins. For each compound within an extract, a total value of 100 % was assigned, and the corresponding signals in the eluted fractions were adjusted accordingly. This helps to focus on the elution profiles for each component within an extract. In order to visualize this complex data set, results are shown as a heatmap in which the grey scale is proportional to the numerical values (Fig. 3). The four components analysed were consistently observed in most of the sub-fractions, meaning that it was difficult to design dedicated extraction conditions. As expected, fucans were mostly eluted by saline conditions, but with a delay in the elution profile towards higher saline solutions in the chemical sequential extraction procedure when compared with the enzymatic procedure (2–5 and 0·5–2 m NaCl. respectively). The remaining components had a similar elution profile between the two extraction procedures. Uronic acids were mostly eluted in the 0·5 and 2 m NaCl AEC fractions. Proteins and phenols have very similar profiles, and in each case two distinct populations were observed: a first population of components was eluted with 0·5–2 m NaCl while the second population was only released after an NaOH treatment. These last NaOH sub-fractions were particularly brown in colour, probably reflecting their high phenol contents.
Fig. 3.
Heatmap showing the distribution of polymers in extracts according to their fractionation patterns by anion-exchange chromatography (A) from the chemical sequential extraction procedure and (B) from the enzymatic sequential extraction procedure. For each polymer within an extract, a total value of 100 % was assigned and the corresponding signals in fractions were adjusted accordingly. Colour intensity was applied to match values. PC, P. canaliculata; AN, A. nodosum; HE, H. elongata; FV, F. vesiculosus; FS, F. serratus; nd, not determined.
In summary, the AEC elution profiles of the cell-wall extracts are particularly similar between all the algal species analysed, and independently of the extraction procedure used beforehand. In all extracts, a substantial portion of cell wall material is observed in the NaOH sub-fractions, meaning that covalent links may exist between these components previously co-extracted. This is particularly true for a population of phenols and proteins. Fucans are mainly eluted by salt solutions, albeit that a population of more anionic fucans is released during the chemical extraction. Fucans are frequently co-eluted with uronic acids, with some populations also enriched in proteins and phenols.
Fucan populations differ in their polymeric size
All extracts from the two extraction procedures were individually applied on a size exclusion chromatography column. From the elution profiles represented in Fig. 4, it can be seen that the two separation methods extracted different populations of polymers, which differed in their size. Polymers of high molecular mass were mostly extracted by the enzymatic sequential extraction procedure, and more specifically by the protease treatment. The cellulase treatment recovered polymers of various sizes. Two distinct populations of polymers of low and high molecular masses were observed in the chemical sequential extraction procedure, with the first solutions (W1, S1) extracting mostly oligomeric compounds. A typical elution profile of the AEC fractions of a P extract is shown in Supplementary Data Fig. S1. Most fucans are eluted by 0·5 and 2 m NaCl and have large sizes, and this was expected considering the observations made from Figs 3 and 4. The profile additionally showed that the 0 m NaCl solution only eluted oligomeric compounds, while there was a switch from the 0·5 m to the 2 m NaCl AEC fractions towards large polymers. Considering the heterogeneity of the fractions, it was not possible to assign specific molecular masses.
Fig. 4.
Size exclusion chromatography profiles of the extracts: (A) from the chemical sequential extraction procedure and (B) from the enzymatic sequential extraction procedure.
Fucans from Himanthalia elongata contain highly regular repeating structures
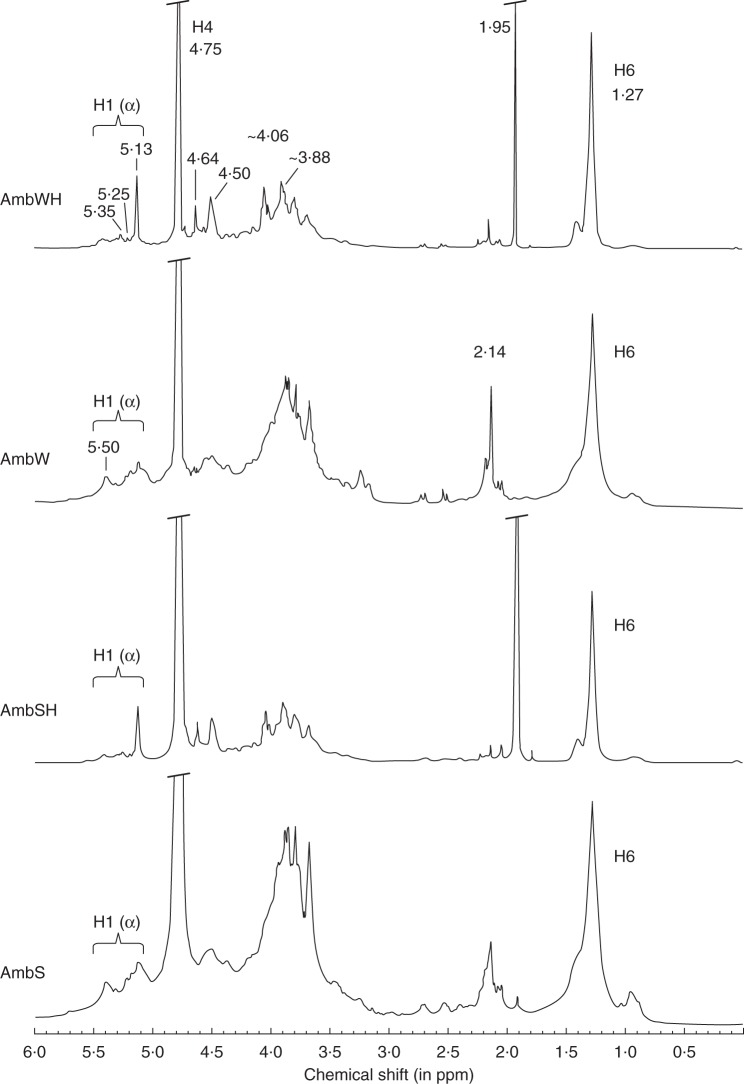
