Abstract
Background and Aims
Plant cell enlargement is unambiguously coupled to changes in cell wall architecture, and as such various studies have examined the modification of the proportions and structures of glucuronoarabinoxylan and mixed-linkage glucan in the course of cell elongation in grasses. However, there is still no clear understanding of the mutual arrangement of these matrix polymers with cellulose microfibrils and of the modification of this architecture during cell growth. This study aimed to determine the correspondence between the fine structure of grass cell walls and the course of the elongation process in roots of maize (Zea mays).
Methods
Enzymatic hydrolysis followed by biochemical analysis of derivatives was coupled with immunohistochemical detection of cell wall epitopes at different stages of cell development in a series of maize root zones.
Key Results
Two xylan-directed antibodies (LM11 and ABX) have distinct patterns of primary cell wall labelling in cross-sections of growing maize roots. The LM11 epitopes were masked by mixed-linkage glucan and were revealed only after lichenase treatment. They could be removed from the section by xylanase treatment. Accessibility of ABX epitopes was not affected by the lichenase treatment. Xylanase treatment released only part of the cell wall glucuronoarabinoxylan and produced two types of products: high-substituted (released in polymeric form) and low-substituted (released as low-molecular-mass fragments). The amount of the latter was highly correlated with the amount of mixed-linkage glucan.
Conclusions
Three domains of glucuronoarabinoxylan were determined: one separating cellulose microfibrils, one interacting with them and a middle domain between the two, which links them. The middle domain is masked by the mixed-linkage glucan. A model is proposed in which the mixed-linkage glucan serves as a gel-like filler of the space between the separating domain of the glucuronoarabinoxylan and the cellulose microfibrils. Space for glucan is provided along the middle domain, the proportion of which increases during cell elongation.
Keywords: Plant cell walls, maize, Zea mays, mixed-linkage glucan, glucuronoarabinoxylan, cellulose, elongation growth, primary root, type II cell walls, masking of polysaccharides, cell expansion
INTRODUCTION
The final size and shape of plant organisms are established during growth by three major processes of cell development: proliferation, expansion and differentiation. The expansion stage of cell life in the axial organs of plants in the majority of cases is presented by a symplastic type of elongation growth when neighbouring cells enlarge in a rapid and synchronous manner, predominantly in one direction. The mechanical properties of cell walls, depending on the cell wall architecture, are considered to be the main determinants of the elongation process. Regulation of the initiation and the realization of growth process are carried out by changing the cell wall compliance rather than by turgor manipulation (Tomos and Pritchard, 1994; Cosgrove, 1997; Kroeger et al., 2011; Kierzkowski et al., 2012).
Cell wall architecture and its dynamics during growth have been extensively studied and discussed. The predominant view for many years has been largely based on the expansin-mediated modification of the interaction between cellulose microfibrils and xyloglucan (Cosgrove, 1997). However, newly obtained data, reviewed by Park and Cosgrove (2012), require a revision of this model. Among the arguments are the results that were obtained by the nuclear magnetic resonance characterization of polymer interactions within the cell wall, which indicated weakly pronounced interactions between cellulose and xyloglucan (Dick-Perez et al., 2011). In addition, the xyloglucan-deficient mutant had a relatively normal phenotype (Cavalier et al., 2008), contradicting the key role of xyloglucan in cell growth.
Cell walls of grasses are of the so-called type II (Carpita and Gibeaut, 1993). The elongation process in grasses is differs from the above mentioned model. Xyloglucan and pectins in grasses are the minor components and are replaced by two major polymers, namely glucuronoarabinoxylan and mixed-linkage glucan. β-Expansins and α-expansins have no profound effect on the creep of type II cell walls (McQueen-Mason et al., 1992; Tabuchi et al., 2011). The substantial involvement of expansins in the elongation growth of the vegetative organs in grasses has not been reported, suggesting the necessity of additional models for the modification of cell wall architecture associated with elongation growth in grasses.
Cell enlargement in grasses is accompanied by the accumulation of glucuronoarabinoxylan and, in particular, mixed-linkage glucan (Carpita, 1984; Kim et al., 2000; Gibeaut et al., 2005). In addition, the degree of glucuronoarabinoxylan backbone substitution reduces so that the Ara/Xyl ratio decreases. Most of these data were obtained on coleoptiles, a convenient model in which most of the cells are at the same stage of elongation (Carpita et al., 2001). However, the coleoptile itself is a transient organ and may have some specificity in its cell wall dynamics. Mixed-linkage glucan is almost totally degraded in coleoptiles after growth cessation and was for decades considered a transient stage-specific polysaccharide (Luttenegger and Nevins, 1985). However, in other plant organs, the polymer remains within the primary cell walls of mature tissues (Trethewey and Harris, 2002; Trethewey et al., 2005; Kozlova et al., 2012a; Vega-Sánchez et al., 2013).
The classical model system for studying cell elongation is the growing primary root, which has consecutive zones with cells at different developmental stages (Ivanov, 1997; Beemster and Baskin, 1998; Anderson et al., 2010). We have used this model to characterize the distribution and structure of mixed-linkage glucan and glucuronoarabinoxylan (Kozlova et al., 2012a, b). Here we attempt to analyse the mutual interposition of these polymers and suggest a hypothesis for the modification of type II cell wall architecture in the course of cell enlargement.
MATERIALS AND METHODS
Plant material
Maize (Zea mays cv. ‘Interkras 375’) plants were grown for 4 d in the dark at 27 °C. An immunohistochemical analysis was performed on primary root segments that were obtained from aetiolated seedlings according to the scheme presented in Fig. 1A. The primary root was dissected into the following zones: meristem zone (0–1 mm, measured from the apical meristem), elongation zone (2–6 mm) and post-elongation zone (7–11 mm) (Fig. 1A). The correspondence between the distance from the apex and the postulated development stage of cells has been previously shown (Kozlova et al., 2012a).
Fig. 1.
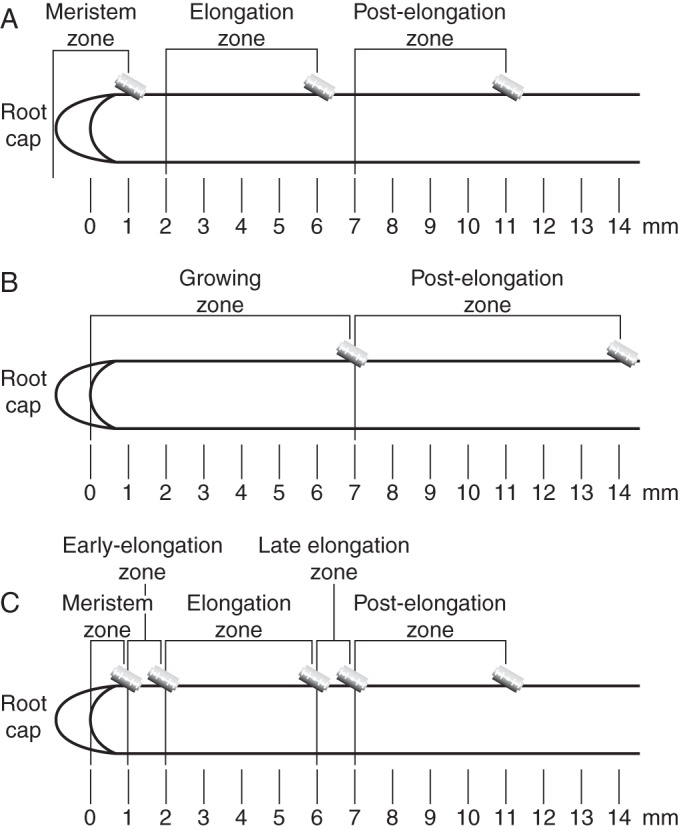
Scheme of sample collection for immunohistochemical (A), biochemical and immunodot (B) experiments and for the establishment of the relationship between the contents of the glucuronoarabinoxylan domains and mixed-linkage glucan (C).