Mabeau and Kloareg (1987) previously reported the variation of the cell wall composition with species zonation. Among all of the brown algal Fucales analysed in the present study, H. elongata is peculiar as it is found in a habitat where Laminariales are usually present and not Fucales. Therefore, one might speculate that H. elongata cell walls are different in terms of composition and/or structure compared with those of the other Fucales under study. The preliminary 1H-NMR spectrum of the FCSP fractions from this species suggested an unusual regular structure. In order to gain more insights into the chemical structure of the constitutive fucans of H. elongata, a dedicated extraction was implemented following the procedure summarized on Fig. 1C. Briefly, after a cellulase treatment, the CaCl2-soluble fraction was applied on an Amberlite IR-77 resin to depolymerize the sulfated polysaccharides by very mild acid hydrolysis (referred to as autohydrolysis; see Anastyuk et al., 2010). The AmbW and AmbS extracts were successively recovered by elution with water and 2 m NaCl. The samples were applied on a size exclusion chromatography column and their elution profiles show that despite the mild acid treatment, the samples still contained high molecular weight polymers (Fig. 5A) which therefore cannot be resolved by C-PAGE analysis (Fig. 5B). The extracts were further depolymerized by stronger acid hydrolysis, and the resulting samples, named AmbWH and AmbSH, respectively, showed a drastic size reduction (Fig. 5). Based on the multiple sharp bands observed in the C-PAGE profiles, well-defined molecular size oligosaccharides were released during the acidic treatment (Fig. 5B). This is an indication that the constitutive sulfated polysaccharides may be of a regular structure defined by specific patterns. The two samples AmbWH and AmbSH gave similar profiles, probably indicating that the same oligosaccharides were produced.
Fig. 5.
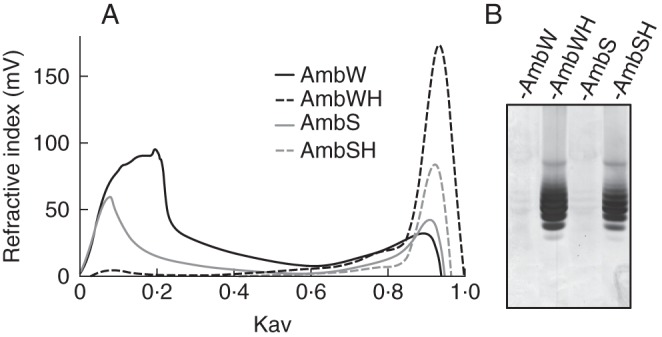
Profiles of the fucan fractions from H. elongata after autohydrolysis (AmbW, AmbS) and subsequent acid hydrolysis (AmbWH, AmbSH): (A) Size exclusion chromatography profiles and (B) C-PAGE profiles.
Himanthalia elongata contains unique variants of α-(1→3)-l-fucans
Sulfated fucans from brown algal Fucales usually give complex NMR spectra, and extensive purification steps are therefore needed to gain structural information. Figure 6 shows the 1H spectra for the four H. elongata fucan fractions. Although some broad signals are observed as expected for carbohydrates of high molecular mass, it is clear that several individual signals can easily be resolved. The acid hydrolysis did not alter the structural features. The four 1H-NMR spectra were all highly similar, although with an increased resolution after the strong acidic treatment (Fig. 6). This shows that the well-defined oligosaccharides obtained have different molecular weights but the same chemical structure, representative of the parent polymer itself. For all the four fucans, the 1H-NMR spectrum displayed characteristic anomeric signals at 5·00–5·50 ppm consistent with the presence of α-linked l-fucopyranosyl units (Fig. 6). After the acidic treatment, the anomeric signal at 5·13 ppm (H1α) increased, indicating that the residue became a reducing terminus and that the polymer was partially hydrolysed. A feature at 4·64 ppm also increased with the acidic treatment, which may be assigned to H1β. Methyl signals are centred at 1·27 ppm, indicative of an α-(1→3)-linked l-fucose. An envelope of acetyl signals centred at 2·14 ppm in the AmbW and AmbS spectrum was strongly reduced after acidic hydrolysis, while a sharp singlet arose at 1·95 ppm. These results may be ascribable to the removal of acetyl groups from the fucans. Finally, a feature at 4·75 ppm can tentatively be assigned to H4 of 4-sulfated fucose.
The proton assignment in the 1H spectrum of the fucans was achieved by analysis of a double-quantum filtered COSY spectrum, as exemplified for AmbWH (Supplementary Data Fig. S2). The protons of the fucose rings were assigned using the anomeric protons as the starting point. The H1–H2 and H5–H6 correlation systems were clear and are indicated in Supplementary Data Fig. S2. The chemical shifts observed (i.e. H1, 5·13 ppm; H2, 3·88 ppm; H3, 4·06 ppm; H4, 4·75 ppm; H5, 4·48 ppm; H6, 1·27 ppm) were similar to those previously recorded for α-(1→3)-linked l-fucans with 4-O-sulfated residues, as encountered in marine echinoderms and brown algal Laminariales. Additional correlations can be traced for another spin system in the acid-treated samples only (i.e. H1′, 5·26 ppm; H2′, 3·95 ppm; H3′, 4·02 ppm; H4′, 4·62 ppm; H5′, 4·20 ppm; H6′, 1·22 ppm), indicating that such correlations were probably masked by an acid-labile structure in the native fucan. The 1H-COSY spectrum confirmed that no signal can be assigned to sulfation at the O-2 and O-3 positions, and that the fucan is entirely sulfated at the O-4 position. The sulfation pattern was also not impacted by the acidic treatments. Altogether, these results are consistent with the sulfated fucan from H. elongata having a regular repeating sequence of residues as follows: [→3]-α-l-Fuc-4(SO3–)-(1)n.
DISCUSSION
Novel insights into the structure of the extracellular matrix of brown algae
With the aim of investigating the various chemical interactions between the main cell wall components of brown algae, a sequence of chemical and enzymatic fractionations combined with extensive chromatographic analyses was applied to five species of the order of Fucales. All fractions comprised a wide continuum of FCSPs. Variations consisted of the size of FCSPs and the chemical nature of the co-released components. In spite of this apparent complexity, the elution profiles were strictly conserved between the algae, suggesting a common cell wall organization in all of the five species under investigation.
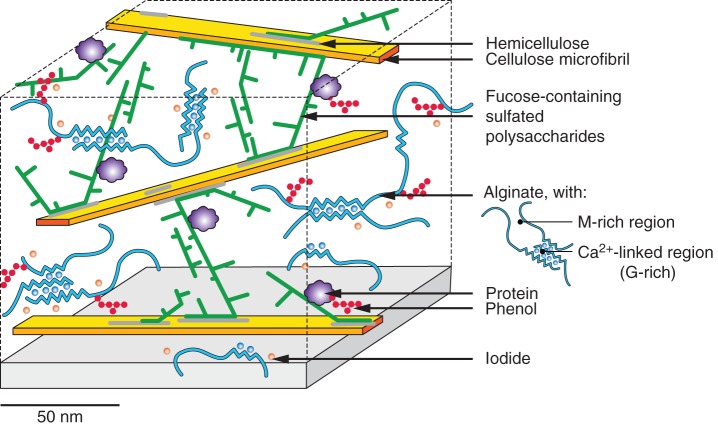
The enzyme treatments revealed that a portion of the FCSPs is tightly associated with cellulose microfibrils. These observations suggest that FCSPs act as major cross-linking glycans in brown algal cell walls, interlocking the cellulosic scaffold (Fig. 7). Considering the charge densities of these polysaccharides, polyanionic and neutral, respectively, the exact nature of the bonds between the sulfated polymers and the cellulose chains still remains elusive. We hypothesize that short-chained hemicellulose molecules might act as intermediaries that would simultaneously interact with the cellulose microfibrils through hydrophobic bonds and bridge the FCSPs (Fig. 7).
Fig. 7.
Cell wall model for brown algae from the order Fucales. Cellulose microfibrils are sparse and with a ribbon shape. Alginates and fucose-containing sulfated polysaccharides (FCSPs; including sulfated fucans) form the greater part of cell wall polymers, the latter acting as cross-linkers between cellulose microfibrils. Considering their respective charge densities, putative short-chained hemicellulose molecules might act as intermediates between the cellulose microfibrils and the FCSPs. Phenols are likely to be associated with alginates and proteins. Large amounts of iodide are also found in the wall, but its association with other polymers remains elusive.
While the fine structure of the proteins of brown algal cell walls has received little attention so far, they have frequently been reported to be present in substantial amounts. Mabeau and Kloareg (1987) mentioned that cell wall proteins accounted for 5–9 % of the total algal dry weight in Fucales and Laminariales brown algae, but contaminations by cytosolic proteins could not be totally excluded. In subsequent studies, the same authors mentioned that purified sulfated fucan fractions were always associated with significant amounts of proteins (Mabeau et al., 1990). Nishino et al. (1994) reported that proteins can account for up to 5 % of the total content in purified FCSP fractions from Fucus vesiculosus. These proteins are likely to be of structural importance, and the present profiles from the enzymatic extraction sequences indicate that they are tightly associated with FCSPs (Fig. 7). The AEC profiles also suggest that the proteins found in the FCSP fractions are covalently attached to phenol compounds, although the latter are more frequently associated with alginate blocks (see below).
As the most abundant gel-forming molecule in brown algal cell walls, alginate is considered as the main cell wall component for the control of ECM rigidity. The regulation of gel strength in alginate gels is based on the frequency of polyguluronate blocks in the polymeric chains through the enzymatic conversion of β-(1→4)-d-mannuronate (M) into α-(1→4)-l-guluronate (G) (Haug et al., 1974). The large number of mannuronan C5-epimerase genes in Ectocarpus siliculosus (Michel et al., 2010) or in Laminaria digitata (Roeder et al., 2005; Tonon et al., 2008) shows that the fine-tuning of alginate structure is an essential trait in brown algae.
Alginate lyase treatment released only a minor fraction of FCSPs, indicating that the cellulose–FCSP network is embedded within the alginate network, with only few, if any, covalent bonds between these two networks. In contrast, significant amounts of polyphenols were always released from the enzymatic degradation of the alginate fractions. Alginates were shown to be able to form high-molecular weight complexes with phenolic substances upon oxidation with haloperoxidases (Bitton et al., 2006; Salgado et al., 2009). Bromo- and/or iodo-peroxidases are known to be present in the apoplasm of a variety of brown algae (Colin et al., 2005; Leblanc et al., 2006; Verhaeghe et al., 2008), where they catalyse the peroxidation of halide ions (Verhaeghe et al., 2008). Our findings confirm that alginate–phenol linkages play an essential role in the brown algal cell wall structure (Vreeland et al., 1998; Schoenwaelder and Wiencke, 2000). Oxidative cross-linking of alginate by polyphenols upon the action of haloperoxidase is thus likely to constitute another mechanism for regulating the rigidity of brown algal cell walls.