For the biochemical and immunodot analyses only the growing (0–7 mm of the decapitated primary root) and post-elongation (7–14 mm of the decapitated primary root) zones were used (Fig. 1B). The experiments were performed in triplicate (cut-offs of 200 roots for each sample).
To check the relationship between the content of the mixed-linkage glucan and the different types of xylanase digestion products, the decapitated maize root was divided into five zones, similar to those of Kozlova et al. (2012a): meristem (0–1 mm), early elongation zone (1–2 mm), elongation zone (2–6 mm), late elongation zone (6–7 mm) and post-elongation zone (7–11 mm) (Fig. 1C). The experiments were performed in triplicate, with each sample containing cut-offs of 450 roots.
Immunohistochemical analysis
Samples of the meristem, elongation and post-elongation zones of maize roots were fixed overnight at 4 °C according to Willats et al. (2004) in 2·5 % (v/v) glutaraldehyde in 0·05 m sodium phosphate buffer (pH 7·4), dehydrated in a graded aqueous ethanol series, and progressively infiltrated with London Resin White (Sigma-Aldrich, St Louis, MO, USA) by increasing the increments of the resin by 25 % each day. The samples were embedded in 100 % resin in Beem capsules and polymerized at 60 °C for 24 h. Semi-thin sections (1 μm thick) were prepared using a glass knife on a LKB 8800 ultramicrotome (LKB Instruments, Stockholm, Sweden) and collected on silane-coated microscope slides.
The immunohistochemical detection of glucuronoarabinoxylans and mixed-linkage glucans was performed using ABX, LM11 and BGI antibodies (Table 1). For immunolocalization, thin sections were (1) blocked with 0·1 m phosphate-buffered saline (PBS, pH 7·2) containing 3 % (w/v) bovine serum albumin (BSA) for 1 h at room temperature; (2) incubated with one of the primary monoclonal antibodies diluted 1 : 10 (LM11), 1 : 20 (BGI) or 1 : 3 (ABX) in PBS with 0·06 % (w/v) BSA for 1 h at room temperature; and (3) incubated with secondary anti-rat or anti-mouse IgG linked to fluorescein isothiocyanate (FITC, Sigma-Aldrich) diluted 1 : 100 in PBS with 0·06 % (w/v) BSA for 1 h at room temperature in the dark. The primary antibody treatment was omitted for the negative controls.
Table 1.
Antibodies used for immunohistochemical and immunodot analyses
| Antibody | Raised against | Target polysaccharide | Manufacturer, country | Reference |
|---|---|---|---|---|
| BGI | Mixed-linkage glucan oligosaccharides | Mixed-linkage glucan | Biosupplies Australia Pty Ltd, Australia | Meikle et al. (1994) |
| ABX (anti-AX1) | Arabinoxylan oligosaccharides | Arabinoxylan | INRA, France | Guillon et al. (2004) |
| LM11 | β-(1→4)-Xylopentaose | Xylan | University of Leeds, UK | McCartney et al. (2005) |
| LM15 | XXXG-motif of xyloglucan | Xyloglucan | University of Leeds, UK | Marcus et al. (2008) |
For the enzymatic pre-treatment, the sections were incubated with lichenase (mixed-linkage glucan-specific enzyme (Megazyme, Bray, Ireland) from Bacillus sp.) or xylanase (β-(1→4)-xylan-specific enzyme (Megazyme) from Neocallimastix patriciarum); 1–1·5 μl of commercial enzyme was diluted in 40 μl of buffer solution and applied to a slide with the root sections. The lichenase treatment was performed in sodium phosphate buffer (pH 6·5) for 1 h at 40 °C, while the xylanase treatment was performed in NaOAc buffer (pH 6·0), also for 1 h at 40 °C.
After the labelling reaction, the sections were washed three times in PBS, mounted in CFM-1 mountant solution (Electron Microscopic Sciences, Hatfield, PA, USA) and observed under a confocal laser scanning microscope (LSM-510, Zeiss, Oberkochen, Germany) using an excitation wavelength of 488 nm and detection wavelengths between 505 and 530 nm. The presented results are from one of five independent experiments with similar regularities. All of the images were recorded under similar instrument settings (laser intensities, pinhole sizes, gain settings and exposure times).
Cell wall fractionation procedure
The cut-offs of the roots were collected in tubes that were placed in liquid nitrogen, fixed by boiling in 70 % (v/v) ethanol for 10 min, dried at 60 °C in a flow of air and weighed. The hemicellulose fragments were obtained according to Kato and Nevins (1984a, b) with the modifications described by Kozlova (2012a, b). Briefly, the plant material was homogenized in a 0·3 % (w/v) solution of NaCl in 0·02 m sodium phosphate buffer (pH 6·5) and washed three times in the same solution. The precipitate was incubated in 3 m LiCl solution in 0·02 m NaOAc buffer (pH 6·0) at 4 °C for 48 h, washed, boiled in water and treated with α-amylase (Sigma-Aldrich). The residual material, which mainly consisted of cell walls, was incubated in 0·02 m sodium phosphate buffer [pH 6·5, with 0·02 % (w/v) NaN3] with 0·03 mg of lichenase per milligram of the material at 40 °C for 24 h. The mixed-linkage glucans that were extracted from the cell walls were quantified as the glucose that was obtained from the supernatant after hydrolysis and monosaccharide analysis.
For extraction of glucuronoarabinoxylan, the pellet remaining after lichenase treatment was incubated in 0·02 m NaOAc buffer [pH 6·0, 0·02 % (w/v) NaN3] with 0·02 mg of endo-xylanase per milligram of the material at 40 °C for 24 h. In some experiments (with the samples described in Fig. 1C), the carbohydrates that were released by xylanase were subjected to gel-filtration on a TSK-65F column (fractionation range for dextrans: 10–1000 kDa, Tosoh Bioscience, Tokyo, Japan) to separate the polymers (glucuronoarabinoxylan fragments that are extractable, but non-degradable, by xylanase) and low-molecular-mass products (glucuronoarabinoxylan fragments that are extractable and degradable by xylanase) (Kozlova et al., 2012b). The yield of high- and low-molecular-mass products was evaluated after the monosaccharide analysis as the total sugar content.
After the enzymatic digestion, the remaining material was treated with 4 m KOH with 3 % (w/v) H3BO3 at room temperature for 24 h to isolate the remaining portions of hemicelluloses. The obtained solution was desalted on a Sephadex G-25 column (10 × 300 mm, Pharmacia) and dried at 60 °C in a flow of air.
Analysis of monosaccharide composition of carbohydrates
Ten micrograms of analysed carbohydrates was incubated in 400 μL of 2 m trifluoroacetic acid (TFA) at 120 °C for 1 h. The hydrolysis products were analysed by anion-exchange high-performance liquid chromatogrpahy on a CarboPac PA-1 column (4 × 250 mm, Dionex, Sunnyvale, CA, USA) using a pulse amperometric detector (Dionex). The rate of elution was 1 ml min–1 with a column temperature of 30 °C. A gradient elution was conducted with buffer A (100 mm NaOH in 1 m NaOAc) and buffer B (15 mm NaOH) according to the following scheme: 0–20 min in 100 % B; 20–21 min in 90 % B and 10 % A; 22–41 min in 50 % B and 50 % A; 42–55 min in 100 % A; and 56–85 min in 100 % B. The results were analysed using PeakNet software according to the calibrations that were obtained for monosaccharide standards that were treated in advance with 2 m TFA at 120 °C for 1 h.
Immunodot analysis
The cell wall fractions obtained by xylanase digestion and KOH extraction were analysed (without the preliminary resolving using gel-permeation chromatography) in an immunodot assay with the BGI, ABX, LM11 and LM15 antibodies (Table 1). The analysed fractions in gradually decreasing concentrations were applied to nitrocellulose membranes (0·2 μm, Sigma-Aldrich). The membranes were allowed to air-dry for 30 min, washed for 5 min in PBST [PBS with 0·05 % (w/v) Triton X-100], blocked for 1 h with PBS containing 3 % (w/v) BSA (Sigma-Aldrich), and then incubated for 40 min with the primary monoclonal antibody. ABX, LM11 and LM15 were applied in a 1 : 40 dilution in PBST, and BGI was applied in a 1 : 1000 dilution. After incubation with the primary antibody, membranes were washed three times for 10 min with PBST and incubated with the secondary biotinylated antibody (Sigma-Aldrich) (anti-rat for LM11 and LM15 and anti-mouse for ABX and BGI) for 40 min. The membranes were washed again three times in PBST with 0·1 % (w/v) BSA for 10 min, incubated for 30 min in the streptavidin conjugated with the alkaline phosphatase diluted 1:3000 and developed with the nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate kit (Sileks, Moscow, Russia). Wheat flour arabinoxylan (Ara/Xyl = 0·6), barley flour mixed-linkage glucan, tamarind seeds xyloglucan (all from Megazyme) and KOH-extracted xylan from poplar normal wood (GlcA/Xyl = 0·1), which were obtained from other investigations in our laboratory from the samples provided by Prof. E. Mellerowicz (Umeå Plant Science Center, Sweden), were used as controls.
Statistical analysis
Results are presented as means with standard errors. Pearson's correlation coefficient was calculated for the relationships between the amounts of mixed-linkage glucan and of the two types of xylanase digestion products: polymeric and low-molecular-weight derivatives of glucuronoarabinoxylan. The significance of the correlation coefficient was determined using a t-test.
RESULTS
Mixed-linkage glucan in different zones of maize roots
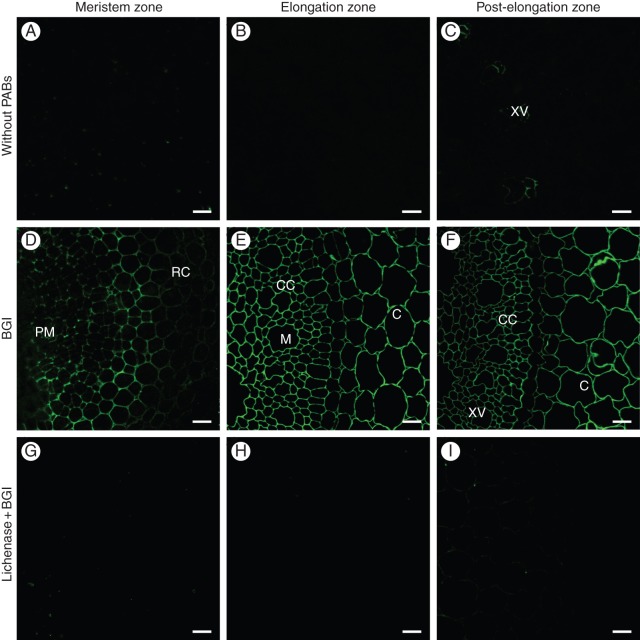
The distribution of mixed-linkage glucan polysaccharides in the cell walls of maize roots was determined using the BGI antibody. In the negative controls (without primary antibody), no fluorescence was detected in cross-sections of the meristem and elongation zones (Fig. 2A, B). In the post-elongation zone, slight highlighting of the xylem vessels was observed (Fig. 2C), probably due to autofluorescence of lignin. Among the types of polysaccharides that potentially could be present in the cell walls of maize roots and that were included in the panel of controls for the immunodot experiments (xylans with various degrees of branching, mixed-linkage glucan and xyloglucan), the BGI antibody bound strongly only to mixed-linkage glucan (Fig. 3C).
Fig. 2.
Indirect immunolabelling analysis of the BGI antibody binding to the cell walls in transverse sections of primary root zones in growing maize seedlings. Cross-sections of primary roots in the meristem (A, D, G), elongation (B, E, H) and post-elongation (C, F, I) zones. Imaging of root sections without treatment with primary antibody (A–C) after labelling with the BGI antibody (D–F) and after labelling with BGI antibody of the lichenase-pretreated sections (G–I). RC, root cap; PM, proximal meristem; M, metaxylem; CC, central cylinder; C, core; XV, xylem vessels. Scale bars = 20 μm.
Fig. 3.
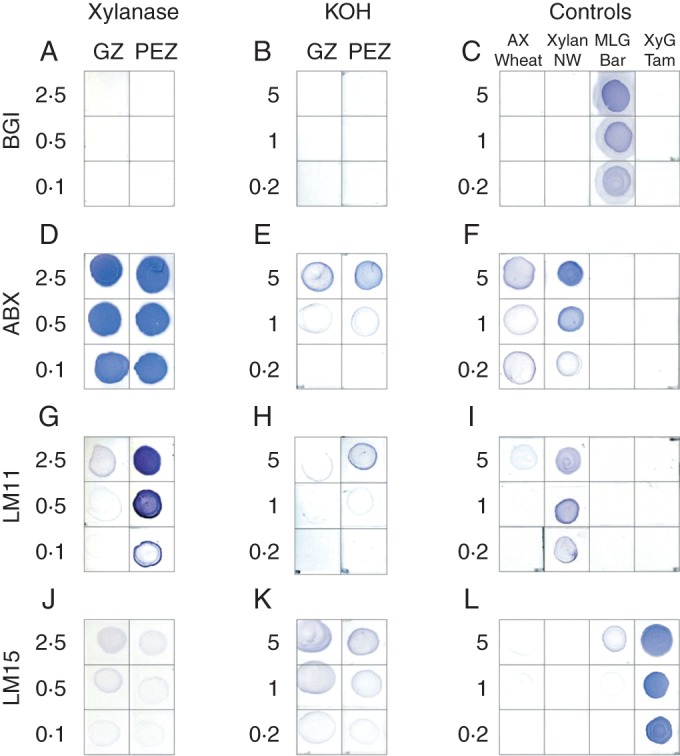
Immunodot analysis with the BGI (A–C), ABX (D–F), LM11 (G–I) and LM15 (J–L) antibodies of the cell wall fractions that were released from the lichenase-pretreated samples by successive xylanase digestion (A, D, G, J) and KOH extraction (B, E, H, K). The samples were collected from the growing (GZ) and post-elongation (PEZ) zones of the maize roots. The carbohydrates that were obtained by xylanase and KOH were not resolved by gel-permeation chromatography. Wheat flour arabinoxylan (AX Wheat), poplar normal wood xylan (Xylan NW), barley mixed-linkage glucan (MLG Bar) and tamarind seed xyloglucan (XyG Tam) were used as controls (C, F, I, L). The amount of carbohydrate in a row is designated at the left of each panel (in µg/dot). All of the immunodot analyses of a given antibody were performed on the same membrane and are artificially separated only for convenience.