Himanthalia elongata features atypical fucans in the order Fucales
The FCSPs are known to be a complex fraction of the cell wall in brown algae, encompassing naturally diverse structures. Sulfated homofucans are one of the main FCSPs. Two backbone motifs are reported. One motif has been described in the orders Laminariales and Ectocarpales, exclusively composed of α-(1→3)-l-fucose residues. The sulfate groups are found at positions O-4 and/or O-2 (Nishino and Nagumo, 1991; Chizhov et al., 1999; Nagaoka et al., 1999; Ponce et al., 2003; Sakai et al., 2003; Anastyuk et al., 2010; Bilan et al., 2010). The second motif consists of an alternation of α-(1→3) and α-(1→4)-l-fucose residues usually bearing two or three sulfate groups per disaccharidic unit. This regular structure, which was isolated in A. nodosum and Fucus species, is frequent in algae in the order Fucales. The sulfate groups occupy positions O-2 and/or O-3 (Pereira et al., 1999; Chevolot et al., 2001; Bilan et al., 2002, 2006; Colin et al., 2006), and a minor part can be located at position O-4 (Marais and Joseleau, 2001; Bilan et al., 2006). In both cases, the core backbones can be further masked by more or less frequent acetylation or branched structures essentially composed of fucosyl residues.
Our results reveal that the homofucan motif isolated from H. elongata differs from the alternating structure frequently described in the order Fucales. It resembles what has been already observed in Laminariales and Ectocarpales in that it contains α-(1→3)-linked fucose residues only.
Plasticity of cell wall composition in relation to habitat
Due to the tidal cycles, the intertidal zone is a dynamic environment which undergoes rapid changes of large amplitude in its physical and chemical environmental parameters. The amounts of sulfated fucans of Fucales brown algae and kelps were shown to be directly correlated to species position in the shore, suggesting that the contents of highly sulfated FCSP structures may confer an adaptive advantage to those species frequently exposed to immersion (Mabeau and Kloareg, 1987). Fucans with a high content of sulfate substituents are highly hygroscopic polyanions and, reminiscent of the sulfated carbohydrate moieties of mammalian glycosaminoglycans, it is thought that they contribute to regulate water potential at the outer cell membrane level (Kloareg, 1981; Kloareg and Quatrano, 1988).
The molecular bases underlying such adaptations can now be investigated by more informative approaches. One Ectocarpus strain isolated from a genuine freshwater environment was shown to have retained its ability to grow in normal (undiluted, 100 %) seawater (Dittami et al., 2012). The transition between normal and low (20-fold dilution of normal seawater, 5 %) salinity is reversible, yet it is accompanied by profound morphological, transcriptomic and metabolic changes. In particular, the re-acclimation from diluted to undiluted seawater involves the upregulation of carbohydrate sulfotransferase genes, of several mannuronan C5-epimerases as well as of a bromoperoxidase (Dittami et al., 2012). We recently confirmed that sulfated fucans are overproduced upon acclimation to 100 % seawater (unpubl. res.). Phloroglucinol accumulation and a 200-fold increase in the gene expression of the related type III polyketide synthase (PSK III) were also observed in the freshwater strain of Ectocarpus incubated in normal seawater (Meslet-Cladière et al., 2013).
The overexpression of the mannuronan C5-epimerases, PSK III and bromoperoxidase genes upon acclimation to normal seawater is likely to result in the reinforcement of polyphenol–alginate complexes. This would consolidate cell wall architecture, a biochemical response to the disruption of ionic linkages by high salinity. Since the cellulose–FCSP network is not, or only loosely, interconnected to the alginate–phenol complexes (Fig. 7), they may not be involved in reinforcing cell wall structure. The increase in the density of sulfate groups in the ECM would, however, significantly affect the ion composition and activity, through Donnan exclusion and/or polyelectrolytic condensation effects (Kloareg et al., 1986, 1987). This response may also regulate water potential in the vicinity of cell membranes.
The ECM structure and the evolution of brown algae
The putative metabolic pathways of the main polysaccharides of brown algal cell walls were reconstructed from the genome sequence of the brown algal model E. siliculosus (Michel et al., 2010). Ectocarpus fucosyl-transferases and sulfotransferases (involved in the substitution of sulfate groups on the glycan backbone) as well as sulfatases (involved in cell wall remodelling) are conserved with animals, indicating that sulfated fucans are of an ancestral origin in eukaryotes, probably pre-dating the divergence between animals and plants. The origin of cellulose biosynthesis in Stramenopiles is more complex. Phylogenetic analyses including the recent genomic sequences of brown and red seaweeds confirmed that the CESAs from oomycetes originate from the red algal endosymbiont (Michel et al., 2010; Collén et al., 2013). However, brown algae appear to have lost this ancestral ‘red algae-like’ CESA and instead possess a distinct type of CESA shared with the Eustigmatophyceae microalgae (Collén et al., 2013). The exact origin of brown algal CESA is nonetheless elusive. A possible scenario may involve a duplication of the ‘red algae-like’ CESA followed by a divergence of the duplicated gene. Consistent with the observed CESA phylogeny (Collén et al., 2013), brown algae and Eustigmatophyceae have probably conserved the divergent CESA gene, while they have lost the gene acquired from the red algal endosymbiont. In the case of alginates, the enzymes responsible for the three last specific steps of its biosynthesis, including mannuronan C5-epimerase, are closely related to enzymes from Actinobacteria (Michel et al., 2010). The PSK IIIs which catalyse the synthesis of phoroglucinol, the constitutive monomer of brown algal polyphenols, were also shown to be of actinobacterian origin (Meslet-Cladière et al., 2013). Taken together, this indicates that while the routes for the assembly of cellulose and FCSPs are ancient pathways, the metabolic machinery for the biosynthesis of alginates and phlorotannins was acquired much later, by HGT from an ancestral actinobacterium (Michel et al., 2010). This massive HGT event occurred rather recently in the scale of evolution, 200–300 million years ago, after the divergence of brown algae and diatoms (which do not feature actinobacterial genes in their genomes).
Based on our current understanding on the molecular organization of the cell walls of extant brown algae (Fig. 7) as well as of the structure–function relationships of their ECM components, we propose that the anatomical organization of brown algae was shaped by recruitment of ECM material as follows (Fig. 8). The ECM of the last eukaryotic common ancestor possibly consisted of a loose, amorphous network of mucilaginous components made of proteins, β-(1→3)-glycans and sulfated fucans (Michel et al., 2010), which probably could not support the building of complex pluricellularity. In Stramenopiles, endosymbiosis with a unicellular red alga brought the capacity to synthesize cellulose, which probably led to the emergence of a denser ECM, consisting of cellulose microfibrils embedded within a soft gel of proteic and FCSP mucilages, allowing the shaping of more complex, filamentous organisms such as the extant oomycetes. About 200–300 million years ago, a massive HGT from an actinobacterium resulted in the acquisition of alginate and of polyphenols. This allowed for the evolution of an extensive ECM, consisting of more tightly assembled cellulose microfibrils within alginate gels strengthened by oxidative cross-linking. We believe that the possibility of regulating the ECM rigidity through the control of alginate fine structure and polyphenol cross-linking brought the capacity to build more complex multicellular organisms, including semi-upright forms with the required plasticity to sustain the physical constraints of the intertidal environment, such as resisting wave action (Fig. 8).
Fig. 8.
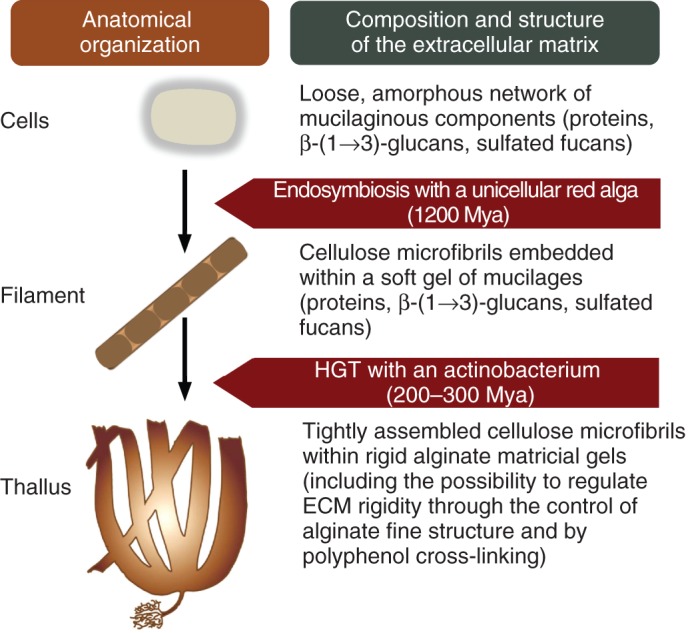
Proposed scenario for the evolution of anatomical organizations in relation to the evolution of the composition and the structure of the ECM within Stramenopiles.
In this scenario, and based on the molecular bases of the acclimation of the freshwater strain of Ectocarpus to incubation in normal seawater, we see the fucose-containing sulfated polysaccharides as more involved in screening brown algal cells from salinity and chemical stress. The evolution in the brown algae from the order Fucales of specific fucans with a higher sulfate density, known as fucoidans, may have been instrumental in the colonization of the higher intertidal zone, which required adaptation to higher osmotic stress.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
The authors would like to thank Delphine Scornet for the purification of the alginate lyase from Haliotis tuberculata.
LITERATURE CITED
- Albuquerque IRL, Queiroz KCS, Alves LG, Santos EA, Leite EL, Rocha HAO. Heterofucans from Dictyota menstrualis have anticoagulant activity. Brazilian Journal of Medical and Biological Research. 2004;37:167–171. doi: 10.1590/s0100-879x2004000200002. [DOI] [PubMed] [Google Scholar]
- Ale MT, Mikkelsen JD, Meyer AS. Important determinants for fucoidan bioactivity: a critical review of structure–function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Marine Drugs. 2011;9:2106–2130. doi: 10.3390/md9102106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastyuk SD, Shevchenko NM, Nazarenko EL, et al. Structural analysis of a highly sulfated fucan from the brown alga Laminaria cichorioides by tandem MALDI and ESI mass spectrometry. Carbohydrate Research. 2010;345:2206–2212. doi: 10.1016/j.carres.2010.07.043. [DOI] [PubMed] [Google Scholar]
- Andersen RA. Biology and systematics of heterokont and haptophyte algae. American Journal of Botany. 2004;91:1508–1522. doi: 10.3732/ajb.91.10.1508. [DOI] [PubMed] [Google Scholar]
- Arun A, Peters NT, Scornet D, Peters AF, Mark Cock J, Coelho SM. Non-cell autonomous regulation of life cycle transitions in the model brown alga Ectocarpus. New Phytologist. 2013;197:503–510. doi: 10.1111/nph.12007. [DOI] [PubMed] [Google Scholar]
- Baba M, Nakajima M, Schols D, Pauwels R, Balzarini J, De Clercq E. Pentosan polysulfate, a sulfated oligosaccharide, is a potent and selective anti-HIV agent in vitro. Antiviral Research. 1988;9:335–343. doi: 10.1016/0166-3542(88)90035-6. [DOI] [PubMed] [Google Scholar]
- Belanger KD, Quatrano RS. Polarity: the role of localized secretion. Current Opinion in Plant Biology. 2000;3:67–72. doi: 10.1016/s1369-5266(99)00043-6. [DOI] [PubMed] [Google Scholar]
- Berger F, Taylor A, Brownlee C. Cell fate determination by the cell wall in early Fucus development. Science. 1994;263:1421–1423. doi: 10.1126/science.263.5152.1421. [DOI] [PubMed] [Google Scholar]
- Berney C, Pawlowski J. A molecular time-scale for eukaryote evolution recalibrated with the continuous microfossil record. Proceedings of the Royal Society B: Biological Sciences. 2006;273:1867–1872. doi: 10.1098/rspb.2006.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilan MI, Grachev AA, Ustuzhanina NE, Shashkov AS, Nifantiev NE, Usov AI. Structure of a fucoidan from the brown seaweed Fucus evanescens C.Ag. Carbohydrate Research. 2002;337:719–730. doi: 10.1016/s0008-6215(02)00053-8. [DOI] [PubMed] [Google Scholar]