BGI labelled cell walls in all of the analysed root zones (Fig. 2D–F); however, in the meristem region, fluorescence was weaker than in the elongation zone (Fig. 2D, E). No decrease in BGI labelling was observed in the post-elongation zone (Fig. 2E, F). These findings are in agreement with our previous results (Kozlova et al., 2012a). The lichenase treatment of root sections completely eliminated the detection of BGI epitopes (Fig. 2G–I). The overwhelming monosaccharide in the products of lichenase hydrolysis was glucose (Table 2). The fractions of cell wall polysaccharides that were obtained from the lichenase-treated root zones by the following incubation with xylanase and KOH extraction were completely free of the BGI epitope (Fig. 3A, B). Thus, the mixed-linkage glucan was completely removed from the cell walls by the lichenase treatment or remained there in the conformation and/or in an amount that was not appropriate for further lichenase digestion or for recognition by BGI.
Table 2.
Monosaccharide composition (mol%, mean ± s.d.) of different fractions of maize root cell walls
| Monosaccharide | Lichenase |
Xylanase |
KOH |
|||
|---|---|---|---|---|---|---|
| Growing zone | Post-elongation zone | Growing zone | Post-elongation zone | Growing zone | Post-elongation zone | |
| Rha | – | – | 2·0 ± 1·0 | 1·9 ± 0·6 | 0·7 ± 0·3 | 0·8 ± 0 |
| Ara | – | – | 27·9 ± 0·1 | 24·1 ± 0·6 | 20·9 ± 0·1 | 23·0 ± 0·3 |
| Gal | 1·4 ± 1·1 | – | 10·5 ± 0·1 | 14·4 ± 0·6 | 19·2 ± 0·4 | 18·9 ± 0·3 |
| Glc | 98·6 ± 1·1 | 100 ± 0 | 11·2 ± 0·2 | 9·5 ± 0·5 | 22·8 ± 0·3 | 18·2 ± 0·7 |
| Xyl | – | – | 44·8 ± 1·0 | 47·8 ± 0·5 | 34·5 ± 0·5 | 37·0 ± 0·2 |
| GalA | – | – | 1·5 ± 0·4 | 1·0 ± 0·1 | 0·8 ± 0·1 | 0·7 ± 0 |
| GlcA | – | – | 2·1 ± 0·3 | 1·3 ± 0·1 | 1·1 ± 0 | 1·4 ± 0·1 |
Different pattern of xylan recognition in maize root cell walls by ABX and LM11 antibodies
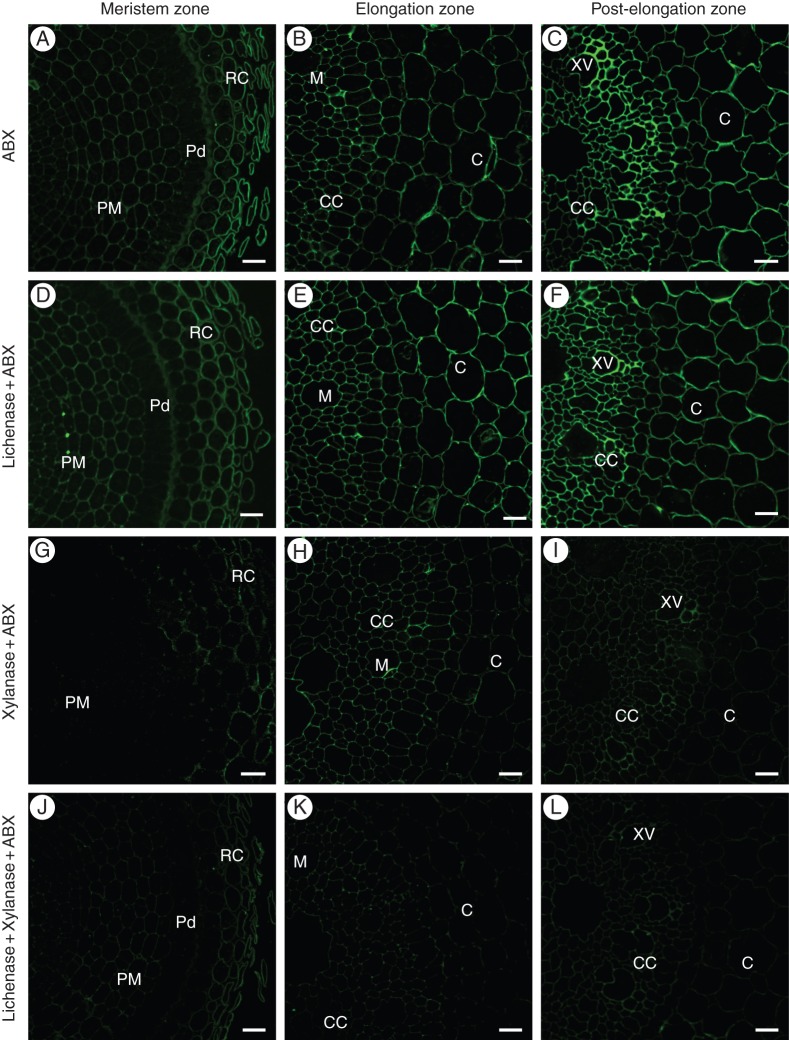
The ABX antibody was raised against grass arabinoxylans and recognizes both the arabinose-substituted fragments of the xylan backbone and the unsubstituted fragments (Guillon et al., 2004) (Table 1). This antibody labelled the cell walls in all of the analysed zones of the maize roots, with a slightly increasing intensity from the meristem to post-elongation zone (Fig. 4A–C). There was no visible variation in the labelling of the cell walls in the different tissues. The only exception was the radial walls of the protoderma cells in the meristem zone, which exhibited a much lower fluorescence (Fig. 4A). Lichenase pre-treatment of the root sections did not affect the labelling patterns. It neither unmasks the new epitopes nor removes the existing epitopes (Fig. 4D–F). Xylanase alone reduced the abundance of ABX labelling significantly but not completely (Fig. 4G–I). Consecutive double pre-treatment with lichenase and xylanase was more effective, but sections still contained some ABX epitopes (Fig. 4J–L).
Fig. 4.
Indirect immunolabelling analysis of ABX antibody binding to cell walls in the transverse sections of primary root zones in growing maize seedlings. Cross-sections of the primary root in the meristem (A, D, G, J), elongation (B, E, H, K) and post-elongation (C, F, I, L) zones. The labelling with ABX antibody (A–C), ABX antibody of the lichenase-pretreated sections (D–F), ABX antibody of the xylanase-pretreated sections (G–I) and ABX antibody of the sections that were successively pretreated with lichenase and xylanase (J–L). RC, root cap; PM, proximal meristem; Pd, protoderm; M, metaxylem; CC, central cylinder; C, core; XV, xylem vessels. Scale bars = 20 μm.
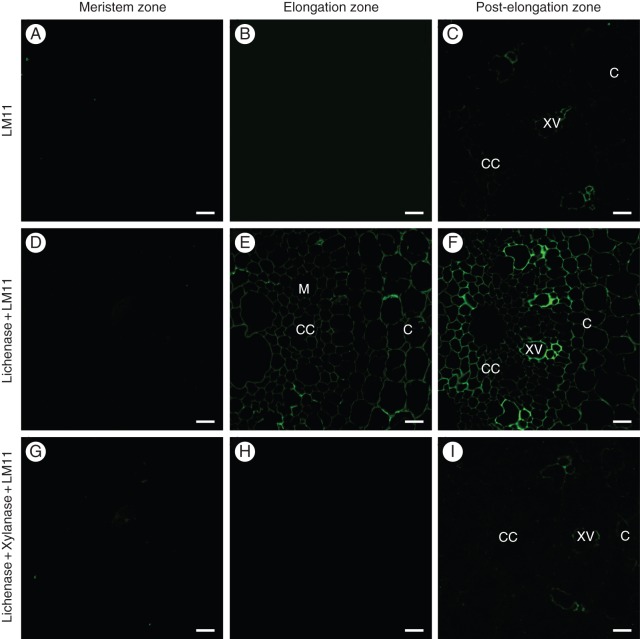
The LM11 antibody was raised against the xylopentaose (McCartney et al., 2005; Table 1), and thus has a stricter requirement than ABX for the xylan backbone to be unsubstituted. LM11 did not bind to the primary cell walls in the cross-sections of maize roots (Fig. 5A–C). The tenuous highlighting of the xylem vessels in the post-elongation zone probably results both from labelling of the secondary cell wall xylans and lignin autofluorescence (Fig. 5C). Labelling of the primary cell walls with LM11 was pronounced after lichenase treatment of the sections and was detected in the elongation (Fig. 5E) and post-elongation (Fig. 5F) zones. Binding of the antibody to the cell walls in the meristem zone remained negligible even after removal of mixed-linkage glucan from the section surface (Fig. 5D). The cell walls in the post-elongation zone were labelled more strongly than those in the elongation zone (Fig. 5E, F). There was no significant difference in labelling between the tissues. Subsequent xylanase treatment completely removed the LM11 epitopes from the cell walls in the lichenase-pretreated sections (Fig. 5G–I).
Fig. 5.
Indirect immunolabelling analysis of LM11 binding to the cell walls in the transverse sections of primary root zones in growing maize seedlings. Cross-sections of the primary root in the meristem (A, D, G), elongation (B, E, H) and post-elongation (C, F, I) zones. The labelling with LM11 antibody (A–C), LM11 antibody of the lichenase-pretreated sections (D–F), LM11 antibody of the sections that were successively pretreated with lichenase and xylanase (G–I). M, metaxylem; CC, central cylinder; C, core; XV, xylem vessels. Scale bar = 20 μm.
The xylanase treatment released carbohydrates that were very intensively labelled by ABX in the immunodot experiments, both in the growing and in the post-elongation zones (Fig. 3D). LM11 bound to the xylanase-released fraction from the post-elongation zone more strongly than that from the growing zone. The Ara/Xyl ratio was higher in the polymers that were released from the growing zone (Table 2). No BGI epitopes were detected in the fraction (Fig. 3A). Some xyloglucan was present, as indicated by faint labelling with LM15 (Fig. 3J) and a small amount of glucose (Table 2), in both the growing and the post-elongation zones.
Xylose was not completely removed from the cell walls of the maize roots by the xylanase treatment; some xylose remained and could be extracted by the KOH treatment (Table 2). The yield of xylose in this fraction constituted 11·5 ± 0·1 % of the cell walls in the growing zone and 13·0 ± 0·2 % in the post-elongation zone. Some of the xylose that remained after the xylanase hydrolysis belonged to xyloglucan, which was detected in the KOH fraction by the LM15 antibody (Fig. 3K). However, some xylans were also present, as indicated by binding of both the ABX and the LM11 antibodies. Therefore, xylan was not completely removed from the cell wall by the xylanase.
Relationship between the contents of the mixed-linkage glucan and of the different types of glucuronoarabinoxylan fragments that were released by xylanase
The carbohydrates that were released from the maize root cell wall by the xylanase were of two types: polymeric (fragments that are extractable, but non-degradable, by xylanase; Mr = 30–400 kDa) and low molecular mass (fragments that are extractable and degradable by xylanase; Mr < 10 kDa); polymeric fragments had higher degree of substitution (Ara/Xyl ≈ 0·7) than the low-molecular mass fragments (Ara/Xyl ≈ 0·4) (Kozlova et al., 2012b). We determined the relationship between the accumulation of the mixed-linkage glucan, that of the high- (polymeric) and that of the low-substituted (oligomeric) glucuronoarabinoxylans (Table 3), analysing five consecutive zones of maize roots (Fig. 1C). There was no significant correlation between the contents of mixed-linkage glucan and that of the polymeric products of xylanase digestion, while the correlation was very high with the amount of degradable fragments.
Table 3.
Correlation between contents of mixed-linkage glucan and different domains of glucuronoarabinoxylan during elongation growth of maize roots
| Cell wall component | Content in cell walls (%CW) |
Correlation coefficient, r | ||||
|---|---|---|---|---|---|---|
| Meristem | Early elongation | Elongation | Late elongation | Post-elongation | ||
| Mixed-linkage glucan | 0·80 ± 0·14 | 5·88 ± 0·68 | 12·61 ± 1·05 | 22·03 ± 1·71 | 22·64 ± 1·01 | – |
| Polymeric fragments of glucuronoarabinoxylan | 1·05 ± 0·01 | 2·16 ± 0·05 | 2·25 ± 0·04 | 2·37 ± 0·22 | 1·72 ± 0·06 | 0·525 |
| Low-molecular-mass fragments of glucuronoarabinoxylan | 0·03 ± 0·01 | 0·29 ± 0·03 | 0·79 ± 0·04 | 1·39 ± 0·06 | 1·51 ± 0·09 | 0·998 |
DISCUSSION
Mixed-linkage glucan arrangement in the cell walls of grasses
The arrangement and function of mixed-linkage glucan, one of the major constituents and quite specific component of the cell walls of grasses, has attracted particluar attention for many years. Originally, mixed-linkage glucan was suggested to participate in the cell wall architecture as an important cross-linking polysaccharide (Huber and Nevins, 1980; Carpita and Gibeaut, 1993). Later, based on the extractability of different cell wall components and the changes in the cell wall image under field-emission scanning electron microscopy, it was proposed that mixed-linkage glucans tightly coat rather than tether the cellulose microfibrils (Carpita et al., 2001). Given the structural properties of mixed-linkage glucans, Fincher (2009) proposed that mixed-linkage glucans constitute a gel-like matrix in the wall, occasionally forming junction zones with cellulose. Vega-Sánchez et al. (2012) analysed the glucan-deficient rice mutant cslf6. The absence of mixed-linkage glucans resulted in a significantly lower strain (deformation on breaking) of the stems, indicating the importance of these polysaccharides for cell wall flexibility. The authors inferred that mixed-linkage glucans did not act as the major load-bearing component and functioned more like a gel matrix. It has also been suggested that mixed-linkage glucans could be used as a sugar resource during growth (Vega-Sánchez et al., 2012).