- Bilan MI, Grachev AA, Shashkov AS, Nifantiev NE, Usov AI. Structure of a fucoidan from the brown seaweed Fucus serratus L. Carbohydrate Research. 2006;341:238–245. doi: 10.1016/j.carres.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Bilan MI, Grachev AA, Shashkov AS, et al. Further studies on the composition and structure of a fucoidan preparation from the brown alga Saccharina latissima. Carbohydrate Research. 2010;345:2038–2047. doi: 10.1016/j.carres.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Bitton R, Ben-Yehuda M, Davidovich M, et al. Structure of algal-born phenolic polymeric adhesives. Macromolecular Bioscience. 2006;6:737–746. doi: 10.1002/mabi.200600073. [DOI] [PubMed] [Google Scholar]
- Bouget FY, Berger F, Brownlee C. Position dependent control of cell fate in the Fucus embryo: role of intercellular communication. Development. 1998;125:1999–2008. doi: 10.1242/dev.125.11.1999. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown JW, Sorhannus U. A molecular genetic timescale for the diversification of autotrophic stramenopiles (Ochrophyta): substantive underestimation of putative fossil ages. PLoS One. 2010;5:e12759. doi: 10.1371/journal.pone.0012759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camara RB, Costa LS, Fidelis GP, et al. Heterofucans from the brown seaweed Canistrocarpus cervicornis with anticoagulant and antioxidant activities. Marine Drugs. 2011;9:124–138. doi: 10.3390/md9010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevolot L, Mulloy B, Ratiskol J, Foucault A, Colliec-Jouault S. A disaccharide repeat unit is the major structure in fucoidans from two species of brown algae. Carbohydrate Research. 2001;330:529–535. doi: 10.1016/s0008-6215(00)00314-1. [DOI] [PubMed] [Google Scholar]
- Chizhov AO, Dell A, Morris HR, et al. A study of fucoidan from the brown seaweed Chorda filum. Carbohydrate Research. 1999;320:108–119. doi: 10.1016/s0008-6215(99)00148-2. [DOI] [PubMed] [Google Scholar]
- Cock JM, Sterck L, Rouze P, et al. The Ectocarpus genome and the independent evolution of multicellularity in brown algae. Nature. 2010;465:617–621. doi: 10.1038/nature09016. [DOI] [PubMed] [Google Scholar]
- Collén J, Porcel B, Carré W, et al. Genome structure and metabolic features in the red seaweed Chondrus crispus shed light on evolution of the Archaeplastida. Proceedings of the National Academy of Sciences, USA; 2013. pp. 5247–5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colin C, Leblanc C, Michel G, et al. Vanadium-dependent iodoperoxidases in Laminaria digitata, a novel biochemical function diverging from brown algal bromoperoxidases. Journal of Biological Inorganic Chemistry. 2005;10:156–166. doi: 10.1007/s00775-005-0626-8. [DOI] [PubMed] [Google Scholar]
- Colin S, Deniaud E, Jam M, et al. Cloning and biochemical characterization of the fucanase FcnA: definition of a novel glycoside hydrolase family specific for sulfated fucans. Glycobiology. 2006;16:1021–1032. doi: 10.1093/glycob/cwl029. [DOI] [PubMed] [Google Scholar]
- Dische Z, Shettles LB. A specific color reaction of methylpentoses and a spectrophotometric micromethod for their determination. Journal of Biological Chemistry. 1948;175:595–603. [PubMed] [Google Scholar]
- Dittami SM, Gravot A, Goulitquer S, et al. Towards deciphering dynamic changes and evolutionary mechanisms involved in the adaptation to low salinities in Ectocarpus (brown algae) The Plant Journal. 2012;71:366–377. doi: 10.1111/j.1365-313X.2012.04982.x. [DOI] [PubMed] [Google Scholar]
- Draget KI, Smidsrød O, Skjåk-Bræk G. Alginates from algae. In: Steinbüchel A, Rhee SK, editors. Polysaccharides and polyamides in the food industry: properties, production, and patents. Oxford: Wiley-VCH; 2005. pp. 1–30. [Google Scholar]
- Haug A, Larsen B, Smidsrød O. Uronic acid sequence in alginate from different sources. Carbohydrate Research. 1974;32:217–225. [Google Scholar]
- Hematy K, Cherk C, Somerville S. Host–pathogen warfare at the plant cell wall. Current Opinion in Plant Biology. 2009;12:406–413. doi: 10.1016/j.pbi.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Heyraud A, Gey C, Leonard C, Rochas C, Girond S, Kloareg B. NMR spectroscopy analysis of oligoguluronates and oligomannuronates prepared by acid or enzymatic hydrolysis of homopolymeric blocks of alginic acid. Application to the determination of the substrate specificity of Haliotis tuberculata alginate lyase. Carbohydrate Research. 1996;289:11–23. doi: 10.1016/0008-6215(96)00060-2. [DOI] [PubMed] [Google Scholar]