The amount of mixed-linkage glucan is quite low in the meristem (Table 3, Fig. 2D). In the cells of the maize root quiescent centre, the BGI epitope was not revealed at all (Kozlova et al., 2012a). The accumulation of mixed-linkage glucan during cell growth was demonstrated biochemically (Carpita, 1984; Kim et al., 2000; Kozlova et al., 2012a) and immunocytochemically, both at the light (Fig. 2; Suzuki et al., 2002) and at the electron microscopy (Kozlova et al., 2012a) level, indicating the importance of mixed-linkage glucans for the elongation process.
The BGI epitope was not detected on root sections after lichenase treatment (Fig. 2); in the KOH fraction of the maize cell walls there was no labelling by BGI antibody in the immunodot experiments (Fig. 3B). The glucose that was present in the KOH fraction (Table 2) could be attributed to xyloglucan according to the immunodot analysis (Fig. 3K). We did not confirm the existence of the glycanhydrolase that is able to completely degrade the cellulose-bound polysaccharide. Some endoglycanases degrade only the polysaccharide parts which are not involved in the interaction with cellulose microfibrils, as was shown for the xyloglucan-degrading enzyme (Pauly et al., 1999). There is also an indication that the situation is similar for the secondary cell wall xylans and their accessibility to xylanase (Teleman et al., 2001). If the same is true for lichenase, all of the mixed-linkage glucan in growing maize roots is situated between the microfibrils and does not interact with cellulose.
Mixed-linkage glucan masks the epitopes of LM11 but not of ABX
In the maize root sections, the LM11 epitopes were masked by mixed-linkage glucan and were revealed only after lichenase treatment (Fig. 5), which was demonstrated to remove this polysaccharide (Table 2). By contrast, the ABX epitopes were present on root sections that were not treated by any enzyme; the presence of these epitopes was not affected by incubation with lichenase (Fig. 4), indicating that removal of the mixed-linkage glucan did not reveal any substantial amount of new ABX epitopes. The LM11 epitopes, which emerged after lichenase treatment, were removed from the walls by the xylanase (Fig. 5).
Masking of the epitopes in plant cell walls by the polysaccharides was previously reported only for polygalacturonic acid. The removal of polygalacturonic acid by a corresponding enzymatic treatment revealed the presence of abundant sets of xyloglucan in the type I cell walls of tamarind and nasturtium seeds (Marcus et al., 2008). A similar treatment of tobacco stem sections containing primary and secondary cell walls strongly increased the recognition of xylans (Hervé et al., 2009). The masking of mannan epitopes by polygalacturonic acid has been reported for the cell walls of a wide range of species (Marcus et al., 2010). The origin of masking has not been well researched, but the gel-like consistency seems to be important, as this effect was previously described only for polygalacturonic acid, the gelling properties of which are well known and widely used.
Domain structure of glucuronoarabinoxylan
Glucuronoarabinoxylan is the second main hemicellulose of cereal cell walls (Carpita and Gibeaut, 1993). In maize root polymer, similar to other cereal glucuronoarabinoxylans, arabinose is the main substituent and is attached predominantly to the O-3 or to both the O-3 and the O-2 positions of xylose residues; small amounts of glucuronic acid and acetyl group substitution are also present (Kozlova et al., 2012b). Domains with different degrees of substitution were described in the native glucuronoarabinoxylans of maize roots. The xylanase treatment released from the cell walls two types of products with different molecular masses and different degrees of xylose substitution by arabinose: polymeric fragments with an Ara/Xyl ratio of approx. 0·7 and low-molecular-mass products with an Ara/Xyl ratio of approx. 0·4 (Kozlova et al., 2012b). Besides, there is a portion of glucuronoarabinoxylan that is not extracted by the xylanase, as evidenced by the high xylose content (Table 2) and by the labelling with xylan-recognizing antibodies (Fig. 3E, H) of the fraction that were extracted by alkali from the cell wall residue obtained after enzymatic treatment. Different accessibility to the enzyme and different degrees of substitution in the different portions of the polysaccharide indicate the existence of the domains in the glucuronoarabinoxylan of maize roots.
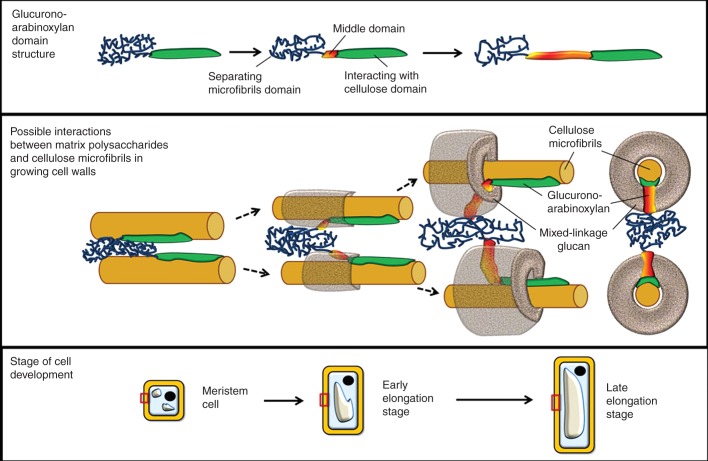
The domain that is released in polymeric form after the xylanase treatment is highly substituted. Such polymers are known to interact poorly with cellulose (Kabel et al., 2007), and these parts of glucuronoarabinoxylan molecules may be termed the separating cellulose microfibrils domain (Fig. 6). The presence of glucuronoarabinoxylan in the residue that remains after the xylanase treatment demonstrates that there is a domain inaccessible to the enzyme but extractable by KOH. Similar to the idea of the molecular domains of the cellulose/xyloglucan network in non-graminaceous cell walls (Pauly et al., 1999), we consider this domain to interact with the cellulose microfibrils and term it the interacting with cellulose domain of the glucuronoarabinoxylan (Fig. 6). Interaction with cellulose may change the conformation of the xylan backbone and make it inaccessible to the enzyme (Teleman et al., 2001).
Fig. 6.
Glucuronoarabinoxylan structure (top) and its arrangement in the cell walls in relation to mixed-linkage glucan and cellulose (middle) at different stages of cell development (bottom). The model does not quantitatively represent the mass ratio of different polymers or their domains. The microfibrils would be longer than is represented here.
The separating and interacting domains are linked by the low-substituted middle domain (Fig. 6), which is degraded by xylanase, making it possible for the separating domain to be released from the cell wall and indicating that the domains are present within the same backbone. The change in the proportion of high- and low-substituted domains in the course of elongation (Table 3) could cause the previously reported general decrease in the Ara/Xyl ratio (Carpita et al., 2001; Obel et al., 2002; Gibeaut et al., 2005). The domain-type structure of plant cell wall polysaccharides is continually receiving support; it was recently reported for glucuronoxylan from the secondary cell walls of Arabidopsis with regard to the patterns of backbone substitution by GlcA (Bromley et al., 2013).
Arrangement of glucuronoarabinoxylan domains and mixed-linkage glucan in the course of cell elongation
With regard to the arrangement of hemicelluloses in growing cells with type II primary cell walls, we consider the following. The amounts of mixed-linkage glucan and of glucuronoarabinoxylan increase significantly during the course of cell elongation (Gibeaut et al., 2005; Kozlova et al., 2012a, b). Mixed-linkage glucan is removed by lichenase completely or at least to levels undetectable by the applied methods (Figs 2 and 3B). The proportion of glucuronoarabixylan domains changes with an increase of cell size, so that the middle domain becomes more pronounced, beginning from virtually zero. The dynamics of middle domain accumulation is very similar to that of mixed-linkage glucan with a high correlation coefficient (Table 3). The molecular mass of the separating domain decreases during elongation growth (Kozlova et al., 2012b).
Mixed-linkage glucan masks the epitope of LM11, while labelling with ABX is not affected by lichenase treatment. The difference between these antibodies is their sensitivity to xylan substitution. The ABX antibody, which was raised specifically against the highly substituted xylans of grasses (Guillon et al., 2004), can bind the separating domain of maize root glucuronoarabinoxylan. LM11 labels such polymers rather poorly (Fig. 3I); it binds only to the middle domain of glucuronoarabinoxylan, as shown by the fact that the epitopes that were uncovered by the lichenase treatment are completely removed by xylanase (Fig. 5). Given the above considerations, it is the middle domain of GAX that is masked by mixed-linkage glucan and is revealed by lichenase treatment.
In summary, we suggest the following arrangement of cell wall polysaccharides in primary cell walls of maize roots (Fig. 6). In very young cells, both the middle domain of glucuronoarabinoxylan and mixed-linkage glucan are virtually absent. The cell wall design is based on cellulose microfibrils, which are kept away by the separating domain of glucuronoarabinoxylan molecules. The separating domain is connected to the middle domain, which is followed by the interacting domain. The interacting domain anchors the glucuronoarabinoxylan molecules in the cell walls via binding to the cellulose microfibrils. At the beginning of elongation, the middle domain appears between the separating and interacting-with-cellulose domains. The proportion of the middle domain increases with time. Concomitantly, mixed-linkage glucan is synthesized, filling the interface between the separating domain of glucuronoarabinoxylan and the cellulose microfibrils, coating the microfibrils and covering the middle domain. A further increase of the distance between the cellulose microfibrils is accompanied by an additional increase of the middle domain and a corresponding accumulation of mixed-linkage glucan (Fig. 6). The amount of mixed-linkage glucan is considerably higher than that of the glucuronoarabinoxylan middle domain (Table 3) because the polymer, while coating the microfibrils, fills a lot of space, while the middle domain only goes through this coat (Fig. 6). The gel-like behaviour of mixed-linkage glucan allows it to play a role as a filler separating cellulose microfibrils and to make the increase in cell size irreversible.
ACKNOWLEDGEMENTS
We would like to express our gratitude to Professor Barry McCleary (Megazyme) for providing lichenase, xylanase, wheat flour arabinoxylan, tamarind xyloglucan and barley mixed-linkage glucan; to Professor Paul Knox (University of Leeds, UK) for LM11 and LM15 antibodies; to Dr Fabienne Guillon (INRA, France) for ABX antibody; and to Professor Takahisa Hayashi (Tokyo University of Agriculture, Japan) for BGI antibody. We thank Dr Natalia Mokshina (Kazan Institute of Biochemistry and Biophysics, Russia) for precise depiction of our ideas. This work was partially supported by the Russian Foundation for Basic Research [grant numbers: 11-04-01016; 14-04-01002] and by a grant for support of Leading Scientific Schools of the Russian Federation [NSh-1890.2014.4].
LITERATURE CITED
- Anderson CT, Carroll A, Akhmetova L, Somerville C. Real-time imaging of cellulose reorientation during cell wall expansion in Arabidopsis roots. Plant Physiology. 2010;152:787–796. doi: 10.1104/pp.109.150128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemster G, Baskin TI. Analysis of cell division and elongation underlying the developmental acceleration of root growth in Arabidopsis thaliana. Plant Physiology. 1998;116:1515–1526. doi: 10.1104/pp.116.4.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromley JR, Busse-Wicher M, Tryfona T, et al. GUX1 and GUX2 glucuronyltransferases decorate distinct domains of glucuronoxylan with different substitution patterns. Plant Journal. 2013;74:423–434. doi: 10.1111/tpj.12135. [DOI] [PubMed] [Google Scholar]
- Carpita NC. Cell wall development in maize coleoptiles. Plant Physiology. 1984;76:205–212. doi: 10.1104/pp.76.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita NC, Gibeaut DM. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant Journal. 1993;3:1–30. doi: 10.1111/j.1365-313x.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Carpita NC, Defernez M, Findlay K, et al. Cell wall architecture of the elongating maize coleoptiles. Plant Physiology. 2001;127:551–565. [PMC free article] [PubMed] [Google Scholar]
- Cavalier DM, Lerouxel O, Neumetzler L, et al. Disrupting two Arabidopsis thaliana xylosyltransferase genes results in plants deficient in xyloglucan, a major primary cell wall component. Plant Cell. 2008;20:1519–1537. doi: 10.1105/tpc.108.059873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ. Assembly and enlargement of the primary cell wall in plants. Annual Review of Cell and Developmental Biology. 1997;13:171–201. doi: 10.1146/annurev.cellbio.13.1.171. [DOI] [PubMed] [Google Scholar]
- Dick-Perez M, Zhang Y, Hayes J, Salazar A, Zabotina OA, Hong M. Structure and interactions of plant cell-wall polysaccharides by two- and three-dimensional magic-angle-spinning solid-state NMR. Biochemistry. 2011;50:989–1000. doi: 10.1021/bi101795q. [DOI] [PubMed] [Google Scholar]
- Fincher GB. Exploring the evolution of (1,3;1,4)-β-d-glucans in plant cell walls: comparative genomics can help! Current Opinion in Plant Biology. 2009;12:140–147. doi: 10.1016/j.pbi.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Gibeaut DM, Pauly M, Bacic A, Fincher GB. Changes in cell wall polysaccharides in developing barley (Hordeum vulgare) coleoptiles. Planta. 2005;221:729–738. doi: 10.1007/s00425-005-1481-0. [DOI] [PubMed] [Google Scholar]
- Guillon F, Tranquet O, Quillien L, Utille J-P, Ordaz-Ortiz JJ, Saulnier L. Generation of polyclonal and monoclonal antibodies against arabinoxylans and their use for immunocytochemical location of arabinoxylans in cell walls of endosperm of wheat. Journal of Cereal Science. 2004;40:167–182. [Google Scholar]