- Jaques LB, Ballieux RE, Dietrich CP, Kavanagh LW. A microelectrophoresis method for heparin. Canadian Journal of Physiology and Pharmacology. 1968;46:351–360. doi: 10.1139/y68-055. [DOI] [PubMed] [Google Scholar]
- Kloareg B. Structure et rôle écophysiologique des parois des algues littorales: contribution à la résistance aux variations de salinité. Physiologie Végétale. 1981;19:427–441. [Google Scholar]
- Kloareg B, Quatrano RS. Structure of the cell walls of marine algae and ecophysiological functions of the matrix polysaccharides. Oceanography and Marine Biology Annual Review. 1988;26:259–315. [Google Scholar]
- Kloareg B, Demarty M, Mabeau S. Polyanionic characteristics of purified sulphated homofucans from brown algae. International Journal of Biological Macromolecules. 1986;8:380–386. [Google Scholar]
- Kloareg B, Demarty M, Mabeau S. Ion-exchange properties of isolated cell walls of brown algae: the interstitial solution. Journal of Experimental Botany. 1987;38:1652–1662. [Google Scholar]
- Knutson CA, Jeanes A. A new modification of the carbazole analysis: application to heteropolysaccharides. Analytical Biochemistry. 1968;24:470–481. doi: 10.1016/0003-2697(68)90154-1. [DOI] [PubMed] [Google Scholar]
- Kropf DL, Kloareg B, Quatrano RS. Cell wall is required for fixation of the embryonic axis in Fucus zygotes. Science. 1988;239:187–190. doi: 10.1126/science.3336780. [DOI] [PubMed] [Google Scholar]
- Kupper FC, Kloareg B, Guern J, Potin P. Oligoguluronates elicit an oxidative burst in the brown algal kelp Laminaria digitata. Plant Physiology. 2001;125:278–291. doi: 10.1104/pp.125.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bail A, Billoud B, Le Panse S, Chenivesse S, Charrier B. ETOILE regulates developmental patterning in the filamentous brown alga Ectocarpus siliculosus. The Plant Cell. 2011;23:1666–1678. doi: 10.1105/tpc.110.081919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc C, Colin C, Cosse A, et al. Iodine transfers in the coastal marine environment: the key role of brown algae and of their vanadium-dependent haloperoxidases. Biochimie. 2006;88:1773–1785. doi: 10.1016/j.biochi.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Mabeau S, Kloareg B. Isolation and analysis of the cell walls of brown algae: Fucus spiralis, F. ceranoides, F. vesiculosus, F. serratus, Bifurcaria bifurcata and Laminaria digitata. Journal of Experimental Botany. 1987;38:1573–1580. [Google Scholar]
- Mabeau S, Kloareg B, Joseleau J-P. Fractionation and analysis of fucans from brown algae. Phytochemistry. 1990;29:2441–2445. [Google Scholar]
- Mahony MC, Clark GF, Oehninger S, Acosta AA, Hodgen GD. Fucoidin binding activity and its localization on human spermatozoa. Contraception. 1993;48:277–289. doi: 10.1016/0010-7824(93)90146-x. [DOI] [PubMed] [Google Scholar]
- Marais M-F, Joseleau J-P. A fucoidan fraction from Ascophyllum nodosum. Carbohydrate Research. 2001;336:155–159. doi: 10.1016/s0008-6215(01)00257-9. [DOI] [PubMed] [Google Scholar]
- Meslet-Cladière L, Delage L, Leroux CJ-J, et al. Structure/function analysis of a type III polyketide synthase in the brown alga Ectocarpus siliculosus reveals a biochemical pathway in phlorotannin monomer biosynthesis. The Plant Cell. 2013;25:3089–3103. doi: 10.1105/tpc.113.111336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel G, Tonon T, Scornet D, Cock JM, Kloareg B. The cell wall polysaccharide metabolism of the brown alga Ectocarpus siliculosus. Insights into the evolution of extracellular matrix polysaccharides in Eukaryotes. New Phytologist. 2010;188:82–97. doi: 10.1111/j.1469-8137.2010.03374.x. [DOI] [PubMed] [Google Scholar]
- Min H, Cowman MK. Combined alcian blue and silver staining of glycosaminoglycans in polyacrylamide gels: application to electrophoretic analysis of molecular weight distribution. Analytical Biochemistry. 1986;155:275–285. doi: 10.1016/0003-2697(86)90437-9. [DOI] [PubMed] [Google Scholar]
- Nagaoka M, Shibata H, Kimura-Takagi I, et al. Structural study of fucoidan from Cladosiphon okamuranus tokida. Glycoconjugate Journal. 1999;16:19–26. doi: 10.1023/a:1006945618657. [DOI] [PubMed] [Google Scholar]
- Nishino T, Nagumo T. The sulfate-content dependence of the anticoagulant activity of a fucan sulfate from the brown seaweed Ecklonia kurome. Carbohydrate Research. 1991;214:193–197. doi: 10.1016/s0008-6215(00)90542-1. [DOI] [PubMed] [Google Scholar]
- Nishino T, Aizu Y, Nagumo T. The influence of sulfate content and molecular weight of a fucan sulfate from the brown seaweed Ecklonia kurome on its antithrombin activity. Thrombosis Research. 1991;64:723–731. doi: 10.1016/0049-3848(91)90072-5. [DOI] [PubMed] [Google Scholar]
- Nishino T, Nishioka C, Ura H, Nagumo T. Isolation and partial characterization of a noval amino sugar-containing fucan sulfate from commercial Fucus vesiculosus fucoidan. Carbohydrate Research. 1994;255:213–224. doi: 10.1016/s0008-6215(00)90980-7. [DOI] [PubMed] [Google Scholar]