- Hervé C, Rogowski A, Gilbert HJ, Knox JP. Enzymatic treatments reveal differential capacities for xylan recognition and degradation in primary and secondary plant cell walls. Plant Journal. 2009;58:413–422. doi: 10.1111/j.1365-313X.2009.03785.x. [DOI] [PubMed] [Google Scholar]
- Huber DJ, Nevins DJ. β-d-glucan hydrolase activity in Zea coleoptile cell wall. Plant Physiology. 1980;65:768–773. doi: 10.1104/pp.65.5.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov VB. Relationship between cell proliferation and transition to elongation in plant roots. Internetional Journal of Developmental Biology. 1997;41:907–915. [PubMed] [Google Scholar]
- Kabel MA, Borne H, Vincken JP, Voragen AGJ, Schols HA. Structural differences of xylans affect their interaction with cellulose. Carbohydrate Polymers. 2007;69:94–105. [Google Scholar]
- Kato Y, Nevins DJ. Enzymic dissociation of zea shoot cell wall polysaccharides II. Dissociation of (1→3), (1→4)-β-d-glucan by purified (1→3), (1→4)-β-d-glucan 4-glucanhydrolase from Bacillus subtilis. Plant Physiology. 1984a;75:745–752. doi: 10.1104/pp.75.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Nevins DJ. Enzymic dissociation of zea shoot cell wall polysaccharides III. Purification and partial characterization of an endo-(1→4)-β-d-xylanase from a Bacillus subtilis enzyme preparation. Plant Physiology. 1984b;75:753–758. doi: 10.1104/pp.75.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierzkowski D, Nakayama N, Routier-Kierzkowska A-L, et al. Elastic domains regulate growth and organogenesis in the plant shoot apical meristem. Science. 2012;335:1096–1099. doi: 10.1126/science.1213100. [DOI] [PubMed] [Google Scholar]
- Kim JB, Olek AT, Carpita NC. Cell wall and membrane-associated exo-β-d-glucanases from developing maize seedlings. Plant Physiology. 2000;123:471–485. doi: 10.1104/pp.123.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlova LV, Snegireva AV, Gorshkova TA. Distribution and structure of mixed linkage glucan at different stages of elongation of maize root cells. Russian Journal of Plant Physiology. 2012a;59:339–347. [Google Scholar]
- Kozlova LV, Mikshina PV, Gorshkova TA. Glucuronoarabinoxylan extracted by treatment with endoxylanase from different zones of growing maize root. Biochemistry (Moscow) 2012b;77:395–403. doi: 10.1134/S0006297912040116. [DOI] [PubMed] [Google Scholar]
- Kroeger JH, Zerzour R, Geitmann A. Regulator or driving force? The role of turgor pressure in oscillatory plant cell growth. PLoS ONE. 2011;6:e18549. doi: 10.1371/journal.pone.0018549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttenegger DG, Nevins DJ. Transient nature of a (1→3), (1→4)-β-d-glucan in Zea mays coleoptile cell walls. Plant Physiology. 1985;77:175–178. doi: 10.1104/pp.77.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus SE, Verhertbruggen Y, Hervé C, et al. Pectic homogalacturonan masks abundant sets of xyloglucan epitopes in plant cell walls. BMC Plant Biology. 2008;8:60. doi: 10.1186/1471-2229-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus SE, Blake AW, Benians TAS, et al. Restricted access of proteins to mannan polysaccharides in intact plant cell walls. Plant Journal. 2010;64:191–203. doi: 10.1111/j.1365-313X.2010.04319.x. [DOI] [PubMed] [Google Scholar]
- McCartney L, Marcus SE, Knox JP. Monoclonal antibodies to plant cell wall xylans and arabinoxylans. Journal of Histochemistry & Cytochemistry. 2005;53:543–546. doi: 10.1369/jhc.4B6578.2005. [DOI] [PubMed] [Google Scholar]
- McQueen-Mason S, Durachko DM, Cosgrove DJ. Two endogenous proteins that induce cell wall expansion in plants. Plant Cell. 1992;4:1425–1433. doi: 10.1105/tpc.4.11.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikle PJ, Hoogenraad NJ, Bonig I, Clarke AE, Stone BA. A (1→3,1→4)-β-glucan-specific monoclonal antibody and its use in the quantitation and immunocytochemical location of (1→3,1→4)-β-glucans. Plant Journal. 1994;5:1–9. doi: 10.1046/j.1365-313x.1994.5010001.x. [DOI] [PubMed] [Google Scholar]
- Obel N, Porchia AC, Scheller HV. Dynamic changes in cell wall polysaccharides during wheat seedling development. Phytochemistry. 2002;60:603–610. doi: 10.1016/s0031-9422(02)00148-6. [DOI] [PubMed] [Google Scholar]
- Park YB, Cosgrove DJ. A revised architecture of primary cell walls based on biomechanical changes induced by substrate-specific endoglucanases. Plant Physiology. 2012;158:1933–1943. doi: 10.1104/pp.111.192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly M, Albersheim P, Darvill A, York WS. Molecular domains of the cellulose/xyloglucan network in the cell walls of higher plants. Plant Journal. 1999;20:629–639. doi: 10.1046/j.1365-313x.1999.00630.x. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Kitamura S, Sone Y, Itoh T. Immunohistochemical localization of hemicelluloses and pectins varies during tissue development in the bamboo culm. Histochemical Journal. 2002;34:535–544. doi: 10.1023/a:1026064816129. [DOI] [PubMed] [Google Scholar]
- Tabuchi A, Li L-C, Cosgrove DJ. Matrix solubilization and cell wall weakening by β-expansin (group-1 allergen) from maize pollen. Plant Journal. 2011;68:546–559. doi: 10.1111/j.1365-313X.2011.04705.x. [DOI] [PubMed] [Google Scholar]
- Teleman A, Larsson PT, Iversen T. On the accessibility and structure of xylan in birch kraft pulp. Cellulose. 2001;8:209–215. [Google Scholar]
- Tomos AD, Pritchard J. Biophysics and biochemistry of single-cell extension growth in roots and leaves. Journal of Experimental Botany. 1994;45:1721–1731. [Google Scholar]
- Trethewey JAK, Harris PJ. Location of (1→3)- and (1→3), (1→4)-β-d-glucans in vegetative cell walls of barley (Hordeum vulgare) using immunogold labeling. New Phytologist. 2002;154:347–358. doi: 10.1046/j.1469-8137.2002.00383.x. [DOI] [PubMed] [Google Scholar]
- Trethewey JAK, Campbell LM, Harris PJ. (1→3), (1→4)-β-d-Glucans in the cell walls of the Poales (sensu lato): an immunogold labeling study using a monoclonal antibody. American Journal of Botany. 2005;92:1660–1674. doi: 10.3732/ajb.92.10.1660. [DOI] [PubMed] [Google Scholar]
- Vega-Sánchez ME, Verhertbruggen Y, Christensen U, Chen X, Sharma V, Varanasi P. Loss of cellulose synthase-like F6 function affects mixed-linkage glucan deposition, cell wall mechanical properties, and defense responses in vegetative tissues of rice. Plant Physiology. 2012;159:56–69. doi: 10.1104/pp.112.195495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Sánchez ME, Verhertbruggen Y, Scheller HV, Ronald PC. Abundance of mixed linkage glucan in mature tissues and secondary cell walls of grasses. Plant Signaling & Behavior. 2013;8:e23143. doi: 10.4161/psb.23143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willats WGT, McCartney L, Steele-King CG, et al. A xylogalacturonan epitope is specifically associated with plant cell detachment. Planta. 2004;218:673–681. doi: 10.1007/s00425-003-1147-8. [DOI] [PubMed] [Google Scholar]