- Nyvall P, Corre E, Boisset C, et al. Characterization of mannuronan C-5-epimerase genes from the brown alga Laminaria digitata. Plant Physiology. 2003;133:726–735. doi: 10.1104/pp.103.025981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paciorek T, Bergmann DC. The secret to life is being different: asymmetric divisions in plant development. Current Opinion in Plant Biology. 2010;13:661–669. doi: 10.1016/j.pbi.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Pereira MS, Mulloy B, Mourão PAS. Structure and anticoagulant activity of sulfated fucans: comparison between the regular, repetitive, and linear fucans from echinoderms with the more heterogeneous and branched polymers from brown algae. Journal of Biological Chemistry. 1999;274:7656–7667. doi: 10.1074/jbc.274.12.7656. [DOI] [PubMed] [Google Scholar]
- Ponce NMA, Pujol CA, Damonte EB, Flores ML, Stortz CA. Fucoidans from the brown seaweed Adenocystis utricularis: extraction methods, antiviral activity and structural studies. Carbohydrate Research. 2003;338:153–165. doi: 10.1016/s0008-6215(02)00403-2. [DOI] [PubMed] [Google Scholar]
- Popper ZA, Michel G, Hervé C, et al. Evolution and diversity of plant cell walls: from algae to flowering plants. Annual Review of Plant Biology. 2011;62:567–590. doi: 10.1146/annurev-arplant-042110-103809. [DOI] [PubMed] [Google Scholar]
- Quatrano RS, Stevens PT. Cell wall assembly in Fucus zygotes: I. Characterization of the polysaccharide components. Plant Physiology. 1976;58:224–231. doi: 10.1104/pp.58.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Prieto A, Weber AP, Bhattacharya D. The origin and establishment of the plastid in algae and plants. Annual Review of Genetics. 2007;41:147–168. doi: 10.1146/annurev.genet.41.110306.130134. [DOI] [PubMed] [Google Scholar]
- Roeder V, Collén J, Rousvoal S, Corre E, Leblanc C, Boyen C. Identification of stress gene transcripts in Laminaria digitata (Phaeophyceae) protoplast cultures by expressed sequence tag analysis. Journal of Phycology. 2005;41:1227–1235. [Google Scholar]
- Sakai T, Ishizuka K, Shimanaka K, Ikai K, Kato I. Structures of oligosaccharides derived from Cladosiphon okamuranus fucoidan by digestion with marine bacterial enzymes. Marine Biotechnology. 2003;5:536–544. doi: 10.1007/s10126-002-0107-9. [DOI] [PubMed] [Google Scholar]
- Salgado LT, Cinelli LP, Viana NB, et al. A vanadium bromoperoxidase catalyzes the formation of high-molecular-weight complexes between brown algal phenolic substances and alginates. Journal of Phycology. 2009;45:193–202. doi: 10.1111/j.1529-8817.2008.00642.x. [DOI] [PubMed] [Google Scholar]
- Schoenwaelder MEA, Wiencke C. Phenolic compounds in the embryo development of several northern hemisphere Fucoids. Plant Biology. 2000;2:24–33. [Google Scholar]
- Silberfeld T, Leigh JW, Verbruggen H, Cruaud C, de Reviers B, Rousseau F. A multi-locus time-calibrated phylogeny of the brown algae (Heterokonta, Ochrophyta, Phaeophyceae): investigating the evolutionary nature of the ‘brown algal crown radiation. Molecular Phylogenetics and Evolution. 2010;56:659–674. doi: 10.1016/j.ympev.2010.04.020. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin–ciocalteu reagent. Methods in Enzymology. 1999;299:152–178. [Google Scholar]
- Tonon T, Rousvoal S, Roeder V, Boyen C. Expression profiling of the mannuronan C5-epimerase multigenic family in the brown alga Laminaria digitata (Phaeophyceae) under biotic stress conditions. Journal of Phycology. 2008;44:1250–1256. doi: 10.1111/j.1529-8817.2008.00580.x. [DOI] [PubMed] [Google Scholar]
- Verhaeghe EF, Fraysse A, Guerquin-Kern JL, et al. Microchemical imaging of iodine distribution in the brown alga Laminaria digitata suggests a new mechanism for its accumulation. Journal of Biological Inorganic Chemistry. 2008;13:257–269. doi: 10.1007/s00775-007-0319-6. [DOI] [PubMed] [Google Scholar]
- Vreeland V, Waite JH, Epstein L. Polyphenols and oxidases in substratum adhesion by marine algae and mussels. Journal of Phycology. 1998;34:1–8. [Google Scholar]
- Yamasaki-Miyamoto Y, Yamasaki M, Tachibana H, Yamada K. Fucoidan induces apoptosis through activation of caspase-8 on human breast cancer MCF-7 cells. Journal of Agricultural and Food Chemistry. 2009;57:8677–8682. doi: 10.1021/jf9010406. [DOI] [PubMed] [Google Scholar]
- Zablackis E, Perez J. A partially pyruvated carrageenan from Hawaiian Grateloupia filicina (Cryptonemiales, Rhodophyta) Botanica Marina. 1990;33:273–276. [Google Scholar]
- Zhu Z, Zhang Q, Chen L, Ren S, Xu P, Tang Y, Luo D. Higher specificity of the activity of low molecular weight fucoidan for thrombin-induced platelet aggregation. Thrombosis Research. 2010;125:419–426. doi: 10.1016/j.thromres.2010.02.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.